Abstract
Silver nanoparticles (Ag-NPs) are used in a wide range of everyday products, leading to increasing concerns regarding their accumulation in soils and subsequent impact on plants. Using single particle inductively coupled plasma mass spectrometry (spICP-MS) and synchrotron-based techniques including X-ray absorption spectroscopy (XAS) and X-ray fluorescence microscopy (XFM), we characterized the uptake, speciation, and translocation of insoluble Ag2S-NPs (an environmentally-relevant form of Ag-NPs in soils) within two plant species, a monocot and a dicot. Exposure to 10 mg Ag L−1 as Ag2S-NPs for one week resulted in a substantial increase in leaf Ag concentrations (3.8 to 5.8 μg Ag g−1 dry mass). Examination using XAS revealed that most of the Ag was present as Ag2S (>91%). Furthermore, analyses using spICP-MS confirmed that these Ag2S particles within the leaves had a markedly similar size distribution to those supplied within the hydroponic solution. These observations, for the first time, provide direct evidence that plants take up Ag2S-NPs without a marked selectivity in regard to particle size and without substantial transformation (dissolution or aggregation) during translocation from roots to shoots. Furthermore, after uptake, these Ag2S-NPs reduced growth, partially due to the solubilisation of Ag+in planta, which resulted in an upregulation of genes involved in the ethylene signalling pathway. Additionally, the upregulation of the plant defense system as a result of Ag2S-NPs exposure may have contributed to the decrease in plant growth. These results highlight the risks associated with Ag-NP accumulation in plants and subsequent trophic transfer via the food chain.
Introduction
Due to their antimicrobial properties, engineered silver nanoparticles (Ag-NPs) are being developed and incorporated into a wide variety of industrial, commercial, medicinal, and everyday products, for example from detergents and textiles to socks and toothpastes.1 Currently, more than 350 manufacturer-identified products containing Ag-NPs have been registered in the Nanotechnology Consumer Product Inventory of the Woodrow Wilson Institute (2016)1 – this being more than any other nanomaterial.1,2 Such extensive use of Ag-NPs increases the likelihood of their release into the environment, and hence it is necessary to understand their fate and their potential impacts.
Recent studies confirm that the majority of Ag in wastewater treatment plants is present as Ag2S-NPs, with this Ag accumulating within the biosolids.3–6 Given the low solubility of Ag2S, this sulfidation significantly decreases the bioavailability of Ag and thus decreases its toxicity.7 Nevertheless, biosolids from wastewater treatment facilities are commonly used as a soil amendments in agricultural lands and rangelands, with this leading to increasing concern regarding the accumulation of Ag2S in soils, and their impact on crops.8,9 Recent studies have investigated the stability of Ag2S and their behaviour within the soil matrix, demonstrating that Ag2S is largely stable in soils over a long aging period (>1 year).10–12 Plants are an important component of the ecological system and serve as a potential pathway for uptake and accumulation of NPs into the food chain.13 Hydroponic plant growth studies have demonstrated that when Ag-NPs and Ag2S-NPs are supplied in the rooting-environment, they are toxic and that Ag accumulates in above-ground tissues.14,15 However, it remains unclear if the chemical and physical properties (such as chemical composition, dissolution and aggregation, and size distribution) of these Ag2S-NPs change after their uptake.
The underlying mechanisms whereby the NPs (and Ag+ ions) are toxic are also unclear. It is possible, for example, they could reduce growth by causing changes in gene expression.16 Indeed, it has been reported that Ag+ can strongly inhibit aquaporin-mediated water flow across plant cell membranes17,18 and interfere with the dynamics of ethylene, an important hormone for plant growth regulation.19 Similarly, miraculin-like proteins (MLPs) in plants, which are involved in defence responses to pathogen and bacteria invasion,20 have been reported to be a NP-specific response.16
The aim of the present study was to characterize the uptake and translocation of the Ag2S-NPs in plants by combining data from complementary techniques: nano-specific analysis by single particle inductively coupled plasma mass spectrometry (spICP-MS) provided size distribution of Ag-containing particles, and bulk synchrotron-based analyses were used to determine their composition/speciation and the distribution of Ag in situ within plant tissues. By using these techniques, we provide direct evidence that intact Ag2S-NPs with sizes up to 120 nm can be taken up and translocated to leaves without a marked selection in regard to particle size. In addition, we also compare the effects of Ag2S-NPs and ionic silver on i) water transport system of plants, determining the gene expression of plasma membrane intrinsic protein (PIP) aquaporins, ii) the expression of genes involved in ethylene signalling pathway in plants, and iii) MLP genes to assess whether plants respond to the presence of NPs within their tissues in a manner similar to their response to the invasion of pathogen/bacteria. Information regarding the behaviour of NPs in plants is of importance in understanding any potential adverse effects of NPs upon their inadvertent release into the broader environment.
Materials and methods
Nanoparticles and chemicals
Silver sulfide NPs were prepared as described previously.15,21 However, in contrast to most studies where monodisperse NPs are prepared, we aimed to obtain a broad size distribution to assess, through spICP-MS, whether uptake of large nanoparticles was less efficient or not and what the uptake cut-off was. The synthesised NPs were characterized by scanning electron microscopy (SEM, Quanta 450 FEG-ESEM, PEI Company) with an energy dispersive X-ray spectroscopy (EDS) analyser, dynamic light scattering (DLS) (Zetasizer Nano, Malvern Instruments, Ltd.), and spICP-MS (Agilent 8800). The dissolution of ionic Ag+ from the NPs was assessed using Amicon Ultra-15 (Amicon Ultracel 3K, Millipore) centrifugal filter units with pore diameters of 1–2 nm. All chemicals were AR-grade.
Plant growth and Ag uptake experiment
Experiments were conducted in nutrient solution to examine the uptake, transformation, and distribution of Ag2S-NPs in dicotyledonous cucumber (Cucumis sativus) and monocotyledonous wheat (Triticum aestivum L.). Seeds of cucumber and wheat were germinated in rolled paper towel suspended in tap water. After 3 d, seedlings were transferred to containers with 11 L of nutrient solution (μM): 680 NO3−–N, 120 NH4+–N, 100 Ca, 100 Mg, 310 K, 350 SO42−–S, 10 P, 10 Fe (supplied as Fe(III)EDTA), 3.0 B, 1.0 Mn, 0.05 Cu, 1.0 Zn, and 0.02 Mo. Solution pH was not adjusted (ca. pH 5.6). Plants were grown at 25 °C with 14 h of light with a photon flux density of 400 μmol m−2 s−1, and 10 h of darkness.
After 7 d, plants were transferred into containers (two plants per container) with 200 ml nutrient solution containing one of three treatments with nominal concentrations of 10 mg Ag L−1 as Ag2S-NPs, 0.5 mg Ag L−1 as AgNO3 plus a control containing no added Ag. These Ag concentrations were selected because a preliminary experiment had shown that they reduced fresh mass of cucumber roots by ca. 45% after one week. The experiment consisted of 18 experimental units, with two plant species (cucumber and wheat), three Ag-treatments, and three replicates. All solutions were continuously aerated and renewed every 3 d, with plants harvested after growth in the treatment solutions for one week. Roots were washed with flowing deionized water for ca. 1 min and blotted dry with filter paper. Plants were separated into roots and shoots/leaves before fresh mass was recorded. Subsamples of each tissue were pooled from replicates, immersed in liquid nitrogen and freeze-dried for later analysis using synchrotron-based X-ray absorption spectroscopy (XAS). Additional tissue subsamples from each replicate were analyzed for spICP-MS and gene expression (see details below).
In order to identify the broad areas of the leaf tissues in which Ag was accumulating, leaves of cucumber were cut into four portions (tip, edge, vein, and interveinal tissues), while the leaves of wheat were evenly divided into three portions (tip, middle, and base) (ESI† Fig. S1A and B). These tissues were oven-dried at 70 °C, digested with 10 ml concentrated HNO3 (70%), and analysed for total Ag using a triple quadrupole ICP-MS (Agilent 8800). Blanks and a certificated concentration of Ag solution were included in the digestion for quality control.
Silver speciation using X-ray absorption spectroscopy (XAS)
The speciation of the Ag in the roots and shoots of cucumber and wheat was examined using Ag K-edge absorption near edge structure (XANES) spectroscopy. The XANES data for all samples were collected using a liquid helium cryostat at the XAS beamline at Australian Synchrotron, Melbourne. Details of this beamline, as used for the analysis of Ag in plant tissues, is given elsewhere.22 Our previous study verified that these XAS analyses did not damage the samples.22 Furthermore, no obvious differences were found between the spectra for freeze-dried specimens and hydrated and frozen specimens.22 Therefore, in the present study, only freeze-dried plant samples were utilized. To prepare samples for the XAS analysis, ca. 1–2 g plant tissues were cut and ground under liquid nitrogen using an agate mortar and pestle, with the homogenized tissues pressed using a hand pellet press. A total of 12 standard compounds (ESI† Fig. S2) were also examined, including seven aqueous compounds15 and five finely ground powders.3 The XANES spectra of tissues (three scans per sample) and standards (two scans per standard) were energy normalized using Athena software.23 Principal component analysis (PCA) of the normalized sample spectra was used to estimate the likely number of species contained in the samples, while target transformation (TT) was used to identify relevant standards for linear combination fitting (LCF) of the sample spectra. PCA and TT were undertaken using SixPack.24 For the LCF, the fitting energy range was −30 to +100 eV relative to the Ag K-edge.
Characterization of Ag-containing NPs in leaves using spICP-MS
The silver-containing NPs within the plant tissues were characterized using spICP-MS (Agilent 8800) as described by Dan, et al.25 Approximately 1–2 g (fresh mass) of leaf tissues from plants grown in nutrient solutions containing no Ag (control), AgNO3 or Ag2S-NPs were cut into 1–2 mm pieces using scissors, and processed using a homogenizer with 16 ml of 2 mM citrate buffer (pH 5.6). After homogenization, 4 ml of fresh enzyme solution, prepared through dissolving 1 g Macerozyme R-10 enzyme (cat. 21560017–3, bioPLUS, USA) into 20 ml of ultrapure water, was added. The samples were shaken at 37 °C for 24 h. After digestion, the solution was allowed to stand for ca. 1 h before the supernatant was diluted to a Ag concentration of ca. 25 ng L−1 immediately before the analysis (except for the control treatment, in which Ag was ≤5 ng L−1) using ultrapure water.
In order to investigate the effects of the enzyme digestion procedure on the size of Ag2S-NPs, additional samples were spiked with Ag2S-NPs. These Ag2S-NPs were either spiked into leaves of control plants in order to obtain a Ag concentration of ca. 25 ng L−1 or they were spiked into the enzyme/buffer solution.
The instrumental parameters were set to obtain the maximum sensitivity for 107Ag and calibration was performed using solutions of dissolved Ag standards (0 to 1 μg L−1) and a 50 ng Ag L−1 suspension of 75 nm Ag-NP standard (NIST 8017) prepared in Milli-Q water. For analysis in time resolved mode the dwell time was set at 3 ms and ca. 20 000 data points were collected for each sample. The 3 ms dwell time is shortest time that can be set on the Agilent 8800, with this dwell time being the most used in the literature.26 By diluting the samples to 2 × 105 NPs L−1, we limited the probability of detecting more than one NP in a single pulse. The transport efficiency was calculated using the particle size method described by Pace et al.,27 ranging from 7 to 7.5%. The contribution of background signal and/or dissolved species was determined by an iterative algorithm, using AVG + 5SD cut-off, and removed from the signal distribution. Mass of each individual particle was calculated, considering transport efficiency, calibration curve slope, and ratio of analyte in the particle. The particle size distribution was determined by converting mass of individual particles into diameter (assuming spherical shape), and binning them to create a frequency distribution. All measurements were performed in triplicates and frequencies normalized by the total number of particles were reported. The spICP-MS analysis has a detection limit (particle size) of ca. 20–25 nm and therefore only particle sizes larger than 25 nm are presented here.
Spatial distribution of Ag in leaf tissues using X-ray fluorescence microscopy (XFM)
Plants were grown in Ag-containing nutrient solutions (see above) for 3 d before transfer to Advanced Photon Source (APS), Argonne National Laboratory (ANL) (Argonne, IL) for analysis. Once at the APS, the plants were grown under the same conditions for a further 3–4 d before analysis by XFM, conducted at beamline 13-ID-E. Following exposure to Ag, whole (hydrated) plant leaves were harvested and placed between two pieces of 7 μm thick polyimide film, which formed a tight seal around the leaf to minimize dehydration. The electron storage ring operated at 7 GeV in top-up mode, with the X-ray source being a 72 pole, 35 mm period undulator. A cooled Si(111) monochromator and Kirkpatrick–Baez focusing mirrors were used to obtain a monochromatic beam focused onto the specimen. The leaf was mounted at a 45-degree angle relative to the incident beam and a 4-element fluorescence detector was positioned normal to the incident beam. Maps were collected at 27 keV with a step-size of 3 μm and a dwell of 50 ms per pixel. The Ag Kα1 fluorescence peak at 22.163 keV was used to generate the Ag specific maps.
Gene expression by quantitative real-time polymerase chain reaction (qRT-PCR)
Quantitative analysis of gene expression was performed using qRT-PCR. Three classes of genes, including (i) plant PIP aquaporins, (ii) genes involved in the ethylene signalling pathway, and (iii) MLPs, were expected to be related to plant responses to Ag+ and/or Ag2S-NPs (see Introduction). qRT-PCRs were conducted for three biological replicates per treatment. In brief, total RNA was extracted from the roots or leaves using an RNeasy plant extraction mini kit (Bioteke) and reverse-transcribed into cDNA using HiScript® II Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme). Quantitative real-time PCR was performed on CFX96 (Bio-Rad). Gene sequences were obtained from the Cucurbit Genomics Database for cucumber (http://www.icugi.org/cgi-bin/ICuGI/index.cgi) and the National Center for Biotechnology Information (NCBI) for wheat (http://www.ncbi.nlm.nih.gov/) and were used to design gene-specific real-time primers using Primer Premier 6.0 with primer sequences (ESI† Table S1). Based on the homology of protein sequences, seven PIP apuaporins were found for wheat, and ten unnamed PIP aquaporins identified for cucumber (ESI† Fig. S3). Among these aquaporin genes, only four PIP aquaporin genes, which were expressed at high levels (ESI† Fig. S4), were reported (in wheat: TaAQP1, TaAQP2, TaAQP3, and TaPIP2; in cucumber: CsaPIP1.1, CsaPIP1.2, CsaPIP2.1, and CsaPIP2.6). For both ethylene signalling genes and MLP genes, only one or two of these genes were found for wheat plant in the NCBI database with limited annotation information. As a result, the primers designed by Primer Premier 6 were not specific during qRT-PCR determination, which resulted in a large uncertainty regarding the analyses of ethylene signalling genes and MLP genes in wheat. Therefore, only results for cucumber are presented. In cucumber, six genes involved in ethylene signalling pathway (Csa6M318160, Csa4M001970, Csa3M164580, Csa3M878200, Csa7M405830, and Csa2M070880) and two MLP genes (Csa1M043200 and Csa2M021500) were found. The actin gene was used as the internal control. The expression level for each gene was quantified with AceQ™ qPCR SYBR® Green Master Mix (Vazyme). The mean relative levels of amplification of the target genes and standard deviations were calculated for comparison among treatments.
Statistical analysis
Treatment differences were tested for significance (P < 0.05) using a one-way analysis of variance (ANOVA) performed with IBM SPSS Statistics 20.
Results
Characterization of Ag2S nanoparticles
The synthesized NPs had a broad size distribution, although there were some differences in the reported distribution depending upon the analytical method used (Fig. 1). Analysis using spICP-MS has been recently developed for determination of NP size, distribution, and particle concentration (in both suspensions and in enzyme-mediated digestion media of plant tissues), with a particle size detection limit of 20–25 nm.25,26 Examination of our NPs using spICP-MS showed that the Ag2S-NPs had two major populations, with sizes of 35 nm and 73 nm (mean = 59 nm). Similarly, the particle size distribution using SEM demonstrated a polydispersity of distribution, ranging from 17 to 120 nm, with a mean size of 70 nm. It should be noted that SEM analyses have been shown previously to overestimate particle size due to the sputter coating of gold during sample preparation28 – this presumably also resulting in a right-shift in size distribution in the present study (Fig. 1C). The DLS analysis determined the hydrodynamic diameter and yielded the largest particle size distribution, with a number-weighted mean size of 92.6 nm and a zeta potential of −13 mV. The chemical composition of the Ag2S-NPs was confirmed by SEM-EDS (Fig. 1B) and also by XAS analysis (Fig. S2†). The concentration of soluble Ag+ in NP-suspension was below the detection limit of the ICP-MS (being 0.007 μg Ag L−1).
Fig. 1.
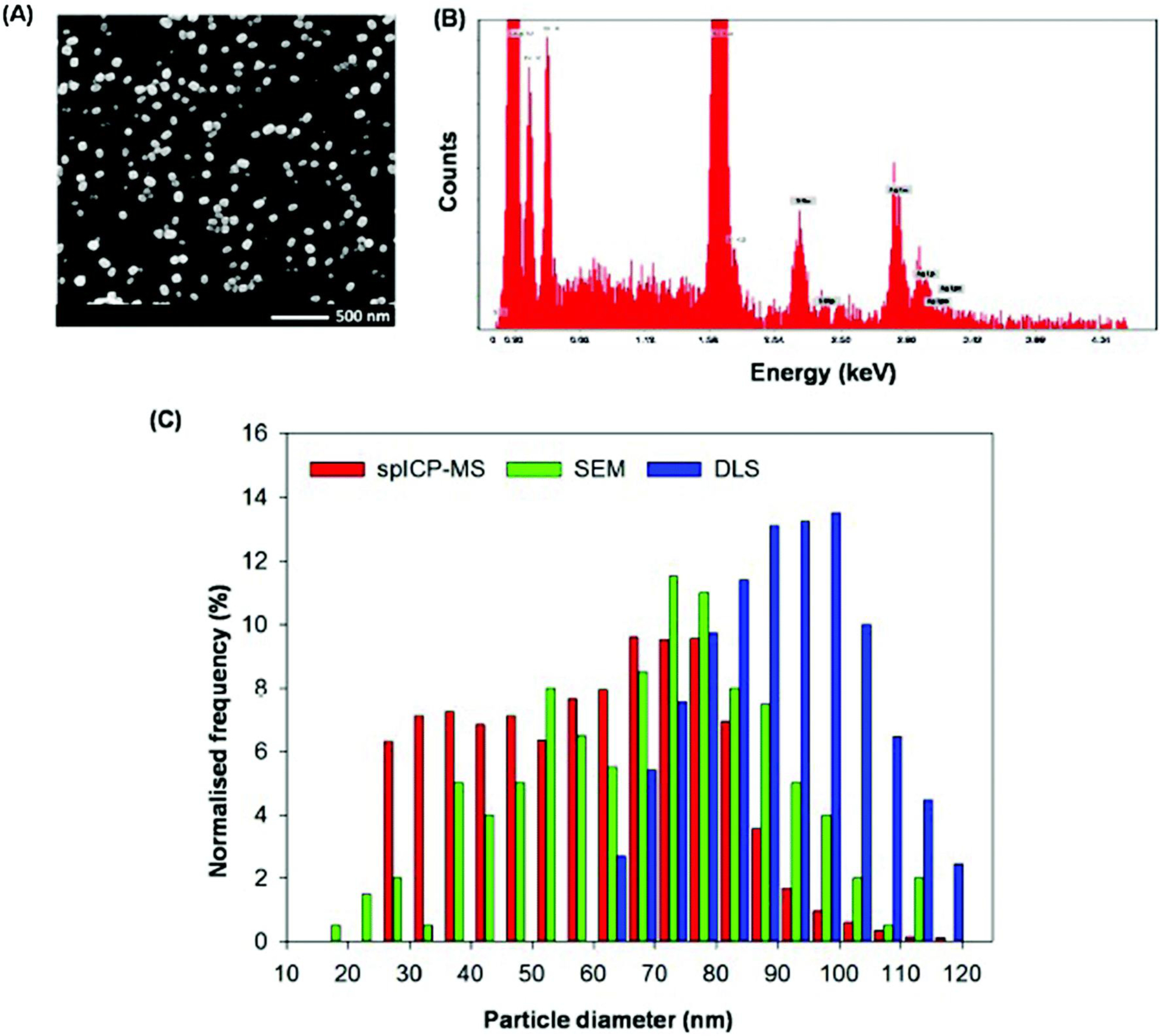
Characterization of Ag2S-NPs suspended in deionized water. (A) Scanning electron micrograph (SEM) and (B) an energy dispersive X-ray spectroscopy (EDS) spectrum. (C) Particle size distributions using SEM, a DLS analyzer, and spICP-MS.
Effects on plant growth and accumulation of Ag in leaf tissues
After one week growth in nutrient solution, exposure to either Ag2S-NPs or AgNO3 significantly reduced plant growth compared to that in the control (Fig. 2). For example, the addition of 10 mg Ag L−1 as Ag2S-NPs decreased fresh tissue biomass of cucumber roots by 42% and shoots by 18%, while in wheat, root fresh mass was reduced by 16% and shoot mass by 15%. However, this concentration (10 mg Ag L−1) of Ag2S-NPs was slightly less toxic than 0.5 mg Ag L−1 as AgNO3 (particularly for wheat), which resulted in reductions of 45–48% for roots and 31–33% for shoots in both species.
Fig. 2.
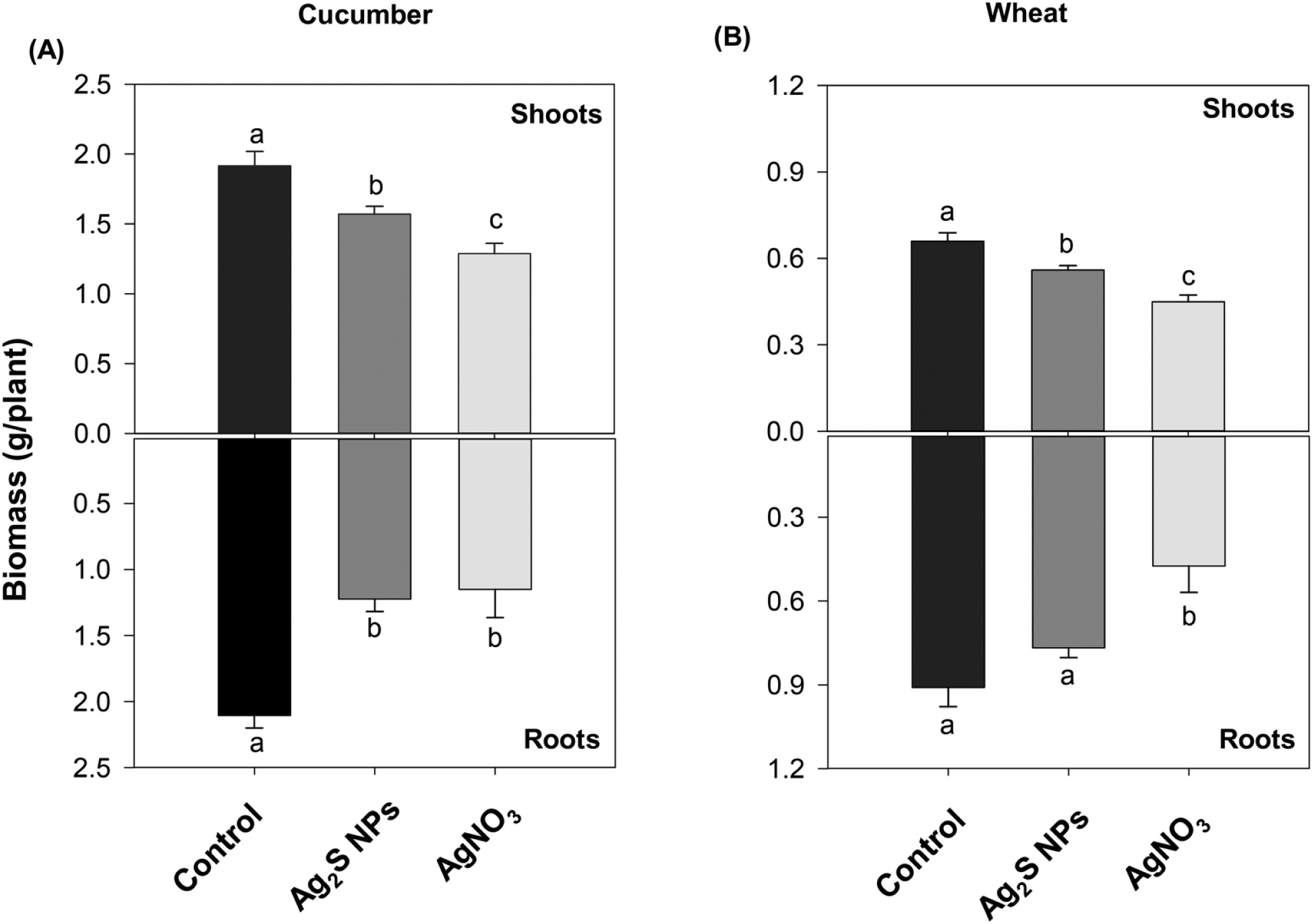
Fresh biomass of cucumber (A) and wheat (B) grown for one week in nutrient solution containing no added Ag (control), 10 mg Ag L−1 as Ag2S-NPs, or 0.5 mg Ag L−1 as AgNO3. Data are presented as the mean ± standard error (n = 4–16 biological replicates). Different letters indicate significant differences.
Exposure of plants to Ag-containing solutions lead to a substantial accumulation of Ag in above-ground tissues (stems and leaves). For example, tissue Ag concentrations in cucumber were 5.8 μg g−1 in the Ag2S-NP treatment and 0.77 μg g−1 in the AgNO3 treatment, with corresponding values for wheat shoots being 3.8 μg g−1 for the Ag2S-NP and 0.71 μg g−1 for the AgNO3 treatments. Tissue Ag concentrations were also determined in dissected leaf tissues. In cucumber plants, concentrations of Ag tended to be higher in the leaf tip and near the edge of the leaf, while for wheat, Ag concentrations tended to be higher in the leaf tip (ESI† Fig. S1C and D). However, these differences were not statistically significant.
Speciation of Ag in leaf tissues
The Ag speciation in leaf tissues was examined in situ using Ag K-edge XANES spectroscopy. First, we compared the XANES spectrum of Ag2S-NPs with that of bulk Ag2S and found that they were similar (ESI† Fig. S5A) – this making it impossible to distinguish between these two forms using this approach (hereafter referred to as ‘Ag2S’ when identified as being present in plant tissues). Similarly, the XANES spectrum of Ag-NPs was almost identical to that of the metallic Ag foil (ESI† Fig. S5B) – hereafter referred to as ‘metallic Ag’. As a result, the XANES analyses were not capable of distinguishing whether the compounds were in bulk form or as NPs. Next, we compared the XANES spectrum of Ag2S-NPs (sulfidation) with that of Ag-glutathione (thiolation) and found that although they were quite similar, there were distinguishable shifts in the edges (at 25.528 keV and 25.577 keV) and k space of k2-weighted extended X-ray absorption fine structure (EXAFS) spectra, which are capable of differentiating the two compounds (ESI† Fig. S6).7,15
When plants were exposed to Ag2S-NPs, the Ag XANES spectra of both the leaves and roots were similar to the spectra of Ag2S-NPs and bulk Ag2S (i.e. ‘Ag2S’) regardless of plant species (Fig. 3, and ESI† Fig. S2). The LCF analysis revealed that the majority of Ag was present as Ag2S (91–99%) with only small amount (1–9%) associated with glutathione (ESI† Table S2). Furthermore, these XANES spectra of Ag2S-NP treated plants differed from those of AgNO3-treated plants (ESI† Fig. S7), where most of the Ag was associated with glutathione (88–98%) with the remaining 2–12% present as metallic Ag (Fig. 3 and ESI† Table S2). This result demonstrated that Ag accumulation in plant tissues upon exposure to Ag2S-NP could not be attributed to the uptake of ionic Ag+ due to the dissolution of Ag2S-NPs in the rooting media and/or on the root surface. However, it remained unclear whether the Ag2S in the leaves of plants exposed to Ag2S-NPs was due to the direct uptake of Ag2S-NPs themselves or due to the dissolution and reformation of Ag2S in planta (although the latter was considered unlikely given the very low solubility of Ag2S and the absence of Ag2S in plants exposed to AgNO3).
Fig. 3.
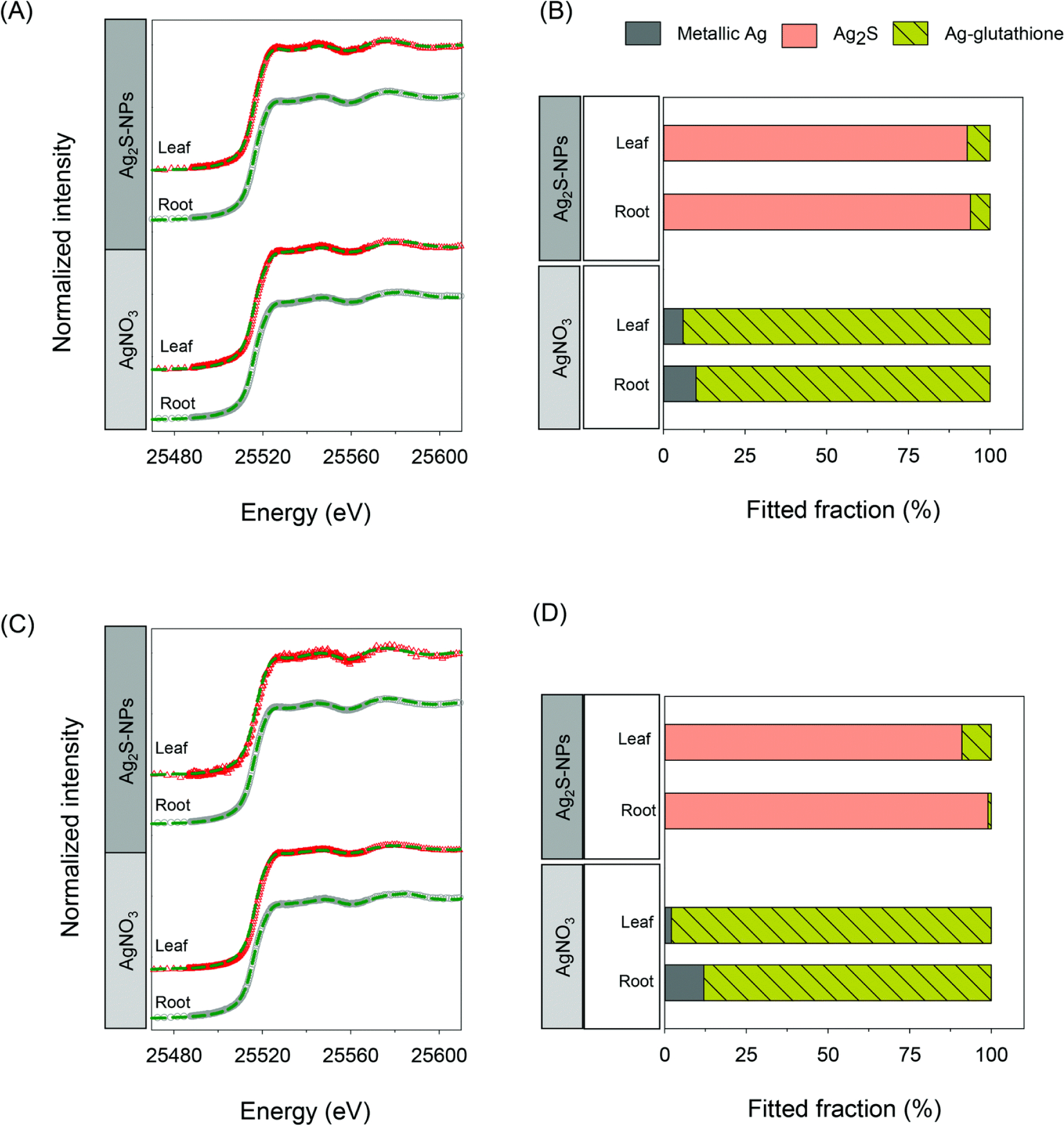
In situ analyses of Ag speciation in tissues of cucumber and wheat using synchrotron-based X-ray absorption spectroscopy. Normalized Ag K-edge XANES spectra for roots and leaves of cucumber (A) and wheat (C) exposed to either Ag2S-NPs or AgNO3 together with the results of linear combination fitting results for cucumber (B) and wheat (D).
Characterization of Ag-containing nanoparticles in leaf tissues
In order to ascertain whether the Ag2S within leaves of plants exposed to Ag2S-NPs was present as NPs and if this was due to the direct uptake of Ag2S-NPs, we used spICP-MS to compare the size distribution of the Ag-containing NPs extracted from the plant tissues with the size distribution of the Ag2S-NPs originally supplied to the hydroponic medium. Initially, we investigated the effects of the enzymatic digestion method on size distribution by directly spiking Ag2S-NPs into three different solutions [(i) enzyme solution, (ii) enzyme plus buffer solution, and (iii) enzyme plus buffer solution with control plant leaves (no Ag treatment)], and applying the standard enzyme incubation procedure. The measured particle size distribution in these controls agreed well with the original Ag2S-NPs suspension (Fig. 4A), indicating that the selected digestion procedure did not cause changes in size distribution of Ag2S-NPs. This is consistent with the previous reports in which the Macerozyme R-10 can release NPs from plant tissues without causing changes in the size of Ag-NPs29 and Au-NPs.25
Fig. 4.
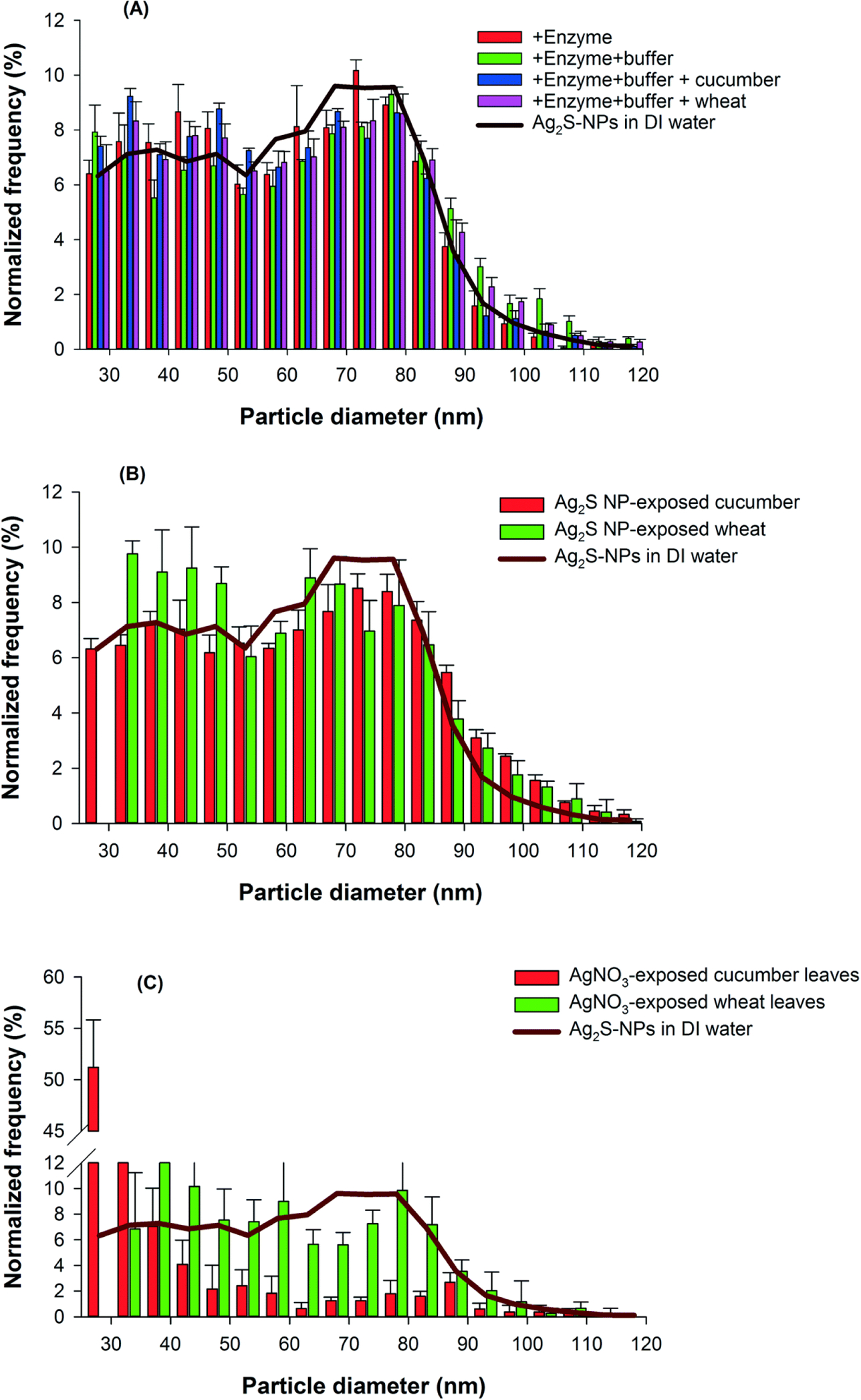
Normalized frequency of Ag-containing nanoparticles in the exposure suspensions and in the enzymatically-digested leaves of cucumber and wheat plants as determined by spICP-MS. (A) Particle size distribution of Ag2S-NPs in the deionized (DI) water in the presence of enzyme, buffer solution, and the leaves of control plants. (B and C) Particle size distribution of Ag-containing nanoparticles in leaves of cucumber and wheat exposed to Ag2S-NPs (B) or AgNO3 (C) for one week in nutrient solution. Data are means ± SD.
Next, we used the spICP-MS to analyze the enzyme-digested leaf tissues of plants exposed to either Ag2S-NPs or AgNO3. The size distribution of recovered Ag-containing NPs differed substantially between these two Ag treatments. For both plant species exposed to Ag2S-NPs, the measured size distribution from the leaf tissues was in good agreement with those of the Ag2S-NPs from the rooting medium (Fig. 4B). These results indicate that the two plant species took up the Ag2S-NPs directly, and that the Ag2S-NPs within the plants existed as intact particles and that did not undergo substantial modification (e.g. dissolution or aggregation).
Interestingly, when tissues of plants exposed to AgNO3 were examined using spICP-MS, many pulse signals were observed, indicating the presence of particles that were distinct from the dissolved Ag. This suggests that Ag in leaves of AgNO3-treated plants existed in Ag-containing particulate form. Importantly, the size distribution of these Ag-containing compounds differed markedly from that of the Ag2S-NPs, with smaller sizes identified (Fig. 4C). Indeed, more than 71% of the Ag-containing NP compounds were found to be smaller than 35 nm in leaves of cucumber (the spICP-MS is not capable of analyzing size less than 20–25 nm). In comparison, for wheat leaves, the size of newly formed Ag-containing NPs appeared to be larger, ranging from 35–85 nm (Fig. 4C).
Spatial distribution of Ag in leaf tissues
Next, we examined the distribution of Ag within intact and hydrated leaves using synchrotron-based XFM. First, consideration is given to cucumber. For leaves of Ag2S-NP-treated cucumber (Fig. 5), elemental mapping showed that the highest concentrations of Ag were in the veins, with some hotspots identified within the interveinal tissues (e.g. hotspots, HS #1–5 in Fig. 5B and C). These Ag-containing hotspots were 200–300 μm in diameter and the presence of Ag in these hotspots was confirmed by examination of the XFM spectra at these specific locations (Fig. 5D). Indeed, the Ag concentrations in these hotspots were much higher than the concentrations in surrounding areas (non-hotspots, NH #5 and 6) and background (BG #7). When cucumber was exposed to AgNO3, the highest Ag concentrations were found in the leaf edge and tip, with lower concentrations in the vein and interveinal tissues (Fig. 6A and B). This distribution pattern was also confirmed by careful examination of the XFM spectra (Fig. 6C). In contrast to the Ag2S-NPs treatment, no obvious Ag-hotspots were identified in leaves exposed to AgNO3.
Fig. 5.
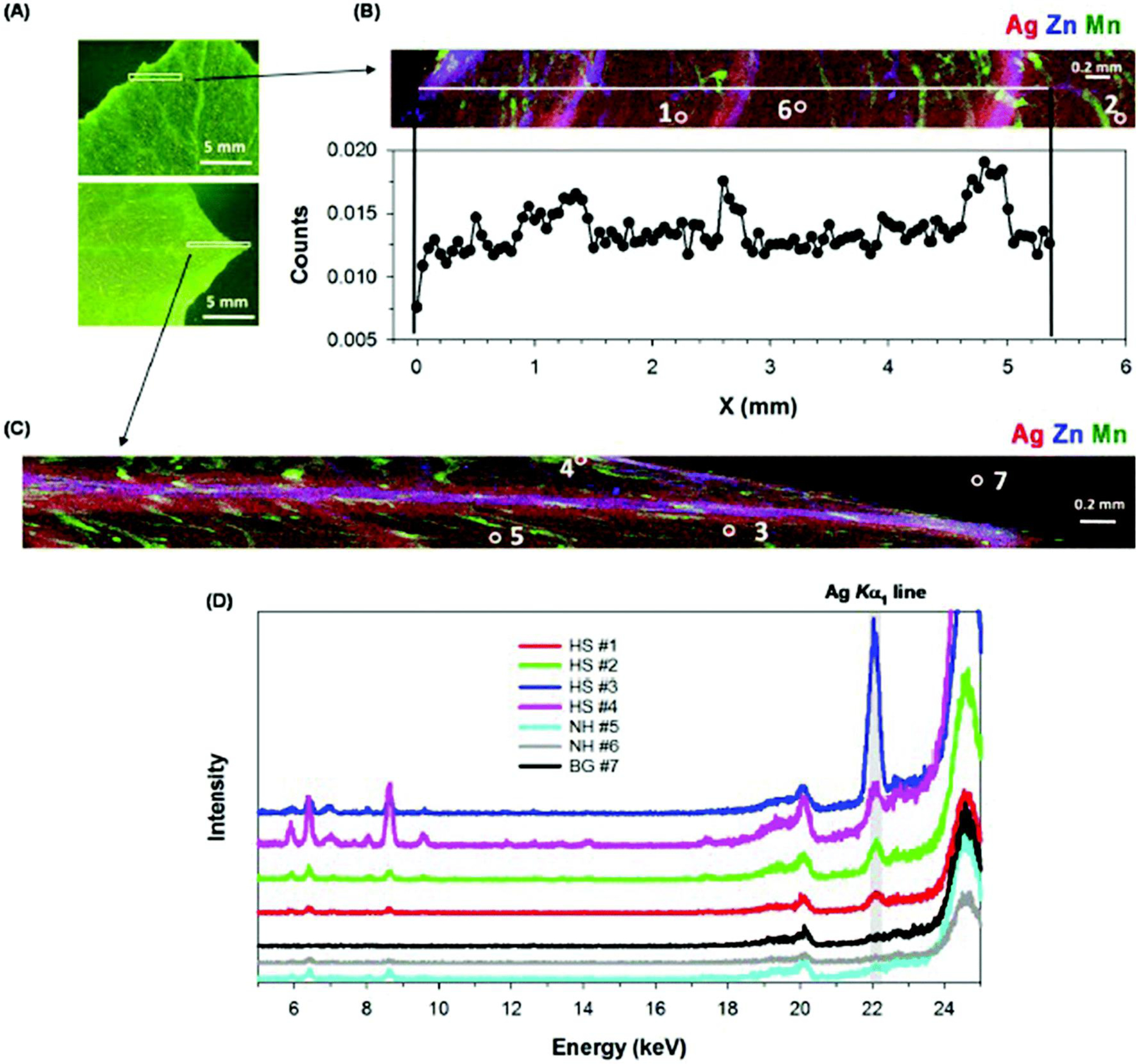
Leaves of cucumber grown in nutrient solution containing 10 mg Ag L−1 as Ag2S-NPs for one week. (A) Light micrograph after XFM analysis, with the white box indicating the area examined by XFM. (B and C) Tricolor XFM map of Ag (red), Zn (blue), Mn (green) distribution, and in (B) with Ag count profile along the white line as shown in the top panel. (D) Fluorescent intensity of XFM spectra collected at 27 keV for hotspots (HS #1–4), non-hotspots (NH #5–6), and background (BG #7) as shown in (B) and (C).
Fig. 6.
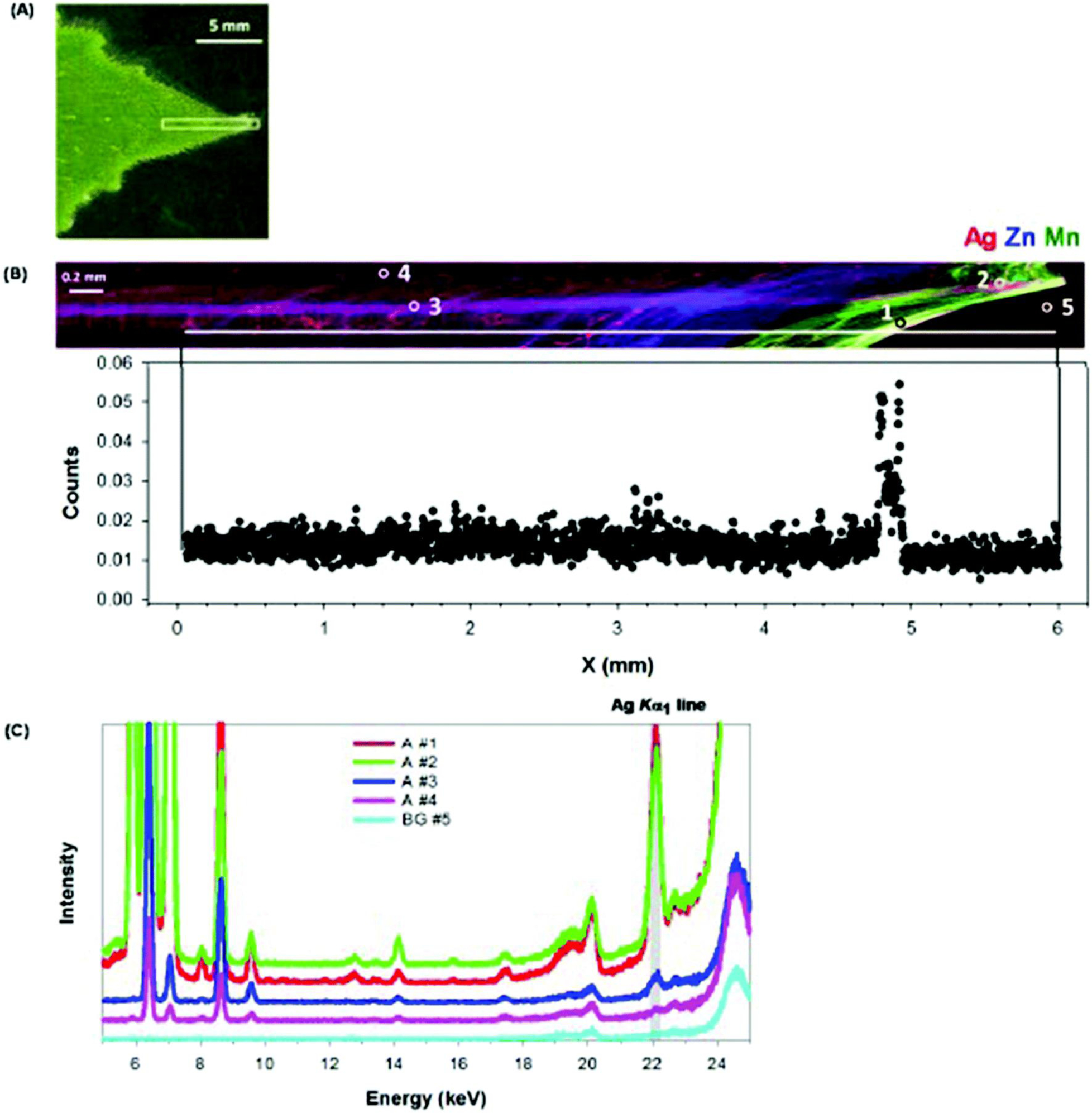
Leaves of cucumber grown in nutrient solution containing 0.5 mg Ag L−1 as AgNO3 for one week. (A) Light micrograph after XFM analysis, with the white box indicating the area examined by XFM. (B) Tricolor XFM map of Ag (red), Zn (blue), Mn (green) distribution (top panel), with Ag count profile along the white line as shown in the top panel (bottom panel). (C) Fluorescent intensity of XFM spectra collected at 27 keV for areas (A # 1–4), and background (BG #5) as shown in top panel of (B).
Next, consideration is given to the leaves of wheat, with the pattern of Ag accumulation being slightly different to that described above for cucumber. Specifically, the Ag was found to be comparatively evenly distributed (ESI† Fig. S8). Again, some Ag-containing hotspots were identified in leaf tissues when exposed to Ag2S-NPs (ESI† Fig. S8B and E), while no hotspots were found in the AgNO3 treatment (ESI† Fig. S8D and F).
Gene expression
It is known that Ag+ is an antagonist of ethylene perception.19 Therefore, the genes involved in the ethylene signalling pathway are likely related to Ag+-specific responses. Indeed, all six of these genes were significantly upregulated (P < 0.05) in cucumber following exposure to either AgNO3 or Ag2S-NPs (Fig. 7A). This finding suggested that both AgNO3 and Ag2S-NPs could potentially reduce plant growth through an interface with the ethylene signalling pathway, and that the effect of Ag2S-NPs was (at least in part) due to the direct interactions of NPs with ethylene signalling or the partial dissolution of the NPs and the release of soluble Ag+ (although Ag2S remained the dominant form within plant tissues).
Fig. 7.
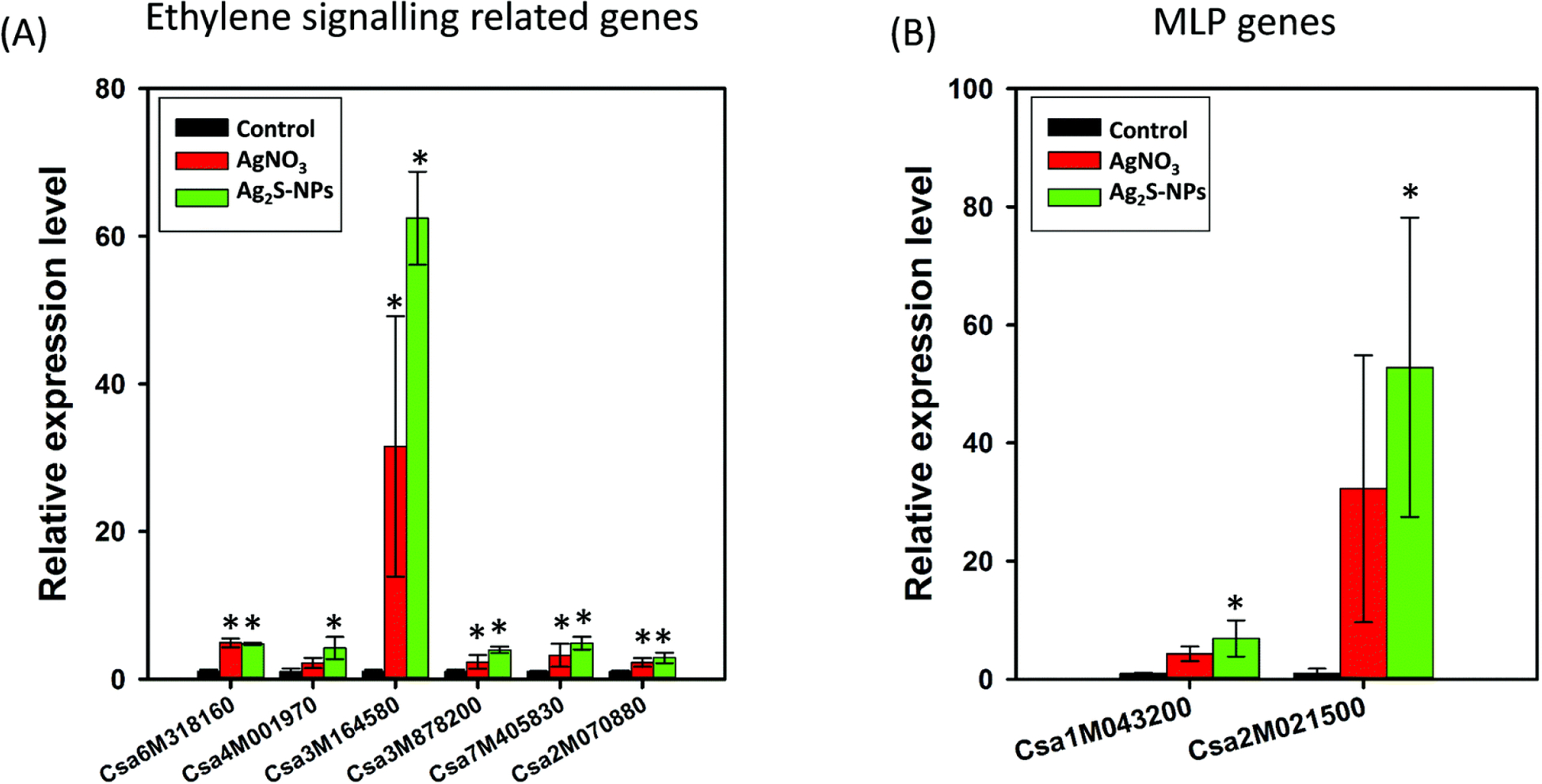
Relative expression of genes involved in ethylene signalling pathways (A) and miraculin-like protein (MLP) genes (B) in shoots of cucumber exposed to Ag2S-NPs or AgNO3 for one week. Data are means ± SD. * indicates significant difference compared to the control.
The MLP genes are hypothesized to reflect NP-specific responses to the presence of NPs within tissues.16 Indeed, two MLP genes in shoots were significantly upregulated following exposure to Ag2S-NPs (Fig. 7B). For plants exposed to AgNO3, the MLP genes were also observed to be upregulated. This result suggests the formation of metallic Ag which was synthesized by plants treated with AgNO3. This observation was in agreement with the results analyzed by XANES and spICP-MS (Fig. 3 and 4). Furthermore, the magnitude of upregulation was substantially higher in the Ag2S-NP treatment than the AgNO3 treatment, despite that the latter was more toxic to plants than the former (Fig. 7B).
In wheat, four aquaporin genes in both roots and shoots were found to be downregulated following exposure to AgNO3 or Ag2S-NPs. The magnitude was less in shoots than in roots (ESI† Fig. S9A and B), with expression of TaAQR1 and TaAQR2 being reduced significantly in roots (P < 0.05). For cucumber, the responses were differed between the roots and shoots (ESI† Fig. S9C and D). For example, CsaPIP1.2 was downregulated in roots but upregulated in the shoots. Similarly, CsPIP2.1 was upregulated and CsPIP 2.6 was downregulated in the shoots, while expression was not affected in the roots. These observations suggested that the effect on aquaporin genes varies with both plant species and with tissue. Interestingly, for wheat, severe leaf dehydration was observed only one day after exposure (data not shown). This symptom was in good accordance with the expression of wheat aquaporins, indicating that the reduction in wheat growth caused by AgNO3 and Ag2S-NPs was likely due to (at least in part) a decrease in upward water transport.
Discussion
Up until now, only a limited number of studies have provided evidence of ‘direct’ uptake of NPs – this being defined as insoluble NPs (e.g. mesoporous silica NPs, quantum dots, gold-NPs, TiO2-NPs, Fe3O4-NPs) that are directly analyzed or visualized within the cytoplasm, vasculature, or after long distance upward translocation in plants.25,30–35 However, it remains unclear once they enter into plant systems if these NPs are subjected to changes in their intrinsic and extrinsic properties, such as their chemical composition, dissolution and aggregation, and size distribution. In the present study, we have provided direct evidence that intact Ag2S-NPs can be taken up by plant roots and subsequently delivered as Ag2S-NPs into leaf tissues without substantial transformation or dissolution/aggregation. In contrast to the paucity of information available regarding direct uptake and transport of insoluble NPs, a large number of studies have investigated the uptake of NPs in plants, for relatively soluble NPs such as Ag-NPs,14 CuO-NPs,36,37 ZnO-NPs,38 (and to a lesser extent CeO2-NPs39–41 and YbO3-NPs42). For these soluble NPs, it remains difficult to determine whether the NPs were taken up intact or the uptake of a soluble form occurs with subsequent formation of NPs in planta. Indeed, this was observed in the present study, for plants exposed to AgNO3, with the reduction of Ag+ to form metallic Ag NPs in planta (Fig. 3 and 4C, and ESI† Table S2). This plant-mediated synthesis of NPs has been reported previously for cobalt (Co), copper (Cu), gold (Au), magnetite (Fe3O4), palladium (Pd), platinum (Pt), silver (Ag), and zinc oxide (ZnO) (see Kuppusamy, et al.22 for a review).
The uptake of intact Ag2S-NPs in the present study was evidenced by the following five observations. First, exposure to a nutrient solution containing Ag2S-NPs (with undetectable concentrations of Ag+) resulted in an increase in concentrations of the Ag in leaf tissues. Second, in situ XAS analyses revealed that the majority of Ag that accumulated within leaf tissues was present as Ag2S (>91%) when plants were exposed to Ag2S-NPs (Fig. 3 and ESI† Table S2). Third, spICP-MS analysis confirmed that the measured size distribution of Ag-containing NPs extracted from leaves of plants exposed to Ag2S-NPs was in good agreement with those of the Ag2S-NP from the rooting medium itself (Fig. 4B). Fourth, XFM analyses demonstrated the existence of hotspots which contained elevated concentrations of Ag due to localization or tissue-specific delivery effects of NPs (Fig. 5 and ESI† Fig. S8). Finally, MLP genes, probably reflecting the presence of NPs within plants, were highly upregulated in the leaves of both plant species (Fig. 7B).
The demonstrated uptake and delivery of intact Ag2S-NPs within plant tissues has important implications. Firstly, the particle size distribution in leaves determined by spICP-MS was in good agreement with the Ag2S-NPs in the rooting medium, ranging from 30 to 120 nm (Fig. 4B). This uptake of intact NPs up to 120 nm was unexpected, given that NPs must traverse a series of chemical and physiological barriers before final delivery into leaf tissues.43 Plant organs differ substantially in their size exclusion limits (SELs).43 Based upon the size distribution of Ag2S-NPs observed in leaves in the present study, it seems that the apoplastic transport pathway through cell walls (with a SEL of 5–20 nm)44–46 and Casparian strip (<1 nm)47 and the symplastic transport pathway through plasmodesmata (3–50 nm)45,48 were the main barriers to Ag2S-NP uptake and translocation. How these Ag2S-NPs, which were larger than these SELs, circumvented these barriers remains unclear. Similarly, a few previous studies have also reported that NPs with particle sizes larger than the SELs of these organs can be internalized.25,35,49–51 These observations suggest that the SELs of plant organs are perhaps somewhat flexible and could possibly be influenced by the NPs themselves. Indeed, it has been reported that TiO2-NPs larger than 20 nm could modify the plasmodesmata structures in wheat roots, which in turn facilitated their transit from cell to cell.51 Clearly, further research is required in order to understand the interactions between Ag2S-NPs and plant organs, and in particular, the mechanism by which Ag2S-NPs circumvent these barriers within plants.
Secondly, our results using XFM analysis have revealed that Ag-containing hotspots (with diameters of 200–300 μm) were identified in the interveinal tissues of cucumber leaves (Fig. 5B and C) and in the tips of wheat leaves (ESI† Fig. S8B) when plants were exposed to Ag2S-NPs. This spatial distribution seems to imply a tissue-related localization in relation to Ag2S-NPs. In particular, this could be important under the context of phyto-nanotechnology because it provides the possibility to design nanoparticles as a kind of nanocarrier to deliver agrochemicals to targeted tissues for insect control or alleviation of pest-caused symptoms.43 As a precursor to the possibility of phyto-nanotechnology, further research is required on the movement and distribution of nanoparticles at the tissue and subcellular levels within plants.
Finally, it is noteworthy that although Ag2S is extremely insoluble, with a solubility constant of Ksp = 6 × 10−51,7 exposure to Ag2S-NPs reduced plant growth significantly (Fig. 2). We propose that this toxicity was partially due to both toxic effects of Ag+ (from the slow dissolution of Ag2S-NPs in planta) and NP-specific effects. Indeed, once the Ag2S-NPs had accumulated in the plant tissues, small amounts of Ag+ could be released in planta. Released Ag+ would bind rapidly to ligands within the plant tissues, as evidenced by the formation of small amounts of Ag-glutathione-like complexes (Fig. 3 and ESI† Table S2). Furthermore, similarly to Ag+, Ag2S-NPs upregulated genes involved in ethylene signalling pathway (in relation to plant growth) (Fig. 7A), suggesting Ag+-relevant effects. The magnitude of upregulation was more pronounced in the Ag2S-NP treatment (Fig. 7A), although the amount of dissolution from NPs (resulting in small amount of Ag-thiol complex) was lower compared to that of the AgNO3 treatment (Fig. 3 and ESI† Table S2). This suggests a potential interaction of Ag2S-NPs with the ethylene signalling pathway or that the localization of the release of Ag+ at tissue or subcellular levels may have an impact on the strength of interactions. Indeed, some differences in Ag distribution were observed between the two treatments (Fig. 5 and 6 and ESI† Fig. S8). In addition, Ag2S-NPs also appeared to exert an NP-specific toxic effect, as demonstrated by the upregulation of the defence systems in relation to pathogen/bacteria and particle invasion (Fig. 7B). The present study revealed the intact uptake of Ag2S-NPs by plants with substantial accumulation within leaf tissues (Fig. 3–5). This indicates a potential risk associated with Ag2S-NPs through dietary uptake or food chain contamination given that plants are the basis of terrestrial food chains.
Conclusions
Although there is a series of barriers in plants that would be anticipated to block the uptake and translocation of NPs within plants, the present study has shown that Ag2S-NPs with sizes up to 120 nm can be directly taken up by plant roots and subsequently translocated to leaves without substantial transformation or dissolution. Some Ag-containing hotspots were identified that suggested tissue-specific delivery. These findings imply that nanoparticles could potentially be developed as a nanocarrier to deliver agrochemicals into tissue-specific targets, providing potential applications of phyto-nanotechnology. This is particularly important given that this area of research is largely unexplored when compared to the progress made in similar areas in the human and animal science domains.43 However, due to the ion-specific and NP-specific toxic effects observed in the present study, consideration must be given to the potential risks of NP toxicity associated with trophic transfer via the food chain.
Supplementary Material
Environmental significance.
In recent years, silver nanoparticles (Ag-NPs) have been used in more consumer products than any other nanomaterial, prompting concerns regarding their release into the broader environment and their subsequent risk. Here we utilize novel methodological approaches (including synchrotron-based spectroscopy, spICP-MS, and qRT-PCR) to characterize the uptake, accumulation and toxicity mechanism of Ag2S-NP in plants. Our data, for the first time, provide the direct evidence that Ag2S-NPs can be taken up by plant roots and subsequently translocated throughout the leaves without substantial transformation or dissolution. These findings demonstrated that Ag2S-NPs pose a risk to plants and through the food chain.
Acknowledgements
Support was provided to P. Wang as a recipient of an Australian Research Council (ARC) DECRA (DE130100943) and to P. Kopittke as a recipient of an ARC Future Fellowship (FT120100277). Parts of this research were carried out at the XAS Beamline at the Australian Synchrotron, Victoria, Australia (AS153/XAS/9937). We acknowledge the support of GeoSoilEnviroCARS (Sector 13), which is supported by the National Science Foundation - Earth Sciences (EAR-1128799), and the Department of Energy, Geosciences (DE-FG02-94ER14466). We acknowledge travel funding provided by the International Synchrotron Access Program (ISAP) managed by the Australian Synchrotron and funded by the Australian Government (ISAP152/ISP10150). The US-EPA contributed to this article but the research was neither performed nor funded by the US-EPA and is not subject to the US-EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in this article are solely those of the authors and do not necessarily reflect or represent the US-EPA’s views or policies. The authors would like to acknowledge Dr Matthew Newville from the APS for his assistance.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c6en00489j
References
- 1.PENI, Project on Emerging Nanotechnologies Inventory, http://www.nanotechproject.org/.
- 2.Forbrugerrådet Tænk The Nanodatabase, http://nanodb.dk/en/.
- 3.Lombi E, Donner E, Taheri S, Tavakkoli E, Jämting ÅK, McClure S, Naidu R, Miller BW, Scheckel KG and Vasilev K, Environ. Pollut, 2013, 176, 193–197. [DOI] [PubMed] [Google Scholar]
- 4.Kaegi R, Voegelin A, Sinnet B, Zuleeg S, Hagendorfer H, Burkhardt M and Siegrist H, Environ. Sci. Technol, 2011, 45, 3902–3908. [DOI] [PubMed] [Google Scholar]
- 5.Kim B, Park C-S, Murayama M and Hochella MF, Environ. Sci. Technol, 2010, 44, 7509–7514. [DOI] [PubMed] [Google Scholar]
- 6.Brunetti G, Donner E, Laera G, Sekine R, Scheckel KG, