Introduction
Reflex syncope, also known as neurocardiogenic or vasovagal syncope (VVS), is the most frequent etiology of syncope in young people without apparent cardiac or neurological pathology.1, 2, 3 Cardioinhibitory response with prolonged asystole and/or transient atrioventricular block induced by a massive vagal reflex is commonly observed in severely symptomatic cases. The treatment of VVS is challenging. Multicenter placebo-controlled trials published to date have shown limited efficacy of pharmacological and interventional therapies as well as cardiac pacing. According to the current guidelines, dual-chamber cardiac pacing is not recommended in young patients (below the age of 40).1, 2, 3
Recently, ablation of the parasympathetic ganglionated plexi (GP), so-called cardioneuroablation, has been shown to provide excellent short- and middle-term results in the treatment of syncope caused by cardioinhibitory functional bradycardia or functional atrioventricular block.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 As a relatively new therapeutic option, cardioneuroablation is considered but not yet recommended for treatment because of a low number of patients and lack of results from specific population-based studies.1, 2, 3,14,15
Case report
We present a case of a patient referred for consultation because of recurrent syncopal episodes without prodromal symptoms. The patient was a 39-year-old white man (weight: 100 kg; height: 176 cm), a farmer, without structural heart disease. Medical history revealed that seizures and syncope reoccurred at least twice a year since childhood, without prodromal symptoms. The episodes of syncope stopped for 4 years after successful zero-fluoroscopy radiofrequency ablation of very frequent idiopathic premature ventricular complexes from the junction of the left and right aortic valve cusps. During this time, the patient stopped antiepileptic therapy and resumed driving.
Within the previous 3 months, the patient experienced 10 prolonged severe syncopal episodes. He stopped driving and professional activity. He was referred to a regional center for cardiovascular diagnostic workup, with a recommendation of cardioneuroablation if the cardioinhibitory mechanism were confirmed. Echocardiography, electrocardiography, and Holter monitoring were normal. Obstructive sleep apnea and neurological disorders were excluded. Well-controlled mild hypertension was noted. However, a head-up tilt test revealed VVS with severe cardioinhibitory response (asystole of 23 seconds). Although cardioneuroablation had already been planned, the regional center proceeded with pacemaker implantation. During the subclavian access for pacemaker implantation, an asystole of about 90 seconds occurred with a seizure-like episode. After periprocedural short resuscitation the atrial lead implantation was abandoned owing to the first operator decision. Therefore, only a VVIR pacemaker (Endurity; Abbott, Abbott Park, IL) was implanted with the distal tip of the lead in the right ventricular apex. Moreover, during hospitalization, a single self-limited episode of paroxysmal atrial fibrillation was reported. Immediately after the VVIR implantation, the patient reported symptoms of pacemaker syndrome (dyspnea, chest discomfort, neck sensation) during resting sinus bradycardia (50–55 beats per minute [bpm]) and ventricular pacing.
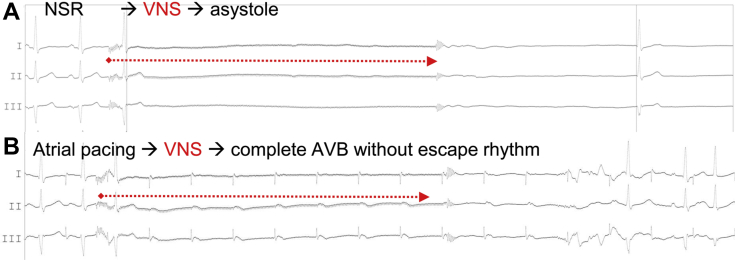
Eight weeks after the implantation, the patient was referred to another institution for cardioneuroablation with endovascular vagus nerve stimulation (VNS).6 One day before cardioneuroablation, autonomic tests (deep breathing test, Valsalva maneuver, carotid sinus massage) showed normal heart rhythm changes (>15 bpm); however, the atropine test (2 mg intravenous) resulted in a sudden increase of heart rhythm from 55 bpm to 120 bpm (>200%). On the next day, cardioneuroablation without pulmonary vein isolation was performed under general anesthesia. Three-dimensional electroanatomic mapping, navigation, catheter ablation, and pacing were performed using an integrated mobile electrophysiological system (EPMap-System; Pulmokard, Herdecke, Germany). Five seconds of VNS (according to Pachon’s approach: frequency: 30 Hz, pulse width: 50 ms, amplitude: 50 V) from the right and left internal jugular veins each time resulted in cardioinhibitory response, with more than 8 seconds of pause and/or atrioventricular block during atrial pacing (Figure 1A and B, Video 1). Ablation was performed using the empirical anatomic modified Pachon's approach with ablation delivered at 3-dimensional anatomical location of the GP and fragmented/fractionated potentials (targeting the GP in the proximity of the left superior, left inferior, right superior, and right inferior pulmonary veins and space between inferior vena cava-LA [IVC–LA] and proximal CS [coronary sinus] as well as superior vena cava and right atrial junction).5,6,15
Figure 1.
