Abstract
Objectives
The main objective of this study was to estimate effects of dementia on Medicaid expenditures in an ethnically diverse community.
Methods
The sample included 1,211 Medicare beneficiaries who did not have any Medicaid coverage and 568 who additionally had full Medicaid coverage enrolled in the Washington Heights-Inwood Columbia Aging Project (WHICAP), a multiethnic, population-based, prospective study of cognitive aging in northern Manhattan (1999–2010). Individuals’ dementia status was determined using a rigorous clinical protocol. Relationship between dementia and Medicaid coverage and expenditures were estimated using a two-part model.
Results
In participants who had full Medicaid coverage, average annual Medicaid expenditures were substantially higher for those with dementia than those without dementia ($50,270 vs. $21,966, p < .001), but Medicare expenditures did not differ by dementia status ($8,458 vs. $9,324, p = .19). In participants who did not have any Medicaid coverage, average annual Medicare expenditures were substantially higher for those with dementia than those without dementia ($12,408 vs. $8,113, p = .02). In adjusted models, dementia was associated with a $6,278 increase in annual Medicaid spending per person after controlling for other characteristics.
Discussion
Results highlight Medicaid’s contribution to covering the cost of dementia care in addition to Medicare. Studies that do not include Medicaid are unlikely to accurately reflect the true cost of dementia.
Keywords: Cost of illness, Dementia, Health care expenditure, Medicare, Medicaid
The costs of care for individuals with Alzheimer’s disease (AD) or other dementias are estimated at $290 billion in the United States in 2019 (in 2019$) (Alzheimer’s Association, 2019). About half of these costs ($146 billion) were paid for by Medicare, and another $47 billion by Medicaid. Direct health care expenditures on dementia care are similar to heart disease and significantly higher than cancer (Hurd, Martorell, Delavande, Mullen, & Langa, 2013). In addition, many individuals with dementia rely on family caregivers to provide assistance with care and other daily activities, resulting in substantial costs related to informal caregiving and lost income (Kasper, Freedman, Spillman, & Wolff, 2015).
Virtually, all adults aged 65 or older in the United States have Medicare, which covers a wide range of acute and post-acute care, including inpatient hospital stays, skilled nursing care after a hospital stay, hospice, lab tests, surgery, and some home health care. Certain physician and other health care providers’ services, outpatient care, medical supplies, and some preventive services are also covered. However, Medicare does not cover custodial (homemaker) or personal (help with bathing, dressing, eating) care that patients need to remain safely in the community and has limited coverage of long-term care. Until 2006, Medicare also did not cover prescription medication. Private insurance for long-term care has not been widely used (Nguyen, 2017). Most older adults have to pay out-of-pocket or rely on other insurance programs or Medicaid to cover these costs.
Medicaid is a joint federal and state program that provides health care coverage to eligible low-income individuals and those with disabilities. In addition, Medicaid has an option that allows individuals to “spend down” to eligibility by incurring medical and/or remedial care expenses to offset their income above eligibility criteria, thereby reducing it to a level below the maximum allowed by the State’s Medicaid plan. More than two-thirds of Medicaid’s budget has historically been spent on the elderly and disabled. The program is the largest single payer of long-term nursing home care, financing about one-third of total nursing home spending (Peter G. Peterson Foundation, 2017). Until Medicare Part D became available in 2006, Medicaid was also the only source of prescription medication coverage for many low-income individuals.
Higher Medicare expenditures associated with dementia have been examined extensively (Ayyagari, Salm, & Sloan, 2007; Hill et al., 2002; Hurd et al., 2013; Jutkowitz et al., 2017; Murman et al., 2002; Oremus & Aguilar, 2011; Yang, Zhang, Lin, Clevenger, & Atherly, 2012; Zhu et al., 2015). For many individuals with dementia who face intense care needs for long durations, Medicaid serves as a particularly critical role to cover the cost of long-term services and support that Medicare and most other payers do not cover. Medicaid beneficiaries with dementia are more likely to be female, more racial/ethnically diverse, have lower income, and have fewer financial resources available to pay for care out-of-pocket than those without dementia (Garfield, Musumeci, Reaves, & Damico, 2015). Nationally, the Alzheimer’s Association reported average annual Medicaid payment per person for individuals with dementia ($8,565) to be 23 times greater than for those without dementia ($365) in 2019 (Alzheimer’s Association, 2019). Several older studies reported annualized per beneficiary Medicaid spending between $7,700 and $9,829 higher in individuals with dementia compared to those without dementia in a number of states (Bharmal et al., 2012; Geldmacher et al., 2013; Martin, Ricci, Kotzan, Lang, & Menzin, 2000; Menzin, Lang, Friedman, Neumann, & Cummings, 1999). In addition to incurring higher costs, individuals with dementia are more than twice as likely to be eligible for Medicaid coverage than those without dementia (dual Medicare and Medicaid eligible, 27% vs. 11%; Alzheimer’s Association, 2019). Dual-eligible beneficiaries are among the most vulnerable older adults, accounting for disproportionate shares of chronic illness and health care costs compared to other Medicare beneficiaries (Garfield et al., 2015; Lied, 2006).
Despite its importance in covering the cost of care for individuals with dementia, Medicaid expenditures for individuals with dementia are not extensively studied. This may be due in part to a lack of availability of comprehensive data that include both Medicare and Medicaid expenditures (Bradley, Dahman, Bataki, & Koroukian, 2010; Hurd et al., 2013; Jutkowitz et al., 2017; Murman et al., 2002; Oremus & Aguilar, 2011; Yang et al., 2012). Before Medicaid Analytic Extract (MAX) files from the Centers for Medicare and Medicaid Services (CMS) became available to researchers, Medicaid data could only be obtained on a state-by-state basis through state Medicaid agencies.
In this study, we examined Medicare and Medicaid utilization and expenditures in a diverse cohort of Medicare beneficiaries residing in New York City. We divided our sample into two separate cohorts by their Medicaid coverage status. For those who were not covered by Medicaid, we examined individuals’ Medicare expenditures similar to what has been reported in the literature. For those who were dual eligible, we examined how much Medicaid contributed to care costs in addition to Medicare expenditures. Analyses were performed by clinically assessed dementia status to estimate excess expenditures to Medicaid due to dementia overall and by specific components of care. We also examined differences in expenditures relative to dementia status and other key clinical and demographic characteristics.
