Abstract
Hyperglycemia is the consequence of blood glucose dysregulation and a driving force of diabetic complications including retinopathy, nephropathy and cardiovascular diseases. The serum and glucocorticoid inducible kinase-1 (SGK1) has been suggested in the modulation of various pathophysiological activities. However, the role of SGK1 in blood glucose homeostasis remains less appreciated. In this review, we intend to summarize the function of SGK1 in glucose level regulation and to examine the evidence supporting the therapeutic potential of SGK1 inhibitors in hyperglycemia. Ample evidence points to the controversial roles of SGK1 in pancreatic insulin secretion and peripheral insulin sensitivity, which reflects the complex interplay between SGK1 activation and blood glucose fluctuation. Furthermore, SGK1 is engaged in glucose absorption and excretion in intestine and kidney and participates in the progression of hyperglycemia-induced secondary organ damage. As a net effect, blockage of SGK1 activation via either pharmacological inhibition or genetic manipulation seems to be helpful in glucose control at varying diabetic stages.
Keywords: SGK1, hyperglycemia, insulin resistance, blood glucose homeostasis
Introduction
Hyperglycemia, the leading indicator of diabetes, refers to a pathological state with abnormally high blood glucose level. Hyperglycemia can be a consequence of either impaired insulin secretion arising from the loss of pancreatic islet β cells or a decline of insulin sensitivity in downstream organs, including liver, skeletal muscle and adipose tissue. Hyperglycemia can also result from inappropriately high endogenous glucose production either via glycogenolysis or gluconeogenesis. Specifically, in the long run, metabolic disorders that are responsible for insulin resistance may also, in turn, damage the insulin-secreting capability of islet β cells through the accumulation of proinflammatory cytokines and toxic lipids (1, 2). Existing therapeutics are aimed at lowering blood glucose level by stimulating insulin secretion (direct: sulfonylureas, nateglinide; indirect: DPP-4 inhibitors/GLP-1), alleviating peripheral insulin resistance (Metformin, TZDs), reducing enteral glucose absorption (alpha-glucosidase inhibitors) and increasing renal glucose excretion (SGLT-2 inhibitors). When mono-drug therapy fails, various combinations of drug administration are required for different clinical conditions. In worsened cases, patients rely on exogenous insulin supplement which cannot control blood glucose levels as accurately as the endogenous counterpart. Around one-third of patients demand special clinical care to blunt hyperglycemia-induced complications such as retinal, renal and cardiovascular diseases (3). To achieve the goal of better blood glucose management, a comprehensive understanding of the potential pathological factors is urgently needed.
The serum and glucocorticoid inducible kinase-1 (SGK1) is a ubiquitously expressed serine/threonine kinase downstream of the phosphatidylinositide-3 kinase (PI3K) signaling pathway (4, 5). It was originally defined as an early transcribed gene in response to serum and glucocorticoid stimulation in rat mammary tumor cells. Subsequent studies, however, indicated that SGK1 is widely expressed in various cell types following a vast array of external stimulations, such as transforming growth factor-β (TGF-β) and interleukin 6 (IL-6) (4). Multiple endocrine hormones including insulin, insulin-like growth factor 1 (IGF-1), hepatic growth factor (HGF), follicle-stimulating hormone (FSH), thrombin and corticosterone are identified as endogenous regulators of SGK1 activation. In addition to PI3K, signaling molecules, such as bone marrow kinase/extracellular signal-regulated kinase 5 (BK/ERK5), p38α, or calcium-sensitive calmodulin-dependent protein kinase kinase (CaMKK), are also critical upstream kinases. Upon activation, SGK1 takes a pivotal part in the modulation of fundamental biological activities, such as ion channel opening, metabolite transport, hormone release and cell survival (4). Excessive expression and activity of SGK1 is implicated in the progression of diverse disorders, including hypertension, obesity, diabetes, thrombosis, stroke, fibrotic diseases, vascular calcification, infertility, autoimmune disease and tumor growth.
Notably, SGK1 is identified as a hub molecule on which the cascade of events elicited by high salt diet (HSD) intake converges (6, 7, 8). As a salt-sensing molecule, SGK1 mediates high sodium chloride-triggered responses in kidney, brain, heart and various immune cell types (9, 10, 11, 12, 13, 14, 15, 16, 17). For a long period of time, dietary management and caloric restriction were generally focused on three major energy sources, carbohydrate, fat and protein, while the role of noncaloric nutrients, namely salt (sodium chloride), is less appreciated. Besides the well-recognized effect on the cardiovascular system, the HSD-SGK1 axis is also suggested to engage with gastroenterological disorders, obesity-related metabolic syndrome and autoimmune diseases (8, 18, 19). Therefore, investigations on the multifaceted effect of SGK1 not only help to enrich our understanding of nutritional regulation but provide valuable targets for clinical interventions as well.
Recently, several lines of evidence support that SGK1 is substantially implicated in the regulation of glucose homeostasis. Excessive activation of SGK1 was found to impair insulin secretion from pancreatic cells but seems to enhance the insulin sensitivity in peripheral organs (20, 21, 22, 23). Moreover, SGK1 modulates glucose uptake and reabsorption in the intestinal tract and proximal renal tubules and it is critically involved in the pathogenesis of hyperglycemia-mediated diabetic complications (4, 24). We, therefore, intend to summarize the links between SGK1 activation and hyperglycemia and indicate the potential usage of small molecule SGK inhibitors in blood glucose control.
