Abstract
Clinical presentation of Takotsubo syndrome (TTS) may range from acute chest pain to dyspnea: the prognostic role of clinical onset is still controversial. Aim of this study was therefore to investigate the prognostic relevance of dyspnea at presentation in patients with TTS. We analyzed 1,071 TTS patients (median age 72 years, 90% female) enrolled in the international multicenter GEIST registry. Patients were divided according to the presence or absence of dyspnea at hospital admission, as clinically assessed by the accepting physician. The primary endpoint was occurrence of in-hospital complications defined as a composite of pulmonary edema, cardiogenic shock and death. Overall, 316 (30%) patients presented with dyspnea at hospital admission. Diabetes, lower left ventricular ejection fraction and presence of pulmonary disease or atrial fibrillation were independently associated with dyspnea. In-hospital pulmonary edema, cardiogenic shock and death (17% vs. 3%, p < 0.001; 12% vs. 7%, p = 0.009; 5% vs. 2%, p = 0.004 respectively) and long-term overall mortality (22% vs. 11%, p < 0.001) occurred more frequently in patients with dyspnea than in those without. At multivariable analysis, dyspnea at presentation remained independently associated to both the composite primary endpoint [odds ratio 2.98 (95% confidence interval (CI) 1.95–4.59, p < 0.001] and all-cause mortality [hazard ratio 2.03 (95% CI 1.37–2.99), p < 0.001]. Dyspnea at presentation is common in TTS and is independently associated with in-hospital complications and impaired long-term prognosis. Thorough symptom assessment including dyspnea therefore represents a valuable tool to potentially optimize risk-stratification models for TTS patients.
Subject terms: Cardiology, Signs and symptoms
Introduction
Takotsubo syndrome (TTS) is an acute heart failure syndrome characterized by transient left ventricular (LV) systolic dysfunction in the absence of identifiable culprit coronary artery stenosis1. Due to the reversible nature of LV dysfunction, it has been initially considered a disorder with a benign prognosis2,3. Nonetheless, several complications during in-hospital course and follow up may occur in TTS with an event rate comparable to that observed in patients with acute coronary syndrome (ACS)4–7. However, both short8,9 and long-term6,10 prognosis remain quite heterogeneous in TTS, which supports the need of improved risk stratification in order to identify those patients who may most benefit from intensive in-hospital management and long-term clinical follow-up11. Dyspnea at presentation is associated with worse prognosis in ACS12, and its assessment was able to improve accuracy of a traditional risk stratification model in these patients13. Thus, aim of the present study was to investigate the prevalence and prognostic relevance of dyspnea at admission in a large cohort of patients with TTS.
Methods
Out of 1,303 patients enrolled in the multicentric international TTS registry GEIST (German Italian STress Cardiomyopathy registry), the final analysis included 1,071 patients (median age 72 years, 90% female) for which the data about dyspnea was available. Detailed description of inclusion and exclusion criteria have been previously reported14,15. Demographic, clinical and instrumental characteristics were recorded at admission. Symptoms at presentation, including the occurrence of dyspnea as a self-reported uncomfortable feeling of breathing, were recorded at admission by the accepting physician13. Ballooning patterns were described as apical (typical), mid-ventricular or basal as previously reported16. Recovery of left ventricular systolic function was documented 3–6 months after the acute event in all surviving patients. The primary end-point (in-hospital complications) was defined as the composite of acute pulmonary edema, cardiogenic shock needing supportive therapy (mechanical ventilator support, intra-aortic balloon pump, catecholamine administration), and in-hospital death. The secondary end-point was defined as all-cause long-term mortality. All events were verified via medical records and evaluated by a clinical events committee. All patients were managed in accordance with the Declaration of Helsinki and signed an informed consent for the processing of personal data for scientific research purpose. This is an observational study not needing full Ethics Committee approval. Data are not publicly available but would be available on request. Permission was obtained from the authorities to use the data.
Statistical analysis
Data were analyzed with SPSS software version 22.0 (SPSS Inc., Chicago, Illinois). Continuous variables were presented as mean ± standard deviation or as median with interquartile range (25°–75°), as appropriate. Categorical variables were compared using a Chi-square analysis or Fisher’s exact test as appropriate. Normally distributed continuous variables were compared using the Student t test for independent samples, in case of non-normally distributed variables, Mann–Whitney U test was used. Univariate logistic regression analysis was used to calculate estimated and 95% confidence intervals odds ratios for variables associated to dyspnea and to in-hospital complications. Univariate Cox-regression analysis was performed to assess variables independently associated to long-term mortality. Variables with p < 0.1 on univariate analysis were entered into a multivariable logistic regression model to identify independent risk factors for the secondary end-point long-term mortality. Kaplan–Meier curve and log-rank test were used to assess survival function at follow-up. Landmark analysis at 30 days was performed to specifically assess long-term mortality excluding deaths occurred during the acute phase.