Our study offers several advantages. First, studies on the cost of dementia care often had to rely on dementia diagnosis recorded in the claims data. Substantial misidentification of dementia status using claims-identification and resultant biases in estimates of cost of dementia care have been documented in a number of studies (Lin et al., 2010; Zhu et al., 2019). Participants in our study have been prospectively followed with careful in-person clinical diagnoses, thereby avoiding such biases. Second, few studies have examined Medicare and Medicaid cost together. Analyses restricted to Medicare claims will miss much of long-term care and medication expenditures that are covered by Medicaid. Alternatively, analyses restricted to Medicaid claims will miss much of hospital and physician expenditures for which Medicare covers and is the first payer. Our study using data with both Medicare and Medicaid claims overcomes these difficulties and offers a unique opportunity to identify a more comprehensive perspective on the expenditures associated with dementia. Third, our cohort is largely minority, older, from diverse educational and socioeconomic backgrounds, and varies widely in their health status. Minority elders are disproportionally poor and more than twice as likely to be eligible for Medicaid than their non-minority counterparts but are underrepresented in most studies that examine brain aging (Administration for Community Living, 2019a, 2019b). As the population of older adults rapidly becomes more ethnically diverse, the importance of examining diverse, vulnerable population cannot be overemphasized.
Method
Participants
Participants were drawn from the Washington Heights-Inwood Columbia Aging Project (WHICAP), a multiethnic, population-based, prospective study of cognitive aging of Medicare beneficiaries aged 65 or older residing in northern Manhattan. Lists of all Medicare or Medicaid recipients living in the area were provided by CMS at the beginning of study enrollment in 1992. An additional cohort was formed in 1999 using similar methods based on an updated beneficiaries list. The original list of names was divided into six strata based on age (65–74, ≥75 years) and ethnicity (Hispanic, non-Hispanic black, non-Hispanic white) groups. These strata were further divided into subsamples so that the distributions by age and ethnicity within each subsample were similar. This provided a means to ensure equal representation of the community during participants’ initial assessment. Detailed descriptions of study methodology have been reported previously (Scarmeas, Levy, Tang, Manly, & Stern, 2001; Stern et al., 1992).
At the time of study entry, each participant underwent an in-person interview on general health and functional ability, followed by a standardized assessment including medical history, physical and neurological examination, and a neuropsychological battery. Participants were then followed at approximately 18-month intervals with similar assessments. Evaluations were conducted in English or Spanish, based on participants’ primary language or preference. Few participants were followed after nursing home placement. Recruitment, informed consent and study procedures were approved by the institutional review boards of Columbia Presbyterian Medical Center and Columbia University Health Sciences, New York State Psychiatric Institute, and CMS Privacy Board. Written informed consent was obtained from all participants. All participants were able to provide informed consent at the initial visit, which included consent for follow-up.
Individuals were matched to Medicare Beneficiary Summary File using social security number, name, and Medicare beneficiary ID. The study period for the current analysis was defined to begin with the individual’s first WHICAP visit or beginning of CMS data availability (January 1, 1999), whichever is later, and to end with individuals’ last WHICAP visit, end of CMS data availability (December 31, 2010 at the time of data acquisition), or death, whichever is earlier to ensure data overlap between WHICAP study visits and Medicare claims. Because Medicare claims from individuals who were covered under managed care plans are incomplete, we followed CMS Chronic Condition Warehouse guidelines and excluded observations from subjects who were not covered by Medicare fee-for-service (FFS) providers for 10 or more months during a calendar year (or had no more than 1 month not covered by FFS during the year of death if the participant died; Buccaneer, 2011).
Individuals may qualify for full, comprehensive Medicaid coverage, or may qualify only for partial/restricted benefits. Under partial/restricted benefits, individuals may receive Medicare and Medicaid premium assistance only, prescription drug coverage only, or other forms of restricted benefits. Because the wide variety of partial/restricted benefits that may affect Medicaid payments (Chronic Condition Data Warehouse, October 2017), a small number of participants (N = 73) who always had partial Medicaid coverage throughout the study were excluded from the analysis.
Medicare and Medicaid Expenditures
Medicare expenditures were obtained from Medicare Standard Analytic Files and included all covered services (inpatient stays, outpatient visits, physician services, durable medical equipment [DME], skilled nursing, home health, and hospice care). Medicaid expenditures were obtained from MAX data from New York State. MAX is a set of person-level data files on Medicaid eligibility, service utilization, and payment information and consists of 1 personal summary file and 4 claims (Inpatient, Long-Term Care, Prescription Drug, and Other Therapy) files. The Other Therapy file contains claim records for a wide variety of Medicaid covered services. We categorized the most frequently occurring types of service into long-term care, inpatient care, personal care, home health, physician services, prescription medications, transportation services, laboratory visits, and outpatient hospital utilizations. All other service types had frequency of claims less than 1% and were grouped together.
Medicare and Medicaid expenditures were computed by summing all payments for each beneficiary during the study period by service type, reflecting actual payments for each beneficiary. Because participants were followed for varying lengths of time, we divided total expenditures a participant incurred during the study by total years of follow-up for that individual to obtain an average annual expenditure for each individual. Expenditures were adjusted to 2018$ using the medical care component of the Consumer Price Index (Bureau of Labor Statistics, 2012).
Clinical Diagnosis of Dementia
At each WHICAP visit, diagnostic conferences were held by a group of neurologists, psychiatrists, and neuropsychologists using results from the neuropsychological battery and evidence of impairment in social or occupational functions (McKhann et al., 1984; Stern et al., 1992). A diagnosis of dementia was determined based on Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria. Diagnosis of probable or possible AD was made based on National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (now Alzheimer’s Association, NINCD/ADRDA) criteria. Because of the epidemiologic nature of the study, neither participants nor their primary care providers were notified of a diagnosis of dementia. Participants were categorized into two groups as dementia (those who were clinically diagnosed with dementia at some point during the study) or non-dementia (those who were never clinically diagnosed with dementia throughout the study period).
Sociodemographic and Clinical Characteristics
Sociodemographic characteristics based on known associations with health care spending included age, sex, race/ethnicity, years of education, and marital status. On the basis of household size and household income reported, we constructed a poverty indicator using federal poverty guidelines (Social Security Administration, October 2018). Because WHICAP did not follow participants after long-stay nursing home placement, only years of living in the community were included in the study. WHICAP did not ask caregiver and caregiving-related questions.
Participant medical history data were obtained via a WHICAP survey to construct a modified version of the Charlson comorbidities index (Zhu et al., 2015, 2006). Comorbidities included myocardial infarction, congestive heart failure, peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, arthritis, gastrointestinal diseases, liver disease, diabetes, chronic renal disease, and systemic malignancy. Only Medicare files were used because the incremental value of using Medicaid claims to identify comorbidities has been reported to be small over what is already obtained from Medicare claims (Bradley et al., 2010). Functional status was measured using the Blessed Dementia Rating Scale (range = 0–13; Blessed, Tomlinson, & Roth, 1968); a higher score indicates worse functioning. Cognitive status was measured by a global cognitive z-score, comprising multiple domains of cognition including memory, abstract reasoning, language, visuospatial functioning, and executive/speed processing (Cosentino et al., 2008); a higher score indicates better cognition. Extrapyramidal signs were assessed using a modified Unified Parkinson’s Disease Rating Scale (UPDRS), whose interrater reliability has already been established (Richards et al., 1991); a total UPDRS score was computed and dichotomized into less than 2 (slight or none) versus at least 2 (moderate or severe). An indicator for the individual within a year before death was included in the models to adjust for effect of high expenditures at the end of life.
