Abstract
Objective
The concepts of mild cognitive impairment (MCI) and subjective cognitive decline (SCD) have been proposed to identify individuals in the early stages of Alzheimer’s disease (AD), or other neurodegenerative diseases. One approach to validate these concepts is to investigate the relationship between pathological brain markers and cognition in those individuals.
Method
We included 126 participants from the Consortium for the Early Identification of Alzheimer’s disease-Quebec (CIMA-Q) cohort (67 SCD, 29 MCI, and 30 cognitively healthy controls [CH]). All participants underwent a complete cognitive assessment and structural magnetic resonance imaging. Group comparisons were done using cognitive data, and then correlated with hippocampal volumes and white matter hyperintensities (WMHs).
Results
Significant differences were found between participants with MCI and CH on episodic and executive tasks, but no differences were found when comparing SCD and CH. Scores on episodic memory tests correlated with hippocampal volumes in both MCI and SCD, whereas performance on executive tests correlated with WMH in all of our groups.
Discussion
As expected, the SCD group was shown to be cognitively healthy on tasks where MCI participants showed impairment. However, SCD’s hippocampal volume related to episodic memory performances, and WMH to executive functions. Thus, SCD represents a valid research concept and should be used, alongside MCI, to better understand the preclinical/prodromal phase of AD.
Keywords: Alzheimer’s disease, Biomarkers, Neuroimaging, Neuropsychology
In spite of major research effort, the exact pathophysiological events leading to Alzheimer’s disease (AD) remain largely unexplained. Yet, one major research advance over the last years was to show that the pathological cascade leading to AD most likely begins decades before the earliest clinical symptoms occur (Bateman et al., 2012; Iturria-Medina et al., 2016; Leuzy, Heurling, Ashton, Schöll, & Zimmer, 2018; Price & Morris, 1999). It is generally believed that a better understanding of the earlier phases of AD will contribute to identifying causal mechanisms and eventually develop effective disease-modifying therapies.
Many authors have described a cognitive continuum from being without symptoms, to expressing a subjective complaint, to being objectively impaired, all in parallel with an accumulation of neuropathological markers and symptoms in a continuum from asymptomatic AD to dementia (Albert et al., 2011; Jack et al., 2010; Sperling et al., 2011). The term mild cognitive impairment (MCI) has been proposed to represent the stage on this continuum when cognitive impairments are present, but not sufficiently severe for the person to meet criteria for AD or other neurodegenerative diseases (Albert et al., 2011; Petersen et al., 1999). More recently, it has been proposed that a subjective cognitive decline (SCD) stage extends the spectrum of AD to an earlier phase than that of MCI (Cheng, Chen, & Chiu, 2017; Jessen, Wolfsgruber, et al., 2014). SCD might also represent a very early phase of other neurodegenerative diseases. Persons with SCD complain about their memory but present scores within the normal range on standardized neuropsychological tests. It has been shown that a significant proportion of those individuals will later develop MCI and AD dementia, particularly if they display biomarker evidence of AD (Eckerström et al., 2017) or express worry about this perceived change in cognitive ability in addition to their memory complaints (Jessen, Amariglio, et al., 2014; Reisberg, Shulman, Torossian, Leng, & Zhu, 2010). Hence, SCD might be considered as an interesting construct to study the first clinico-pathological manifestations of AD (Dubois et al., 2016; Sperling et al., 2011). However, it is a new classification and remains to be better understood and validated. In particular, it might prove useful to compare persons with SCD with those meeting criteria for MCI as the latter is now fairly well characterized. Furthermore, SCD, and to a lesser extent MCI, both constitute heterogeneous groups (Mendonça, Alves, & Bugalho, 2016). Determining that there are coherent relationships between neural markers of age-related neurodegenerative diseases and cognition would support these concepts as early manifestations of AD neurodegeneration, and possibly contribute to defining a pattern of brain–cognition relationships within these groups.
Hippocampal atrophy, the degree of which can be measured with anatomical magnetic resonance imaging (MRI), is often used as a biomarker of neurodegeneration in prodromal AD. Indeed, the medial temporal lobe, which includes the hippocampus and surrounding cortices, is known to be one of the earliest sites of pathological changes in AD (Price et al., 2001). MRI studies have highlighted a reduction of hippocampal volume in MCI (Du et al., 2001; van de Pol et al., 2007). Several studies also demonstrated that SCD is associated with longitudinal hippocampal atrophy (Cherbuin, Sargent-Cox, Easteal, Sachdev, & Anstey, 2015; Perrotin et al., 2015). As the hippocampus plays an important role in episodic memory (Squire & Zola-Morgan, 1991), it is expected that in the preclinical AD phase, inter-individual variability in memory performance would be related to anatomical modifications associated with hippocampal volume loss, even if the measured memory changes remain within the range of normality. For instance, Cantero, Iglesias, Van Leemput, and Atienza (2016) and Flier and coworkers (2004) both reported an association between lower volumes in specific hippocampal regions and cognitive vulnerability in SCD. However, both studies were done with relatively small samples, 48 in the former and 20 self-referred memory complainers from hospital outpatient services in the latter.
In addition, white matter hyperintensities (WMHs) represent a common feature in the typical elderly population (Birdsill et al., 2014), but also in patients with a stroke and in pathological aging (Wardlaw, Smith, & Dichgans, 2013). WMH would reflect microstructural damages to the white matter that are typically localized around the ventricles or in the deep white matter (DeCarli, Fletcher, Ramey, Harvey, & Jagust, 2005). It is generally believed that WMHs signal non-AD-related small-vessel diseases as they are associated with vascular risk factors and vascular diseases. However, a large number of recent studies suggest that WMH may play a role in early AD (for review, see Radanovic et al., 2013) because they frequently co-occur with the AD pathology. WMH might also contribute to dementia appearance and symptomatology, considering that there is an accelerating cognitive decline in persons with MCI who present with this marker (Wolf, Ecke, Bettin, Dietrich, & Gertz, 2000). For these reasons, WMHs are often studied in early AD (Birdsill et al., 2014; Liu, Braidy, Poljak, Chan, & Sachdev, 2018), over and above their possible association with non-AD pathologies (Wardlaw et al., 2013). Despite a growing number of studies on WMHs in AD and MCI (Hirono, Kitagaki, Kazui, Hashimoto, & Mori, 2000; Jorm et al., 2004; van Rooden et al., 2018), their effect on the cognition of persons with SCD remains unclear. Most studies have reported that a larger volume of WMH is related to executive functioning deficits (de Groot et al., 2001; Villeneuve & Belleville, 2012), while some point to a link between WMHs and memory abilities (Li et al., 2016; van Rooden et al., 2018). Thus, it is important to include WMH in studies identifying the relation between cognition and MRI brain markers in SCD and MCI.