Results
The incidence of dyspnea at admission was 30% (316 pts). Baseline demographic, clinical and instrumental characteristics of the whole study population and according to the presence or absence of dyspnea at presentation are reported in Table 1. Patients with dyspnea were older (78 vs. 74 years, p = 0.001), more frequently male (13% vs. 8%, p = 0.035), and less often presenting with chest pain (40% vs. 63%, p < 0.001). Among patients with dyspnea, a physical trigger was more often identified (38% vs. 31%, p = 0.03) and prevalence of comorbidities such as diabetes, pulmonary disease, and atrial fibrillation was higher (26% vs. 16%, p < 0.001; 38% vs. 13%, p < 0.001; 17% vs. 9%, p = 0.001 respectively) while LV ejection fraction (LVEF) was lower (36% vs. 40%, p < 0.001) compared to patients without dyspnea. Prevalence of dyspnea within specific physical trigger subgroups has been reported in supplementary Table 1.
Table 1.
Baseline characteristics of study population.
| Variable | Overall (n = 1,071) |
Dyspnea (n = 316) |
No dyspnea (n = 755) |
p value |
|---|---|---|---|---|
| Age (years) | 72 (63, 79) | 78 (65, 80) | 74 (63, 78) | 0.001 |
| Male | 104 (10%) | 40 (13%) | 64 (8%) | 0.035 |
| Coronary risk factors | ||||
| Hypertension | 725 (68%) | 216 (69%) | 509 (68%) | 0.756 |
| Diabetes mellitus | 200 (19%) | 82 (26%) | 118 (16%) | < 0.001 |
| Dyslipidemia | 455 (42%) | 129 (41%) | 326 (43%) | 0.490 |
| Current smoker | 230 (22%) | 74 (24%) | 156 (21%) | 0.286 |
| Clinical presentation | ||||
| Chest pain | 603 (56%) | 128 (40%) | 475 (63%) | < 0.001 |
| Hystory of cancer | 141 (13%) | 51 (16%) | 90 (12%) | 0.073 |
| Pulmonary disease | 215 (20%) | 122 (38%) | 93 (13%) | < 0.001 |
| Stressful triggera | ||||
| Emotional | 435 (41%) | 112 (35%) | 323 (43%) | 0.026 |
| Physical | 355 (33%) | 120 (38%) | 235 (31%) | 0.030 |
| None | 285 (27%) | 83 (26%) | 202 (27%) | 0.869 |
| Admission electrocardiographic findings | ||||
| Atrial fibrillation | 124 (12%) | 54 (17%) | 70 (9%) | 0.001 |
| ST-segment elevation | 499 (47%) | 132 (42%) | 367(49%) | 0.052 |
| ST-segment depression | 71 (7%) | 26 (8%) | 45 (6%) | 0.240 |
| T-wave inversion | 501 (47%) | 136 (56%) | 365 (43%) | < 0.001 |
| Laboratory data | ||||
| NT-Pro-BNP (161/1,071)b | 7,561 (2,696, 15,500) | 8,321 (3,431, 17,150) | 7,511 (2,120, 15,337) | 0.270 |
| TnI peak (ng/ml) (433/1,071)b | 2.9 (1.1, 6.3) | 2.3 (0.68 – 5.1) | 3.2 (1.38 – 6.8) | 0.013 |
| Admission echocardiographic findings | ||||
| Apical ballooning | 942 (88%) | 279 (88%) | 663 (86%) | 0.827 |
| Mid-ventricular ballooning | 111 (10%) | 34 (11%) | 77 (10%) | 0.784 |
| Basal ballooning | 18 (2%) | 3 (1%) | 15 (2%) | 0.228 |
| Mitral Insufficiency (moderate to severe) (671/1,071)b | 112 (17%) | 50 (23%) | 62 (14%) | 0.002 |
| EF (%) | 40 (32, 45) | 36 (30, 45) | 40 (34, 45) | < 0.001 |
| Angiographic findings | ||||
| CAD (721/1,071)b | 114 (16%) | 25 (13%) | 89 (17%) | 0.192 |
| Discharge therapy | ||||
| Aspirin (774/1,071)b | 551 (71%) | 153 (62%) | 398 (75%) | < 0.001 |
| DAPT (336/1,071)b | 43 (13%) | 10 (8%) | 33 (16%) | 0.035 |
| Anticoagulant (589/1,071)b | 113 (19%) | 60 (28%) | 53 (14%) | < 0.001 |
| Beta-Blocker (669/1,071)b | 559 (84%) | 177 (82%) | 382 (84%) | 0.336 |
| Ace-inhibitor/ARBs (778/1,071)b | 622 (80%) | 198 (79%) | 424 (80%) | 0.720 |
Data are presented as no. (%), mean ± standard deviation, median (interquartile range).
