Abstract
Objectives
Although educational attainment is related to cognitive function in later life, little is known about the mechanisms involved. This study assessed the independent mediating effects of two behavioral variables, physical and cognitive activity, on the association between educational attainment and cognitive function and change.
Methods
Data were derived from the three waves of the Midlife in the United States (MIDUS) study. Predictors (educational attainment) were from the 1995 baseline, mediators (physical and cognitive activities) were from the 2004 wave, and outcomes (cognitive function) were from the 2004 and 2013 waves. Conditional process modeling was applied using PROCESS in SPSS.
Results
There were both direct and indirect effects of educational attainment on level and change of executive function (EF) and episodic memory (EM). Physical activity and cognitive activity were both significant mediators for cognitive level. For mediators of change, however, cognitive activity was significant for EF and physical activity was significant for EM.
Discussion
Physical and cognitive activity are discussed as possible factors for protecting against cognitive decline in later life. The findings have implications for advancing supportive policies and practices related to maximizing the benefits of education and physical and cognitive activities for cognition in middle age and later life.
Keywords: Cognition, Education, Health, Mediation analysis
There is considerable evidence for wide individual differences in the extent of cognitive change in later life (Mella, Fagot, Renaud, Kliegel, & de Ribaupierre, 2018; Schaie, Willis, & Caskie, 2004). Studies investigating change in multiple cognitive domains reported individual differences in both the rate of change and variations in the patterns of change across a number of cognitive abilities (Mungas et al., 2010; Tucker-Drob, Johnson, & Jones, 2009). Many risk and protective factors for cognitive aging have been examined, including genetic, health, physical, behavioral, lifestyle, and sociodemographic contributors such as educational attainment (e.g., Alley, Suthers, & Crimmins, 2007; Hertzog, Kramer, Wilson, & Lindenberger, 2008; Salthouse, 2014).
Education and Cognitive Change
It has been suggested that educational experiences provide the foundation for continued intellectual stimulation across the life course, resulting in improved cognitive functioning in late adulthood (Wilson et al., 2009). However, there is still considerable controversy about the relationship between educational attainment and cognitive change. It remains unclear whether higher educational attainment slows the rate of cognitive decline over time in middle age and later life (Fritsch et al., 2001; Glymour et al. 2005). Epidemiologic evidence suggests that individuals with higher educational or occupational attainment have a great degree of cognitive reserve (CR) and show a reduced risk of developing Alzheimer’s disease and other forms of dementia (Hall et al., 2007; Stern, 2012). CR has been suggested to account for the delayed onset of behavioral manifestations of dementia among those with brain pathology (Stern, 2012). However, there is mixed evidence as to whether education is associated with the timing and extent of cognitive declines in normal aging (Stern, 2009, 2012). Some research suggests that higher educational attainment does not protect against cognitive decline (Le Carret, Lafont, Mayo, & Fabrigoule, 2003) or even results in a slightly faster rate of cognitive decline among those who develop dementia (Wilson et al., 2009).
Cognitive Benefits of Physical and Cognitive Activity
Of interest is whether there are modifiable factors that are related to educational attainment that can account for differences in cognitive aging. Education may cultivate the knowledge, skills, and ability necessary for continued participation in intellectually demanding activities (e.g., reading, taking courses) or health-promoting behaviors (e.g., physical exercise) well into middle and later adulthood. Higher educational attainment has been associated with greater participation in various lifestyle activities (Allet et al., 2016; Thrane, 2006), including physical activities and activities that are cognitively demanding (Lachman, Agrigoroaei, Murphy, & Tun, 2010).
The beneficial effects of maintaining an engaged lifestyle have been demonstrated across several studies, even when activities are introduced later in life (Bherer et al. 2013). On the basis of findings from cohort studies and short-term clinical trials, previous studies indicated that individuals who continuously engage in high level of physical activity and place significant demands on their intellectual resources may maintain or even enhance cognitive potential (Fratiglioni, Paillard-Borg, & Winblad, 2004; Lövdén, Bäckman, Lindenberger, Schaefer, & Schmiedek, 2010; Park et al., 2014). Compared to other forms of lifestyle activity, greater participation in intellectually demanding activities may be especially beneficial for cognitive function (Stine-Morrow et al., 2014). For instance, results of the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study, the first large-scale, randomized trial to test the long-term outcomes of cognitive training effects on prevention of decline in daily function, support the effectiveness of cognitive intervention in maintaining cognitive health over the long-term and indicate modest but detectable far transfer to instrumental activities of daily living, health-related quality of life, and driving outcomes (Tennstedt & Unverzagt, 2013). Conversely, activities low in cognitive stimulation, such as watching television, have been related to an increased risk of cognitive impairment (Wang et al., 2006). Moreover, activities low in cognitive demand may be more prevalent among those with lower educational attainment (Wilson et al., 2009).
The benefits of physical activity for older adults are well established (Kirk-Sanchez & McGough, 2014). There is compelling evidence that an active lifestyle has broad benefits for cognitive, physical, and psychological health among older adults (Smith et al., 2010). Physical activity can delay or prevent many chronic diseases, including heart disease, type 2 diabetes, some cancers and dementia, which have been associated with cognitive declines. The cognitive benefits of physical activity are also well documented (Erickson, Hillman, & Kramer, 2015). Zhu and colleagues (2017) investigated the association of objectively measured physical activity with incidence of cognitive impairment and longitudinal cognition among older adults using data from 6,452 participants in the United States and indicated that high level of physical activity was associated with lower risk of cognitive impairment and better maintenance of memory and executive function over time, particularly in white adults. Using nationally representative samples of participants aged 50 years and older from 11 European countries (Austria, Germany, Sweden, Denmark, Switzerland, the Netherlands, Belgium, France, Spain, Italy, and Greece), Aichberger and colleagues (2010) found cognitive benefits from both light intensity activity, such as leisurely walking, and higher intensity aerobic activity. Individuals who participated in any type of regular physical activity showed less cognitive decline after 2.5 years, especially when they engaged in vigorous activities more than once a week (Aichberger et al., 2010). In another study, Albinet, Boucard, Bouquet, and Audiffren (2010) reported that 12 weeks of aerobic training led to enhanced performance in executive control and increased heart rate variability in older men and women aged 65–78 years. These results suggest that aerobic exercise may be an important brain protective factor as people age. Across cognitive domains, there is evidence for exercise-related improvements for both executive functioning (e.g., processing speed; Frederiksen et al., 2015) and memory (e.g., spatial/episodic memory; Erickson et al., 2015). However, there is some evidence to suggest that these functions are distinctly influenced by physical activity, in that processes that require executive control, in contrast to memory, tend to exhibit more robust findings (Kramer et al., 1999; Smith et al., 2010).