Analysis
Bivariate comparisons by dementia status were performed using chi-square test for categorical variables and Kruskal–Wallis test for continuous variables, separately for individuals with Medicare only and those with Medicare and Medicaid.
The relationships between participant characteristics and Medicaid coverage and expenditures were estimated using a two-part model (Deb & Norton, 2018). In the first part of this model, a logistic regression was used to estimate the probability of Medicaid coverage using the full sample. Conditional on having Medicaid coverage, the second part of the model estimated characteristics associated with Medicaid expenditures using a generalized linear model (GLM) with gamma family and log link. The two-part model allows for separate investigation of the effect of covariates on the extensive margin (logit model) and on the intensive margin (GLM, amount of expenditures if any). Average marginal effects for each part of the model and the combined overall effects from both parts of the model were reported (Cameron & Trivedi, 2010). For categorical variables, marginal effects estimate the discrete change in the predicted probabilities in one category relative to the reference category, for example, from female to male, Hispanic to non-Hispanic. For continuous variables, marginal effects estimate the rate of change in the predicted probabilities for a small amount of change in an independent variable, providing a good estimate to the amount of change in the predicted probabilities produced by a 1-unit change in the independent variable. Adjusted difference between dementia and nondementia groups and 95% CI were estimated, controlling for the sociodemographic and clinical characteristics described earlier. Analyses were conducted using Stata statistical software (version 13.1; StataCorp, College Station, TX).
Results
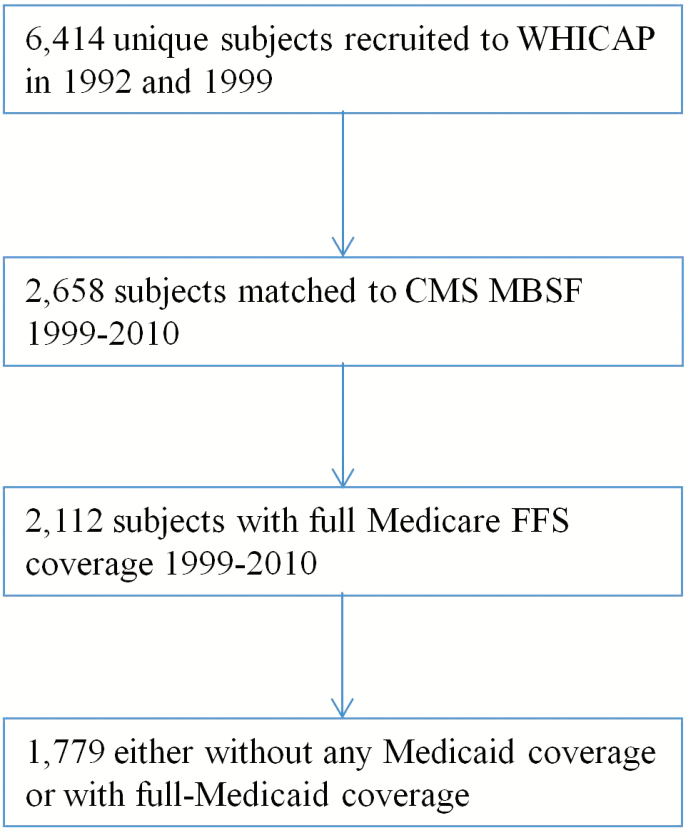
The analysis sample included 1,779 Medicare beneficiaries with full FFS coverage, of whom 568 (31.9%) qualified for full Medicaid coverage (full-benefit dual eligible) and 1,211 (68.1%) who did not have any Medicaid coverage. Figure 1 summarizes the study’s sample selection process.
Figure 1.
Sample selection. CMS = Centers for Medicare and Medicaid Services; MBSF = Medicare Beneficiary Summary file; FFS = Fee for Service.
Medicare Only Sample
Among participants who were not covered by Medicaid, those with dementia were older, less likely to be white, and had fewer years of education, lower household income, higher poverty rate, and worse functional and cognitive status than those without dementia (Table 1, all ps < .001). There were no differences in gender, marital status, household size, or number of comorbidities by dementia status. Compared to those without dementia, average follow-up years were slightly shorter for those with dementia (3.4 ± 2.8 vs. 4.0 ± 3.2 years, p = .07). Of those with dementia, 14.1% died within a year from the date of last follow-up compared to 3.9% in those without dementia, p < .001).
Table 1.
Baseline Characteristics by Dementia and Medicaid Coverage Status
| Variable | Medicare only (No Medicaid) | Dual Medicare and Medicaid covered | ||||
|---|---|---|---|---|---|---|
| Dementia | No dementia | p | Dementia | No Dementia | p | |
| N | 142 | 1,069 | 228 | 340 | ||
| Age, mean (SD) | 82.1 | 76.5 | <.001 | 82.1 | 76.1 | <.001 |
| (7.2) | (6.6) | (7.2) | (6.5) | |||
| Female, (%) | 62.0 | 61.6 | .94 | 71.5 | 69.7 | .648 |
| White, (%) | 34.5 | 50.3 | <.001 | 2.6 | 6.2 | .152 |
| Black, (%) | 33.1 | 27.4 | 23.7 | 22.9 | ||
| Hispanic, (%) | 32.4 | 21.2 | 73.2 | 69.7 | ||
| Married, (%) | 32.3 | 37.4 | .256 | 21.9 | 25.2 | .394 |
| Years of schooling, mean (SD) | 10.1 | 12.3 | <.001 | 5.9 | 7.5 | <.001 |
| (4.4) | (4.1) | (4.3) | (4.1) | |||
| Household size, mean (SD) | 1.6 | 1.5 | .099 | 1.6 | 1.6 | .963 |
| (0.6) | (0.6) | (0.6) | (0.6) | |||
| Household income ($), mean (SD) | 23,179 | 34,749 | <.001 | 10,531 | 11,192 | <.001 |
| (21,387) | (27,432) | (4,924) | (7,073) | |||
| Household income missing, (%) | 45.8 | 32.5 | <.001 | 41.7 | 17.9 | <.001 |
| Below Federal Poverty Level, (%) | 45.1 | 21.7 | <.001 | 89.6 | 90.2 | .847 |
| Last follow-up <1 year before death, (%) | 14.1 | 3.9 | <.001 | 7.9 | 5.0 | .16 |
| Follow-up years, mean (SD) | 3.4 | 4.0 | .069 | 3.4 | 3.5 | .531 |
| (2.8) | (3.2) | (2.2) | (2.4) | |||
| # Comorbidities, mean (SD) | 2.33 | 2.30 | .94 | 2.83 | 2.80 | .07 |
| (1.93) | (1.78) | (1.82) | (2.08) | |||
| # Extrapyramidal signs, mean (SD) | 2.34 | 0.74 | <.001 | 2.21 | 0.84 | <.001 |
| (4.13) | (1.70) | (3.61) | (1.73) | |||
| BDRS, mean (SD) | 1.50 | 0.27 | <.001 | 2.36 | 0.43 | <.001 |
| (2.63) | (0.74) | (3.37) | (0.82) | |||
| Cognitive z-score, mean (SD) | –0.58 | 0.27 | <.001 | –1.07 | –0.29 | <.001 |
| (0.63) | (0.57) | (0.64) | (0.55) | |||
Note. BDRS = Blessed Dementia Rating Scale.
