Introduction
Conduction system pacing ensures rapid activation of both ventricles, resulting in synchronized contraction. His bundle pacing (HBP) is the most physiological pacing modality but is limited by higher thresholds and lower success rates in patients with wide QRS. Left bundle branch pacing (LBBP) has been proposed by Huang and colleagues1 as an alternative strategy to overcome the limitations of HBP. Since the pacing lead is positioned deep in the basal interventricular septum, the possibility of injuring coronary artery branches is a concern. In this report, we describe a case of aborted ST-elevation myocardial infarction during LBBP lead implantation.
Case report
A 65-year-old woman with nonischemic cardiomyopathy, left ventricular (LV) ejection fraction of 30%, left bundle branch block (LBBB), QRS duration of 160 ms (Figure 1A), and recurrent heart failure was referred for cardiac resynchronization therapy. Coronary angiography performed 2 years ago was normal. LBBP was attempted using a 3830 SelectSecure pacing lead and C315His sheath (Medtronic Inc, Minneapolis, MN) at a right ventricular septal site 1 cm apical and inferior to the distal His region. The lead was advanced deep into the septum with 4–5 rapid turns. The paced QRS demonstrated qR morphology in lead V1 (Supplemental Figure S1). Unipolar pacing impedance increased gradually from 350 ohms on the right side of the septum to 700 ohms before it decreased to 400 ohms. The patient developed angina, diaphoresis, and hypotension. Electrocardiogram (ECG) showed significant ST elevation in leads I, aVL, and V1–V3 with reciprocal changes in II, III, and aVF (Figure 1B). The lead was immediately withdrawn and repositioned 1.5 cm posterior and apical to the initial location. Urgent coronary angiography showed a major septal branch at the initial lead placement site (deep septum) with TIMI 3 flow in the left anterior descending (LAD) artery (Figure 2, Videos 1 and 2). The ST-segment changes resolved within 10 minutes. During threshold testing at the second pacing site, nonselective-to-selective left bundle branch capture was demonstrated with paced QRS duration of 110 ms (Figure 3A). The pacing threshold was 0.3 V at 0.6 ms pulse width, unipolar lead impedance 650 ohms, and sensed R wave 10 mV. ECG showed symmetrical T-wave inversion in precordial leads suggestive of T-wave memory from prior LBBB (Figure 3B). Postprocedure echocardiogram did not show new wall motion abnormalities. Peak troponin I was 0.23 ng/mL (normal 0.01–0.08 ng/mL). Hospital course was uneventful, and the patient was discharged the next day. The patient’s functional status improved from New York Heart Association class III at baseline to class II during follow-up. LV ejection fraction had improved to 45% at 1 month.
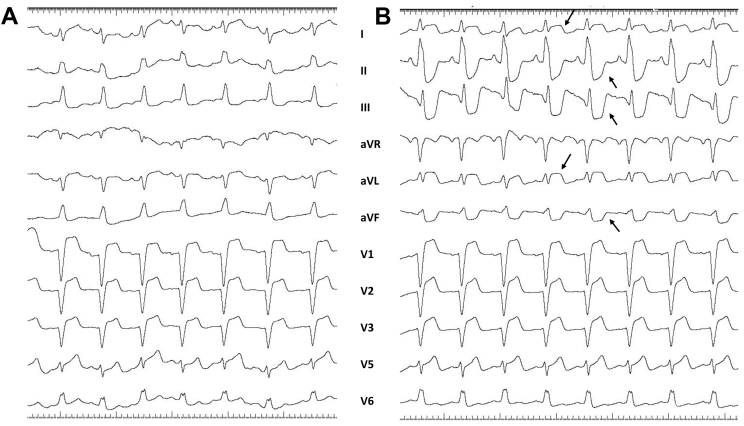
Figure 1.
A: Baseline electrocardiogram (ECG) showing complete left bundle branch block with QRS duration of 160 ms. B: ECG during anginal pain showed ST-segment elevation in leads I, aVL, and V1–V3 with reciprocal ST depression in inferior leads.
