Introduction
The circumferential electrical isolation of the pulmonary vein (PV) antra represents the cornerstone of all atrial fibrillation (AF) treatments. Traditionally, PV isolation by means of radiofrequency (RF) ablation is achieved using a point-by-point technique, which makes it difficult to create circular and contiguous lesions, potentially allowing conduction gaps and incomplete (nontransmural) lesions in cases of poor catheter-tissue contact. Cryoballoon ablation is now emerging as an important alternative to RF ablation.1
In a recent multicenter randomized single-blinded trial (CIRCA-DOSE), contact force RF ablation and 2 different regimens of cryoenergy application resulted in no difference in 1-year efficacy endpoints.2
The cornerstone of a successful PV isolation by cryoballoon is the “complete” contact between the inflated balloon and the antrum of the PV, since the presence of blood flow will prevent adequate cooling and the subsequent lesion formation.
Injecting contrast medium into the lumen at the tip of the balloon is the method actually used to confirm PV occlusion during cryoballoon ablation and is mandatory to obtain an effective PV isolation.
Other techniques were tested in order to avoid contrast injection (in case of renal insufficiency or contrast allergy) and to reduce fluoroscopy exposure, such as intracardiac echocardiography and transesophageal echocardiography color flow Doppler. Those are considered reliable methods for the evaluation of mechanical PV occlusion in this kind of procedure.
Recently, a new software add-on (KODEX [Best, Netherlands]-EPD occlusion tool; EPD Solutions) was introduced to give a real-time evaluation of the PV occlusion.
In this experience we describe the use of this new “occlusion tool” software, using the novel KODEX-EPD mapping system,3 to verify the degree of PV occlusion during a cryoballoon ablation without the use of contrast injection.
Case report
A 61-year-old man presented with a history of 1-year symptomatic paroxysmal AF, not amenable to drug therapy. He was referred to our institution for AF ablation.
He was considered for 1-shot cryoballoon ablation with Arctic Front Advance Pro (AFA-Pro) 28 mm cryoballoon (Medtronic, Minneapolis, MN) guided by the KODEX-EPD Navigation mapping system.
The patient signed a written informed consent before the procedure.
The procedure was conducted under local anesthesia with moderate sedation as per our center protocol.
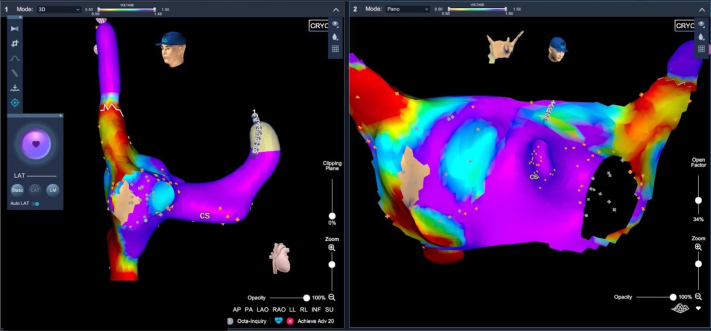
Once a coronary sinus multipolar catheter (Livewire 8-pole, St Jude Medical, St Paul, MN) was inserted via left femoral vein access, a brief, nonfluoroscopic right atrium (RA) map was performed to delineate the coronary ostium, the fossa ovalis, and the phrenic nerve position in the superior vena cava (Figure 1) (RA volume: 104.8 mL; RA mapping time: 4 minutes, 30 seconds). Next, transseptal access was obtained under fluoroscopic guidance.
Figure 1.
Right atrial map in left anterior oblique view (left) and PANO (panoramic) view (right). Right atrial mapping was performed with the use of an 8-pole deflectable coronary sinus (CS) catheter (phrenic nerve tag and CS positioning) without the use of fluoroscopy.
The cryoballoon system (AFA Pro 28 mm; Medtronic) was advanced in the left atrium (LA). A brief LA posterior wall map, with PV ostia, was reconstructed with the use of the circular mapping catheter (Medtronic Achieve; Medtronic) with the aid of the navigation system and brief fluoroscopy usage.
Once the mapping catheter was inserted in each PV, the cryoballoon was inflated and then the occlusion tool software was activated.