SGK1 impairs insulin secretion of pancreatic β cells
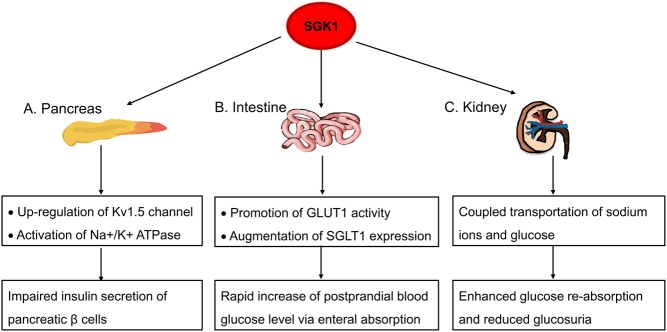
Islet β cells take charge for synthesizing and secreting insulin, which is committed to maintaining blood glucose level within a normal range. Genetic polymorphisms in 3′-UTR region of SGK1 are associated with the insulin secretion capacity and the risk of T2D development, suggesting a regulatory role of SGK1 in human β-cell function (25). Even though the mRNA transcripts of the human serine/threonine kinase SGK are highly enriched in human pancreas, low level of SGK is detected in corresponding islet β cells (20, 26). Consistent with this observation, INS-1 cell, an in vitro β cell line, only shows a modest expression of SGK1 protein at the steady state (20). Notably, the expression level of SGK1 sharply increases in the presence of glucocorticoids (4). Abnormally elevated SGK1 fuels blockage of insulin secretion in the mouse model. The damaging consequence of SGK1 on insulin production was further confirmed in human islets and human EndoC-βH1 β cells (27). Following SGK1 activation, upregulation of voltage-sensitive Kv1.5 channel improperly hyperpolarizes the β cell plasma membrane, which ultimately contributes to the impairment of insulin release (20). Moreover, elevated SGK1 also potentiates the activation of Na+/K+ ATPase during plasma membrane repolarization, thus leading to an attenuated insulin secretion (28).
SGK1 influences glucose level by acting on intestine and kidney
Intestine and kidney, responsible for glucose absorption and reabsorption, respectively, are subjected to the regulation of SGK1. Glucose transporter 1 (GLUT1) and sodium-glucose cotransporter 1 (SGLT1) are two important intestinal glucose transporters that are upregulated by SGK1 upon dietary sugar intake (4, 24). Palmada et al. found that SGK1 can promote GLUT1 trafficking and activity, although the detailed mechanisms remain unclear (44). Besides, diabetic mice and patients have been shown to exhibit an augmented SGLT1 expression in the intestine, which is attributed to the overactivation of SGK1 (29, 30). This can, at least in part, account for the rapid increase of postprandial blood glucose level in diabetic individuals. As for kidney, under physiologic condition, the expression of SGK1 is largely confined to distal nephron and glomerulus while it is nearly undetectable in the proximal renal tubule, the site for glucose reabsorption (31). Diabetes leads to dramatically increased SGK1, which in turn mediates the coupled transportation of sodium ions and glucose via SGLT1 channel, so as to reduce glucosuria (32). Genetic ablation of SGK1 in Akita mice, a spontaneously developed diabetes model, was found to cause reduced glucose reabsorption along with elevated urinary excretion of glucose. Strangely, the plasma glucose level did not change with the glucosuria alteration and the underlying mechanisms are still unknown. Overall, extra-pancreatic glucose regulating function of SGK1 provides another rationale for its application in diabetes treatment. For example, inactivation of SGK1 by fibroblast growth factor 21 (FGF-21) exerts hypoglycemic effect by lowering the expression of SGLT1 and blocking the intestinal route of glucose entry (33).
SGK1 impacts on the insulin sensitivity of major downstream organs
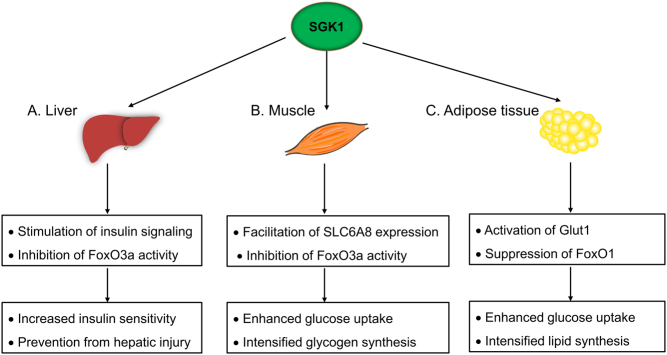
Hepatic insulin resistance partially accounts for the occurrence of hyperglycemia and previous studies on SGK1 provide evidence for its vital effect on reinforcing insulin sensitivity in the liver (7, 21). For instance, liver specific SGK1 knockout mice (LKO mice) demonstrated impaired glucose clearance and attenuated insulin sensitivity (21). Following insulin stimulation, major components of insulin signaling including IRS1, AKT and GSK-3β were hypophosphorylated in LKO hepatocytes, which subsequently blunt glucose uptake (21). SGK1 also exerts dramatic liver protection effect. Existing researches revealed that SGK1 alleviates hepatocyte apoptosis via repressing the transcriptional activity of FoxO3a (34, 35). Besides, SGK1-triggered VDCA-1 (voltage-dependent anion channel) degradation negatively regulates the mitochondrial permeability transition pore (mPTP) opening and prevents from hepatic injury (36).