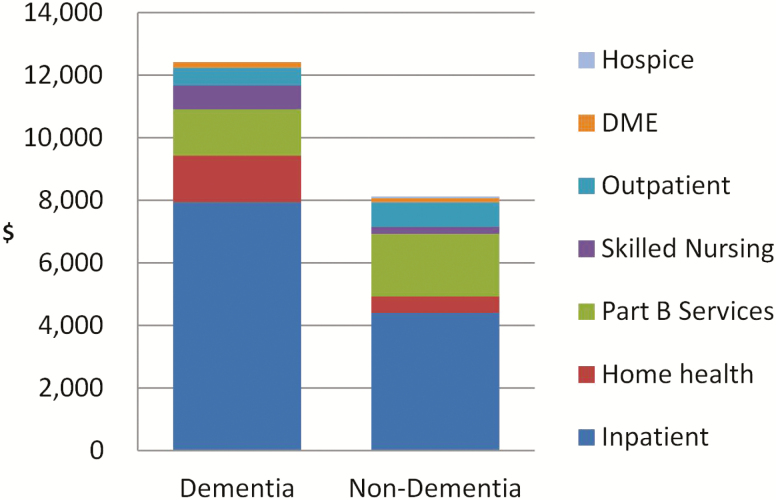
In this sample of participants who did not have Medicaid coverage, average annual Medicare expenditures were substantially higher in dementia than those without dementia ($12,408 vs. $8,113, p = .02; Figure 2). Specifically, compared with those without dementia, individuals with dementia incurred higher expenditures for inpatient ($7,939 vs. $4,405, p = .004) and home health services ($1,494 vs. $515, p < .001), but lower expenditures for outpatient and part B services (2,041 vs. 2,800, p = .02). Expenditures for other services (skilling nursing, DME, hospice) were similar between those with and without dementia.
Figure 2.
Average annual Medicare expenditures per person among individuals without Medicaid coverage, by dementia status. Part B services include fee-for-service claims submitted by professional providers including physicians, physician assistants, clinical social workers, nurse practitioners, and claims for some organizational providers such as free-standing facilities.
Dual Medicare–Medicaid Sample
Among participants with full Medicaid coverage, those with dementia were older, had fewer years of education, lower household income, and worse functional and cognitive status than those without dementia (Table 1, all ps < .001). There were no differences in gender, race/ethnicity, marital status, household size, or number of comorbidities by dementia status. Average follow-up years were similar between those with and without dementia (3.4 ± 2.4 vs. 3.4 ± 2.5 years, p = .531).
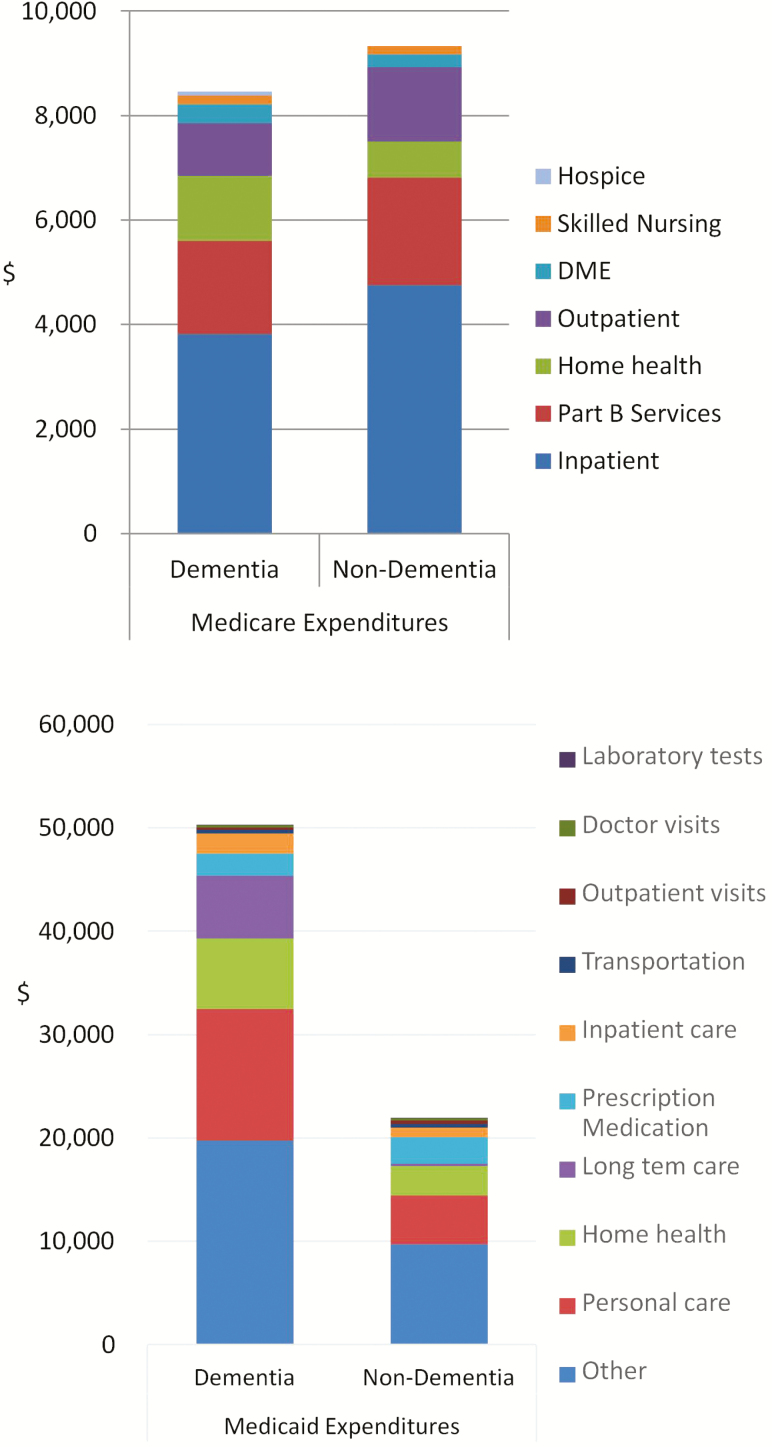
In this sample of dual-eligible beneficiaries, total Medicaid expenditures were substantially higher for those with dementia ($50,270) than those without dementia ($21,966, p < .001; Figure 3). At $12,693, personal care accounted for the largest share of Medicaid costs (25%) in individuals with dementia, but only $4,713 (21%) in those without dementia (p < .001). Home health care accounted for a substantial share of Medicaid costs as well, with $6,838 for individuals with dementia and $2,850 for those without dementia (p < .001). Even though most individuals lived in the community, long-term care costs were the third-highest cost category among individuals with dementia, at $6,055, compared to only $203 among those without dementia (p = .016). Inpatient costs also were high among individuals with dementia, at $1,979, compared with $905 for those without dementia (p = .005). Cost of prescription medications however was slightly lower among individuals with dementia ($2,138 vs. $2,594, p = .034). Interestingly, in this sample of individuals with dual Medicare–Medicaid coverage, average annual Medicare expenditures did not differ by dementia status ($8,458 vs. $9,324, p = .19; Figure 3). Except for higher expenditures for home health services in dementia compared to non-dementia ($1,240 vs. $695, p < .001), all individual components of Medicare expenditure did not differ significantly by dementia status. Taken together, average annual total Medicare and Medicaid expenditures were $58,728 for individuals with dementia, substantially higher than $31,290 for those without dementia (p < .001).