The goal of the present study is to investigate the relationship between episodic memory, executive functions, and MRI markers of neurodegeneration defined by hippocampal volume and vascular integrity in SCD and MCI. More precisely, we predicted that in these two groups, memory performance would be specifically related to hippocampal volume and that executive function performance would be specifically related to WMH.
Method
Participants
This study relied on the data collected by the Consortium pour l’identification précoce de la maladie d’Alzheimer – Québec (CIMA-Q – Quebec Consortium for the Early Identification of Alzheimer’s disease). Participants in the CIMA-Q cohort lived in the community and were recruited from different sources such as memory clinics, advertisements in electronic media, and ads posted in the community. Participants for this study were recruited from the cities of Montréal, Sherbrooke, and Québec City. The study has received approval from the coordinating ethic committee of the IUGM. Participants gave their written informed consent before enrollment in the study, and for the different components of the project.
Inclusion/Exclusion Criteria
CIMA-Q participants were at least 65 years old and native French or English speakers to represent the Canadian bilingual context. The CIMA-Q study recruited individuals along the spectrum of AD, from cognitively healthy controls (CH), to SCD, MCI, and clinically probable AD.
Participants met criteria for MCI based on the National Institute on Aging and the Alzheimer’s Association (NIA-AA) clinical criteria for MCI (Albert et al., 2011): (a) a reported decline of their memory; (b) an objective memory impairment according to education-adjusted normative values on the Logical Memory II subtest of the Wechsler Memory Scale (WMS III; Weschler, 1997), that is, a score between 2 and 6 for 0–7 years of education, 4 and 9 for 8–15 years of education, or 8 and 11 for ≥16 years of education; (c) a score between 20 and 26 on the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005); and (d) a score of 0.5 on the Clinical Dementia Rating Scale.
The criteria for cognitively healthy participants were: (a) no memory complaint or worry; (b) a normal score on education-adjusted normative values on the WMS-III Logical Memory II subtest, that is, a score of >3 for 0–7 years of education, >5 for 8–15 years of education, or >9 for ≥16 years of education; (c) a performance above 26/30 on the MoCA; and (d) a score of 0 on the Clinical Dementia Rating Scale (Morris, 1993).
Participants with SCD had to have scores within the same normality range on the Logical Memory II subtest, MoCA, and Clinical Dementia Rating Scale (see above for CH), but had to have reported that their memory was not as good as it used to be and that it worried them.
Other inclusion and all exclusion criteria (e.g., for AD participants) are listed in Supplementary Material S1. The CIMA-Q cohort is constructed to reflect the type of patients encountered in a typical clinical setting. We thus restricted the number of exclusion criteria based on co-morbidities to a minimum (see list of exclusions) as long as participants met the accepted parameters indicated by the diagnostic criteria.
Design
CIMA-Q developed a large-scale, multicenter, longitudinal protocol, which includes standardized neuroimaging, cognitive assessment (for additional information, see website, http://www.cima-q.ca/, and design paper, Belleville et al., under review), and biological sampling with training and quality control procedures. All material was prepared in French and English and when possible, cognitive tests were selected or developed to have equivalent versions in both languages. The first wave of enrollment and testing was done between 2015 and 2017 and included 290 participants in total. A telephone pre-screening interview was first conducted and the Telephone – Mini-Mental State Examination (t-MMSE; Newkirk et al., 2004) was administered to review exclusion/inclusion criteria. Then, participants were invited to a clinical diagnosis assessment completed by a nurse and a physician (via standardized checklists using accepted criteria). They then came for a session where they received neuropsychological testing. MRI-compatible participants were also invited to participate in a third session for MRI examination. For this study, we present data from the 126 non-demented participants (67 SCD, 29 MCI, and 30 CH) who completed the cognitive test battery, as well as the anatomical MRI, of the first CIMA-Q wave.
Cognitive Assessment of Episodic Memory and Executive Function
The study was designed to reduce issues of circularity, as we used tasks that were different for inclusion criteria from those used to assess our hypotheses. Two tasks from the CIMA-Q battery were used to assess the relationship between episodic memory and hippocampal volume. To reduce the number of comparisons, we used delayed word recall and Face-Name association, two tasks that were reported to be among the most sensitive predictors of progression from MCI to dementia based on a meta-analysis of prospective predictive studies (Belleville, Fouquet, Duchesne, Collins, & Hudon, 2014). The Memoria Word Recall test (Chatelois et al., 1993) is a 15-word memory task measured with free and cued recall. The Face-Name test (Brambati, for CIMA-Q) is an associative memory test where participants learn the association between a face and a first name (see Figure 1) and are then asked for immediate and delayed recall of the name associated with each face. We analyzed the free recall portion of the word recall and the delayed portion of the Face-Name association task. In both cases, larger scores represent better performance.
Figure 1.
Face-Name task (Brambati, for CIMA-Q).
Three tasks examined the relationship between executive functions and WMHs. First, we used the Semantic Fluency test, which reflects semantic integrity while imposing demands upon executive control processes, as it requires lexical search, effortful retrieval, attention-shifting, and sequencing (Belleville et al., 2017; Zhao, Guo, & Hong, 2013). In this task, participants are asked to give as many animal names as possible within 60 s. A larger score represents better performance. The ratio between completion time of Trail B and Trail A was used as a measure of reactive flexibility (Strauss, Sherman, Spreen, & Spreen, 2006). Here, a larger score represents poorer performance. Finally, a computerized version of the Hayling test (Bélanger & Belleville, 2009; Belleville, Rouleau, & Van der Linden, 2006; from the original Hayling test Burgess & Shallice, 1997) was used to assess inhibition (inhibition score). In this test, participants are presented with sentences that are missing the last word and are asked to complete it with an unrelated word. A larger inhibition score represents better performance.
Anatomical MRI
Brain imaging followed the standardized Canadian Dementia Imaging Protocol (https://www.cdip-pcid.ca/), which includes T1-weighted, PD-T2-weighted, T2*, FLAIR, 30-direction diffusion, and T2*-weighted gradient-echo EPI at rest acquisitions. As this is a multicentric cohort, different MRI apparatuses were used from GE Medical Systems (Discovery), Philips Healthcare (Achieva; Ingenia), and Siemens Healthcare (Tim Trio; Prisma). Each scanner used a 20-channel head coil. The parameters for the different sequences were harmonized across MRI models to reduce variability and increase comparability (for details, see design paper, Duchesne et al., (2019), and Supplementary Table S1). For the purposes of this study, we specifically analyzed the T1-weighted and FLAIR acquisitions.
Image Processing and Analysis
Image preprocessing included magnetic field-related signal inhomogeneity correction and linear affine registration of FLAIR to the T1-weighted sequence. Hippocampal segmentation was performed using the FreeSurfer 5.3.0 software (http://surfer.nmr.mgh.harvard.edu/), a procedure fully described in Fischl and coworkers (2004). Once segmented, visual quality control was used to determine segmentation accuracy. The remaining raw left and right hippocampal volumes in standardized space were transformed into z-scores according to normative data adjusting for age, sex, estimated total intracranial volume, scanner type, and scanner strength (Potvin, Mouiha, Dieumegarde, & Duchesne, 2016). As for WMH analysis, neuroimaging data processing was performed on FLAIR sequences using the volBrain pipeline (Manjón & Coupé, 2016), a method available through the volBrain online web interface (http://volbrain.upv.es). To correct for head size difference between each subject, total WMH volume was expressed as a percentage of the intracranial cavity volume. A more general measure of the vascular burden was also proposed through the Hachinski scale (Hachinski, Lassen, & Marshall, 1974), providing an ischemia score and differentiating neurodegenerative dementia from vascular dementia using only clinical data.
Behavioral and MRI Comparisons and Multimodal Analyses
Group comparisons of clinical, behavioral, and brain measures, as well as multimodal analyses, were done with the Statistical Package for the Social Sciences (IBM-SPSS Statistics, version 25). Group comparisons were done for demographic values and hippocampal measures using analyses of variance (ANOVAs) for continuous variables or chi-squared analyses for discrete ones. Behavioral performances for each task and WMH were analyzed with separate analyses of covariance (ANCOVAs) using Group (MCI, SCD, and CH) as a between-subject factor and controlling for age. When there was a Group main effect, Bonferroni’s post hoc analyses were conducted to locate the source of the group difference. In order to assess the relationship between cognition and MRI brain markers, we computed Pearson correlations (one-tailed due to a priori hypotheses on the directionality of the effect) between performance on cognitive tests and brain markers separately for each group. We focused our interest on the correlations between memory and hippocampal volumes, and between executive functions and WMH. We also analyzed the correlation between memory and WMH, and between executive functions and hippocampal volumes, to be sure that the first set of relationships were specific and not reflecting some general pattern of poor brain integrity being associated with poorer cognition. To compare the relative contribution of MRI markers, these were entered in a multiple linear regression model with backward elimination of nonsignificant variables as predictors of cognition.
Results
Demographic Data, Cognitive Performance, and MRI Measures
Demographic data, cognitive performances, and MRI measures are presented in Table 1. The groups were comparable with regards to sex and years of education. However, participants with MCI were slightly older than CH and SCD, therefore age was controlled for in our analyses of behavioral performance. Individuals with SCD were unimpaired on measures of episodic memory and executive function relative to CH, whereas MCI differed from CH on both memory tasks (Memoria free recall, p < .05; Face-Name delayed recall, p < .05), and on two measures of executive functions (Trail B/A, p < .05; Semantic Fluency, p < .05). MCI also showed lower performance than SCD on memory and executive tasks, p <.05, with the exception of the Face-Name and Hayling tasks. The group-wise analysis of MRI measures highlighted lower left and right hippocampal volume in MCI than in SCD and CH (left: p ≤ .001 and right: p < .05), but no significant difference between SCD and CH. As for WMH, there was no significant difference between groups, but MCI and CH differed on the Hachinski score (p < .05).
Table 1.
Demographic, WMH, and Hippocampal Data, and Performance on Selected Neuropsychological Measures of Participants With Subjective Cognitive Decline, Mild Cognitive Impairment, and Cognitively Healthy Controls
| P values | ||||||
|---|---|---|---|---|---|---|
| CH | SCD | MCI | SCD vs. CH | MCI vs. CH | SCD vs. MCI | |
| N | 30 | 67 | 29 | |||
| Sex (Male/Female) | 9/21 | 25/42 | 14/15 | ns | ns | ns |
| Age | 71.9 ± 5.7 | 72.3 ± 5.1 | 76.3 ± 5.3 | 1.000 | 0.005* | 0.003* |
| Education (Years) | 16.1 ± 3.8 | 15.2 ± 3.2 | 15.0 ± 3.0a | ns | ns | ns |
| WMH (%) | 0.2 ± 0.1 | 0.3 ± 0.3 | 0.4 ± 0.3 | 0.334 | 0.101 | 1.000 |
| Left hippocampal volume (z-score) | -0.2 ± 0.9 | -0.2 ± 1.1 | -1.3 ± 1.3 | 1.000 | < 0.001** | < 0.001** |
| Right hippocampal volume (z-score) | -0.03 ± 1.0 | -0.2 ± 1.2 | -1.1 ± 1.3 | 1.000 | 0.002* | 0.002* |
| Hachinski | 0.6 ± 1.0 | 1.1 ± 1.2a | 1.5 ± 1.6 | 0.260 | 0.022* | 0.420 |
| MoCA | 28.5 ± 1.4 | 27.9 ± 1.3 | 24.8 ± 2.1 | 0.364 | < 0.001** | < 0.001** |
| MMSE | 25.2 ± 1.0 | 24.4 ± 1.9 | 24.3 ± 1.6 | ns | ns | ns |
| Logical Memory | 14.7 ± 4.6 | 13.4 ± 4.4 | 9.6 ± 4.3 | 0.528 | < 0.001** | 0.001* |
| Memoria free word recall (/15) | 13.8 ± 1.4 | 13.0 ± 1.9 | 11.3 ± 3.9 | 0.523 | 0.004* | 0.033* |
| Face-Name delayed recall (/9) | 5.4 ± 1.7 | 4.4 ± 2.4b | 2.8 ± 2.3 | 0.151 | 0.002* | 0.099 |
| Hayling inhibition test (/30) | 18.6 ± 4.1c | 19.9 ± 4.9d | 19.0 ± 4.9d | ns | ns | ns |
| Semantic Fluency test | 21.1 ± 5.0 | 19.5 ± 4.7 | 16.4 ± 4.0 | 0.373 | 0.003* | 0.039* |
| Trail test Time B/ Time A | 1.9 ± 0.6 | 2.0 ± 0.7 | 2.8 ± 1.9 | 1.000 | 0.013* | 0.007* |
Note: CH = cognitively healthy; ns = nonsignificant analysis before post hoc comparisons; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; MoCA = Montréal Cognitive Assessment; SCD = subjective cognitive decline; WMH = white matter hyperintensities. Unless otherwise indicated, values are mean ± SD. p Values refer to significant analysis of variance (demographic and imaging data) and analysis of covariance models (neuropsychological tests controlled for age), followed by post hoc pairwise comparisons with Bonferroni correction.
aData missing for one subject. bData missing for two subjects. cData missing for three subjects. dData missing for five subjects.
*p < .05. **p < .001.