Electrocardiogram leads I, II, and III during 5-second vagus nerve stimulation (VNS) before cardioneuroablation. A: Normal sinus rhythm (NSR). B: Atrial pacing. VNS resulted in prolonged asystole or complete atrioventricular block (AVB).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hrcr.2020.04.021
The following is the Supplementary data to this article
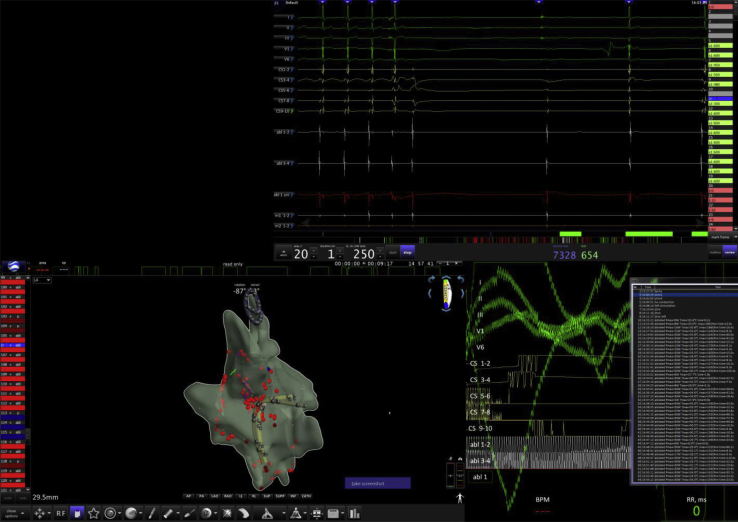
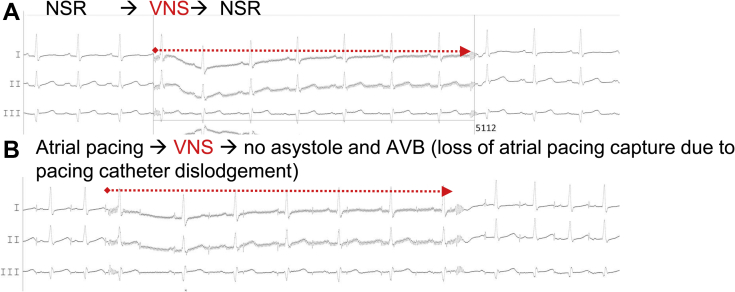
Afterwards, left and right atrial catheter ablation led to an increase in resting sinus heart rhythm (from 55 to 80 bpm) (Figure 2) and complete disappearance of cardioinhibitory response after VNS (Figure 3A and B, Videos 2 and 3). The patient became unresponsive to several atropine tests (2 mg intravenous infusion, performed immediately after the procedure and then at 2, 4, 12, 14, 56, and 60 weeks after the procedure; an increase of less than 20% from baseline 75–80 bpm after 10–20 minutes of observation). After the procedure, the patient suffered from recurrent pericarditis (at day 1 and after 1 and 3 months) associated with prior mild influenza-like symptoms, with noninvasive or invasive evacuation of pericardial effusion with transparent fluid of up to 800 mL. Although repeated pacemaker controls did not reveal lead perforation parameters, a computed tomography scan after 3 months confirmed drainage of ventricular lead tip into the pericardium. Therefore, recurrence of pericarditis was suspected to be associated with ventricular lead. Owing to persistent cardioneuroablation effect, the permanent pacemaker and ventricular lead were explanted. Then, as part of our standard approach after cardioneuroablation and pacemaker extraction, the patient was offered a 2-week cardiac rehabilitation program to confirm cardioneuroablation results as well as repeat autonomic testing.
Figure 2.
Three-dimensional electroanatomical simplified mapping of both left and right atrium for cardioneuroablation and electrocardiogram during vagus nerve stimulation.
Figure 3.