Bold values are statistically significant.
NT-pro-BNP N-terminal prohormone of brain natriuretic peptide, TnI troponin I, EF left ventricular ejection fraction, CAD coronary artery disease, DAPT dual antiplatelet therapy, ARBs angiotensin II receptor blockers.
aPatients n = 22 experienced both emotional and physical trigger.
bNumber of patients with available data.
At adjusted multivariable regression analysis, diabetes [OR 1.69 (95% confidence interval (CI) 1.15–2.48); p = 0.007], presence of pulmonary diseases [OR 4.1 (95% CI 2.83–5.9); p < 0.001], lower LVEF (per 10% decrease) [OR 1.26 (95% CI 1.06–1.5); p = 0.009] and presence of atrial fibrillation [OR 2.17 (95% CI 1.35–3.49); p = 0.001] were identified as factors independently associated with the occurrence of dyspnea (Table 2).
Table 2.
Univariate and multivariable analysis for factors associated to dyspnea.
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (per 10 year) | 1.23 (1.09–1.38) | < 0.001 | NS | NS |
| Male | 1.56 (1.03–2.38) | 0.036 | NS | NS |
| Physical trigger | 1.35 (1.03–1.78) | 0.03 | NS | NS |
| History of cancer | 1.41 (0.97–2) | 0.074 | NS | NS |
| Diabetes | 1.9 (1.38–2.62) | < 0.001 | 1.69 (1.15–2.48) | 0.007 |
| Pulmonary disease | 4.35 (3.11–6.1) | < 0.001 | 4.1 (2.83–5.9) | < 0.001 |
| EF (per 10% decrease) | 1.32 (1.14–1.51) | < 0.001 | 1.26 (1.06–1.5) | 0.009 |
| Atrial fibrillation | 1.99 (1.33–2.98) | 0.001 | 2.17 (1.35–3.49) | 0.001 |
| Apical ballooning | 1.11 (0.75–1.65) | 0.596 | – | – |
Bold values are statistically significant.
OR odds ratio, CI confidence interval, EF left ventricular ejection fraction, NS non-significant.
Overall, 167 (16%) patients experienced complications during the in-hospital course (Table 3). Patients with dyspnea at admission had higher in-hospital complications rates (29% vs. 10%; p < 0.001) compared with those with other or no symptoms at presentation; specifically, during hospital stay they suffered more frequently of both pulmonary edema and cardiogenic shock (17% vs. 3%, p < 0.001 and 12% vs. 7%, p = 0.009 respectively); moreover, median length of hospitalization was longer and in-hospital mortality higher (8 vs. 6 days, p < 0.001 and 5% vs. 2%, p = 0.004 respectively). After adjustment for other clinical risk factors in multivariable analysis, dyspnea was independently associated with the primary end-point in-hospital complications [OR 3 (95% CI 1.96–4.62); p < 0.001], along with male gender, lower LVEF, presence of pulmonary diseases, and atrial fibrillation (Table 4).
Table 3.