Current Study
Although a number of studies have explored the independent contributions of physical or cognitive activities on cognition, only a few studies have explored these factors in combination (Sturman et al., 2005). Ghisletta, Bickel, & Lövdén (2006) found that activities such as reading a book and playing games were related to changes in perceptual speed, whereas other forms of engagement, for example, physical and social activities, were not associated with such changes. Using data from a large biracial community of older adults, Sturman and colleagues (2005) found that the beneficial effects of physical activity on the rate of cognitive decline over 6 years were reduced and no longer statistically significant when cognitive activity was adjusted and in analyses that eliminated persons with the lowest cognitive performance at baseline. Cognitive function was measured by the East Boston Tests of Immediate Memory and Delayed Recall, the Mini-Mental State Examination, and the Symbol Digit Modalities Test. They argued that physical activity alone does not protect against cognitive decline among older adults (Sturman et al., 2005). With data from 250 participants aged between 18 and 44 years old, the results from the INSIGHT study, a comprehensive, multidisciplinary brain training system, indicated that physical activity was more important than cognitive activity for adaptive reasoning and problem solving (Daugherty et al., 2018).
Given the varied findings about the relative benefits of physical and cognitive activity, the current study examined both forms of activity together to consider whether they have independent contributions to cognitive level and changes more than 9 years. In addition, although some research has examined mechanisms for exercise interventions, little attention has been paid to possible mediators in prospective longitudinal studies (Hertzog et al., 2008). Our study also considered whether physical and cognitive activity are mediators of the relationship between educational attainment and cognitive level and change. Those with higher education are expected to engage more frequently in both cognitive and physical activity. Specifically, we tested the following hypotheses: (a) education was expected to be positively related to both level and change in cognition, such that individuals with higher levels of educational attainment would demonstrate better performance on cognitive function and show less cognitive decline; (b) participants with higher levels of educational attainment were expected to report being more physically and cognitively active; (c) more frequent cognitive and physical activity was expected to be related to better cognitive performance, independent of education; and (d) finally, we predicted that the association between educational attainment and cognition would be mediated by both physical and cognitive activities.
Method
Participants
This study includes the three waves of the Midlife in the United States (MIDUS) national database. The first wave (MIDUS 1) was collected between 1995 and 1996 with 7,108 noninstitutionalized participants in the 48 contiguous states selected via random digit phone dialing (Brim, Ryff, & Kessler, 2004). The original participants ranged in age from 24 to 75 years (M = 46.40, SD = 13.00), had a mean education level of 13.21 years, and women made up 48.3% of the sample. Nine years later, the second wave (MIDUS 2) included data from about 75% (N= 4,963) of the respondents who participated in the follow-up study. As is typically found, those who participated at the second wave showed some differences on MIDUS 1 variables compared with those who dropped out of the study (Radler & Ryff, 2010). Compared to the dropouts, longitudinal participants were more highly educated, t(6,757) = 12.48, p < .001, mean years of education 14.06 versus 13.21; were more likely to be women, 53.8% versus 48.3%, χ2(1) = 17.49, p < .001; and had higher self-rated health, t(6,759) = 10.42, p < .001, 3.61 versus 3.33 on a 5-point scale where 1 = poor, 5 = excellent. Dropouts did not differ from longitudinal participants in terms of age at MIDUS 1, t(6,711) = .70, p = .48, 46.14 versus 46.39 years old. The average age of the longitudinal participants (the sample we included in this study) was 58.69 (SD = 11.37), with 53% women. A majority of the participants (93%) were white, and more than 70% of the participants were married or cohabiting. The average education level of the participants at MIDUS 2 (M2) was 14.32 years (SD = 2.62). MIDUS 3 (M3) was conducted 9.12 years later, on average (SD = 0.53). Of the sample from M2, 76.9% of those eligible (N = 3,294) were retested (Hughes, Agrigoroaei, Jeong, Bruzzese, & Lachman, 2018). The descriptive statistics for the M2 sample and the participants who had longitudinal data for cognitive variables are shown in Supplementary Table A.
At M3, participants ranged in age from 42 to 92 years (M = 64.30, SD = 11.2) and had a mean education level of 14.6 years (SD = 2.6). Women made up 55.3% of the sample. The Brief Test of Adult Cognition by Telephone (BTACT; the psychometric properties of the BTACT are reported in Lachman, Agrigoroaei, Tun, & Weaver, 2014) was administered for the first time at M2, in a separate telephone interview, with a completion rate of 86% (N = 4,206) of eligible participants. As with M2, at M3 the BTACT was administered in a separate telephone interview, with a completion rate of 82% (N = 2,693) of eligible participants. The cognitive tests at M2 and M3 were conducted on average 9.32 years apart (SD = 0.45). About 85% of the survey sample at M2 (4,206 of 4,963 participants) and about 82% (2,693 of 3,294 participants) of the survey sample at M3 completed the cognitive phone interview. There were no significant demographic differences, including age, gender, education, race, marital status, and health, between participants who completed the cognitive phone interview and those who did not. Those who participated at the third wave showed some differences on M2 variables compared with those who dropped out of the study. Compared to the dropouts at M3, longitudinal participants were more highly educated, t(4,198) = 10.53, p < .001; M = 14.69 versus 13.83 years of education; were younger, t(4,204) = 5.11, p < .01, M = 55.20 versus 57.18 years old; and had higher self-rated health, t(4,204) = 11.09, p < .001, M = 3.68 versus 3.34 on a 5-point scale where 1 = poor, 5 = excellent. Dropouts did not differ from longitudinal participants in sex, 55.3% versus 52.5% female, χ2(1) = .14, p =.71. Compared to dropouts, longitudinal participants performed significantly better on all cognitive tests and factors at M2 (Hughes et al., 2018).
Dependent Variables
Episodic memory (EM) was measured by immediate and delayed free recall. Following exploratory and confirmatory factor analysis (Lachman et al., 2010, 2014), an EM composite factor score was computed as a standardized mean of the z-scored measures loading on the factor.
Executive function (EF) was measured by working memory (measured by backward digit span), verbal fluency (measured by category fluency), reasoning (measured by number series completion), executive functioning (measured by task-switching [Stop and Go Switch Task]) and speed of processing (measured by 30 seconds and counting task, or 30-SACT). An EF composite score was computed following exploratory and confirmatory factor analysis (Lachman et al., 2010). The EF factor score was computed as a standardized mean of the z-scored measures loading on the factor.
Both factor scores at M3 were standardized using the means and standard deviation from M2 to allow for examination of change. Change in EM and EF was analyzed by using M3 scores as the dependent variable and including M2 scores as a predictor.