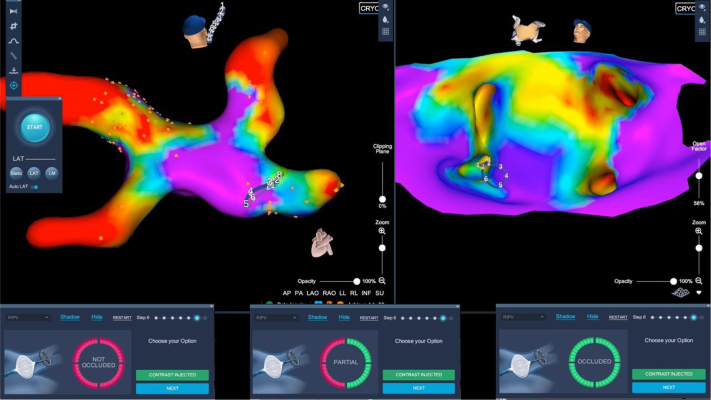
The occlusion feature workflow includes a baseline impedance recording by the circular catheter inside the PVs and a comparison with the impedances recorded when the inflated balloon is advanced against the PV ostium. The voltage comparison is calculated for each of the 8 electrodes of the circular catheter in order to obtain an estimation of the dielectric coefficient change in the tissue surrounding the mapping catheter and subsequently show in real time the occlusion degree: a green electrode indicates good occlusion in the proximity of the same electrode, while red color indicates an incomplete occlusion. In case of partial occlusion, shown by having more than 1 red electrode, the balloon was repositioned and the effect of the maneuvers, in order to obtain a better and complete occlusion, is shown in real time, without the need of several checks using multiple die injections and angiography. The complete occlusion shown by all green electrodes (Figure 2) in the software tool suggested the presence of a good occlusion. Occlusion evaluation obtained by the software was used as the only methodology to evaluate a pre-cryoapplication complete vein sealing. Minimal movements were required when partial occlusion occurred before delivering cryoenergy.
Figure 2.
Occlusion tool verification in right inferior pulmonary vein (RIPV). Top left: Posteroanterior view of the left atrium after cryoablation of left superior, left inferior, and right superior pulmonary veins. Achieve mapping catheter (Medtronic, Minneapolis, MN) in RIPV. Top right: PANO (panoramic) view of the posterior wall with the Achieve mapping catheter engaging the RIPV. Bottom panels show the stepwise, real-time occlusion of the vein, confirmed by the software.
Once total occlusion was achieved, cryoapplication started. The veins were ablated following this sequence: left superior PV, left inferior PV, right inferior PV, right superior PV (Figure 3). When the right PVs were ablated, the right phrenic nerve was paced through the superior vena cava catheter and diaphragmatic movements were closely monitored, as well as C-MAP potential, in order to immediately stop freezing in case of any degree of phrenic nerve palsy.
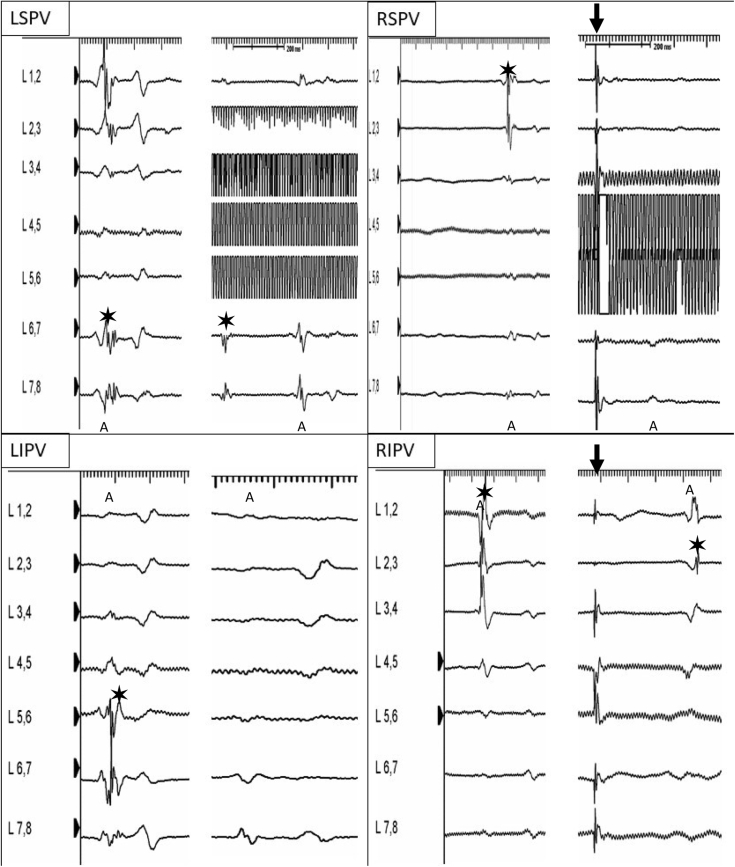
Figure 3.
Electrograms (EGMs) before and during/after the cryoablation, recorded by the Achieve mapping catheter (Medtronic, Minneapolis, MN). “A” indicates atrial far field. Asterisk designates pulmonary vein potential. Black arrow indicates phrenic nerve stimulation. Artifact EGMs in left superior and right superior pulmonary veins are due to ice formation on the electrodes of the circular mapping catheter. Note that for right veins, the Achieve mapping catheter was advanced deep in the vein to maximize system stability during cryoenergy delivery. During right inferior pulmonary vein cryoapplication note the breakthrough change. LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
Cryoapplication time for each vein was 180 seconds. Left superior PV time to isolation was 50 seconds at 38°C; temperature nadir was -43°. Left inferior PV time to isolation was 35 seconds at 28°C; temperature nadir was -40°. Right superior PV time to isolation was not annotated; temperature nadir was -48°. Right inferior PV time to isolation was not annotated; temperature nadir was -40°.
In order to verify an effective cryoballoon application, entrance and exit blocks were tested for each vein at the end of the cryoapplication. After the ablation, all the catheters were retrieved and the groin sutured with a figure-of-8 stitch.
The patient was discharged the day after the procedure with no complications, without any antiarrhythmia drug and with a full dose of rivaroxaban (20 mg).
Total procedure time (from groin puncture to last catheter removal) was 64 minutes. Total LA dwell time was 39 minutes. Fluoroscopy time was 3 minutes, 1 second. Total DAP was 1130 mGy/cm2 and air kerma 6.89 mGy. Total LA volume mapped was 43.6 mL with 7 minutes LA mapping time.
Discussion
This is the first case report that demonstrates the feasibility of a new occlusion tool software, using the novel KODEX-EPD system, to verify the presence and the degree of PV occlusion during a cryoballoon ablation, without the use of contrast injection.
The KODEX-EPD system was used to obtain images of the RA and LA anatomy, to navigate inside those structures, and to assess the balloon PV occlusion. The KODEX-EPD system technical characteristics have been previously described. The system creates high-resolution images of cardiac anatomy by exploiting the distinct dielectric properties of biological tissue. The system receives and analyzes the subtle electrical field simultaneous transmission and detection from all catheter electrodes as they are manipulated in the cardiac chambers and from the exterior skin patches. Structures such as the endocardial atrial surface, cardiac veins, and heart valves cause marked gradients in the electrical field. This “bending of the electrical field” is sensed by the system and is used to calculate the geometric characteristics of the 3-dimensional image. With this technique, KODEX can collect anatomic information without a strict physical surface contact up to a few millimeters around the catheter electrodes, resulting in a certain degree of “far-field imaging.” RA morphology image, phrenic nerve capture site tag, and voltage map were created by the KODEX-EPD system using the Livewire 8-electrode catheter (St Jude) while LA morphology image and voltage map were created with the Achieve mapping catheter (Medtronic).
As reported in the case, all of the veins were targeted. Mean nadir temperature was optimal for each vein, as well as time to isolation for left veins.
During right PV ablation, in order to ensure a better stability of the system and to obtain a stable circular mapping catheter position when acquiring impedance baseline, the Achieve mapping catheter was pushed deep into the veins. This could have not allowed us to obtain the real-time recording of vein potentials, to evaluate the presence of electrical isolation and time to isolation interval.
Additionally, all the veins were tested for entrance and exit block at the end of the cryoapplication to verify the effective acute isolation.
The system allows a nonfluoroscopic and real-time detection of the PV occlusion grade during cryoballoon ablation of AF. One of the major arguments in favor of the technique is the possibility to have a direct and real-time feedback of the occlusion grade, which allows a quick repositioning of the cryoballoon system, with minimal movements. Moreover, the utilization of the KODEX-EPD navigation system permits to have biatrial maps and more information about the electrical properties of the LA. The PANO view (panoramic view) clearly shows both the pulmonary ostia, giving a highly confident imaging of the posterior wall.
This case report highlights the promising possibility to avoid the use of contrast dye or at least to reduce its use in some circumstances, as well as to reduce the total amount of fluoroscopy exposure and radiation dosage. Future studies with a larger sample are needed to confirm this hypothesis.
Conclusion
This case suggests that a new software (occlusion tool – KODEX-EPD), designed to determine the occlusion of the pulmonary veins, gives reliable feedback in defining the grade of vein sealing during cryoballoon ablation of AF. The KODEX-EPD navigation system integrated with the cryoballoon platform can significantly reduce fluoroscopy time.
Key Teaching Points.
-
•
Although the cornerstone of a successful pulmonary vein (PV) isolation by cryoballoon is the “complete” contact between the inflated balloon and the antrum of the PVs, some other techniques were tested in order to avoid contrast injection.
-
•
The KODEX-EPD occlusion tool software (EPD Solutions) allows to detect real-time pulmonary vein occlusion and to avoid contrast dye injection for cryoballoon ablation of atrial fibrillation.
-
•
The KODEX-EPD navigation system permits reduction of the use of fluoroscopy exposure and total radiation dosage during cryoballoon ablation of atrial fibrillation.
Footnotes
Carlo Maria Giannitti is an employee of EPD Solutions, which manufactures the KODEX-EPD system. The other authors have no conflict of interest related to the manuscript.
References
- 1.Calkins H., Hindricks G., Cappato R. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Heart Rhythm. 2017;10:445–494. doi: 10.1016/j.hrthm.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Andrade J.G., Champagne J., Dubuc M. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779–1788. doi: 10.1161/CIRCULATIONAHA.119.042622. [DOI] [PubMed] [Google Scholar]
- 3.Romanov A., Dichterman E., Shwartz Y. High-resolution, real-time, and nonfluoroscopic 3-dimensional cardiac imaging and catheter navigation in humans using a novel dielectric-based system. Heart Rhythm. 2019;16:1883–1889. doi: 10.1016/j.hrthm.2019.06.020. [DOI] [PubMed] [Google Scholar]