In-hospital course and short- and long-term outcome.
| Variable | Overall (n = 1,071) |
Dyspnea (n = 316) |
No dyspnea (n = 755) |
p value |
|---|---|---|---|---|
| In-hospital course | ||||
| In-hospital complications | 167 (16%) | 90 (29%) | 77 (10%) | < 0.001 |
| Pulmonary edema | 77 (7%) | 54 (17%) | 23 (3%) | < 0.001 |
| Cardiogenic shock | 89 (8%) | 37 (12%) | 52 (7%) | 0.009 |
| In-hospital death | 30 (3%) | 16 (5%) | 14 (2%) | 0.004 |
| In-hospital treatment | ||||
| Invasive ventilation (960/1,071)a | 141 (15%) | 75 (27%) | 66 (10%) | < 0.001 |
| IABP (1,056/1,071)a | 15 (1.4%) | 5 (1.6%) | 10 (1.3%) | 0.778 |
| Lenght of stay | 7 (5, 10) | 8 (5, 11) | 6 (5, 9) | < 0.001 |
| All-cause mortality | ||||
| Follow up (days) | 576 (27,1668) | 486 (14,1607) | 605 (36,1733) | 0.193 |
| Long-term | 155 (14%) | 69 (22%) | 86 (11%) | < 0.001 |
Data are presented as no. (%), median (interquartile range).
Bold values are statistically significant.
IABP intra-aortic balloon pump.
aNumber of patients with available data.
Table 4.
Univariate and multivariable logistic regression analysis for prediction of in-hospital complications.
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (per 10 year) | 1.17 (1.01–1.35) | < 0.034 | NS | NS |
| Male | 2.74 (1.74–4.31) | < 0.001 | 2.9 (1.65–5.1) | < 0.001 |
| Dyspnea | 3.51 (2.49–4.92) | < 0.001 | 3 (1.96–4.62) | < 0.001 |
| Physical trigger | 1.23 (0.87–1.74) | 0.235 | – | – |
| History of cancer | 1.07 (0.66–1.74) | 0.770 | – | – |
| Diabetes | 2.01 (1.37–2.93) | < 0.001 | NS | NS |
| Pulmonary disease | 2.44 (1.66–3.59) | < 0.001 | 1.75 (1.1–2.8) | 0.02 |
| EF (per 10% decrease) | 2.21 (1.8 2.71) | < 0.001 | 2.13 (1.66–2.74) | < 0.001 |
| Atrial fibrillation | 2.96 (1.89–4.63) | < 0.001 | 2.27 (1.31–3.93) | 0.004 |
| Apical ballooning | 1.31 (0.77–2.21) | 0.317 | - | - |
Bold values are statistically significant.
OR odds ratio, CI confidence interval, EF left ventricular ejection fraction, NS non-significant.
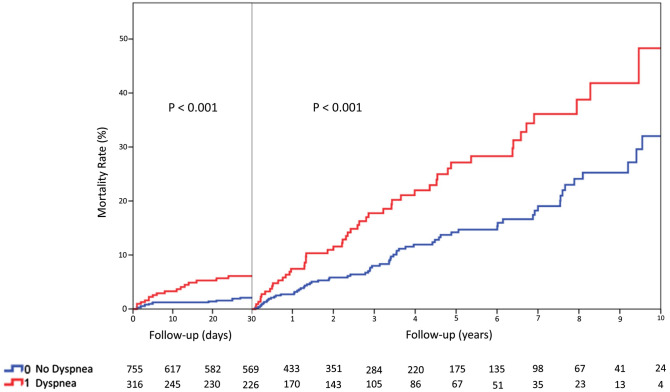
During a median follow up of 576 days (interquartile range: 27,2), TTS patients with dyspnea at admission had significantly higher long-term mortality rates compared with those without (22% vs. 11%; p < 0.001) (Table 2). Landmark analysis at 30 days showed significant increase in mortality rate among patients presenting with dyspnea both in the acute phase and in the long term (p < 0.001 for both, Fig. 1). Kaplan–Meier plot illustrates mortality curves progressively diverging during the length of follow-up (Fig. 1). In multivariable Cox-regression analysis, dyspnea at admission was also a significant predictor of the secondary end-point long-term mortality [HR 2.02 (95% CI 1.37–2.98); p < 0.001] (Table 5). In addition, age, male sex, the presence of malignancies or pulmonary diseases, lower LVEF and the occurrence of cardiogenic shock during the acute phase were identified as secondary end-point determinants.
Figure 1.
Kaplan–Meier curves showing survival rate at 30 days and long-term follow-up among patients admitted with takotsubo syndrome with or without dyspnea.
Table 5.
Univariate and multivariable Cox-regression analysis for predictors of long-term mortality.