Mediating Variables
Frequency of engaging in cognitive activities
The cognitive activity variable, from M2, was created by averaging the self-reported frequencies on a 6-point scale (1 = never, 2 = once a month, 3 = several times a month, 4 = once a week, 5 = several times a week, and 6 = daily) of engaging in four cognitive activities: reading books, magazines, or newspapers; doing word games such as crossword puzzles or Scrabble; attending educational lectures or courses; and writing (e.g., letters, journal entries, or stories; Lachman et al., 2010).
Frequency of physical activity
Physical activity, from M2, was created by twelve questions assessing the participants’ frequency of vigorous (e.g., competitive sports such as running, vigorous swimming, or high intensity aerobics; digging in the garden or lifting heavy objects) and moderate intensity (e.g., leisurely sports such as light tennis, slow or light swimming, low-impact aerobics, or golfing without a power cart; brisk walking or mowing the lawn with a walking lawnmower). These questions referred to frequency of physical activities separately for the summer and winter months, in three different settings (i.e., home, work, and leisure), with ratings from 1 = never, 2 = less than once a month, 3 = once a month, 4 = several times a month, 5 = once a week, and 6 = several times a week. Higher score indicates more frequent physical activity. We computed the mean score across summer and winter in all three settings for both moderate and vigorous intensity. We selected the activity intensity and setting with the maximum value to represent the highest frequency of physical activity across all intensity levels and domains (Cotter & Lachman, 2010).
Independent Variable and Covariates
Education, the independent variable from M1, was operationalized as the total number of years of formal schooling. Covariates, which were from M2, included age (coded in years), gender (men coded as 1, women coded as 2), marital status (married coded as 1, separated, divorced, widowed, and never married all coded as 0), race (Caucasian coded as 1, African American, and others coded as 0), and self-rated physical health, which was reported by participants on a 5-point scale ranging from 1 (poor) to 5 (excellent).
Statistical Analysis
Descriptive information and correlations were computed for all study variables. Before conducting main analyses, continuous predictor variables were mean-centered for moderation analyses so that the intercepts could be interpreted as the average scores. Conditional process modeling was applied using PROCESS in SPSS. Three criteria need to be satisfied to indicate a mediation relationship (Baron & Kenny, 1986): (a) the predictor variable needs to significantly predict the outcome variable, (b) the predictor variable must significantly predict the mediator variable(s), and (c) the mediator variable(s) must significantly predict the outcome variable while controlling for the predictor variable. If both direct and indirect effects remain significant, the association is said to be partially mediated (Hayes & Preacher, 2014).
Multiple mediation analyses were based on 1,000 bootstrapped samples using Hayes’ PROCESS Macro v2.15 (Hayes & Preacher, 2014), allowing for formal tests of the total, direct, and indirect effects of educational attainment on cognitive function at M2 and cognitive change from M2 to M3. The predictor variable was educational attainment at M1, the two mediator variables were physical activity and cognitive activity at M2, and covariates were from M2; outcomes were cognitive function at M2 and the change of cognitive function from M2 to M3.
Results
Findings of Univariate and Bivariate Analyses
The descriptive statistics for the sample at M2 and longitudinal sample are displayed in Supplementary Table A. On average, participants’ cognitive function level at M3 (EM: M = −0.04, SD = 0.98; EF: M = −0.15, SD = 0.74) was lower than their cognitive function level at M2 (EM: M = 0.02, SD = 0.99; EF: M = 0.06, SD = 0.97).
Means and correlations between all variables are shown in Table 1. Participants who were older, non-white, had lower income and education, poorer physical health, and lower physical and cognitive activity were more likely to show lower levels of EF and EM. Women had lower EF and men had lower EM.
Table 1.
Correlations for All Variables at Midlife in the United States (MIDUS) 2, N = 4,206
| Variables | M(SD) or % | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 55.43 (12.45) | — | |||||||||
| 2. Sex (% female) | 55.3 | .004 | — | ||||||||
| 3. Physical activity | 4.40 (1.31) | −.287** | −.055** | — | |||||||
| 4. Cognitive activity | 3.94 (0.85) | .027 | .028 | .118** | — | ||||||
| 5. Health | 3.54 (1.01) | −.184** | −.024 | .209** | .160** | — | |||||
| 6. Education | 14.28 (2.62) | −.144** | −.103** | .215** | .240** | .260** | — | ||||
| 7. Race/ethnicity (% white) |
91.9 | .095** | .000 | .077** | .065** | .065** | .040** | — | |||
| 8. Episodic memory | 0.02 (0.99) | −.339** | .222** | .187** | .135** | .183** | .213** | .058** | .157** | — | |
| 9. Executive function | 0.06 (0.97) | −.431** | −.113** | .300** | .238** | .298** | .412** | .128** | .317** | .433** | — |
Note: **p < .05; SD = standard deviation.
Findings From Mediation Models
Level of cognition
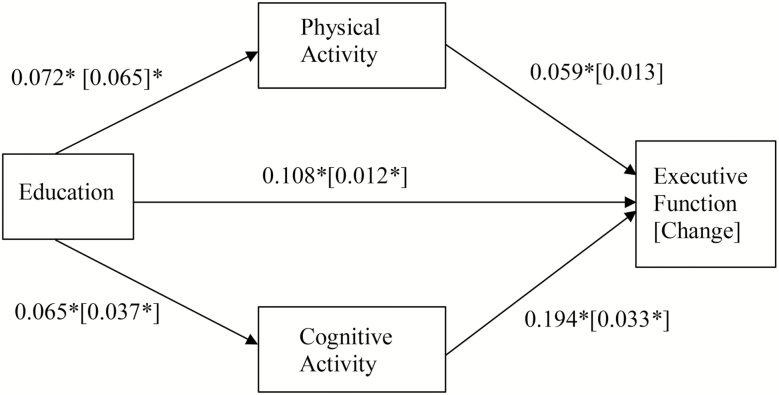
For the mediation models, coefficients and 95% confidence intervals (CIs) are provided (see Table 2). Model 1 tested whether educational attainment was related to EF at M2 and whether this relationship was mediated by physical activity and cognitive activity. For the mediational effect, kappa squared (κ2) is provided as a measure of effect size, as recommended by Preacher and Kelley (2011). With the guidelines of Cohen (1988), small, medium, and large effect sizes are stated as 0.01, 0.09, and 0.25, respectively, for mediation analysis. The total effects model that does not consider the effect of the mediator demonstrated that educational attainment was significantly related to EF (total effect: 0.124, 95% CI: 0.114, 0.134). As shown in Table 2, while controlling for age, sex, race, self-reported health, and household income, educational attainment was positively related to physical activity (0.072, 95% CI: 0.056, 0.087) and cognitive activity (0.065, 95% CI: 0.055, 0.075). Both physical activity and cognitive activity were also positively related to EF, and the direct path between educational attainment and EF was significant (direct effect: 0.108, 95% CI: 0.097, 0.118). The mediation analysis demonstrated that both physical activity (indirect effect: 0.004, 95% CI: 0.002, 0.006, κ2 = 0.012) and cognitive activity (indirect effect: 0.013, 95% CI: 0.009, 0.016, κ2 = 0.035) had small but significant partial mediation effects on the relationship between educational attainment and level of EF.