Figure 3.
Average annual Medicare and Medicaid expenditures per person among individuals with dual Medicare and Medicaid coverage, by dementia status.
Participant Characteristics Associated With Medicaid Coverage and Expenditure
Table 2 shows parameter estimates and marginal effects using two-part models of participant characteristics associated with Medicaid coverage and expenditures. Results show that after controlling for participant characteristics, individuals with dementia were more likely to be covered by Medicaid. Among those who had Medicaid, individuals with dementia also incurred higher spending than those without dementia. Combining both parts of the model, the overall effect of dementia was a $6,278 increase in average annual Medicaid spending after controlling for other characteristics.
Table 2.
Two-part Model Estimates of Average Annual Medicaid Expenditures
| Logistic regressionCoef. (SE) [95% CI] |
p | Generalized linear modelCoef. (SE) [95% CI] |
p | Average marginal effects$ (SE) [95% CI] |
p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Dementia | 0.853 | *** | <.001 | 0.391 | ** | .003 | 6,278 | *** | <.001 |
| (0.230) | (0.130) | (1,518) | |||||||
| [0.401, 1.304] | [0.136, 0.646] | [3,302, 9,253] | |||||||
| Age | –0.004 | .780 | 0.058 | *** | <.001 | 527 | *** | <.001 | |
| (0.014) | (0.008) | (112) | |||||||
| [–0.031, 0.023] | [0.041, 0.074] | [307, 747] | |||||||
| Female | –0.208 | .298 | 0.714 | *** | <.001 | 6,003 | *** | <.001 | |
| (0.200) | (0.132) | (1,579) | |||||||
| [–0.601, 0.184] | [0.456, 0.973] | [2,908, 9,098] | |||||||
| Black | –0.784 | *** | <.001 | 0.052 | .738 | –1,943 | .212 | ||
| (0.217) | (0.155) | (1,557) | |||||||
| [–1.208, –0.359] | [–0.252, 0.355] | [–4,994, 1,109] | |||||||
| Hispanic | 1.253 | *** | <.001 | 0.077 | .805 | 4,590 | .132 | ||
| (0.333) | (0.310) | (3,048) | |||||||
| [0.600, 1.906] | [–0.531, 0.684] | [–1,385, 10,564] | |||||||
| Married | 1.777 | *** | <.001 | –0.022 | .941 | 5,289 | .075 | ||
| (0.333) | (0.304) | (2,973) | |||||||
| [1.124, 2.430] | [–0.618, 0.574] | [–5,371, 1,116] | |||||||
| Years of schooling | –0.075 | *** | .001 | 0.033 | * | .031 | 71 | .650 | |
| (0.023) | (0.015) | (156) | |||||||
| [–0.120, –0.031] | [0.003, 0.062] | [–235, 377] | |||||||
| Below Federal Poverty Level | 3.002 | *** | <.001 | –0.774 | *** | .001 | 2,083 | .254 | |
| (0.232) | (0.223) | (1,826) | |||||||
| [2.547, 3.456] | [–1.211, –0.337] | [–1,496, 5,661] | |||||||
| <1 year before death | 0.569 | * | .042 | 0.448 | ** | .006 | 5,930 | ** | .002 |
| (0.280) | (0.164) | (1,896) | |||||||
| [0.021, 1.117] | [0.126, 0.770] | [2,213, 9,646] | |||||||
| # Comorbidities | 0.091 | * | .050 | 0.188 | *** | <.001 | 2,027 | *** | <.001 |
| (0.047) | (0.031) | (421) | |||||||
| [0.001, 0.182] | [0.126, 0.249] | [1,202, 2,853] | |||||||
| # Extrapyramidal signs | 0.005 | .886 | 0.054 | ** | .009 | 517 | * | .027 | |
| (0.034) | (0.021) | (233) | |||||||
| [–0.062, 0.072] | [0.013, 0.095] | [60, 974] |
In the first part of this model, a logistic regression was used to estimate the probability of Medicaid coverage using the full sample. Conditional on having Medicaid coverage, the second part of the model estimated characteristics associated with Medicaid expenditures using a generalized linear model (GLM) with gamma family and log link.
* p<.05, ** p<.01, *** p<.001.
Other characteristics associated with Medicaid spending included a small increase ($527) in Medicaid spending with increasing age, and $6,003 higher Medicaid spending in women than men, but no difference in Medicaid spending by ethnicity groups and marital status. Education and living below the poverty level had opposite effects on the likelihood of Medicaid coverage and Medicaid expenditures, resulting in nonsignificant relationships between these variables and overall Medicaid spending. Each additional comorbid condition was associated with a $2,027 increase in annual Medicaid spending. Finally, Medicaid spending was $5,930 higher among those who were observed within a year of death.
Discussion
In this study, we compared Medicare and Medicaid expenditures in a largely minority cohort of Medicare beneficiaries in New York City who have been clinically assessed for dementia. Before discussing results from the study, it is important to consider the community characteristics from which this sample is drawn. The Washington Heights-Inwood area is an ethnically diverse community that despite economic and environmental improvements since the 1970s continues to be a vulnerable community with limited income, poor health, low health literacy, and low insurance or insurance knowledge (US Census Bureau, 2019). The proportion of adults aged 18 or older who report not getting needed medical care (17%) is one of the highest in the city (Naidoo et al., 2018). Among those aged 65 years or older, 18.5% have a disability (US Census Bureau, 2019). Although not a representative sample of all Medicare beneficiaries in the United States, the WHICAP study participants were matched to US Census data of older adults living in the community (Mayeux et al., 2011). Our study adds to the handful of well-designed cohort studies that have been able to overcome major challenges in conducting population-based studies of dementia in minority communities to include sufficient diversity (Evans et al., 2003; Haan et al., 2003; Hall et al., 2009; Noble et al., 2017).