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (per 10 year) | 1.75 (1.47–2.01) | < 0.001 | 1.57 (1.28–1.91) | < 0.001 |
| Male | 2.61 (1.69–4) | < 0.001 | 1.89 (1.13–3.18) | 0.015 |
| Dyspnea | 2.19 (1.58–3.02) | < 0.001 | 2.02 (1.37–2.98) | < 0.001 |
| Physical trigger | 1.35 (0.97–1.89) | 0.078 | NS | NS |
| History of cancer | 2.65 (1.82–3.89) | < 0.001 | 2.91 (1.8–4.72) | < 0.001 |
| Diabetes | 1.68 (1.12–2.42) | 0.005 | NS | NS |
| Pulmonary disease | 2.53 (1.62–3.41) | < 0.001 | 1.67 (1.1–2.51) | 0.014 |
| EF (per 10% decrease) | 1.68 (1.4–2) | < 0.001 | 1.77 (1.43–2.2) | < 0.001 |
| Atrial fibrillation | 2.26 (1.51–3.39) | < 0.001 | NS | NS |
| Apical ballooning | 1.9 (1.11–3.24) | 0.018 | NS | NS |
| Cardiogenic shock | 4.65 (3.22–6.72) | < 0.001 | 4.1 (2.64–6.39) | < 0.001 |
Bold values are statistically significant.
HR hazard ratio, CI confidence interval, EF left ventricular ejection fraction, NS non-significant.
Discussion
We report incidence of dyspnea and prognostic implication among a multicenter international registry of patients admitted with TTS. The main results of the present study are as follows: (I) dyspnea at hospital admission is present in one third of patients admitted with TTS; (II) it is associated with coexisting comorbidities and worse cardiac function during the acute phase; and (III) dyspnea is an independent predictor of in-hospital complications and long-term mortality in TTS patients.
Incidence and determinants of dyspnea
In our analysis, approximately one third of TTS patients had dyspnea at admission, a result in keeping with previous TTS studies17 and comparable to what observed among ACS patients13. Several cardiac mechanisms could be responsible for dyspnea in the setting of an acute heart failure syndrome18. In TTS, these include systolic and diastolic dysfunction19, mitral insufficiency due to papillary muscles dysfunction20, atrial fibrillation21, and heart rate9. Accordingly, in our population we found that lower LVEF was independently associated with dyspnea. On the other hand, the symptom dyspnea not only represents underlying cardiovascular dysfunction, but it could reflect more variegate interactions, being influenced by the presence of coexisting comorbidities22 which are quite common in patients with TTS17. Consistently, we found TTS patients with dyspnea to be older and to have higher prevalence of physical triggers (i.e. underlying illness as a cause of the acute TTS episode), which may be directly linked to the symptom breathlessness22. Moreover, pulmonary diseases and diabetes were independently associated with the occurrence of dyspnea, with the latter possibly contributing through myocardial fibrosis, dysfunctional remodeling and associated diastolic dysfunction23, leading to augmented LV filling pressures and pulmonary fluid accumulation as previously reported in the setting of TTS14.
Prognostic relevance of dyspnea
In the present study, patients with dyspnea at admission had increased rates of in-hospital complications. Though dyspnea is intuitively linked to some of the components of the composite primary end-point that could have been already present at the time of symptom assessment, it is essential to highlight that complications could also develop later during hospitalization24. Therefore, earlier risk stratification might identify high-risk individuals that could most benefit from a more intensive and prolonged management11.
Moreover, long-term mortality rate was higher in patients exhibiting dyspnea at presentation, even after excluding those events during the first month, with mortality curves progressively diverging during follow-up. These findings indicate that TTS patients admitted with symptoms of decompensated heart failure suffer a worse prognosis even after LV systolic function is expected to have largely recovered, in keeping with a significant body of evidence suggesting that in TTS the severity of cardiac involvement correlates with worse prognosis in the long-term24–30. One potential explanation is that dyspnea at presentation, being associated to higher degree of cardiac dysfunction, represents a marker of more severe TTS episodes that can lead to long-term sequelae and subsequent heart failure phenotype31. Indeed, impaired cardiac mechanics32, diffuse fibrosis31 and inflammatory activation33 have been described after the acute phase, suggesting that a subgroup of patients might be characterized by incomplete recovery. Additionally, it has been suggested that TTS individuals prone to develop hemodynamic instability in the acute phase may be characterized by a pre-existing concealed decreased cardiac reserve24, possibly influencing long-term outcome34. Hence we hypothesize that, in the vulnerable subset of patients with dyspnea at presentation, comorbidities may act in a synergistic fashion with TTS functional abnormalities, resulting in greater cardiac dysfunction and decompensation during the acute phase, and worse prognosis in the long-term. Accordingly, the prognostic relevance of comorbidities is supported by results of both our and other studies14,35–37, and further corroborated by the fact that long-term mortality in TTS is mainly non-cardiovascular6,10,24,29.