Table 2.
Coefficients, Standard Errors, and 95% Confidence Intervals for the Mediation Model for Executive Function and Episodic Memory
| Variables | Direct effects | Path coefficients | Indirect effect for EF | |||||
|---|---|---|---|---|---|---|---|---|
| EF | PA | CA | ||||||
| b(SE) | CI | b(SE) | CI | b(SE) | CI | b(SE) | CI | |
| Age | −0.029***(0.001) | −0.032,−0.027 | −0.027***(0.002) | −0.030, −0.024 | 0.005***(0.001) | 0.003, 0.006 | ||
| Sex (female) | −0.147***(0.026) | −0.198,−0.095 | −0.111***(0.041) | −0.190, −0.032 | 0.076***(0.024) | 0.028, 0.124 | ||
| Race/ethnicity (white) | 0.391***(0.045) | 0.304,0.479 | 0.323***(0.069) | 0.189, 0.461 | 0.070(0.042) | −0.025, 0.157 | ||
| Education | 0.108***(0.006) | 0.097,0.118 | 0.072***(0.008) | 0.056, 0.087 | 0.065***(0.005) | 0.055, 0.075 | ||
| Health | 0.116***(0.014) | 0.089,0.142 | 0.169*** (0.022) | 0.126, 0.207 | 0.067***(0.013) | 0.044, 0.093 | ||
| PA | 0.059***(0.011) | 0.038,0.079 | 0.004***(0.001) | 0.002, 0.006 | ||||
| CA | 0.194***(0.019) | 0.159,0.2228 | 0.013***(0.001) | 0.009, 0.016 | ||||
| EM | PA | CA | Indirect effect for EM | |||||
| b(SE) | CI | b(SE) | CI | b(SE) | CI | b(SE) | CI | |
| Age | −0.024***(0.001) | −0.026,−0.021 | −0.027***(0.002) | −0.030, −0.024 | 0.005***(0.001) | 0.003, 0.006 | ||
| Sex (female) |
0.484***(0.027) | 0.425,0.542 | −0.109***(0.041) | −0.188, −0.029 | 0.076***(0.024) | 0.028, 0.124 | ||
| Race/ethnicity (white) | 0.223***(0.048) | 0.122,0.324 | 0.323***(0.069) | 0.195, 0.468 | 0.070(0.042) | −0.025, 0.157 | ||
| Education | 0.060***(0.006) | 0.047,0.071 | 0.072***(0.008) | 0.056, 0.087 | 0.065***(0.005) | 0.055, 0.075 | ||
| Health | 0.057**(0.014) | 0.081,0.137 | 0.168*** (0.022) | 0.126, 0.207 | 0.067***(0.013) | 0.044, 0.093 | ||
| PA | 0.044***(0.013) | 0.020,0.068 | 0.003**(0.001) | 0.001, 0.005 | ||||
| CA | 0.107***(0.021) | 0.067,0.146 | 0.007***(0.001) | 0.004, 0.009 |
Notes: CA = cognitive activity; EF = executive function; EM = episodic memory; PA = physical activity.
*p < .1, **p < .05, ***p < .01.
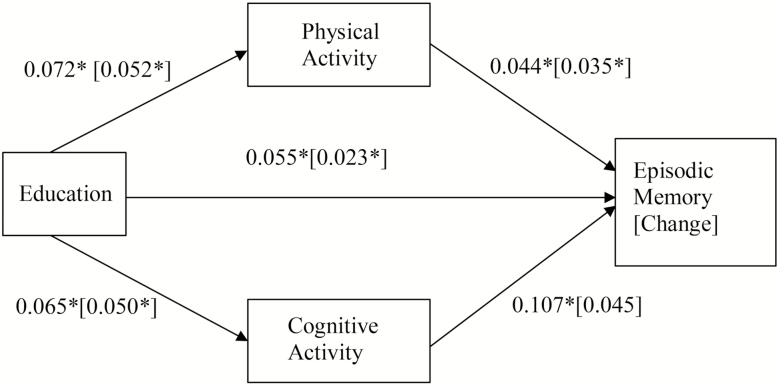
As shown in Table 2, the results also supported the partial mediation effects of physical activity and cognitive activity on the association between educational attainment and EM at M2. The direct effect between educational attainment and EM was significant (direct effect: 0.060, 95% CI: 0.047, 0.071). The indirect effects between educational attainment and EM through both physical activity (indirect effect: 0.003, 95% CI: 0.001, 0.005, κ2 = 0.008) and cognitive activity (indirect effect: 0.007, 95% CI: 0.004, 0.009, κ2 = 0.033) were also significant, indicating that both physical activity and cognitive activity had small but significant partial mediation effects on the relationship between educational attainment and level of EM.
Change in cognition
Model 3 and Model 4 tested whether physical activity and cognitive activity mediated the relationship of educational attainment and 9-year change in cognitive function (EM and EF; see Tables 3). To accomplish this, we added M2 cognition as a predictor variable, in addition to age, sex, race, marital status, and self-reported health, and used M3 cognition as dependent variables. The direct effects model found a significant relationship between educational attainment and EF change (0.012, 95% CI: 0.004, 0.020) and a significant relationship between cognitive activity and EF change (0.033, 95% CI: 0.003, 0.062). Participants with higher education showed less decline in EF and those with more frequent cognitive activity showed less decline in EF. The indirect effects for EF change through cognitive activity (indirect effect: 0.001, 95% CI: 0.001, 0.002, κ2 = 0.006) was also significant, indicating that cognitive activity had a small but significant mediation effects on the relationship between educational attainment and change in EF.
Table 3.
Coefficients, Standard Errors, and 95% Confidence Intervals for the Mediation Model for EF and EM Change
| Variables | Direct effects | Path coefficients | Indirect effect for EF | |||||
|---|---|---|---|---|---|---|---|---|
| EF | PA | CA | ||||||
| b(SE) | CI | b(SE) | CI | b(SE) | CI | b(SE) | CI | |
| Age | −0.015***(0.004) | −0.017,−0.013 | −0.016***(0.002) | −0.021, −0.011 | 0.007***(0.001) | 0.005, 0.010 | ||
| Sex (female) |
−0.028(0.020) | −0.065,0.009 | −0.092*(0.049) | −0.187, 0.004 | 0.057**(0.024) | 0.006, 0.108 | ||
| Race/ethnicity (white) |
0.094***(0.038) | 0.024,0.164 | 0.231**(0.092) | 0.051, 0.411 | 0.115**(0.042) | 0.019, 0.212 | ||
| Education | 0.012**(0.004) | 0.004,0.020 | 0.065***(0.011) | 0.043, 0.084 | 0.037***(0.005) | 0.026, 0.048 | ||
| Health | 0.033***(0.011) | 0.012,0.053 | 0.156*** (0.022) | 0.103, 0.208 | 0.014(0.013) | −0.014, 0.042 | ||
| PA | 0.013*(0.008) | −0.002,0.029 | 0.001(0.001) | −0.001, 0.002 | ||||
| CA | 0.033**(0.015) | 0.003,0.062 | 0.001***(0.001) | 0.001, 0.002 | ||||
| EM | PA | CA | Indirect effect for EM | |||||
| b(SE) | CI | b(SE) | CI | b(SE) | CI | b(SE) | CI | |
| Age | −0.014***(0.001) | −0.029,−0.008 | −0.017***(0.002) | −0.018, −0.009 | 0.007***(0.001) | 0.005, 0.010 | ||
| Sex (female) |
0.316***(0.042) | 0.183,0.124 | −0.153*(0.049) | −0.157, 0.0 | 0.057**(0.024) | 0.006, 0.108 | ||
| Race/ethnicity (white) |
0.056***(0.075) | 0.288,0.120 | 0.264**(0.092) | 0.051, 0.411 | 0.115**(0.042) | 0.019, 0.212 | ||
| Education | 0.023***(0.009) | 0.009,0.036 | 0.052***(0.011) | 0.043, 0.084 | 0.050***(0.005) | 0.026, 0.048 | ||
| Health | 0.056***(0.019) | 0.021,0.092 | 0.168*** (0.022) | 0.103, 0.208 | 0.025(0.013) | −0.003, 0.053 | ||
| PA | 0.035**(0.011) | 0.034,0.078 | 0.003***(0.001) | 0.001, 0.005 | ||||
| CA | 0.045*(0.019) | −0.159,0.233 | 0.002(0.001) | −0.001, 0.004 |
Notes. CA = cognitive activity; EF = executive function; EM = episodic memory; PA = physical activity.
*p < .1, **p < .05, ***p < .01.
The direct effect of educational attainment on EM change was significant as well (0.023, 95% CI: 0.009, 0.036), indicating that participants with higher education showed less decline in EM. As shown in Table 3, there was a significant relationship between physical activity and EM change (0.035, 95% CI: 0.034, 0.078), indicating that those with more frequent physical activity showed less decline in EM. The indirect effect for EM change through physical activity (indirect effect: 0.003, 95% CI: 0.001, 0.005, κ2 = 0.037) was also significant, indicating that physical activity had small but significant mediation effects on the relationship between educational attainment and change in EM. The results of the models for EF and EM level and change are presented in Figures 1 and 2, respectively. Note that for all four models we tested whether age was a moderator, and found no significant moderating effects for age.
Figure 1.
Mediation models for executive function (Model 1) and executive function change (Model 2). Model 1: the relationship of education and level of executive function, mediated by physical activity and cognitive activity. *p < .05. Model 2: the relationship of education and change in executive function, mediated by physical activity and cognitive activity. Model 2 parameters are presented in square brackets. *p < .05. Indirect effect of physical activity: 0.004, 95% CI: 0.002, 0.006, κ2 = 0.012 [0.001, 95% CI: −0.001, 0.002, κ2 = 0.001]; indirect effect of cognitive activity: 0.013, 95% CI: 0.009, 0.016, κ2 = 0.035 [0.001, 95% CI: 0.001, 0.002, κ2 = 0.006].
Figure 2.
Mediation models for episodic memory (Model 3) and episodic memory change (Model 4). Model 3: the relationship of education and level of episodic memory, mediated by physical activity and cognitive activity. *p < .05. Model 4: the relationship of education and change in episodic memory, mediated by physical activity and cognitive activity. Model 4 parameters are presented in square brackets. *p < .05. Indirect effect of physical activity: 0.003, 95% CI: 0.001, 0.005, κ2 = 0.008 [0.003, 95% CI: 0.001, 0.005, κ2 = 0.037]; indirect effect of cognitive activity: 0.007, 95% CI: 0.004, 0.009, κ2 = 0.033 [0.002, 95% CI: −0.001, 0.004, κ2 = 0.001].
Discussion
This study demonstrated the role of both physical and cognitive activity in the relationship between educational attainment and individual differences in cognitive function and change therein at middle age and later life in a large cohort from across the United States. The results were consistent with previous findings that individuals with higher educational attainment had better cognitive functioning in later adulthood (Wilson et al., 2009). Given the importance of understanding the disparate findings in previous literature on the association between educational attainment and cognitive change in later life, our study added to this discussion by showing both direct and indirect effects for the association between educational attainment and cognitive change.
Previous studies have shown wide variability in aging-related changes in cognition (Mella et al., 2018; Schaie et al., 2004). The present study examined two modifiable behavioral factors, both physical and cognitive activity, together as possible mediators that could mitigate cognitive decline in old age and clarify mechanisms linking education and cognition (Lachman et al., 2010). In line with our hypotheses and previous work (Hall et al., 2007; Stern, 2012), participants with higher levels of educational attainment reported being more active both physically and cognitively. The results provided support for the independent effects of physical activity and cognitive activity on level of both EF and EM. Consistent with previous findings that higher educational attainment was associated with greater participation in various lifestyle activities (Allet et al., 2016; Hess, 2014; Lachman et al., 2010; Thrane, 2006), our study also found that higher educational attainment was associated with greater levels of physical and cognitive activities. Education can provide advantages to older adults by increasing access to resources and opportunities for engaging in various physical activities and continued intellectual stimulation across the life course, thereby resulting in improved cognitive functioning in late adulthood (Wilson et al., 2009).
Although the effect sizes were small, and we cannot make direct causal inferences from the data, the findings that cognitive activity was significant as a predictor and mediator of EF change and that physical activity was a significant predictor and mediator for EM change adds to this literature, supporting the notion that physical and cognitive activity may play a role in protecting against age-related declines in cognition. We found no moderating effects of age on the mediating effects of physical activity and cognitive activity, suggesting that, contrary to predictions, the effects were of equal magnitude across the adult life span.
Physical activity has been found in other studies to be related to both cognitive function and cognitive change (Barnes, Yaffe, Satariano, & Tager, 2003; Renaud, Bherer, & Maquestiaux, 2010; Robinson & Lachman, 2018). Within the cognitive domain, there is evidence for exercise-related improvements for both executive functioning (e.g., processing speed; Frederiksen et al., 2015) and memory (e.g., spatial/episodic memory; Erickson et al., 2015). Older adults who have completed a physical activity program that produces significant increases in cardiorespiratory fitness often show enhanced EM (Barnes et al., 2003; Kramer et al., 1999; Smith et al., 2010). Although previous evidence shows EF is affected by physical activity (Kramer et al., 1999; Smith et al., 2010), our findings indicated that physical activity mediated the relationship between educational attainment and change in EM but not EF.