Substantial misidentification of dementia status using self-reported or claims-identification have been documented in a number of studies (Lin et al., 2010; Zhu et al., 2019). An earlier article using data from the same cohort showed that Medicare claims correctly identified only about half of clinically diagnosed dementia cases (Zhu et al., 2019). If we consider cost estimates derived from clinical diagnosis of dementia as true costs of disease, the study showed important and nuanced effects of claims misidentification of dementia on estimation of Medicare expenditures: On a per-person level, the study found an overestimation of $3,487 per person annually when Medicare claims were used to identify dementia. On an aggregated level, however, total annual expenditures for all beneficiaries with claims-identified dementia were $258,707 lower than that for all those who were clinically diagnosed, suggesting an overall underestimation of total Medicare expenditures if claims were used to identify dementia. Results from these studies contribute to the literature by using data from participants prospectively followed with careful in-person clinical diagnoses, thereby avoiding such biases.
We began the study by restricting our analyses to using Medicare claims only, similar to existing studies in which Medicaid coverage status was not taken into account. Not surprisingly, results showed substantially higher average annual Medicare expenditures among individuals with dementia compared to those without dementia. Results also showed that the proportion of people diagnosed with dementia was significantly higher among those with full Medicaid coverage than among those without Medicaid coverage, and individuals with dual Medicare and Medicaid coverage differed substantially from those who did not have Medicaid. We therefore examined expenditures separately by individuals’ Medicaid coverage.
In the sample of Medicare beneficiaries with no Medicaid coverage, Medicare expenditures were substantially higher among individuals with dementia than those without dementia, largely due to substantially higher expenditures for inpatient and home health services incurred by those with dementia. The excess expenditures in Medicare spending for those with dementia were lessened by somewhat lower expenditures for outpatient and physician services. These findings are consistent with those reported in existing studies (Ayyagari et al., 2007; Hill et al., 2002).
Our study adds to the literature by highlighting Medicaid’s contribution to covering the cost of dementia care. Among dually covered beneficiaries, average annual total Medicare and Medicaid expenditures was $58,728 for individuals with dementia, nearly twice as much as that for those without dementia. Regardless of dementia status, the majority of the expenditures were paid for by Medicaid. Differences in expenditures for individuals with dementia compared to those without dementia were almost entirely born by Medicaid. The largest differences were from services critically important to dementia patients but not covered by Medicare, including personal care and home health visits, which together accounted for 39% of total Medicaid costs among individuals with dementia. Although the WHICAP study did not follow participants after long-stay nursing home placement, costs of short-stay nursing home care were still the third most expensive category among individuals with dementia. Although inpatient care is covered by Medicare, dually covered individuals with dementia incurred an additional $1,979 for inpatient services, more than twice as much as individuals without dementia. Unlike the cost differences in Medicaid, we found no overall differences in Medicare expenditures among individuals with dementia versus those without dementia in this sample. Of all types of services covered by Medicare, only home health expenditures were higher in individuals with dementia than those without dementia, although the amount of Medicare expenditure was low.
Focusing on the effects of dementia on Medicaid spending, our results showed that dementia had strong independent effects on increased likelihood of Medicaid coverage. Among those with Medicaid coverage, dementia also was associated with higher Medicaid spending after controlling for participants’ demographic and socioeconomics characteristics. Despite differences in geography and time, estimates of annual excess Medicaid spending of $6,278 per person due to dementia from the current study are similar in magnitude to previous estimates reported from several other states (Bharmal et al., 2012; Geldmacher et al., 2013; Martin et al., 2000; Menzin et al., 1999). In New York State, an estimated 400,000 individuals aged 65 or older have AD in 2018 (New York State Coordinating Council for Services Related to Alzheimer’s Disease and Other Dementias, 2017), with Medicaid covering about one in four Medicare beneficiaries (Kaiser Family Foundation, November 2018). Our estimate suggests that excess Medicaid spending due to dementia at $628 million in NYS.
These results have important policy implications. It is well known that separate funding streams and misalignment in the programs’ benefits and services offer little incentives to deliver care efficiently, leading to little to no coordination of care for dually covered beneficiaries. To make informed decisions on where cost savings might be achieved, it is important to simultaneously characterize Medicare and Medicaid expenditures in dementia. This will be especially key to calculations of cost-effectiveness of home and community-based services, a priority as states are shifting Medicaid resources away from institutional care. Our study highlights the significant added contribution of Medicaid spending for dementia care. Delineating Medicare and Medicaid expenditures is particularly important in the current contentious health care policy debates. The vast majority of studies that do not include Medicaid spending may substantially underestimate increased total payer costs related to dementia care. As long-term care shifts away from the nursing home setting, we need to have complete understanding of the costs of care in the community which includes Medicare and Medicaid expenses. This is especially critical amid a growing dementia population that requires long-term care.
The study has several limitations. First, we emphasize that there may be unique patterns of health care use and expenditures in this sample drawn from an ethnically diverse, predominantly Hispanic and black population living in one urban community in New York City. For example, Hispanic caregivers have historically been less likely than non-Hispanic caregivers to place their family members in nursing homes, even after accounting for health and socioeconomic characteristics (Thomeer, Mudrazija, & Angel, 2015). As the WHICAP study did not follow participants after nursing home placement, there may be underestimation of Medicaid expenditures. The magnitude of the underestimation will be an important topic for future investigation. As home-based care expands and individuals with dementia live at home for longer periods of time, Medicaid spending for patients with dementia among those who reside in the community can only become more important. It is imperative that we examine health care expenditures beyond Medicare. Second, individuals in this study all had full Medicare FFS coverage for the majority of time during the study. Caution should be exercised in generalizing our results to other populations, such as those enrolled in Medicare Advantage. New York is one of the most generous states in terms of Medicaid coverage and services, with few or no limitations on service availability (Albert, Simone, Brassard, Stern, & Mayeux, 2005). Variation in Medicaid eligibility rules, benefits and payment rates, clinical practice patterns, and other parameters differ across states. Comparisons to other states are not advised. Third, we only had data on Medicaid coverage, not Medicaid eligibility. We are unable to address who is eligible or why some who may be eligible did not enroll. Fourth, we note that expenditures reported in this study only reflect Medicare and Medicaid payments and do not include expenditures by private insurance, informal caregiving, and opportunity costs to patients or caregivers and out-of-pocket costs, which exert tremendous burden. Last, it should always be noted that the relationships reported in this study are associations, not causal.
One of the important developments in the health care sector in recent years was the implementation of the Affordable Care Act (ACA), which included major expansions of health insurance coverage through Medicaid and private health insurance Marketplaces in 2014 (Lassman et al., 2017; Martin, Hartman, Washington, Catlin, & National Health Expenditure Accounts, 2017). CMS recently funded demonstration projects to improve care and control costs for dual-eligible beneficiaries (Kaiser Commission of Medicaid and the Uninsured, 2015). Ten of the 13 states, New York among them, agreed to test capitated models that provide the full range of Medicare and Medicaid benefit to enrollees (Centers for Medicare & Medicaid Services, 2017). Although the demonstrations offer potential opportunities to improve care coordination, lower program costs, and achieve outcomes such as better health and the increased use of home and community-based care, changes in care delivery systems may also increase the vulnerability of dual eligible. Our data predates the ACA and precludes us from examining its effects on utilization and expenditures of this population. Future studies with more updated data will be needed to examine the effects of ACA on dementia spending.