Multimodal Analyses
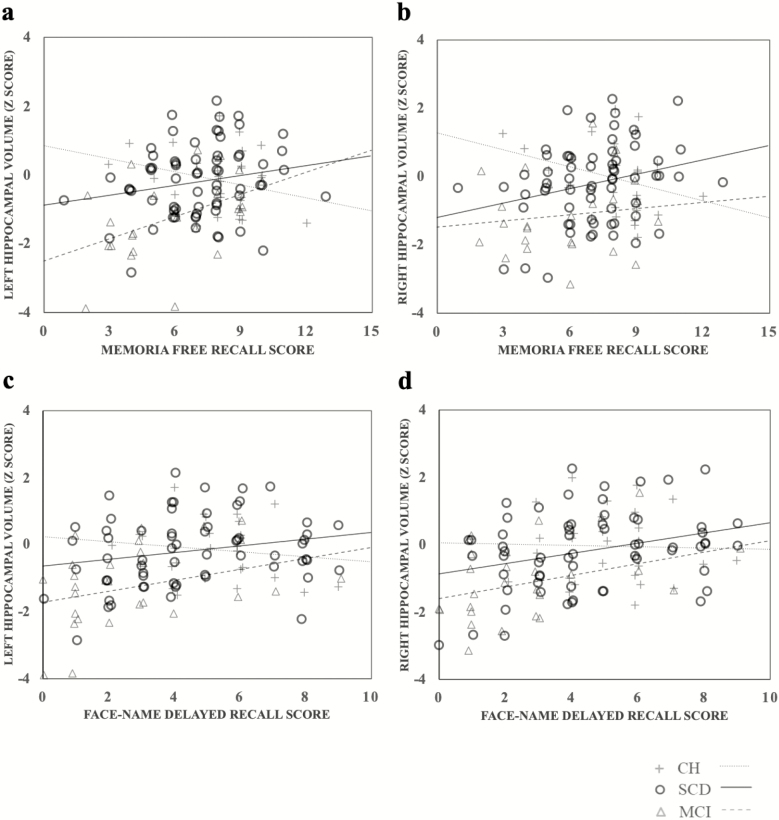
After false discovery rate control, Pearson tests (see Table 2 for details) revealed significant correlations between memory performance and hippocampal volumes (see Figure 2). More precisely, in MCI, smaller left hippocampal volume was associated with lower performance on the Memoria free recall (r = .427; p = .010) and lower delayed face-name association, just short of significance (r = .308; p = .052). In SCD, a smaller right hippocampal volume was associated with lower Memoria free recall (r = .267; p = .015) and lower delayed performance in Face-Name association (r = .319; p = .005), and smaller left hippocampal volume was associated with lower performance on the Memoria free recall just short of significance (r = .202; p = .052). In CH, lower performance on the Memoria free recall was associated with bigger left hippocampal volume (r = −.314; p = .049). Importantly, hippocampal volumes were not associated with performance on executive measures.
Table 2.
Pearson Correlations (one-tailed significance) Between Performance on Cognitive Tests and Brain Markers Separately for Each Group
| Memoria | Face-Name | Hayling | Fluency | Trail | ||||
|---|---|---|---|---|---|---|---|---|
| L-Hcp | R-Hcp | L-Hcp | R-Hcp | WMH | ||||
| CH | Pearson | −0.281 | −0.314* | −0.144 | −0.032 | 0.183 | 0.175 | 0.518* |
| p values | 0.070 | 0.049 | 0.228 | 0.870 | 0.186 | 0,183 | 0,002 | |
| SCD | Pearson | 0.202t | 0.267* | 0.228* | 0.319* | −0.301* | −0.257* | 0.181 |
| p values | 0.052 | 0.016 | 0.034 | 0.005 | 0,009 | 0.018 | 0.071 | |
| MCI | Pearson | 0.427* | 0.125 | 0.292 | 0.308t | −0.520* | −0.269 | 0.436* |
| p values | 0.010 | 0.258 | 0.062 | 0.052 | 0.007 | 0.092 | 0.013 | |
Note: Pearson correlation r scores and p values. CH = cognitively healthy; L-Hcp = left hippocampus; MCI = mild cognitive impairment; R-Hcp = right hippocampus; SCD = subjective cognitive decline; WMH = white matter hyperintensities.
*p < .05. tp = .052.
Figure 2.
Scatter plots and lines of best fit for the relation between (a) left hippocampal volume and Memoria free recall score; (b) right hippocampal volume and memoria free recall score; (c) left hippocampal volume and Face-Name recall score; and (d) right hippocampal volume and Face-Name recall score. CH = cognitively healthy; MCI = mild cognitive impairment; SCD = subjective cognitive decline.
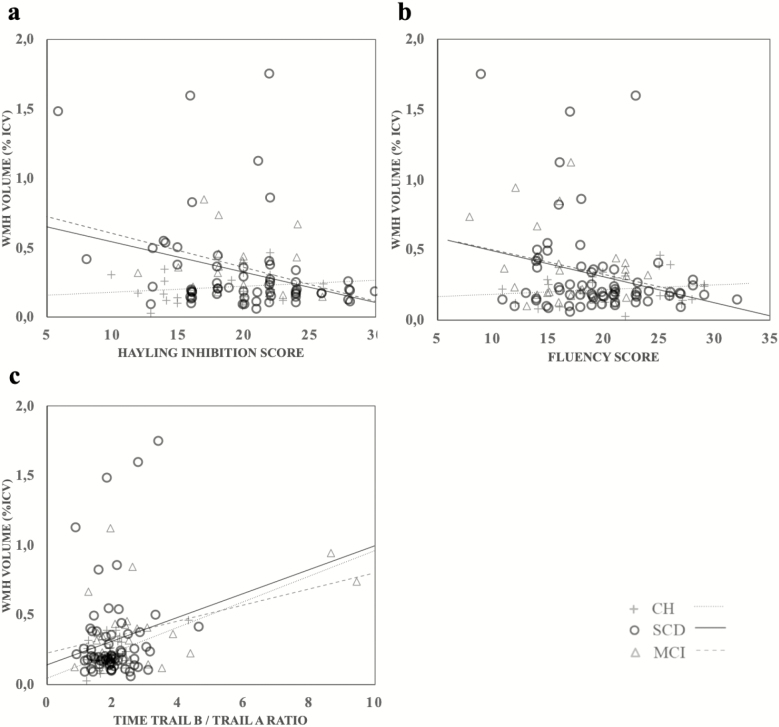
There were significant negative correlations between WMH and executive functions (see Figure 3). In the MCI group, larger volumes of WMH were associated with lower Hayling scores (r = −.520; p = .007), and with a higher ratio between completion time of Trail B/Trail A (r = .436; p = .013). In SCD individuals, larger volumes of WMH were associated with lower Fluency scores (r = −.257; p = .018) and with lower scores on the Hayling test (r = −.301; p = .009). In CH, larger volumes of WMH were only associated with a higher ratio between completion time of Trail B/Trail A (r = .518; p = .002). No significant correlation was found between WMH and any of the memory measures for all groups.