The results from our study that cognitive activity was an important factor for EF and change therein, beyond educational attainment and physical activity, supported and extended previous findings that environmental conditions—such as cognitive stimulation in the home—are most robustly associated with aspects of cognition such as language, attention, and other EMs (Greenfield & Moorman, 2018; Noble et al., 2015; Peyre et al., 2016).
The present results suggest the importance of taking a developmental and longitudinal approach to investigate individual differences in antecedent variables that lead to greater decrements for some persons and maintenance of high levels of functioning for others. Educational attainment is related to better cognitive functioning and physical and cognitive activity were supported as possible mechanisms. Because we were able to examine both the direct and indirect effects, we can conclude that educational attainment has not only a direct effect but also indirect effects through physical and cognitive activity. Furthermore, the results provide evidence that both cognitive and physical activity have independent effects for level and change. In previous research these two forms of activity have not typically been studied together. Moreover, this study was conducted with a large longitudinal sample to test whether physical and cognitive activity are unique contributors to cognitive function and change in middle age and later life. The study also includes a test battery that covers multiple key aspects of cognition that are associated with cognitive aging and sensitive to change across the adult life span (Lachman & Tun, 2008).
Limitations and Future Research
There are some limitations that should be considered as future studies continue and expand on this work. One limitation is a lack of objective measurements of both physical and cognitive activity. Although self-report measurements are useful to help gain insight into one’s level of physical and cognitive activity, they possess several limitations in terms of reliability and validity, such as a capacity to over- or underestimate true physical and cognitive activity and potential issues of recall and response bias (e.g., social desirability, inaccurate memory; Prince et al., 2008; Shephard, 2003). In addition, participants’ level of EF and EM may differentially influence the reporting of physical and cognitive activities. There is also the possibility that errors in reporting these activities may be correlated, so that any confounding between the self-report measures may be increased by relying on self-report. In future studies, mediation analysis with latent variables could include a correlated error term between cognitive and physical activities. Future work could also use objective assessments of activity and more items at each wave of assessment to help reduce measurement error (Barnett, van der Pols, & Dobson, 2005).
Another limitation is that the participants in the cognitive sample were not screened for cognitive impairment or dementia; all participants were included in the analysis. Although only a small percentage of participants had stroke, heart disease, or other factors that might affect cognitive function, a goal for future waves of MIDUS is to screen for cognitive impairment. In addition, there was a lack of racial diversity in the MIDUS sample; the vast majority of participants were non-Hispanic whites. Thus, additional research is needed to examine the generalizability of the findings in more diverse samples.
Implications for Policy and Practice
As Hertzog and colleagues (2008) indicated, previous research and practice focus has been on short-term gains in older adults, rather than proactive intervention at younger ages to produce long-term effects. In general, more attention should be given to how interventions in midlife could be structured to promote and enhance health and well-being, productivity, and cognitive development (Hertzog et al., 2008). Our findings regarding the mediating effect of physical activity and cognitive activity on the association between educational attainment and cognitive function over time have important implications for understanding the factors predicting cognitive functioning and cognitive change associated with aging. The study results suggest that engaging in physical and cognitive activity in midlife may be one explanation for how educational attainment affects level of cognitive functioning at old age. This study highlights the importance of engaging in physical and cognitive activity in midlife and older adulthood and can inform future policy work and intervention development aimed at enhancing physical and cognitive health in an aging population. However, a recent report suggests that only about 20% of adults meet the recommended guidelines for physical activity (Clarke, Ward, Norris, & Schiller, 2017). Policies and programs are needed to promote regular physical activity among older adults as a means to maintaining cognitive health.
This study also provided support for the benefits of cognitive activities for older adults including formal cognitive training and informal cognitive interventions. Park and colleagues (2014) found that older adults who learned quilting or digital photography had more memory improvement than those who only socialized or did less cognitively demanding activities. Formal cognitive trainings such as the ACTIVE should be supported and promoted among older adults (Brodziak, Wolińska, Kołat, & Różyk-Myrta, 2015). In the ACTIVE trials, healthy adults 65 years and older participated in 10 sessions of memory training, reasoning training, or processing-speed training. The sessions improved participants’ cognitive skills in the area in which they were trained. Most of these improvements persisted 10 years after the training was completed (Rebok et al., 2014).
Many physicians view mild cognitive impairment, age-related decline in memory, and other cognitive processes as a transitional phase between normal cognitive aging and dementia, although data regarding the actual rate of crossover to dementia are inconclusive (Blieszner, Roberto, Wilcox, Barham, & Winston, 2007). Understanding the factors that relate to cognitive function and cognitive change in midlife may be important for improving early interventions for dementia and related disorders. Consistent with previous findings that physical activity reduces the rate of cognitive decline in word list delayed recall and clinical dementia rating among older adults at risk (Lautenschlager et al., 2008), our findings indicated that physical activity reduced the rate of decline in EM. This is particularly important as the transition from midlife to older adulthood typically coincides with the transition from working to retirement, where regular activity from a steadier schedule can be disrupted and there is a need to find strategies to maintain consistent activity (Robinson & Lachman, 2018).
In summary, this study was a strong test of the independent mediation effects of physical activity and cognitive activity on the relationship between educational attainment and cognitive function. This study adds to the literature on the importance of both physical and cognitive activity in the cognitive function and cognitive change in middle age and later life. The longitudinal findings suggest that educational attainment, physical activity, and cognitive activity may be particularly important precursors to cognitive function that may have the potential for mitigating cognitive declines in middle age and later life.
Funding
This study was funded by the National Institute on Aging (NIA) Grants no. P01-AG020166 and U19-AG051426.
Conflict of Interest
None reported.