Electrocardiogram leads I, II, III during 5-second vagus nerve stimulation (VNS) after cardioneuroablation. A: Normal sinus rhythm (NSR). B: Atrial pacing. VNS resulted in prolonged asystole or complete atrioventricular block (AVB).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hrcr.2020.04.021
The following are the Supplementary data to this article
The patient’s resting sinus rhythm (SR) was over 70 bpm, and he showed negative response to the atropine test as well as autonomic tests (deep breathing test, Valsalva maneuver, carotid sinus massage; 3-minute orthostatic challenge showed heart rhythm changes <15 bpm), and no recurrence of presyncope and syncope. Ambulatory 24-hour Holter monitoring revealed normal SR (average 77 bpm, range 67–97 bpm, no atrial fibrillation) with appropriate chronotropic response during the exercise stress test (normal SR at rest: 91 bpm → maximum sinus tachycardia 141 bpm: 10 METs). Three months after explantation, the patient experienced transient influenza-like symptoms without significant pericardial effusion. Five months after cardioneuroablation and 1 month after pacemaker removal and cardiac rehabilitation, the patient was allowed to drive a car and a farming vehicle on public roads. He was scheduled for long-term monitoring with regular checkups every 6 months with noninvasive autonomic tests and atropine test, as needed. Within 14-month follow-up after cardioneuroablation, the patient did not report syncope or presyncope. One year after the procedure, the results of the head-up tilt test were negative.
Our case illustrates a complex and challenging management of a young patient with cardioinhibitory VVS and complications associated with pacemaker therapy. Invasive and noninvasive tests as well as a cardiac rehabilitation program showed persistent effects of cardioneuroablation that allowed the patient to return to his professional activity and driving.
Discussion
Recently, cardioneuroablation has been proposed as an effective and alternative method for the treatment of VVS, especially caused by cardioinhibitory or mixed reflex. Different research groups using various approaches to cardioneuroablation reported excellent short- and long-term outcomes in patients with VVS, functional atrioventricular block, or sick sinus syndrome.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 The 2018 European Society of Cardiology guidelines for syncope were the first official document to mention cardioneuroablation as a potential method for VVS treatment, but it did not receive any recommendation class.1
The endpoints of cardioneuroablation were different in all reported studies.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Pachon and colleagues4,5,15 defined the endpoints as elimination of the specific fractionated potentials in the right and left atrial regions overlapping the GPs and venous insertions. In the studies by Aksu and colleagues, the endpoints included elimination (<0.1 mV) of the atrial fractionated potentials above 300 Hz, elimination of parasympathetic response to high-frequency stimulation, a persistent increase in SR and the Wenckebach point, and complete elimination of functional atrioventricular block.10,14 Finally, Sun and colleagues9 validated the elimination of all vagal responses at each identified target as study endpoint.
Endovascular VNS has been proposed as a new method for an acute invasive assessment of vagal response during the procedure.6,15 However, the optimal technique for VNS, cardioneuroablation, and identification of GP location has not been established yet. For the identification of GP sites, spectral mapping, high-frequency stimulation, or anatomic approach have been used.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 We believe that in our case the previous catheter ablation might have had an unintentional effect on the parasympathetic aortic ganglion, which stopped syncopal recurrences for 4 years.
There are no widely accepted targets for acute SR acceleration following cardioneuroablation and for an increase in heart rhythm after the atropine test. In the latter, a 20% increase in SR (or above 100 bpm) is often used as a cutoff value. However, sinus rate obtained by atropine plus propranolol infusion performed prior to cardioneuroablation (at least 24–48 hours) could probably provide more accurate data, as it estimates the denervated, intrinsic heart rate.
Therefore, the extent of cardioneuroablation and its clinical effects should consider denervation, innervation, or complex modulation of the intrinsic cardiac neural system with an evaluation of parasympathetic and sympathetic balance.13, 14, 15 The results of a meta-analysis on cardioneuroablation confirmed that it may be considered an alternative treatment method; however, large-scale randomized controlled trials and registries are needed to provide more evidence for its use in patients with sick sinus syndrome, VVS, atrial fibrillation alone, and tachy-brady syndrome.1,8,10, 11, 12, 13, 14, 15
Moreover, cardioneuroablation could be an option in other patients with VVS and complications or failure of pacemaker therapy. Finally, VNS requires a standardized approach with a minimally invasive test protocol, dedicated neurostimulator, and/or catheter, as well as training of interventional electrophysiologists and cardiac surgeons.
Acknowledgments
The authors thank Professor Andrzej Kutarski from Zamość Cardiovascular Center for his consultation and pacemaker explantation.
Footnotes
Victor Ton is an employee of Pulmokard and chief engineer for the development and training program of the EPMap-System. Sebastian Stec is the coauthor of several patents for diagnostic and ablation catheters and a stockholder of Medinice S.A.; no products of this company were used during the reported procedure.
Key Teaching Points.
-
•Cardioinhibitory vasovagal syncope (VVS) in young patients remains challenging and requires patient-tailored therapy.
-
•Cardioneuroablation is a currently evolving technique that can be offered to patients with severe cardioinhibitory VVS who do not respond to or who experience complications from a recommended therapy.
-
•Vagus nerve stimulation (VNS), atropine test, and Holter monitoring are valuable tools to evaluate the efficacy of cardioneuroablation.