Figure 3.
Scatter plots and lines of best fit for the relation between (a) white matter hyperintensities and the Hayling inhibition score; (b) White matter hyperintensities and the Fluency score; and (c) White matter hyperintensities and Memoria free recall score. CH = cognitively healthy; MCI = mild cognitive impairment; SCD = subjective cognitive decline; WMH = white matter hyperintensities.
In order to be sure that our correlations were robust and not driven by some potential outliers, we used fully adjusted regression models of the relationship between MRI brain measures and cognition. Results are provided in Supplementary Table S2. The majority of the associations mentioned above using Pearson correlation analyses were confirmed by the adjusted models. In particular, they confirmed the relation between the right hippocampus and Face-Name memory in SCD, the relation between the left hippocampus and the delayed word recall in MCI, and the relation between WMH and executive functions in SCD (Fluency and Hayling) and in CH (Trail).
Discussion
The aim of this study was to assess whether memory and executive functions were related to the MRI anatomical features that are typically associated with AD in persons with SCD and MCI. Our results indicate a specific relation between lower memory performance and smaller hippocampal volume in MCI and SCD, and a specific relation between larger volumes of WMH and lower executive functions in SCD, MCI, and CH. We acknowledge the importance of reducing circularity issues in this study. In doing so, the tests employed to measure cognition were independent from those used for diagnosis and inclusion criteria. Nonetheless, the CIMA-Q cohort is meant to focus on AD and, by design, criteria for MCI require the presence of a memory problem. Thus, our study might miss MCI participants with non-memory problems, a pattern that could characterize the prodromal phase of non-AD neurodegenerative diseases. Even though persons with SCD are not expected to be impaired on typical neuropsychological measures, we predicted a correlation between brain markers and cognition in this group, similar to what we would observe in MCI individuals. The same correlation pattern suggests that similar brain changes occur during SCD and MCI stages and that these brain changes account for inter-individual cognitive differences in both groups. Our results thus support the presence of a continuum between SCD and MCI. In contrast, we did not expect such a correlational pattern in CH. WMH was also expected to be associated with executive functions in both SCD and MCI.
Thus, an important goal was to assess the relation between MRI markers and cognition in SCD. The rationale is that if the complaint of persons with SCD reflects a cognitive change which is felt by the individual, but remains undetected by traditional tests, one would expect inter-individual variability on brain measures to relate to inter-individual variability in cognitive performance. This was assessed by examining correlations between MRI markers and cognitive performance. As expected, we found positive correlations between hippocampal volume and memory performance in both MCI and SCD. Smaller right hippocampal volume was associated with worse Face-Name recall in SCD, and in MCI to a lesser extent. This is consistent with studies showing that Face-Name recall is a sensitive measure in the early stages of AD (Rubiño & Andrés, 2018) and that lower performance on Face-Name recall in MCI (Rentz et al., 2011) and SCD (Sanabria et al., 2018) is associated with beta amyloid burden in brain regions implicated in memory processes. We also found that, smaller left hippocampal volume was associated with worse delayed word recall in MCI and in SCD to a lesser extent. However, in CH, we observe an inverse association, in which smaller left hippocampal volume was associated with better performance during this task. Although this seems counterintuitive, it is not entirely unexpected based on prior findings (for review and meta-analysis, see Van Petten, 2004). Although the loss of hippocampal volume is often associated with memory decline in AD, studies examining the relationship between hippocampal volume and memory have found both positive and negative relationships (for review and meta-analysis, see Van Petten, 2004).
When examining group differences of hippocampal volume, we found a preferential reduction of bilateral hippocampi in MCI compared to CH, and no significant difference was highlighted between SCD and CH. This finding leads us to think that, despite the fact that cerebral changes in SCD already seem to influence their memory performance, their anatomy is more similar to that of healthy controls, than to that of MCI individuals when examining group effects. Noteworthily, inspection of Figure 2 indicates that a fair proportion of SCD individuals have hippocampal volumes in the low range value overlapping with that of MCI, particularly for the right hippocampus. Given that these individuals also display lower memory scores, it is consistent with the notion that these early brain modifications produce subtle cognitive changes, which bring on a complaint before becoming clinically measurable (Cherbuin et al., 2015).
We found an association between WMH and executive functions in all three groups: WMH correlated with the Fluency and Trail tests in SCD, with the Hayling and Trail tests in MCI, and with the Trail in CH. Thus, the relation between WMH and executive functions does not seem to be specific to MCI and SCD and may be relevant to a broader range of individuals. This is consistent with numerous studies showing that increased vascular burden is associated with lower executive functions in older adults (Au et al., 2006; Smith et al., 2011; Van Petten et al., 2004). It is important to acknowledge that Fluency tests are multidimensional, whereby reflecting multiple cognitive processes at once. Thus, our findings could represent a link between WMH and other cognitive processes, not restricted to executive functioning. Some studies have indicated that white matter abnormalities have an effect on executive performance, but can also impair episodic memory by affecting connections between the cortex and subcortical structures (Smith et al., 2011). This would be particular to older adults at risk of developing AD (SCD or individuals with abnormal amyloid-β, for example, Freeze et al., 2016). However, we did not find evidence of a relationship between WMH volumes and memory in this study.
As expected, the SCD group did not differ from CH on cognitive measures. This is unsurprising as it corresponds with this group’s definition (Perrotin et al., 2017; Sperling et al., 2011). Even if subtle cognitive modifications take place within the SCD phase, they do not seem sufficiently severe to be revealed by a standard cognitive evaluation. In addition, the SCD participants report a cognitive decline compared to their own past performances, but this intra-individual change is not typically assessed by a standard battery of tests. It is of note that there is some cognitive heterogeneity within the SCD group (Mendonça et al., 2016), and this is highlighted in Figures 2 and 3, where some SCD individuals show mild cognitive deficits. Furthermore, the performance of persons with SCD on the Face-Name association task stands between that of MCI and CH and did not significantly differ from either of them, suggesting that they have a very mild deficit in this task. Associative memory is known to be impaired early in MCI. Therefore, in the present case, we used an association between a face and a first name because it is particularly challenging and not easily amenable to any form of semantic encoding. This might make the task particularly sensitive to the early memory problems that persons with SCD experience. It is important to acknowledge that we used the Logical Memory subtest of the Wechsler Memory Scale to diagnose unimpaired cognition in SCD. This test might be less sensitive to the very mild symptoms of SCD than the more demanding face-name memory test. Yet, given that the SCD group is closer to CH than to MCI overall, it is fair to say that the subjective complaint of these individuals remains as one of the few directly observable symptoms (Jessen, Wolfsgruber, et al., 2014).