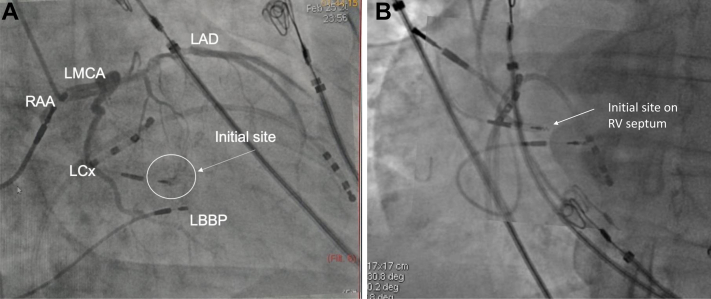
Figure 2.
A: Right anterior oblique 30° fluoroscopy view showing the intial pacing site (right ventricular septal) superimposed on the final pacing site and its relation to the septal branch of the left anterior descending artery (LAD). LBBP = left bundle branch pacing lead; LCx = left circumflex artery; LMCA = left main coronary artery; RAA = right atrial appendage. B: Left anterior oblique 30° fluoroscopy view showing the initial and final pacing sites. RV = right ventricular.
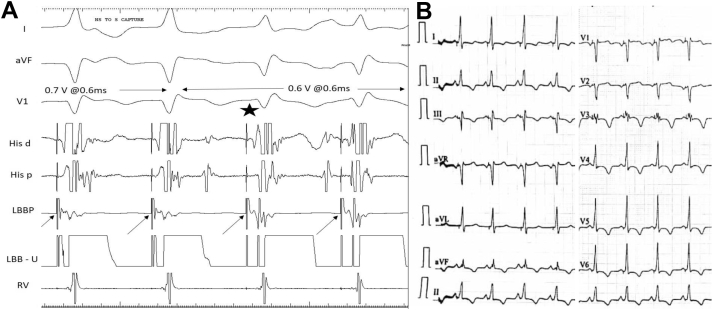
Figure 3.
A: Demonstration of nonselective-to-selective left bundle capture during threshold testing; note the change in QRS morphology from qR to rSR pattern along with discrete local ventricular electrogram after the pacing spike during selective capture. B: Twelve-lead electrocardiogram after left bundle branch block correction by left bundle branch pacing (LBBP) showing qR pattern in V1 with T-wave memory changes.
Discussion
The differential diagnosis for the aborted nonatherosclerotic ST-elevation myocardial infarction in our patient is (1) coronary vasospasm resulting from trauma to the septal arterial branch or (2) micro-emboli into the coronary artery from the lead tip at the LV endocardium with subsequent spontaneous resolution.
There was a large septal branch at the implanted site (Figure 3) and it is likely that the trauma caused by the pacing lead might have produced vasospasm of the LAD artery. No arterial injury or bleeding into the myocardium could be demonstrated by coronary angiography. The ECG changes had resolved within 10 minutes, prior to the angiography. The other possibility is perforation of the LBBP lead into the LV cavity followed by embolization of thrombus from the lead tip into the LAD branches. Spontaneous resolution of micro-thrombus may have occurred, as both ST-segment elevation and anginal pain had resolved by the time angiography was done. Although there was a significant decrease in pacing impedance from 700 to 400 ohms, we did not observe high pacing threshold or loss of R waves at this site. In our experience, acute perforation of the lead tip into the LV cavity during LBBP lead implantation is seen in approximately 3%.2 Perforation can be recognized by a decrease in unipolar pacing impedance to <400 ohms, significant reduction in R-wave amplitudes, and increase in capture thresholds to >3 V. The electrograms from the LBBP lead will also demonstrate loss of myocardial injury current. In our patient, none of these features other than a decrease in pacing impedance was observed. Air embolism into the diagonal artery is another possibility, but angiography failed to demonstrate air bubble or slow flow owing to increase in microcirculatory resistance in response to air. Once recognized, acute septal perforation is managed by removal of the pacing lead and repositioning at a different site. Late perforation of the LBBP lead is a major concern, as this may cause thromboembolic complications. So far, thromboembolic complication has not been reported in literature.