Supplementary Material
References
- Aichberger M. C., Busch M. A., Reischies F. M., Ströhle A., Heinz A., & Rapp M. A (2010). Effect of physical inactivity on cognitive performance after 2.5 years of follow-up. Longitudinal results from the Survey of Health, Ageing and Retirement (SHARE). GeroPsych, 23, 7–15. doi:10.1024/1662-9647/a000003 [Google Scholar]
- Albinet C. T., Boucard G., Bouquet C. A., & Audiffren M (2010). Increased heart rate variability and executive performance after aerobic training in the elderly. European Journal of Applied Physiology, 109, 617–624. doi:10.1007/s00421-010-1393-y [DOI] [PubMed] [Google Scholar]
- Allet L., Giet O., Barral J., Junod N., Durrer D., Amati F., … Puder J. J (2016). Educational level is related to physical fitness in patients with type 2 diabetes—a cross-sectional study. PloS One, 11, e0164176. doi:10.1371/journal.pone.0164176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley D., Suthers K., & Crimmins E (2007). Education and cognitive decline in older Americans: Results from the AHEAD sample. Research on Aging, 29, 73–94. doi:10.1177/0164027506294245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. M., & Kenny D. A (1986). The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. doi:10.1037%2F0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Barnes D. E., Yaffe K., Satariano W. A., & Tager I. B (2003). A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. Journal of the American Geriatrics Society, 51, 459–465. doi:10.1046/j.1532-5415.2003.51153.x [DOI] [PubMed] [Google Scholar]
- Barnett A. G., van der Pols J. C., & Dobson A. J (2005). Regression to the mean: What it is and how to deal with it. International Journal of Epidemiology, 34, 215–220. doi:10.1093/ije/dyh299 [DOI] [PubMed] [Google Scholar]
- Bherer L., Erickson K. I., & Liu-Ambrose T (2013). A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. Journal of Aging Research, 2013, 657508. doi:10.1155/2013/657508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blieszner R., Roberto K. A., Wilcox K. L., Barham E. J., & Winston B. L (2007). Dimensions of ambiguous loss in couples coping with mild cognitive impairment. Family Relations, 56, 196–209. doi:10.1111/j.1741-3729.2007.00452.x [Google Scholar]
- Brim O. G., Ryff C. D., & Kessler R. C. (Eds.). (2004). How healthy are we?: A national study of well-being at midlife. Chicago, IL: University of Chicago Press. [Google Scholar]
- Brodziak A., Wolińska A., Kołat E., & Różyk-Myrta A (2015). Guidelines for prevention and treatment of cognitive impairment in the elderly. Medical Science Monitor, 21, 585–597. doi:10.12659/MSM.892542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke T. C., Ward B. W., Norris T., & Schiller J. S (2017). Early release of selected estimates based on data from the National Health Interview Survey, January–September 2016: Lack of health insurance coverage and type of coverage. Hyattsville, ML: Center for Disease Control and Prevention. [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Cotter K. A., & Lachman M. E (2010). No strain, no gain: Psychosocial predictors of physical activity across the adult lifespan. Journal of Physical Activity and Health, 7, 584–594. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2972196/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A. M., Zwilling C., Paul E. J., Sherepa N., Allen C., Kramer A. F., … Barbey A. K (2018). Multimodal fitness and cognitive training to enhance fluid intelligence. Intelligence, 66, 32–43. doi:10.1016/j.intell.2017.11.001 [Google Scholar]
- Erickson K. I., Hillman C. H., & Kramer A. F (2015). Physical activity, brain, and cognition. Current Opinion in Behavioral Sciences, 4, 27–32. doi:10.1016/j.cobeha.2015.01.005 [Google Scholar]
- Fratiglioni L., Paillard-Borg S., & Winblad B (2004). An active and socially integrated lifestyle in late life might protect against dementia. Lancet. Neurology, 3, 343–353. doi:10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Frederiksen K. S., Verdelho A., Madureira S., Bäzner H., O’Brien J. T., Fazekas F., … Waldemar G.; LADIS Study (2015). Physical activity in the elderly is associated with improved executive function and processing speed: The LADIS Study. International Journal of Geriatric Psychiatry, 30, 744–750. doi:10.1002/gps.4220 [DOI] [PubMed] [Google Scholar]
- Fritsch T., McClendon M. J., Smyth K. A., Lerner A. J., Chen C. H., Petot G. J., … Friedland R. P (2001). Effects of educational attainment on the clinical expression of Alzheimer’s disease: Results from a research registry. American Journal of Alzheimer’s Disease and Other Dementias, 16, 369–376. doi:10.1177/153331750101600606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P., Bickel J. F., & Lövdén M (2006). Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 61, 253–261. doi:10.1093/geronb/61.5.P253 [DOI] [PubMed] [Google Scholar]
- Glymour M. M., Weuve J., Berkman L. F., Kawachi I., & Robins J. M (2005). When is baseline adjustment useful in analyses of change? An example with education and cognitive change. American Journal of Epidemiology, 162, 267–278. doi:10.1093/aje/kwi187 [DOI] [PubMed] [Google Scholar]
- Greenfield E. A., & Moorman S. M (2018). Childhood socioeconomic status and later life cognition: Evidence from the Wisconsin longitudinal study. Journal of Aging and Health. doi:10.1177/0898264318783489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. B., Derby C., LeValley A., Katz M. J., Verghese J., & Lipton R. B (2007). Education delays accelerated decline on a memory test in persons who develop dementia. Neurology, 69, 1657–1664. doi:10.1212/01.wnl.0000278163.82636.30 [DOI] [PubMed] [Google Scholar]
- Hayes A. F., & Preacher K. J (2014). Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology, 67, 451–470. doi:10.1111/bmsp.12028 [DOI] [PubMed] [Google Scholar]
- Hertzog C., Kramer A. F., Wilson R. S., & Lindenberger U (2008). Enrichment Effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest, 9, 1–65. doi:10.1111/j.1539-6053.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Hess T. M. (2014). Selective engagement of cognitive resources: Motivational influences on older adults’ cognitive functioning. Perspectives on Psychological Science, 9, 388–407. doi:10.1177/1745691614527465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M. L., Agrigoroaei S., Jeon M., Bruzzese M., & Lachman M. E (2018). Change in cognitive performance from midlife into old age: Findings from the Midlife in the United States (MIDUS) study. Journal of the International Neuropsychological Society, 24, 805–820. doi:10.1017/S1355617718000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk-Sanchez N. J., & McGough E. L (2014). Physical exercise and cognitive performance in the elderly: Current perspectives. Clinical Interventions in Aging, 9, 51–62. doi:10.2147/CIA.S39506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A. F., Hahn S., Cohen N. J., Banich M. T., McAuley E., Harrison C. R., … Colcombe A (1999). Ageing, fitness and neurocognitive function. Nature, 400, 418–419. doi:10.1038/22682 [DOI] [PubMed] [Google Scholar]