Skeletal muscle is another major insulin target where SGK1 promotes the utilization of glucose. Creatine supplementation can be facilitated through SGK1 mediated expression of CreaT (SLC6A8), which contributes to skeletal muscle sugar uptake and glycogen synthesis (37, 38). The transcription level of SGK1 was found not just to be compensatorily upregulated in skeletal muscle from patients with insulin resistance but also to be restored after exercise (23). On the other hand, hyperglycemia could induce skeletal muscle atrophy via the WWP1/KLF15 axis (39). SGK1 acts as a guarder of skeletal muscle metabolism, as overexpression of SGK1 reverses muscle atrophy. At least two mechanisms are involved in this process: (1) SGK1 inactivates FoxO3a to hamper the expression of Atrogin1 and MuRF1 and (2) SGK-1 and its substrate, N-myc downstream-regulated gene 1 (NDRG1), block Smad2/3 activation in muscle cells (40, 41, 42). The expression and activity of muscle SGK1 is susceptible to blood glucose fluctuation; however, it is unclear whether SGK1 is implicated and what role it plays in hyperglycemia-induced muscle wasting.
Adipose tissue is deemed to be a key modulator of fuel metabolism and glucose homeostasis. Despite high salt diet being shown to activate SGK1 in many other tissues, it suppresses adipocyte SGK1 activation and thus hinders glucose deposition in the fat tissue (7). Research on mouse 3T3-L1 cells proved that adipocyte differentiation was enhanced via distinct metabolic pathways in response to high glucose stimulation, while genetic ablation of Sgk1 attenuates glucose uptake and adipocyte differentiation (43). Mechanistically, SGK1 markedly enhances Glut1 activity via boosting the transporter’s appearance in the plasma membrane instead of potentiating de novo protein synthesis (44). FoxO1, the negative regulator of adipogenesis, is phosphorylated and suppressed by SGK1; meanwhile, the transcription of PPARγ is activated to foster lipid synthesis (22, 45, 46, 47). Although SGK1 serves to transform the blood glucose into storage lipid, excessive SGK1 activation resulting from stimulation of hormones, such as aldosterone and glucocorticoids, is responsible for the development of obesity and the related metabolic disorders (48).
SGK1 exaggerates hyperglycemia-induced diabetic complications
Uncontrolled chronic hyperglycemia is prone to cause long-term damage in various organs, including the eyes, kidneys, heart and blood vessels. SGK1 has been shown to accelerate the progression of these common diabetic complications through distinct molecular pathways (Table 1).
Table 1.
Pathophysiological roles of SGK1 on hyperglycemia-induced complications.
| Complications | Actions of SGK1 | Functional outcomes |
|---|---|---|
| Diabetic retinopathy | Facilitate the accumulation of sorbitol | Retinal microvascular lesions |
| Enhance the expression of EDB+FN | Dysregulated angiogenesis | |
| Hinder the clearance of glutamate | Neurotoxicity | |
| Diabetic nephropathy | Fuel the Na+ reabsorption | Secondary hypertension |
| Trigger the production of profibrotic mediators | Renal fibrosis | |
| Diabetic cardiomyopathy | Accelerate Na+ entry into myocytes | Arrhythmia and heart failure |
| Promote myofibroblast maturation | Cardiac remodeling |
Diabetic retinopathy results from the damage to small blood vessels and retinal neurons. Almost all T1D patients suffer from different forms of retinopathy by the first decade of disease onset. A growing body of evidence suggests that SGK1 is involved in the pathogenesis of retinopathy. First, SGK1 tend to benefit increased conversion of glucose to sorbitol, the excessive deposition of which causes severe retinal microvascular lesions (49). Oncofetal extra domain B fibronectin (EDB+FN), preferentially expressed in diabetic retinopathy and the culprit for dysregulated angiogenesis, can be activated by glucose-induced SGK1 signaling (50). In addition, SGK1 is likely to hinder the clearance of glutamate in synaptic space by affecting glutamate transporters (4). Accumulation of glutamate plays a neurotoxic role and eventually leads to the death of retinal neurons.
Diabetic patients frequently develop nephropathy, which presents as edema, proteinuria, low urinary sodium excretion and hypertension caused by sodium retention. SGK1, as a signal hub of sodium transport, regulates various renal Na+ transporters, such as epithelial sodium channel (ENaC), voltage-gated sodium channel (Nav1.5), sodium hydrogen exchanger 1/3 (NHE1 and NHE3), sodium chloride cotransporter (NCC), sodium chloride potassium cotransporter 2 (NKCC2), Na+/K+-ATPase and type A natriuretic peptide receptor (NPR-A) (4, 51, 52). Exposure to hyperglycemia can dramatically stimulate the expression and activity of SGK1 in proximal tubule cells and mesangial cells (53, 54). Enhanced SGK1 potentiates the activity of ENaC, NCC, NKCC2 and Na+/K+-ATPase, thereby exerting a significant effect on Na+ reabsorption and the occurrence of secondary hypertension (55). Beyond its impact on renal electrolyte excretion, SGK1 also involves in the development of renal fibrosis. Overexpression of SGK1 alone has little effect on the formation of fibronectin in human mesangial cells (56). However, under high glucose stimulation, the pro-fibrotic function of SGK1 is markedly turned on (56). The secreted pro-fibrotic mediators that drive extracellular matrix deposition, such as TGF-β and EGF, in turn, trigger SGK1 activity (56). Such a positive feedback loop plays a deteriorating role in the development of diabetic nephropathy, which may ultimately lead to renal failure.