In conclusion, dyspnea at presentation, reflecting both the TTS patient’s cardiac function and comorbidity burden, might potentially be a simple and easily evaluable parameter to identify those individuals with a worse prognosis.
Conclusions
The occurrence of dyspnea at hospital admission is associated with higher degree of cardiac dysfunction and comorbidity burden. Furthermore, presence of dyspnea is associated with complicated in-hospital course and worse long-term prognosis, potentially making symptom assessment an easy and valuable tool in the search of reliable risk-stratification models for TTS patients.
Limitations
Though the present observational study included one of the largest cohort of TTS patients to date, the generalizability might be limited, and our results should be considered hypothesis generating to be confirmed by further and targeted studies. Self-reported symptoms assessment is a mere qualitative measure of patient’s decompensation, nonetheless this is a key parameter to investigate a suspected heart failure condition and is commonly used in daily clinical practice. Though dyspnea and chest pain are by far the commonest symptoms in TTS, we could not provide data on other affection, such as neurovegetative symptoms, in the whole of our cohort. We do not have specific data on time to onset of in-hospital complications, hence we cannot exclude their presence at the same time as the dyspnea variable was collected; Due to the large number of centers involved in the registry, with a quite heterogeneous approach to blood samples analysis, we could not provide a detailed analysis of biomarkers profile, including inflammatory markers, in the whole of our cohort.
Supplementary information
Acknowledgements
LRL and LA were supported by a “type 2 Start Research Grant” (AR21816436B0B884) by Sapienza University of Rome, Italy.
Author contributions
L.A., M.B.M., and F.S. wrote the paper and analyzed data. I.Eitel, I.El-Battrawy, H.T., I.A., and N.B. supervised the work. P.C., F.G., E.M., M.V., F.R., T.S., C.M., G.N., L.L., L.C., and R.S. collected data. L.A., M.B.M. designed the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Luca Arcari, Maria Beatrice Musumeci, Ingo Eitel and Francesco Santoro.
Supplementary information
is available for this paper at 10.1038/s41598-020-70445-9.
References
- 1.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 2.Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J. Am. Coll. Cardiol. 2007;50:448–452. doi: 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Cacciotti L, Passaseo I, Marazzi G, Camastra G, Campolongo G, Beni S, et al. Observational study on Takotsubo-like cardiomyopathy: Clinical features, diagnosis, prognosis and follow-up. BMJ Open. 2012;2:e001165. doi: 10.1136/bmjopen-2012-001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 5.Tornvall P, Collste O, Ehrenborg E, Järnbert-Petterson H. A case-control study of risk markers and mortality in Takotsubo stress cardiomyopathy. J. Am. Coll. Cardiol. 2016;67:1931–1936. doi: 10.1016/j.jacc.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Stiermaier T, Moeller C, Oehler K, Desch S, Graf T, Eitel C, et al. Long-term excess mortality in Takotsubo cardiomyopathy: Predictors, causes and clinical consequences. Eur. J. Heart Fail. 2016;18:650–656. doi: 10.1002/ejhf.494. [DOI] [PubMed] [Google Scholar]
- 7.Redfors B, Vedad R, Angerås O, Råmunddal T, Petursson P, Haraldsson I, Ali A, Dworeck C, Odenstedt J, Ioaness D, Libungan B, Shao Y, Albertsson P, Stone GW, Omerovic E. Mortality in Takotsubo syndrome is similar to mortality in myocardial infarction—A report from the SWEDEHEART registry. Int. J. Cardiol. 2015;185:282–289. doi: 10.1016/j.ijcard.2015.03.162. [DOI] [PubMed] [Google Scholar]
- 8.Ando K, Sukekawa H, Takahata A, Kobari Y, Tsuchiya H, Ishigaki D, et al. Renal dysfunction indicative of outcomes in hospitalized patients with Takotsubo syndrome. Eur. Heart J. Acute Cardiovasc. Care. 2017 doi: 10.1177/2048872617715019. [DOI] [PubMed] [Google Scholar]
- 9.Arcari L, Limite LR, Cacciotti L, Sclafani M, Russo D, Passaseo I, et al. Admission heart rate and in-hospital course of patients with Takotsubo syndrome. Int. J. Cardiol. 2018 doi: 10.1016/j.ijcard.2018.07.145.16. [DOI] [PubMed] [Google Scholar]
- 10.Looi J-L, Lee M, Webster MWI, To ACY, Kerr AJ. Postdischarge outcome after Takotsubo syndrome compared with patients post-ACS and those without prior CVD: ANZACS-QI 19. Open Heart. 2018;5:e000918. doi: 10.1136/openhrt-2018-000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, et al. Current state of knowledge on Takotsubo syndrome: A position statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 12.Thang ND, Karlson BW, Sundström BW, Karlsson T, Herlitz J. Pre-hospital prediction of death or cardiovascular complications during hospitalisation and death within one year in suspected acute coronary syndrome patients. Int. J. Cardiol. 2015;185:308–312. doi: 10.1016/j.ijcard.2015.03.143. [DOI] [PubMed] [Google Scholar]
- 13.Hellenkamp K, Darius H, Giannitsis E, Erbel R, Haude M, Hamm C, et al. The German CPU Registry: Dyspnea independently predicts negative short-term outcome in patients admitted to German Chest Pain Units. Int. J. Cardiol. 2015;181:88–95. doi: 10.1016/j.ijcard.2014.11.199. [DOI] [PubMed] [Google Scholar]
- 14.Stiermaier T, Santoro F, El-Battrawy I, Möller C, Graf T, Novo G, et al. Prevalence and prognostic impact of diabetes in Takotsubo syndrome: Insights from the international, Multicenter GEIST Registry. Diabetes Care. 2018;41:1084–1088. doi: 10.2337/dc17-2609. [DOI] [PubMed] [Google Scholar]
- 15.El-Battrawy I, Santoro F, Stiermaier T, Möller C, Guastafierro F, Novo G, et al. Incidence and clinical impact of recurrent Takotsubo syndrome: Results from the GEIST Registry. J. Am. Heart Assoc. 2019 doi: 10.1161/JAHA.118.010753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 17.Pelliccia F, Parodi G, Greco C, Antoniucci D, Brenner R, Bossone E, et al. Comorbidities frequency in Takotsubo syndrome: An international collaborative systematic review including 1109 patients. Am. J. Med. 2015;128:11–654. doi: 10.1016/j.amjmed.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Gheorghiade M, Braunwald E. A proposed model for initial assessment and management of acute heart failure syndromes. JAMA. 2011;305:1702. doi: 10.1001/jama.2011.515. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros K, O’Connor MJ, Baicu CF, Fitzgibbons TP, Shaw P, Tighe DA, et al. Systolic and diastolic mechanics in stress cardiomyopathy. Circulation. 2014;129:1659–1667. doi: 10.1161/CIRCULATIONAHA.113.002781. [DOI] [PubMed] [Google Scholar]
- 20.Citro R, Rigo F, D’Andrea A, Ciampi Q, Parodi G, Provenza G, et al. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in Tako-Tsubo cardiomyopathy. JACC Cardiovasc. Imaging. 2014;7:119–129. doi: 10.1016/j.jcmg.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Stiermaier T, Santoro F, Eitel C, Graf T, Möller C, Tarantino N, et al. Prevalence and prognostic relevance of atrial fibrillation in patients with Takotsubo syndrome. Int. J. Cardiol. 2017;245:156–161. doi: 10.1016/j.ijcard.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 22.Manning HL, Schwartzstein RM. Pathophysiology of Dyspnea. N. Engl. J. Med. 1995;333:1547–1553. doi: 10.1056/NEJM199512073332307. [DOI] [PubMed] [Google Scholar]
- 23.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almendro-Delia M, Núñez-Gil IJ, Lobo M, Andrés M, Vedia O, Sionis A, et al. Short- and long-term prognostic relevance of cardiogenic shock in Takotsubo syndrome. JACC Heart Fail. 2018;6:928–936. doi: 10.1016/j.jchf.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Stiermaier T, Santoro F, Graf T, Guastafierro F, Tarantino N, De Gennaro L, et al. Prognostic value of N-terminal Pro-B-type natriuretic peptide in Takotsubo syndrome. Clin. Res. Cardiol. 2018 doi: 10.1007/s00392-018-1227-1. [DOI] [PubMed] [Google Scholar]
- 26.Böhm M, Cammann VL, Ghadri JR, Ukena C, Gili S, Di Vece D, et al. Interaction of systolic blood pressure and resting heart rate with clinical outcomes in Takotsubo syndrome: Insights from the International Takotsubo Registry. Eur J Heart Fail. 2018 doi: 10.1002/ejhf.1162. [DOI] [PubMed] [Google Scholar]
- 27.Citro R, Radano I, Parodi G, Di Vece D, Zito C, Novo G, et al. Long-term outcome in patients with Takotsubo syndrome presenting with severely reduced left ventricular ejection fraction. Eur. J. Heart Fail. 2019 doi: 10.1002/ejhf.1373. [DOI] [PubMed] [Google Scholar]
- 28.Stiermaier T, Eitel C, Desch S, Fuernau G, Schuler G, Thiele H, et al. Incidence, determinants and prognostic relevance of cardiogenic shock in patients with Takotsubo cardiomyopathy. Eur. Heart J. Acute Cardiovasc. Care. 2016;5:489–496. doi: 10.1177/2048872615612456. [DOI] [PubMed] [Google Scholar]
- 29.Scudiero F, Arcari L, Cacciotti L, De Vito E, Marcucci R, Passaseo I, et al. Prognostic relevance of GRACE risk score in Takotsubo syndrome. Eur. Heart J. Acute Cardiovasc. Care. 2019 doi: 10.1177/2048872619882363. [DOI] [PubMed] [Google Scholar]
- 30.El-Battrawy I, Ansari U, Lang S, Behnes M, Schramm K, Fastner C, et al. Impact and management of left ventricular function on the prognosis of Takotsubo syndrome. Eur. J. Clin. Investig. 2017;47:477–485. doi: 10.1111/eci.12768. [DOI] [PubMed] [Google Scholar]
- 31.Scally C, Rudd A, Mezincescu A, Wilson H, Srivanasan J, Horgan G, et al. Persistent long-term structural, functional, and metabolic changes after stress-induced (Takotsubo) cardiomyopathy. Circulation. 2018;137:1039–1048. doi: 10.1161/CIRCULATIONAHA.117.031841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz K, Ahearn T, Srinivasan J, Neil CJ, Scally C, Rudd A, et al. Alterations in cardiac deformation, timing of contraction and relaxation, and early myocardial fibrosis accompany the apparent recovery of acute stress-induced (Takotsubo) cardiomyopathy: An end to the concept of transience. J. Am. Soc. Echocardiogr. 2017;30:745–755. doi: 10.1016/j.echo.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Scally C, Abbas H, Ahearn T, Srinivasan J, Mezincescu A, Rudd A, et al. Myocardial and systemic inflammation in acute stress-induced (Takotsubo) cardiomyopathy. Circulation. 2019;139:1581–1592. doi: 10.1161/CIRCULATIONAHA.118.037975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limite LR, Arcari L, Cacciotti L, Russo D, Musumeci MB. Cardiogenic shock in Takotsubo syndrome. JACC Heart Fail. 2019;7:175–176. doi: 10.1016/j.jchf.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Brunetti ND, Tarantino N, Guastafierro F, De Gennaro L, Correale M, Stiermaier T, et al. Malignancies and outcome in Takotsubo syndrome: A meta-analysis study on cancer and stress cardiomyopathy. Heart Fail Rev. 2019;24:481–488. doi: 10.1007/s10741-019-09773-6. [DOI] [PubMed] [Google Scholar]
- 36.Santoro F, Ferraretti A, Ieva R, Musaico F, Fanelli M, Tarantino N, et al. Renal impairment and outcome in patients with Takotsubo cardiomyopathy. Am. J. Emerg. Med. 2016;34:548–552. doi: 10.1016/j.ajem.2015.12.065. [DOI] [PubMed] [Google Scholar]
- 37.Santoro F, Núñez Gil IJ, Stiermaier T, El-Battrawy I, Guerra F, Novo G, Guastafierro F, Tarantino N, Novo S, Mariano E, Romeo F, Romeo F, Capucci A, Bahlmann E, Zingaro M, Cannone M, Caldarola P, Marchetti MF, Montisci R, Meloni L, Thiele H, Di Biase M, Almendro-Delia M, Sionis A, Akin I, Eitel I, Brunetti ND. Assessment of the German and Italian stress cardiomyopathy score for risk stratification for in-hospital complications in patients with Takotsubo syndrome. JAMA Cardiol. 2019 doi: 10.1001/jamacardio.2019.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.