In contrast, persons with MCI differ from both CH and SCD on two measures of memory and on two measures of executive functions. The finding of an executive impairment is worth noting because MCI subjects from the CIMA-Q cohort are so-called amnestic MCI, in that they were defined on the basis of a memory impairment. Our results confirm prior studies indicating that amnestic MCI is often a multiple-domain MCI, due to additional impairment on measures of executive functions (Bélanger & Belleville, 2009; Belleville, Gilbert, et al., 2006; Johns et al., 2012).
This study has some limitations that call for future work. First, the three groups presented in this study are unequal and therefore do not have the same statistical power. This may partly explain the smaller number of significant correlations in the MCI group. It would therefore be interesting to test the same hypotheses in a larger cohort. Recruitment source is another important aspect to consider because the majority of our participants were recruited from the community. Many studies have shown that MCI participants recruited from the community are less impaired and less likely to progress to dementia than those recruited from memory clinics, as demonstrated in a study by Farias, Mungas, Reed, Harvey, and DeCarli (2009), among others. Studies examining the effect of recruitment source in SCD individuals are scarce but one recent study indicated an impact on the degree of progression from SCD to an MCI diagnosis (Snitz et al., 2018). It remains to be seen whether the recruitment methods in the present study influenced our study outcomes. Finally, as this was a cross-sectional study, we are unable to examine causal relationships between neuroimaging markers and cognitive decline. A follow-up may identify those who progress to AD dementia among SCD and MCI subjects, and thus truly stand in the prodromal phase of AD. A 2-year follow-up assessment is currently underway with the CIMA-Q cohort, hence contributing to longitudinal information on progression of the disease.
Conclusion
This study consolidates the notion that SCD represents a valid research target to better understand the preclinical phase of AD and other neurodegenerative diseases. The notion of SCD complements the criteria that were established to characterize MCI by bringing an even earlier dimension to AD and similar age-related neurodegeneration. This may have important implications, as studying this population might contribute to identifying the processes that contribute to AD at a very early stage. This is critical because disease-modifying treatments should ideally be offered as early as possible (Cheng et al., 2017). Thus, the SCD and MCI periods may be fundamental for drug development and implementation of very early secondary prevention approaches.
Funding
CIMA-Q is supported by the Fonds de recherche du Québec – Santé (FRQ-S), Pfizer Innovation Program (to S. Brambati, S. Duchesne, and C. Hudon), the Quebec Network for Research on Aging, a network supported by the FRQ-S, the Fondation Courtois (Neuromod project; S. Brambati), the Consortium for the Neurodegeneration Associated With Aging (CCNA/CCNV, to S. Brambati, S. Duchesne, and N. Phillips) and Canadian Institutes of Health Research (CIHR) Foundation Grant (to S. Brambati). S. Brambati holds a Canada Research Chair on Cognitive Neuroscience of Aging and Brain Plasticity. J.-F. Gagnon holds a Canada Research Chair on Cognitive Decline in Pathological Aging. S. Duchesne is a Research Scholar from the Fonds de recherche du Québec – Santé (#30801). M. Caillaud holds a post-doctoral fellowship grant from the CRIUGM.
Conflict of Interest
None reported.
Supplementary Material
References
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., … Phelps C. H (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7, 270–279. doi:10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au R., Massaro J. M., Wolf P. A., Young M. E., Beiser A., Seshadri S., … DeCarli C (2006). Association of white matter hyperintensity volume with decreased cognitive functioning. Archives of Neurology, 63, 246. doi:10.1001/archneur.63.2.246 [DOI] [PubMed] [Google Scholar]
- Bateman R. J., Xiong C., Benzinger T. L., Fagan A. M., Goate A., Fox N. C., … Morris J. C.; Dominantly Inherited Alzheimer Network (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. The New England Journal of Medicine, 367, 795–804. doi:10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger S., & Belleville S (2009). Semantic inhibition impairment in mild cognitive impairment: A distinctive feature of upcoming cognitive decline? Neuropsychology, 23, 592–606. doi:10.1037/a0016152 [DOI] [PubMed] [Google Scholar]
- Belleville S., Fouquet C., Duchesne S., Collins D. L., & Hudon C (2014). Detecting early preclinical Alzheimer’s disease via cognition, neuropsychiatry, and neuroimaging: Qualitative review and recommendations for testing. Journal of Alzheimer’s Disease, 42(Suppl. 4), S375– S382. doi:10.3233/JAD-141470 [DOI] [PubMed] [Google Scholar]
- Belleville S., Fouquet C., Hudon C., Zomahoun H. T. V., & Croteau J.; Consortium for the Early Identification of Alzheimer’s Disease-Quebec (2017). Neuropsychological measures that predict progression from mild cognitive impairment to Alzheimer’s type dementia in older adults: A systematic review and meta-analysis. Neuropsychology Review, 27, 328–353. doi:10.1007/s11065-017-9361-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S., Gilbert B., Fontaine F., Gagnon L., Ménard E., & Gauthier S (2006). Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: Evidence from a cognitive intervention program. Dementia and Geriatric Cognitive Disorders, 22, 486–499. doi:10.1159/000096316 [DOI] [PubMed] [Google Scholar]
- Belleville S., Rouleau N., & Van der Linden M (2006). Use of the Hayling task to measure inhibition of prepotent responses in normal aging and Alzheimer’s disease. Brain and Cognition, 62, 113–119. doi:10.1016/j.bandc.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Birdsill A. C., Koscik R. L., Jonaitis E. M., Johnson S. C., Okonkwo O. C., Hermann B. P., … Bendlin B. B (2014). Regional white matter hyperintensities: Aging, Alzheimer’s disease risk, and cognitive function. Neurobiology of Aging, 35, 769–776. doi:10.1016/j.neurobiolaging.2013.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P., & Shallice T (1997). The Hayling and Brixton tests. Bury St. Edmunds, UK: Thames Valley Test Company; Retrieved from http://discovery.ucl.ac.uk/5457/ [Google Scholar]
- Cantero J. L., Iglesias J. E., Van Leemput K., & Atienza M (2016). Regional hippocampal atrophy and higher levels of plasma amyloid-beta are associated with subjective memory complaints in nondemented elderly subjects. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 71, 1210–1215. doi:10.1093/gerona/glw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelois J., Pineau H., Belleville S., Peretz I., Lussier I., Fontaine F. S., & Renaseau-Leclerc C (1993). Batterie informatisée d’évaluation de la mémoire inspirée de l’approche cognitive. Canadian Psychology/Psychologie Canadienne, 34, 45–63. doi:10.1037/h0078803 [Google Scholar]