-
•Standardized criteria for patient selection and assessment of long-term outcomes of cardioneuroablation, as well as for VNS protocol, need to be further developed and validated.
Appendix. Supplementary data
Right vagus nerve stimulation before cardioneuroablation during sinus rhythm. Vagus nerve stimulation resulted in prolonged asystole.
Right vagus nerve stimulation before cardioneuroablation during atrial pacing. Vagus nerve stimulation resulted in complete AV block.
Right vagus nerve stimulation after cardioneuroablation during sinus rhythm. Vagus nerve stimulation have not been associated with prolonged asystole neither complete AV block.
References
- 1.Brignole M., Moya A., de Lange F.J., ESC Scientific Document Group 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883–1948. doi: 10.1093/eurheartj/ehy037. [DOI] [PubMed] [Google Scholar]
- 2.Writing Committee Members. Kusumoto F.M., Schoenfeld M.H., Barrett C. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. Heart Rhythm. 2019;16:e227–e279. doi: 10.1016/j.hrthm.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Shen W.K., Sheldon R.S., Benditt D.G. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017;136:e25–e59. doi: 10.1161/CIR.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 4.Pachon J.C., Pachon E.I., Pachon J.C. “Cardioneuroablation” – new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7:1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Pachon J.C., Pachon E.I., Cunha Pachon M.Z., Lobo T.J., Pachon J.C., Santillana T.G. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: cardioneuroablation long-term results. Europace. 2011;13:1231–1242. doi: 10.1093/europace/eur163. [DOI] [PubMed] [Google Scholar]
- 6.Pachon M.J.C., Pachon M.E.I., Santillana P.T.G. Simplified method for vagal effect evaluation in cardiac ablation and electrophysiological procedures. JACC Clin Electrophysiol. 2015;1:451–460. doi: 10.1016/j.jacep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Yao Y., Shi R., Wong T. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. 2012;5:279–286. doi: 10.1161/CIRCEP.111.966465. [DOI] [PubMed] [Google Scholar]
- 8.Hu F., Zheng L., Liang E. Right anterior ganglionated plexus: the primary target of cardioneuroablation? Heart Rhythm. 2019;16:1545–1551. doi: 10.1016/j.hrthm.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Sun W., Zheng L., Qiao Yu. Catheter ablation as a treatment for vasovagal syncope: long-term outcome of endocardial autonomic modification of the left atrium. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aksu T., Golcuk E., Yalin K., Guler T.E., Erden I. Simplified cardioneuroablation in the treatment of reflex syncope, functional AV block, and sinus node dysfunction. Pacing Clin Electrophysiol. 2016;39:42–53. doi: 10.1111/pace.12756. [DOI] [PubMed] [Google Scholar]
- 11.Osório T.G., Paparella G., Stec S., Chierchia G.B., de Asmundis C. Cardiac parasympathetic modulation in the setting of radiofrequency ablation for atrial fibrillation. Arch Med Sci. 2019 doi: 10.5114/aoms.2019.84717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klank-Szafran M., Stec S., Sledź J., Janion M. Radiofrequency ablation and cardioneuroablation for AVNRT and atrioventricular block [in Polish] Kardiol Pol. 2010;68:720–724. [PubMed] [Google Scholar]
- 13.Piotrowski R., Baran J., Kułakowski P. Cardioneuroablation using an anatomical approach: a new and promising method for the treatment of cardioinhibitory neurocardiogenic syncope. Kardiol Pol. 2018;76:1736–1738. doi: 10.5603/KP.a2018.0200. [DOI] [PubMed] [Google Scholar]
- 14.Aksu T., Güler T.E., Bozyel S., Özcan K.S., Yalın K., Mutluer F.O. Cardioneuroablation in the treatment of neurally mediated reflex syncope: a review of the current literature. Turk Kardiyol Dern Ars. 2017;45:33–41. doi: 10.5543/tkda.2016.55250. [DOI] [PubMed] [Google Scholar]
- 15.Pachon -M.E.I., Pachon-Mateos J.C., Higuti C. Relation of fractionated atrial potentials with the vagal innervation evaluated by extracardiac vagal stimulation during cardioneuroablation. Circ Arrhythm Electrophysiol. 2020 Mar 19 doi: 10.1161/CIRCEP.119.007900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Right vagus nerve stimulation before cardioneuroablation during sinus rhythm. Vagus nerve stimulation resulted in prolonged asystole.
Right vagus nerve stimulation before cardioneuroablation during atrial pacing. Vagus nerve stimulation resulted in complete AV block.
Right vagus nerve stimulation after cardioneuroablation during sinus rhythm. Vagus nerve stimulation have not been associated with prolonged asystole neither complete AV block.