Despite these limitations, our work provides important insight regarding the cost of dementia in diverse, extremely vulnerable population and allows us to understand cost differences due to dementia-related to Medicaid. Results highlight the importance of Medicaid in covering the cost of care of dementia patients. Estimates are further enhanced by using clinical diagnosis to more accurately reflect the true cost of dementia. In the coming decades, policymakers will be challenged to meet the needs of this growing population while managing cost.
Funding
This research was supported by grants from the National Institute on Aging (AG07370, AG037212). Dr. Zhu is also supported by the Department of Veterans Affairs, Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. All authors report no conflict of interest.
Declarations of Interest
None.
References
- Administration for Community Living (2019a). Profile of African Americans Age 65 and Over: 2017. Washington DC: 20201 Retrieved from https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2017OAProfileAfAm508.pdf. [Google Scholar]
- Administration for Community Living (2019b). Profile of Hispanic Americans Age 65 and Over: 2017. Washington DC: 20201 Retrieved from https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2017OAProfileHA508.pdf. [Google Scholar]
- Albert S. M., Simone B., Brassard A., Stern Y., & Mayeux R (2005). Medicaid home care services and survival in New York City. The Gerontologist, 45, 609–616. doi:10.1093/geront/45.5.609 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association (2019). Alzheimer’s disease facts and figures. Alzheimers Dement, 15, 321–387. doi:10.1016/j.jalz.2019.01.010 [Google Scholar]
- Ayyagari P., Salm M., & Sloan F. A (2007). Effects of diagnosed dementia on Medicare and Medicaid program costs. Inquiry, 44, 481–494. doi:10.5034/inquiryjrnl_44.4.481 [DOI] [PubMed] [Google Scholar]
- Bharmal M. F., Dedhiya S., Craig B. A., Weiner M., Rosenman M., Sands L. P., … Thomas J (2012). Incremental dementia-related expenditures in a medicaid population. American Journal of Geriatric Psychiatry, 20, 73–83. doi:10.1097/JGP.0b013e318209dce4 [DOI] [PubMed] [Google Scholar]
- Blessed G., Tomlinson B. E., & Roth M (1968). The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry, 114, 797–811. doi:10.1192/bjp.114.512.797 [DOI] [PubMed] [Google Scholar]
- Bradley C. J., Dahman B., Bataki P. M., & Koroukian S (2010). Incremental value of using Medicaid claim files to study comorbid conditions and treatments in dually eligible beneficiaries. Medical Care, 48, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccaneer (2011). Chronic Condition Data Warehouse: Getting Started with CMS Medicare Administrative Research Files: A Technical Guidance Paper https://www.ccwdata.org/web/guest/technical-guidance-documentation
- Bureau of Labor Statistics (2012). Consumer price index Retrieved from http://www.bls.gov/cpi/home.htm
- Cameron A., & Trivedi P (2010). Microeconometrics using stata: revised edition (2nd ed). College Station, TX: Stata Press; (March 9, 2010). [Google Scholar]
- Centers for Medicare and Medicaid Services (2017). Financial alignment initiative Retrieved from https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-Medicaid-Coordination-Office/FinancialAlignmentInitiative/FinancialModelstoSupportStatesEffortsinCareCoordination.html.
- Chronic Condition Data Warehouse (October 2017). Medicaid Analytic eXtract Files (MAX) User Guide Retrieved from https://www.ccwdata.org/documents/10280/19002246/ccw-max-user-guide.pdf
- Cosentino S., Scarmeas N., Helzner E., Glymour M. M., Brandt J., Albert M., … Stern Y (2008). APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology, 70, 1842–1849. doi:10.1212/01.wnl.0000304038.37421.cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb P., & Norton E. C (2018). Modeling health care expenditures and use. Annual Review of Public Health, 39, 489–505. doi:10.1146/annurev-publhealth-040617-013517 [DOI] [PubMed] [Google Scholar]
- Evans D. A., Bennett D. A., Wilson R. S., Bienias J. L., Morris M. C., Scherr P. A., … Schneider J (2003). Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Archives of Neurology, 60, 185–189. doi:10.1001/archneur.60.2.185 [DOI] [PubMed] [Google Scholar]
- Garfield R., Musumeci M., Reaves E., & Damico A (2015). Medicaid’s role for Medicare beneficiaries. Menlo Park, CA: Retrieved from: https://www.kff.org/medicaid/issue-brief/medicaids-role-for-medicare-beneficiaries/ [Google Scholar]
- Geldmacher D. S., Kirson N. Y., Birnbaum H. G., Eapen S., Kantor E., Cummings A. K., & Joish V. N (2013). Pre-diagnosis excess acute care costs in Alzheimer’s patients among a US Medicaid population. Applied Health Economics and Health Policy, 11, 407–413. doi:10.1007/s40258-013-0038-9 [DOI] [PubMed] [Google Scholar]
- Haan M. N., Mungas D. M., Gonzalez H. M., Ortiz T. A., Acharya A., & Jagust W. J (2003). Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. Journal of the American Geriatrics Society, 51, 169–177. doi:10.1046/j.1532-5415.2003.51054.x [DOI] [PubMed] [Google Scholar]
- Hall K. S., Gao S., Baiyewu O., Lane K. A., Gureje O., Shen J., … Hendrie H. C (2009). Prevalence rates for dementia and Alzheimer’s disease in African Americans: 1992 versus 2001. Alzheimer’s & Dementia, 5, 227–233. doi:10.1016/j.jalz.2009.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. W., Futterman R., Duttagupta S., Mastey V., Lloyd J. R., & Fillit H (2002). Alzheimer’s disease and related dementias increase costs of comorbidities in managed Medicare. Neurology, 58, 62–70. doi:10.1212/wnl.58.1.62 [DOI] [PubMed] [Google Scholar]
- Hurd M. D., Martorell P., Delavande A., Mullen K. J., & Langa K. M (2013). Monetary costs of dementia in the United States. New England Journal of Medicine, 368, 1326–1334. doi:10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkowitz E., Kane R. L., Gaugler J. E., MacLehose R. F., Dowd B., & Kuntz K. M (2017). Societal and family lifetime cost of dementia: implications for policy. Journal of the American Geriatrics Society, 65, 2169–2175. doi:10.1111/jgs.15043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Commission of Medicaid and the Uninsured (2015). Financial and Administrative Alignment Demonstrations for Dual Eligible Beneficiaries Compared: States with Memoranda of Understanding Approved by CMS Retrieved from https://www.kff.org/medicaid/issue-brief/financial-and-administrative-alignment-demonstrations-for-dual-eligible-beneficiaries-compared-states-with-memoranda-of-understanding-approved-by-cms/
- Kaiser Family Foundation (November 2018). Medicaid in New York Retrieved from http://files.kff.org/attachment/fact-sheet-medicaid-state-NY