- Cheng Y.-W., Chen T.-F., & Chiu M.-J. (2017). From mild cognitive impairment to subjective cognitive decline: Conceptual and methodological evolution. Neuropsychiatric Disease and Treatment, 13, 491–498. doi:10.2147/NDT.S123428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N., Sargent-Cox K., Easteal S., Sachdev P., & Anstey K. J (2015). Hippocampal atrophy is associated with subjective memory decline: The PATH Through Life study. The American Journal of Geriatric Psychiatry, 23, 446–455. doi:10.1016/j.jagp.2014.07.009 [DOI] [PubMed] [Google Scholar]
- DeCarli C., Fletcher E., Ramey V., Harvey D., & Jagust W. J (2005). Anatomical mapping of white matter hyperintensities (WMH): Exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke, 36, 50–55. doi:10.1161/01.STR.0000150668.58689.f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot J. C., de Leeuw F. E., Oudkerk M., Hofman A., Jolles J., & Breteler M. M (2001). Cerebral white matter lesions and subjective cognitive dysfunction: The Rotterdam Scan Study. Neurology, 56, 1539–1545. doi:10.1212/wnl.56.11.1539 [DOI] [PubMed] [Google Scholar]
- Du A. T., Schuff N., Amend D., Laakso M. P., Hsu Y. Y., Jagust W. J., … Weiner M. W (2001). Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 71, 441–447. doi:10.1136/jnnp.71.4.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B., Hampel H., Feldman H. H., Scheltens P., Aisen P., Andrieu S., … Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA. (2016). Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s & Dementia, 12, 292–323. doi:10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne S., Chouinard I., Potvin O., Fonov V. S., Khademi A., Bartha R., … Black S. E.; CIMA-Q Group and the CCNA Group (2019). The Canadian dementia imaging protocol: Harmonizing national cohorts. Journal of Magnetic Resonance Imaging, 49, 456–465. doi:10.1002/jmri.26197 [DOI] [PubMed] [Google Scholar]
- Eckerström M., Göthlin M., Rolstad S., Hessen E., Eckerström C., Nordlund A., … Wallin A (2017). Longitudinal evaluation of criteria for subjective cognitive decline and preclinical Alzheimer’s disease in a memory clinic sample. Alzheimer’s & Dementia (Amsterdam, Netherlands), 8, 96–107. doi:10.1016/j.dadm.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias S. T., Mungas D., Reed B. R., Harvey D., & DeCarli C (2009). Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Archives of Neurology, 66, 1151–1157. doi:10.1001/archneurol.2009.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Ségonne F., Salat D. H., … Dale A. M (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex (New York, N.Y.: 1991), 14, 11–22. doi:10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Flier W., Buchem M., Weverling-Rijnsburger A. E., Mutsaers E., Bollen E. E. M., Admiraal-Behloul F., … Middelkoop H. M (2004). Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. Journal of Neurology, 251, 671–675. doi:10.1007/s00415-004-0390-7 [DOI] [PubMed] [Google Scholar]
- Freeze W. M., Jacobs H. I. L., Gronenschild E. H., Jansen J. F. A., Burgmans S., Aalten P., … Verhey F. R (2016). White matter hyperintensities potentiate hippocampal volume reduction in non-demented older individuals with abnormal amyloid-β. Journal of Alzheimer’s Disease, 55, 333–342. doi:10.3233/JAD-160474 [DOI] [PubMed] [Google Scholar]
- Hachinski V. C., Lassen N. A., & Marshall J (1974). Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet (London, England), 2, 207–210. doi:10.1016/s0140-6736(74)91496-2 [DOI] [PubMed] [Google Scholar]
- Hirono N., Kitagaki H., Kazui H., Hashimoto M., & Mori E (2000). Impact of white matter changes on clinical manifestation of Alzheimer’s disease: A quantitative study. Stroke, 31, 2182–2188. doi:10.1161/01.str.31.9.2182 [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y., Sotero R. C., Toussaint P. J., Mateos-Pérez J. M., & Evans A. C.; Alzheimer’s Disease Neuroimaging Initiative (2016). Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nature Communications, 7, 11934. doi:10.1038/ncomms11934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R. Jr, Knopman D. S., Jagust W. J., Shaw L. M., Aisen P. S., Weiner M. W., … Trojanowski J. Q (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology, 9, 119–128. doi:10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Amariglio R. E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G., … Wagner M.; Subjective Cognitive Decline Initiative (SCD-I) Working Group (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10, 844–852. doi:10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Wolfsgruber S., Wiese B., Bickel H., Mösch E., Kaduszkiewicz H., … German Study on Aging, Cognition and Dementia in Primary Care Patients. (2014). AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimer’s & Dementia, 10, 76–83. doi:10.1016/j.jalz.2012.09.017 [DOI] [PubMed] [Google Scholar]
- Johns E. K., Phillips N. A., Belleville S., Goupil D., Babins L., Kelner N., … Chertkow H (2012). The profile of executive functioning in amnestic mild cognitive impairment: Disproportionate deficits in inhibitory control. Journal of the International Neuropsychological Society, 18, 541–555. doi:10.1017/S1355617712000069 [DOI] [PubMed] [Google Scholar]
- Jorm A. F., Butterworth P., Anstey K. J., Christensen H., Easteal S., Maller J., … Sachdev P (2004). Memory complaints in a community sample aged 60–64 years: Associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychological Medicine, 34, 1495–1506. doi:10.1017/S0033291704003162 [DOI] [PubMed] [Google Scholar]
- Leuzy A., Heurling K., Ashton N. J., Schöll M., & Zimmer E. R (2018). In vivo detection of Alzheimer’s disease. The Yale Journal of Biology and Medicine, 91, 291–300. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/30258316 [PMC free article] [PubMed] [Google Scholar]
- Li X. Y., Tang Z. C., Sun Y., Tian J., Liu Z. Y., & Han Y (2016). White matter degeneration in subjective cognitive decline: A diffusion tensor imaging study. Oncotarget, 7, 54405–54414. doi:10.18632/oncotarget.10091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Braidy N., Poljak A., Chan D. K. Y., & Sachdev P (2018). Cerebral small vessel disease and the risk of Alzheimer’s disease: A systematic review. Ageing Research Reviews, 47, 41–48. doi:10.1016/j.arr.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Manjón J. V, & Coupé P (2016). volBrain: An online MRI brain volumetry system. Frontiers in Neuroinformatics, 10, 30. doi:10.3389/fninf.2016.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça M. D., Alves L., & Bugalho P (2016). From subjective cognitive complaints to dementia: Who is at risk?: A systematic review. American Journal of Alzheimer’s Disease and Other Dementias, 31, 105–114. doi:10.1177/1533317515592331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. C. (1993). The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology, 43, 2412–2412. doi:10.4067/S0718-34292016005000011 [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. doi:10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Newkirk L. A., Kim J. M., Thompson J. M., Tinklenberg J. R., Yesavage J. A., & Taylor J. L (2004). Validation of a 26-point telephone version of the Mini-Mental State Examination. Journal of Geriatric Psychiatry and Neurology, 17, 81–87. doi:10.1177/0891988704264534 [DOI] [PubMed] [Google Scholar]