Theoretically, coronary artery injury can occur when the lead is placed deep in the proximal septum. This is the first reported case of ST elevation during LBBP lead implantation. Based on the above findings, we believe that coronary artery spasm induced by the LBBP lead is the likely cause for the transient ST elevation observed in this patient.
LBBP is characterized by capture of left bundle or its fascicular branches along with septal myocardium at low output (<1 V at 0.5 ms pulse width). Huang and colleagues1 first reported successful LBBP for a patient with heart failure and LBBB. Deep septal placement of the lead below the His bundle region resulted in LBBB correction at low pacing output (0.5 V at 0.5 ms). The LV ejection fraction had improved from 34% to 62% with regression of LV dimensions. LBBP overcomes many of the limitations of HBP, as it provides low and stable thresholds, lead stability, and ability to correct distal conduction system disease. LBBP has the potential to be an effective alternative for cardiac resynchronization therapy. Zhang and colleagues3 demonstrated that LBBP can improve LV dyssynchrony in patients with systolic heart failure and LBBB. Huang and colleagues4 reported 97% success rate in a multicenter prospective study involving 63 patients with nonischemic cardiomyopathy and LBBB. In our patient, LBBB could be successfully corrected by LBBP, with subsequent improvement in functional status and LV ejection fraction. The long-term effects of deep septal placement of the lead and the challenges of lead extraction from this site are currently unknown and need further evaluation in long-term clinical trials.
Footnotes
Shunmuga Sundaram Ponnusamy has no disclosures. Pugazhendhi Vijayaraman discloses the following: honoraria, consultant, research, fellowship support: Medtronic; consultant: Boston Scientific, Abbott, Biotronik, and Eaglepoint LLC.
Key Teaching Points.
-
•Left bundle branch pacing (LBBP) overcomes many of the limitations of His bundle pacing, as it provides conduction system capture at low and stable threshold.
-
•Though LBBP has the potential to be an effective alternative to cardiac resynchronization therapy, the safety of LBBP needs continued evaluation.
-
•Injury to coronary artery branches (septal perforators) during LBBP lead implantation is a concern.
-
•We describe an interesting case of aborted ST-segment elevation myocardial infarction during LBBP likely due to arterial spasm induced by trauma during implantation.
Appendix. Supplementary data
Coronary angiogram in Cranial 45 degree view demonstrating the septal perforator branches and the final LBBP lead location.
Coronary angiogram in Right Anterior Oblique (RAO) 36 degree view and the final LBBP lead location.
Paced morphology and impedances during initial lead implantation. A. Paced QRS morphology during pacing from the initial right ventricular septal site with pacing impedance of 350 Ohms. B. During pacing from the mid-septal site, narrowing of QRS with pacing impedance of 700 Ohms. C. At the deep LV septal location paced QRS morphology of qR pattern in lead V1 is seen with a decrease in pacing impedance of 400 Ohms.
References
- 1.Huang W., Su L., Wu S. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1736.e1–1736.e3. doi: 10.1016/j.cjca.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Huang W., Chen X., Su L., Xia X., Vijayaraman P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16:1791–1796. doi: 10.1016/j.hrthm.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J., Wang Z., Cheng L. Immediate clinical outcomes of left bundle branch area pacing vs conventional right ventricular pacing. Clin Cardiol. 2019;42:768–773. doi: 10.1002/clc.23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W., Wu S., Vijayaraman P. Cardiac resynchronization therapy in patients with non-ischemic cardiomyopathy utilizing left bundle branch pacing. JACC Clin Electrophysiol. 2020 In press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronary angiogram in Cranial 45 degree view demonstrating the septal perforator branches and the final LBBP lead location.
Coronary angiogram in Right Anterior Oblique (RAO) 36 degree view and the final LBBP lead location.
Paced morphology and impedances during initial lead implantation. A. Paced QRS morphology during pacing from the initial right ventricular septal site with pacing impedance of 350 Ohms. B. During pacing from the mid-septal site, narrowing of QRS with pacing impedance of 700 Ohms. C. At the deep LV septal location paced QRS morphology of qR pattern in lead V1 is seen with a decrease in pacing impedance of 400 Ohms.