Diabetic cardiomyopathy is described as a clinical condition of ventricular dysfunction in individuals with diabetes mellitus but free of other cardiac risk factors, such as coronary atherosclerosis. SGK1 is moderately expressed in human cardiomyocyte and the heart resident fibroblast. In STZ-induced diabetic mouse model, glucose-induced cardiomyocyte hypertrophy is associated with the further upregulation and activation of SGK1, which is then confirmed in diabetic patient samples (57, 58). Activated SGK1 accelerates Na+ entry through the NHE1-mediated pathway, thereby disrupting ion homeostasis and leading to arrhythmia and even heart failure (59). Besides, SGK1 has been similarly suggested to be an important mediator in myocardial fibrosis. Activation of cardiac fibroblast SGK1 promotes the differentiation and maturation of myofibroblast which generates massive pro-fibrotic factors that participate in ventricular remodeling.
Conclusion and discussion
As easily seen, SGK1 plays a dual role during the entire process of blood glucose regulation. For one thing, it serves to strengthen insulin sensitivity (glucose reuptake in particular) of liver, skeletal muscle and fat tissue (Fig. 1). For another, aberrant activation of SGK1 damages insulin-secreting β cells, augments intestinal and renal glucose absorption and aggravates the progression of hyperglycemia-induced diabetic complications (Fig. 2). The functional duality of SGK1 seems organ dependent and the spatial counterregulation helps to maintain the blood glucose homeostasis. However, despite a certain level of SGK1 activity being helpful and necessary for insulin target organs, overactivation of SGK1 has been proven harmful in adipocytes. It is thus possible that the dose-dependent effect of SGK1 dictates the physiological and pathological roles of SGK1, and such concept deserves support from more experimental investigations. As a net effect, inhibition of SGK1 has proven its effectiveness in various pre-clinical studies. Chemical inhibition of SGK1 exhibits a favorable effect on counteracting hyperglycemia, as demonstrated by decreased fasting blood glucose (FBP), glycosylated hemoglobin (HbA1c) and small intestine SGLT1 transcription in diabetic mice (60). Administration of EMD638683 blunts SGK1 activity, thereby restoring blood glucose level and preventing diet-induced weight gain. Moreover, EMD638683 may play a helpful role in reducing the risk of diabetic cardiomyopathy. The cardiac protective effect has been shown in an angiotensin-II (AngII)-induced cardiac inflammation and fibrosis model (61). Although the small molecule inhibitor was found to have little effect on the elevated blood pressure in AngII-perfused mice, it helps to normalize blood pressure in high salt-induced hypertension (61, 62, 63). The discrepancy most likely derives from the distinct roles of SGK1 in different hypertensive models, whereas the part of SGK1 that takes in diabetic nephropathy is quite similar to that in high salt-induced hypertension. Additionally, SGK1 inhibitors have been reported to inhibit epithelial to mesenchymal transformation and promote renal tubular epithelial cell autophagy, which is of benefit to slow down the development of renal fibrosis (64). Until now, several SGK1 inhibitors have been developed and EMD638683 ranks the most effective one, which has been proved for its long-term safety in animal experiments and shows potential in glucose management (60, 65).
Figure 1.
The blood glucose lowering effect of SGK1 in insulin target organs. (A) In liver, SGK1 stimulates insulin signaling and inhibits FoxO3a activity, thus increasing insulin sensitivity and preventing hepatic injury. (B) In muscle, SGK1 facilitates SLC6A8 expression and inhibits FoxO3a activity, resulting in enhanced glucose uptake and glycogen synthesis. (C) In adipose tissue, SGK1 promotes Glut1 expression and suppresses FoxO1, which leads to intensified glucose uptake and lipid synthesis.
Figure 2.
SGK1 exacerbates hyperglycemia by acting on pancreas, intestine and kidney. (A) In pancreas, SGK1 abnormally activates Kv1.5 channel and Na+/K+ ATPase to interfere with the membrane polarization and repolarization process. As a result, the insulin-secreting activity of pancreatic β cell is severely impaired. (B) In intestine, SGK1 promotes the activity of GLUT1 and SGLT1, causing a rapid increase of postprandial blood glucose level via enteral absorption. (C) In kidney, SGK1 facilitates coupled transportation of sodium ions and glucose, which enhances glucose reabsorption and reduces glucosuria.
Though ample studies support the active involvement of SGK1 in T2D-related hyperglycemia, the straight link between SGK1 activation and T1D is less defined. The damage of insulin-secreting cells, including mass reduction and functional impairment, is now acknowledged as a crucial pathophysiological factor immersed on hyperglycemia. Glucose-stimulated insulin secretion (GSIS) is impaired in SGK1 overactive islet β cells, especially in the presence of glucocorticoids released from adrenal gland with the possible aim to quench the enduring inflammatory response, which would be much more prominent under T1D conditions (20). SGK1 was discovered to enhance the effector function of DCs and macrophages (14, 66, 67, 68). At the same time, mounting evidence points to the fact that high salt concentration boosts the polarization of Th17 lineage while limiting the suppressive function of Treg cells (9, 69, 70). Importantly, the immune disturbing effect induced by sodium chloride largely attributes to the activation of SGK1 and high salt intake has been proved to accelerate diabetes development in both LADA patients (latent autoimmune diabetes in adults) and NOD mice (6, 8; https://www.sciencedaily.com/releases/2017/09/170914210621.htm). These findings imply that SGK1 is central to the vicious cycle formed by immune activation, islet β cell impairment and blood glucose dysregulation, which together push T1D into an irreversible direction.
Collectively, SGK1 is central to blood glucose regulation and hyperglycemia-induced diabetic complications. Inhibition of SGK1 represents a comprehensive strategy to blunt and even block the development of hyperglycemia, while the detailed mechanisms remain to be determined.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
The research was supported by the National Natural Science Foundation of China (NSFC) grant number 81904015.
Author contribution statement
Y C L performed conceptualization and wrote the original draft. L J Y performed literature collection and wrote, reviewed and edited the manuscript. S F and Y J wrote, reviewed and edited the manuscript. Z H F performed plotting. Y C performed supervision and wrote, reviewed and edited the manuscript.
References
- 1.Oh YS, Bae GD, Baek DJ, Park EY, Jun HS. Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Frontiers in Endocrinology 2018. 9 384 ( 10.3389/fendo.2018.00384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordmann TM, Dror E, Schulze F, Traub S, Berishvili E, Barbieux C, Böni-Schnetzler M, Donath MY. The role of inflammation in β-cell dedifferentiation. Scientific Reports 2017. 7 6285 ( 10.1038/s41598-017-06731-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, et al Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Science Translational Medicine 2015. 7 315ra189 ( 10.1126/scitranslmed.aad4134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiological Reviews 2006. 86 1151–1178. ( 10.1152/physrev.00050.2005) [DOI] [PubMed] [Google Scholar]
- 5.Lang F, Artunc F, Vallon V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Current Opinion in Nephrology and Hypertension 2009. 18 439–448. ( 10.1097/MNH.0b013e32832f125e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigaux J, Semerano L, Favre G, Bessis N, Boissier MC. Salt, inflammatory joint disease, and autoimmunity. Joint Bone Spine 2018. 85 411–416. ( 10.1016/j.jbspin.2017.06.003) [DOI] [PubMed] [Google Scholar]
- 7.Boini KM, Hennige AM, Huang DY, Friedrich B, Palmada M, Boehmer C, Grahammer F, Artunc F, Ullrich S, Avram D, et al Serum- and glucocorticoid-inducible kinase 1 mediates salt sensitivity of glucose tolerance. Diabetes 2006. 55 2059–2066. ( 10.2337/db05-1038) [DOI] [PubMed] [Google Scholar]
- 8.Sharif K, Amital H, Shoenfeld Y. The role of dietary sodium in autoimmune diseases: the salty truth. Autoimmunity Reviews 2018. 17 1069–1073. ( 10.1016/j.autrev.2018.05.007) [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013. 496 513–517. ( 10.1038/nature11984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, et al Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. Journal of Clinical Investigation 2015. 125 4212–4222. ( 10.1172/JCI81151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoi W, Niisato N, Sawabe Y, Miyazaki H, Tokuda S, Nishio K, Yoshikawa T, Marunaka Y. Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt-sensitively hypertensive rats. Cell Biology International 2007. 31 1288–1291. ( 10.1016/j.cellbi.2007.03.036) [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Fang S, Wan C, Kong Q, Wang G, Wang S, Zhang H, Zou H, Sun B, Sun W, et al Excess salt exacerbates blood-brain barrier disruption via a p38/MAPK/SGK1-dependent pathway in permanent cerebral ischemia. Scientific Reports 2015. 5 16548 ( 10.1038/srep16548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakata F, Ito Y, Mizuno M, Sawai A, Suzuki Y, Tomita T, Tawada M, Tanaka A, Hirayama A, Sagara A, et al Sodium chloride promotes tissue inflammation via osmotic stimuli in subtotal-nephrectomized mice. Laboratory Investigation 2017. 97 432–446. ( 10.1038/labinvest.2017.4) [DOI] [PubMed] [Google Scholar]
- 14.Van Beusecum JP, Barbaro NR, McDowell Z, Aden LA, Xiao L, Pandey AK, Itani HA, Himmel LE, Harrison DG, Kirabo A. High salt activates CD11c(+) antigen-presenting cells via SGK (serum glucocorticoid kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension 2019. 74 555–563. ( 10.1161/HYPERTENSIONAHA.119.12761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Yao G, Chen W, Tang X, Feng X, Sun L. Exacerbation of lupus nephritis by high sodium chloride related to activation of SGK1 pathway. International Immunopharmacology 2015. 29 568–573. ( 10.1016/j.intimp.2015.09.027) [DOI] [PubMed] [Google Scholar]
- 16.Rao AD, Sun B, Saxena A, Hopkins PN, Jeunemaitre X, Brown NJ, Adler GK, Williams JS. Polymorphisms in the serum- and glucocorticoid-inducible kinase 1 gene are associated with blood pressure and renin response to dietary salt intake. Journal of Human Hypertension 2013. 27 176–180. ( 10.1038/jhh.2012.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farjah M, Roxas BP, Geenen DL, Danziger RS. Dietary salt regulates renal SGK1 abundance: relevance to salt sensitivity in the Dahl rat. Hypertension 2003. 41 874–878. ( 10.1161/01.HYP.0000063885.48344.EA). [DOI] [PubMed] [Google Scholar]
- 18.Lanaspa MA, Kuwabara M, Andres-Hernando A, Li N, Cicerchi C, Jensen T, Orlicky DJ, Roncal-Jimenez CA, Ishimoto T, Nakagawa T, et al High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. PNAS 2018. 115 3138–3143. ( 10.1073/pnas.1713837115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Sun F, Guo Y, Fan H. High-salt diet gets involved in gastrointestinal diseases through the reshaping of gastroenterological milieu. Digestion 2019. 99 267–274. ( 10.1159/000493096) [DOI] [PubMed] [Google Scholar]