- Kasper J. D., Freedman V. A., Spillman B. C., & Wolff J. L (2015). The disproportionate impact of dementia on family and unpaid caregiving to older adults. Health Affairs (Project Hope), 34, 1642–1649. doi:10.1377/hlthaff.2015.0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassman D., Sisko A. M., Catlin A., Barron M. C., Benson J., Cuckler G. A., … Whittle L (2017). Health spending by state 1991-2014: measuring per capita spending by payers and programs. Health Affairs (Project Hope), 36, 1318–1327. doi:10.1377/hlthaff.2017.0416 [DOI] [PubMed] [Google Scholar]
- Lied T. R. (2006). Dually eligible enrollees: 2002. Health Care Financing Review, 27, 137–144. [PMC free article] [PubMed] [Google Scholar]
- Lin P. J., Kaufer D. I., Maciejewski M. L., Ganguly R., Paul J. E., & Biddle A. K (2010). An examination of Alzheimer’s disease case definitions using Medicare claims and survey data. Alzheimer’s & Dementia, 6, 334–341. doi:10.1016/j.jalz.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Martin A. B., Hartman M., Washington B., & Catlin A.; National Health Expenditure Accounts Team (2017). National health spending: faster growth in 2015 as coverage expands and utilization increases. Health Affairs (Project Hope), 36, 166–176. doi:10.1377/hlthaff.2016.1330 [DOI] [PubMed] [Google Scholar]
- Martin B. C., Ricci J. F., Kotzan J. A., Lang K., & Menzin J (2000). The net cost of Alzheimer disease and related dementia: a population-based study of Georgia Medicaid recipients. Alzheimer Disease and Associated Disorders, 14, 151–159. doi:10.1097/00002093-200007000-00006 [DOI] [PubMed] [Google Scholar]
- Mayeux R., Reitz C., Brickman A. M., Haan M. N., Manly J. J., Glymour M. M., … Morris J. C (2011). Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment-Part 1. Alzheimer’s & Dementia, 7, 15–34. doi:10.1016/j.jalz.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., & Stadlan E. M (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34, 939–944. doi:10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- Menzin J., Lang K., Friedman M., Neumann P., & Cummings J. L (1999). The economic cost of Alzheimer’s disease and related dementias to the California Medicaid program (“Medi-Cal”) in 1995. The American Journal of Geriatric Psychiatry, 7, 300–308. doi:10.1097/00019442-199911000-00005 [PubMed] [Google Scholar]
- Murman D. L., Chen Q., Powell M. C., Kuo S. B., Bradley C. J., & Colenda C. C (2002). The incremental direct costs associated with behavioral symptoms in AD. Neurology, 59, 1721–1729. doi:10.1212/01.wnl.0000036904.73393.e4 [DOI] [PubMed] [Google Scholar]
- Naidoo M., Traore K., Culp G., King L., Lopez C., Hinterland K., ... Gwynn R (2018). Community Health Profiles 2018 Map Atlas Retrieved from https://www1.nyc.gov/assets/doh/downloads/pdf/data/2018-chp-atlas.pdf.
- New York State Coordinating Council for Services Related to Alzheimer’s Disease and Other Dementias (2017). Report to Governor Andrew M. Cuomo and the New York State Legislature Retrieved from https://www.alznys.org/get-involved/2017%20coord%20council%20annaul%20report.pdf.
- Nguyen V.(March 2017). Fact Sheet: Long-Term Support and Services Retrieved from https://www.aarp.org/content/dam/aarp/ppi/2017-01/Fact%20Sheet%20Long-Term%20Support%20and%20Services.pdf
- Noble J. M., Schupf N., Manly J. J., Andrews H., Tang M. X., & Mayeux R (2017). Secular trends in the incidence of dementia in a multi-ethnic community. Journal of Alzheimer’s Disease: JAD, 60, 1065–1075. doi:10.3233/JAD-170300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremus M., & Aguilar S. C (2011). A systematic review to assess the policy-making relevance of dementia cost-of-illness studies in the US and Canada. Pharmacoeconomics, 29, 141–156. doi:10.2165/11539450-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Peter G. Peterson Foundation. (2017). Budget basics: Medicaid. https://www.pgpf.org/budget-basics/budget-explainer-medicaid. Accessed September 7, 2019. [Google Scholar]
- Richards M., Marder K., Bell K., Dooneief G., Mayeux R., & Stern Y (1991). Interrater reliability of extrapyramidal signs in a group assessed for dementia. Archives of neurology, 48, 1147–1149. doi:10.1001/archneur.1991.00530230055021 [DOI] [PubMed] [Google Scholar]
- Scarmeas N., Levy G., Tang M. X., Manly J., & Stern Y (2001). Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology, 57, 2236–2242. doi:10.1212/wnl.57.12.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Social Security Administration (October 2018). Annual statistical report on the social security disability insurance program, 2017. Washington, DC: Retrieved from https://www.ssa.gov/policy/docs/statcomps/supplement/2014/3e.html#table3.e8. [Google Scholar]
- Stern Y., Andrews H., Pittman J., Sano M., Tatemichi T., Lantigua R., & Mayeux R (1992). Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Archives of Neurology, 49, 453–460. doi:10.1001/archneur.1992.00530290035009 [DOI] [PubMed] [Google Scholar]
- Thomeer M. B., Mudrazija S., & Angel J. L (2015). How do race and Hispanic ethnicity affect nursing home admission? Evidence from the Health and Retirement Study. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 70, 628–638. doi:10.1093/geronb/gbu114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Census Bureau (2019). American Community Survey, 2013–2017 American Community Survey 5-Year Estimates, SELECTED Demographics, Social, Economic, Housing, and Demographic Characteristics, generated using American FactFinder; <http://factfinder.census.gov>, (May 9, 2019).
- Yang Z., Zhang K., Lin P. J., Clevenger C., & Atherly A (2012). A longitudinal analysis of the lifetime cost of dementia. Health Services Research, 47, 1660–1678. doi:10.1111/j.1475-6773.2011.01365.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. W., Cosentino S., Ornstein K., Gu Y., Scarmeas N., Andrews H., & Stern Y (2015). Medicare utilization and expenditures around incident dementia in a multiethnic cohort. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70, 1448–1453. doi:10.1093/gerona/glv124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. W., Ornstein K. A., Cosentino S., Gu Y., Andrews H., & Stern Y (2019). Misidentification of dementia in medicare claims and related costs. Journal of the American Geriatrics Society, 67, 269–276. doi:10.1111/jgs.15638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. W., Scarmeas N., Torgan R., Albert M., Brandt J., Blacker D., … Stern Y (2006). Clinical features associated with costs in early AD: baseline data from the Predictors Study. Neurology, 66, 1021–1028. doi:10.1212/01.wnl.0000204189.18698.c7 [DOI] [PubMed] [Google Scholar]