- Lachman M. E., Agrigoroaei S., Murphy C., & Tun P. A (2010). Frequent cognitive activity compensates for education differences in episodic memory. American Journal of Geriatric Psychiatry, 18, 4–10. doi:10.1097/JGP.0b013e3181ab8b62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman M. E., Agrigoroaei S., Tun P. A., & Weaver S. L (2014). Monitoring cognitive functioning: psychometric properties of the Brief Test of Adult Cognition by Telephone. Assessment, 21, 404–417. doi:10.1177/1073191113508807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman M. E., & Tun P. A (2008). Cognitive testing in large-scale surveys: Assessment by telephone. In Hofer S. M. & Alwin D. F., (Eds.), Handbook of cognitive aging: Interdisciplinary perspectives(pp. 506–523). Thousand Oaks, CA: Sage. [Google Scholar]
- Lautenschlager N. T., Cox K. L., Flicker L., Foster J. K., van Bockxmeer F. M., Xiao J., … Almeida O. P (2008). Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA, 300, 1027–1037. doi:10.1001/jama.300.9.1027 [DOI] [PubMed] [Google Scholar]
- Le Carret N., Lafont S., Mayo W., & Fabrigoule C (2003). The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Developmental Neuropsychology, 23, 317–337. doi:10.1207/S15326942DN2303_1 [DOI] [PubMed] [Google Scholar]
- Lövdén M., Bäckman L., Lindenberger U., Schaefer S., & Schmiedek F (2010). A theoretical framework for the study of adult cognitive plasticity. Psychological Bulletin, 136, 659–676. doi:10.1037/a0020080 [DOI] [PubMed] [Google Scholar]
- Mella N., Fagot D., Renaud O., Kliegel M., & de Ribaupierre A (2018). Individual differences in developmental change: Quantifying the amplitude and heterogeneity in cognitive change across old age. Journal of Intelligence, 6, 10. doi:10.3390/jintelligence6010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D., Beckett L., Harvey D., Farias S. T., Reed B., Carmichael O., … DeCarli C (2010). Heterogeneity of cognitive trajectories in diverse older persons. Psychology and Aging, 25, 606–619. doi:10.1037/a0019502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K. G., Engelhardt L. E., Brito N. H., Mack L. J., Nail E. J., Angal J., … Elliott A. J.; PASS Network (2015). Socioeconomic disparities in neurocognitive development in the first two years of life. Developmental Psychobiology, 57, 535–551. doi:10.1002/dev.21303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. C., Lodi-Smith J., Drew L., Haber S., Hebrank A., Bischof G. N., & Aamodt W (2014). The impact of sustained engagement on cognitive function in older adults: The Synapse Project. Psychological Science, 25, 103–112. doi:10.1177/0956797613499592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre H., Bernard J. Y., Hoertel N., Forhan A., Charles M.-A., De Agostini M., & Ramus F (2016). Differential effects of factors influencing cognitive development at the age of 5-to-6 years. Cognitive Development, 40, 152–162. doi:10.1016/j.cogdev.2016.10.001 [Google Scholar]
- Preacher K. J., & Kelley K (2011). Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods, 16, 93–115. doi:10.1037/a0022658 [DOI] [PubMed] [Google Scholar]
- Prince, S. A., Adamo, K. B., Hamel, M. E., Hardt, J., Connor Gorber, S., & Tremblay M. (2008). A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. The International Journal of Behavioral Nutrition and Physical Activity, 5, 56. doi:10.1186/1479-5868-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radler B. T., & Ryff C. D (2010). Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. Journal of Aging and Health, 22, 307–331. doi:10.1177/0898264309358617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok G. W., Ball K., Guey L. T., Jones R. N., Kim H.-Y., King J. W., … & Willis S. L (2014). Ten-year effects of the ACTIVE cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society, 62, 16–24. doi:10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud M., Bherer L., & Maquestiaux F (2010). A high level of physical fitness is associated with more efficient response preparation in older adults. TheJournals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 317–322. doi:10.1093/geronb/gbq004 [DOI] [PubMed] [Google Scholar]
- Robinson S. A., & Lachman M. E (2018). Perceived control and cognition in adulthood: The mediating role of physical activity. Psychology and Aging, 33, 769–781. doi:10.1037/pag0000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. A. (2014). Correlates of cognitive change. Journal of Experimental Psychology. General, 143, 1026–1048. doi:10.1037/a0034847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie K. W., Willis S. L., & Caskie G. I (2004). The Seattle longitudinal study: Relationship between personality and cognition. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 11, 304–324. doi:10.1080/13825580490511134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard R. J. (2003). Limits to the measurement of habitual physical activity by questionnaires. British Journal of Sports Medicine, 37, 197–206. doi:10.1136/bjsm.37.3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. J., Blumenthal J. A., Hoffman B. M., Cooper H., Strauman T. A., Welsh-Bohmer K., … Sherwood A (2010). Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine, 72, 239–252. doi:10.1097/PSY.0b013e3181 d14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2009). Cognitive reserve. Neuropsychologia, 47, 2015–2028. doi:10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet. Neurology, 11, 1006–1012. doi:10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow E. A. L., Payne B. R., Roberts B. W., Kramer A. F., Morrow D. G., Payne L., … Parisi J. M (2014). Training versus engagement as paths to cognitive enrichment with aging. Psychology and Aging, 29, 891–906. doi:10.1037/a0038244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman M. T., Morris M. C., Mendes de Leon C. F., Bienias J. L., Wilson R. S., & Evans D. A (2005). Physical activity, cognitive activity, and cognitive decline in a biracial community population. Archives of Neurology, 62, 1750–1754. doi:10.1001/archneur.62.11.1750 [DOI] [PubMed] [Google Scholar]
- Tennstedt S. L., & Unverzagt F. W (2013). The ACTIVE study: Study overview and major findings. Journal of Aging and Health, 25, 3S–20S. doi:10.1177/0898264313518133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrane C. (2006). Explaining educational-related inequalities in health: Mediation and moderator models. Social Science and Medicine (1982), 62, 467–478. doi:10.1016/j.socscimed.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Tucker-Drob E. M., Johnson K. E., & Jones R. N (2009). The cognitive reserve hypothesis: A longitudinal examination of age-associated declines in reasoning and processing speed. Developmental Psychology, 45, 431–446. doi:10.1037/a0014012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Zhou D. H., Li J., Zhang M., Deng J., Tang M., … Chen M (2006). Leisure activity and risk of cognitive impairment: The Chongqing aging study. Neurology, 66, 911–913. doi:10.1212/01.wnl.0000192165.99963.2a [DOI] [PubMed] [Google Scholar]
- Wilson R. S., Beckett L. A., Barnes L. L., Schneider J. A., Bach J., Evans D. A., & Bennett D. A (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging, 17, 179–193. doi:10.1037/0882-7974.17.2.179 [PubMed] [Google Scholar]
- Wilson R. S., Hebert L. E., Scherr P. A., Barnes L. L., Mendes de Leon C. F., & Evans D. A (2009). Educational attainment and cognitive decline in old age. Neurology, 72, 460–465. doi:10.1212/01.wnl.0000341782.71418.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Wadley V. G., Howard V. J., Hutto B., Blair S. N., & Hooker S. P (2017). Objectively measured physical activity and cognitive function in older adults. Medicine and Science in Sports and Exercise, 49, 47–53. doi:10.1249/MSS.0000000000001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.