- 20.Ullrich S, Berchtold S, Ranta F, Seebohm G, Henke G, Lupescu A, Mack AF, Chao CM, Su J, Nitschke R, et al Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes 2005. 54 1090–1099. ( 10.2337/diabetes.54.4.1090) [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Yu J, Xia T, Xiao Y, Zhang Q, Liu B, Guo Y, Deng J, Deng Y, Chen S, et al Hepatic serum- and glucocorticoid-regulated protein kinase 1 (SGK1) regulates insulin sensitivity in mice via extracellular-signal-regulated kinase 1/2 (ERK1/2). Biochemical Journal 2014. 464 281–289. ( 10.1042/BJ20141005) [DOI] [PubMed] [Google Scholar]
- 22.Ding L, Zhang L, Biswas S, Schugar RC, Brown JM, Byzova T, Podrez E. Akt3 inhibits adipogenesis and protects from diet-induced obesity via WNK1/SGK1 signaling. JCI Insight 2017. 2 e95687 ( 10.1172/jci.insight.95687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Z, Zhou L, He T. Potential effect of exercise in ameliorating insulin resistance at transcriptome level. Journal of Sports Medicine and Physical Fitness 2019. 59 116–125. ( 10.23736/S0022-4707.17.07862-8) [DOI] [PubMed] [Google Scholar]
- 24.Lang F, Gorlach A, Vallon V. Targeting SGK1 in diabetes. Expert Opinion on Therapeutic Targets 2009. 13 1303–1311. ( 10.1517/14728220903260807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedrich B, Weyrich P, Stancakova A, Wang J, Kuusisto J, Laakso M, Sesti G, Succurro E, Smith U, Hansen T, et al Variance of the SGK1 gene is associated with insulin secretion in different European populations: results from the TUEF, EUGENE2, and METSIM studies. PLoS One 2008. 3 e3506 ( 10.1371/journal.pone.0003506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klingel K, Warntges S, Bock J, Wagner CA, Sauter M, Waldegger S, Kandolf R, Lang F. Expression of cell volume-regulated kinase h-sgk in pancreatic tissue. American Journal of Physiology: Gastrointestinal and Liver Physiology 2000. 279 G998–G1002. ( 10.1152/ajpgi.2000.279.5.G998) [DOI] [PubMed] [Google Scholar]
- 27.Esguerra JLS, Ofori JK, Nagao M, Shuto Y, Karagiannopoulos A, Fadista J, Sugihara H, Groop L, Eliasson L. Glucocorticoid induces human beta cell dysfunction by involving riborepressor GAS5 LincRNA. Molecular Metabolism 2020. 32 160–167. ( 10.1016/j.molmet.2019.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ullrich S, Zhang Y, Avram D, Ranta F, Kuhl D, Haring HU, Lang F. Dexamethasone increases Na+/K+ ATPase activity in insulin secreting cells through SGK1. Biochemical and Biophysical Research Communications 2007. 352 662–667. ( 10.1016/j.bbrc.2006.11.065) [DOI] [PubMed] [Google Scholar]
- 29.Ogata H, Seino Y, Harada N, Iida A, Suzuki K, Izumoto T, Ishikawa K, Uenishi E, Ozaki N, Hayashi Y, et al KATP channel as well as SGLT1 participates in GIP secretion in the diabetic state. Journal of Endocrinology 2014. 222 191–200. ( 10.1530/JOE-14-0161) [DOI] [PubMed] [Google Scholar]
- 30.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. American Journal of Physiology: Gastrointestinal and Liver Physiology 2002. 282 G241–G248. ( 10.1152/ajpgi.00310.2001) [DOI] [PubMed] [Google Scholar]
- 31.Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. American Journal of Physiology: Renal Physiology 2001. 280 F675–F682. ( 10.1152/ajprenal.2001.280.4.F675) [DOI] [PubMed] [Google Scholar]
- 32.Ackermann TF, Boini KM, Volkl H, Bhandaru M, Bareiss PM, Just L, Vallon V, Amann K, Kuhl D, Feng Y, et al SGK1-sensitive renal tubular glucose reabsorption in diabetes. American Journal of Physiology: Renal Physiology 2009. 296 F859–F866. ( 10.1152/ajprenal.90238.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang N, Li S, Guo XC, Li JY, Ren GP, Li DS. Fibroblast growth factor 21 improves glucose homeostasis partially via down-regulation of Na(+)-d-glucose cotransporter SGLT1 in the small intestine. Biomedicine and Pharmacotherapy 2019. 109 1070–1077. ( 10.1016/j.biopha.2018.10.198) [DOI] [PubMed] [Google Scholar]
- 34.Huang CK, Yu T, de la Monte SM, Wands JR, Derdak Z, Kim M. Restoration of Wnt/beta-catenin signaling attenuates alcoholic liver disease progression in a rat model. Journal of Hepatology 2015. 63 191–198. ( 10.1016/j.jhep.2015.02.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao GZ, Lehwald N, Jang KY, Baek J, Xu B, Omary MB, Sylvester KG. Wnt/beta-catenin signaling protects mouse liver against oxidative stress-induced apoptosis through the inhibition of forkhead transcription factor FoxO3. Journal of Biological Chemistry 2013. 288 17214–17224. ( 10.1074/jbc.M112.445965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou B, Kreuzer J, Kumsta C, Wu L, Kamer KJ, Cedillo L, Zhang Y, Li S, Kacergis MC, Webster CM, et al Mitochondrial permeability uncouples elevated autophagy and lifespan extension. Cell 2019. 177 299–314.e16. ( 10.1016/j.cell.2019.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shojaiefard M, Christie DL, Lang F. Stimulation of the creatine transporter SLC6A8 by the protein kinases SGK1 and SGK3. Biochemical and Biophysical Research Communications 2005. 334 742–746. ( 10.1016/j.bbrc.2005.06.164) [DOI] [PubMed] [Google Scholar]