- Perrotin A., De Flores R., Lamberton F., Poisnel G., La Joie R., De La Sayette V., … Chételat G (2015). Hippocampal subfield volumetry and 3D surface mapping in subjective cognitive decline. Journal of Alzheimer’s Disease, 48(S1), S141–S150. doi:10.3233/JAD-150087 [DOI] [PubMed] [Google Scholar]
- Perrotin A., La Joie R., de La Sayette V., Barré L., Mézenge F., Mutlu J., … Chételat G (2017). Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: Differential affective and imaging correlates. Alzheimer’s & Dementia, 13, 550–560. doi:10.1016/j.jalz.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Smith G. E., Waring S. C., Ivnik R. J., Tangalos E. G., & Kokmen E (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56, 303–308. [DOI] [PubMed] [Google Scholar]
- Potvin O., Mouiha A., Dieumegarde L., & Duchesne S (2016). Normative data for subcortical regional volumes over the lifetime of the adult human brain. NeuroImage, 137, 9–20. doi:10.1016/j.neuroimage.2016.05.016 [DOI] [PubMed] [Google Scholar]
- Price J. L., Ko A. I., Wade M. J., Tsou S. K., McKeel D. W., & Morris J. C (2001). Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Archives of Neurology, 58, 1395–1402. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11559310 [DOI] [PubMed] [Google Scholar]
- Price J. L., & Morris J. C (1999). Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Annals of Neurology, 45, 358–368. doi: 10.1002/1531-8249(199903)45:3<358::AID-ANA12>3.0.CO;2-X [DOI] [PubMed] [Google Scholar]
- Radanovic M., Pereira F. R., Stella F., Aprahamian I., Ferreira L. K., Forlenza O. V., & Busatto G. F (2013). White matter abnormalities associated with Alzheimer’s disease and mild cognitive impairment: A critical review of MRI studies. Expert Review of Neurotherapeutics, 13, 483–493. doi:10.1586/ern.13.45 [DOI] [PubMed] [Google Scholar]
- Reisberg B., Shulman M. B., Torossian C., Leng L., & Zhu W (2010). Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s & Dementia, 6, 11–24. doi:10.1016/j.jalz.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz D. M., Amariglio R. E., Becker J. A., Frey M., Olson L. E., Frishe K., … Sperling R. A (2011). Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia, 49, 2776–2783. doi:10.1016/j.neuropsychologia.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubiño J., & Andrés P (2018). The Face-Name Associative Memory Test as a tool for early diagnosis of Alzheimer’s disease. Frontiers in Psychology, 9, 1464. doi:10.3389/fpsyg.2018.01464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria A., Alegret M., Rodriguez-Gomez O., Valero S., Sotolongo-Grau O., Monté-Rubio G., … FACEHBI Study Group. (2018). The Spanish version of Face-Name Associative Memory Exam (S-FNAME) performance is related to amyloid burden in subjective cognitive decline. Scientific Reports, 8, 3828. doi:10.1038/s41598-018-21644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. E., Salat D. H., Jeng J., McCreary C. R., Fischl B., Schmahmann J. D., … Greenberg S. M (2011). Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology, 76, 1492–1499. doi:10.1212/WNL.0b013e318217e7c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz B. E., Wang T., Cloonan Y. K., Jacobsen E., Chang C. H., Hughes T. F., … Ganguli M (2018). Risk of progression from subjective cognitive decline to mild cognitive impairment: The role of study setting. Alzheimer’s & Dementia, 14, 734–742. doi:10.1016/j.jalz.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. A., Aisen P. S., Beckett L. A., Bennett D. A., Craft S., Fagan A. M., … Phelps C. H (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7, 280–292. doi:10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L. R., & Zola-Morgan S (1991). The medial temporal lobe memory system. Science, 253, 1380–1386. doi: 10.1126/science.1896849. Retrieved from www.sciencemag.org [DOI] [PubMed] [Google Scholar]
- Strauss E., Sherman E. M. S., Spreen O., & Spreen O (2006). A compendium of neuropsychological tests : Administration, norms, and commentary. New York: Oxford University Press; Retrieved from https://global.oup.com/academic/product/a-compendium-of-neuropsychological-tests-9780195159578?cc=us&lang=en& [Google Scholar]
- van de Pol L. A., Korf E. S. C., van der Flier W. M., Brashear H. R., Fox N. C., Barkhof F., & Scheltens P (2007). Magnetic resonance imaging predictors of cognition in mild cognitive impairment. Archives of Neurology, 64, 1023. doi:10.1001/archneur.64.7.1023 [DOI] [PubMed] [Google Scholar]
- Van Petten C. (2004). Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia, 42, 1394–1413. doi:10.1016/j.neuropsychologia.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Van Petten C., Plante E., Davidson P. S., Kuo T. Y., Bajuscak L., & Glisky E. L (2004). Memory and executive function in older adults: Relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia, 42, 1313–1335. doi:10.1016/j.neuropsychologia.2004.02.009 [DOI] [PubMed] [Google Scholar]
- van Rooden S., van den Berg-Huysmans A. A., Croll P. H., Labadie G., Hayes J. M., Viviano R., … Damoiseaux J. S (2018). Subjective cognitive decline is associated with greater white matter hyperintensity volume. Journal of Alzheimer’s Disease, 1, 1–12. doi:10.3233/JAD-180285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S., & Belleville S (2012). The nature of memory failure in mild cognitive impairment: Examining association with neurobiological markers and effect of progression. Neurobiology of Aging, 33, 1967–1978. doi:10.1016/j.neurobiolaging.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Wardlaw J. M., Smith C., & Dichgans M (2013). Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. The Lancet Neurology, 12, 483–497. doi:10.1016/S1474-4422(13)70060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. (1997). Wechsler Adult Intelligence Scale® - third edition. San Antonio, TX: The Psychological Corporation; Retrieved from https://www.pearsonclinical.com/psychology/products/100000243/wechsler-adult-intelligence-scale--third-edition-wais-iii.html [Google Scholar]
- Wolf H., Ecke G. M., Bettin S., Dietrich J., & Gertz H. J (2000). Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. International Journal of Geriatric Psychiatry, 15, 803–812. doi: 10.1002/1099-1166(200009)15:9<803::AID-GPS190>3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- Zhao Q., Guo Q., & Hong Z (2013). Clustering and switching during a semantic verbal fluency test contribute to differential diagnosis of cognitive impairment. Neuroscience Bulletin, 29, 75–82. doi:10.1007/s12264-013-1301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.