- 38.Young JC, Young RE. The effect of creatine supplementation on glucose uptake in rat skeletal muscle. Life Sciences 2002. 71 1731–1737. ( 10.1016/s0024-3205(02)01941-0) [DOI] [PubMed] [Google Scholar]
- 39.Hirata Y, Nomura K, Senga Y, Okada Y, Kobayashi K, Okamoto S, Minokoshi Y, Imamura M, Takeda S, Hosooka T, et al Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 2019. 4 e124952 ( 10.1172/jci.insight.124952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo J, Liang A, Liang M, Xia R, Rizvi Y, Wang Y, Cheng J. Serum glucocorticoid-regulated kinase 1 blocks CKD-induced muscle wasting via inactivation of FoxO3a and Smad2/3. Journal of the American Society of Nephrology 2016. 27 2797–2808. ( 10.1681/ASN.2015080867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andres-Mateos E, Brinkmeier H, Burks TN, Mejias R, Files DC, Steinberger M, Soleimani A, Marx R, Simmers JL, Lin B, et al Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Molecular Medicine 2013. 5 80–91. ( 10.1002/emmm.201201443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuleger T, Heinzelbecker J, Takacs Z, Hunter C, Voelkl J, Lang F, Proikas-Cezanne T. SGK1 inhibits autophagy in murine muscle tissue. Oxidative Medicine and Cellular Longevity 2018. 2018 4043726 ( 10.1155/2018/4043726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson RM, Griesel BA, Gurley JM, Szweda LI, Olson AL. Glucose availability controls adipogenesis in mouse 3T3-L1 adipocytes via up-regulation of nicotinamide metabolism. Journal of Biological Chemistry 2017. 292 18556–18564. ( 10.1074/jbc.M117.791970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmada M, Boehmer C, Akel A, Rajamanickam J, Jeyaraj S, Keller K, Lang F. SGK1 kinase upregulates GLUT1 activity and plasma membrane expression. Diabetes 2006. 55 421–427. ( 10.2337/diabetes.55.02.06.db05-0720) [DOI] [PubMed] [Google Scholar]
- 45.Di Pietro N, Hayes S, Bagattin A, Meruvu S, Pandolfi A, Hugendubler L, Fejes-Tóth G, Naray-Fejes-Tóth A, Mueller E.E. Serum- and glucocorticoid-inducible kinase 1 (SGK1) regulates adipocyte differentiation via forkhead box O1. Molecular Endocrinology 2010. 24 370–380. ( 10.1210/me.2009-0265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor FoxO1 regulates adipocyte differentiation. Developmental Cell 2003. 4 119–129. ( 10.1016/s1534-5807(02)00401-x) [DOI] [PubMed] [Google Scholar]
- 47.Kim JJ, Li P, Huntley J, Chang JP, Arden KC, Olefsky JM. FoxO1 haploinsufficiency protects against high-fat diet-induced insulin resistance with enhanced peroxisome proliferator-activated receptor gamma activation in adipose tissue. Diabetes 2009. 58 1275–1282. ( 10.2337/db08-1001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li P, Pan F, Hao Y, Feng W, Song H, Zhu D. SGK1 is regulated by metabolic-related factors in 3T3-L1 adipocytes and overexpressed in the adipose tissue of subjects with obesity and diabetes. Diabetes Research and Clinical Practice 2013. 102 35–42. ( 10.1016/j.diabres.2013.08.009) [DOI] [PubMed] [Google Scholar]
- 49.Hills CE, Squires PE, Bland R. Serum and glucocorticoid regulated kinase and disturbed renal sodium transport in diabetes. Journal of Endocrinology 2008. 199 343–349. ( 10.1677/JOE-08-0295) [DOI] [PubMed] [Google Scholar]
- 50.Khan ZA, Barbin YP, Farhangkhoee H, Beier N, Scholz W, Chakrabarti S. Glucose-induced serum- and glucocorticoid-regulated kinase activation in oncofetal fibronectin expression. Biochemical and Biophysical Research Communications 2005. 329 275–280. ( 10.1016/j.bbrc.2005.01.135) [DOI] [PubMed] [Google Scholar]
- 51.Lou Y, Zhang F, Luo Y, Wang L, Huang S, Jin F. Serum and glucocorticoid regulated kinase 1 in sodium homeostasis. International Journal of Molecular Sciences 2016. 17 1307 ( 10.3390/ijms17081307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng Y, Xiao Y, Yuan F, Liu Y, Jiang X, Deng J, Fejes-Toth G, Naray-Fejes-Toth A, Chen S, Chen Y, et al SGK1/FOXO3 signaling in hypothalamic POMC neurons mediates glucocorticoid-increased adiposity. Diabetes 2018. 67 569–580. ( 10.2337/db17-1069) [DOI] [PubMed] [Google Scholar]
- 53.Saad S, Stevens VA, Wassef L, Poronnik P, Kelly DJ, Gilbert RE, Pollock CA. High glucose transactivates the EGF receptor and up-regulates serum glucocorticoid kinase in the proximal tubule. Kidney International 2005. 68 985–997. ( 10.1111/j.1523-1755.2005.00492.x) [DOI] [PubMed] [Google Scholar]
- 54.Wang Q, Zhang A, Li R, Liu J, Xie J, Deng A, Feng Y, Zhu Z. High glucose promotes the CTGF expression in human mesangial cells via serum and glucocorticoid-induced kinase 1 pathway. Journal of Huazhong University of Science and Technology: Medical Sciences 2008. 28 508–512. ( 10.1007/s11596-008-0504-z) [DOI] [PubMed] [Google Scholar]
- 55.Hills CE, Bland R, Bennett J, Ronco PM, Squires PE. High glucose up-regulates ENaC and SGK1 expression in HCD-cells. Cellular Physiology and Biochemistry 2006. 18 337–346. ( 10.1159/000097611) [DOI] [PubMed] [Google Scholar]
- 56.Feng Y, Wang Q, Wang Y, Yard B, Lang F. SGK1-mediated fibronectin formation in diabetic nephropathy. Cellular Physiology and Biochemistry 2005. 16 237–244. ( 10.1159/000089849) [DOI] [PubMed] [Google Scholar]
- 57.Feng B, Chen S, George B, Feng Q, Chakrabarti S. miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes/Metabolism Research and Reviews 2010. 26 40–49. ( 10.1002/dmrr.1054) [DOI] [PubMed] [Google Scholar]
- 58.Das S, Aiba T, Rosenberg M, Hessler K, Xiao C, Quintero PA, Ottaviano FG, Knight AC, Graham EL, Bostrom P, et al Pathological role of serum- and glucocorticoid-regulated kinase 1 in adverse ventricular remodeling. Circulation 2012. 126 2208–2219. ( 10.1161/CIRCULATIONAHA.112.115592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voelkl J, Lin Y, Alesutan I, Ahmed MS, Pasham V, Mia S, Gu S, Feger M, Saxena A, Metzler B, et al Sgk1 sensitivity of Na(+)/H(+) exchanger activity and cardiac remodeling following pressure overload. Basic Research in Cardiology 2012. 107 236 ( 10.1007/s00395-011-0236-2) [DOI] [PubMed] [Google Scholar]
- 60.Li P, Hao Y, Pan FH, Zhang M, Ma JQ, Zhu DL. SGK1 inhibitor reverses hyperglycemia partly through decreasing glucose absorption. Journal of Molecular Endocrinology 2016. 56 301–309. ( 10.1530/JME-15-0285) [DOI] [PubMed] [Google Scholar]
- 61.Du YN, Tang XF, Xu L, Chen WD, Gao PJ, Han WQ. SGK1-FoxO1 signaling pathway mediates Th17/Treg imbalance and target organ inflammation in angiotensin II-induced hypertension. Frontiers in Physiology 2018. 9 1581 ( 10.3389/fphys.2018.01581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ackermann TF, Boini KM, Beier N, Scholz W, Fuchss T, Lang F. EMD638683, a novel SGK inhibitor with antihypertensive potency. Cellular Physiology and Biochemistry 2011. 28 137–146. ( 10.1159/000331722) [DOI] [PubMed] [Google Scholar]
- 63.Gan W, Ren J, Li T, Lv S, Li C, Liu Z, Yang M. The SGK1 inhibitor EMD638683, prevents angiotensin II-induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochimica and Biophysica Acta: Molecular Basis of Disease 2018. 1864 1–10. [DOI] [PubMed] [Google Scholar]
- 64.Zhuang L, Jin G, Hu X, Yang Q, Shi Z. The inhibition of SGK1 suppresses epithelial-mesenchymal transition and promotes renal tubular epithelial cell autophagy in diabetic nephropathy. American Journal of Translational Research 2019. 11 4946–4956. [PMC free article] [PubMed] [Google Scholar]
- 65.Towhid ST, Liu GL, Ackermann TF, Beier N, Scholz W, Fuchss T, Toulany M, Rodemann HP, Lang F. Inhibition of colonic tumor growth by the selective SGK inhibitor EMD638683. Cellular Physiology and Biochemistry 2013. 32 838–848. ( 10.1159/000354486) [DOI] [PubMed] [Google Scholar]
- 66.Schernthaner-Reiter MH, Kiefer F, Zeyda M, Stulnig TM, Luger A, Vila G. Strong association of serum- and glucocorticoid-regulated kinase 1 with peripheral and adipose tissue inflammation in obesity. International Journal of Obesity 2015. 39 1143–1150. ( 10.1038/ijo.2015.41) [DOI] [PubMed] [Google Scholar]
- 67.Borst O, Schaub M, Walker B, Schmid E, Munzer P, Voelkl J, Alesutan I, Rodriguez JM, Vogel S, Schoenberger T, et al Pivotal role of serum- and glucocorticoid-inducible kinase 1 in vascular inflammation and atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology 2015. 35 547–557. ( 10.1161/ATVBAHA.114.304454) [DOI] [PubMed] [Google Scholar]
- 68.Sun JY, Li C, Shen ZX, Zhang WC, Ai TJ, Du LJ, Zhang YY, Yao GF, Liu Y, Sun S, et al Mineralocorticoid receptor deficiency in macrophages inhibits neointimal hyperplasia and suppresses macrophage inflammation through SGK1-AP1/NF-kappaB pathways. Arteriosclerosis, Thrombosis, and Vascular Biology 2016. 36 874–885. ( 10.1161/ATVBAHA.115.307031) [DOI] [PubMed] [Google Scholar]
- 69.Wu C, Chen Z, Xiao S, Thalhamer T, Madi A, Han T, Kuchroo V. SGK1 governs the reciprocal development of Th17 and regulatory T cells. Cell Reports 2018. 22 653–665. ( 10.1016/j.celrep.2017.12.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013. 496 518–522. ( 10.1038/nature11868) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a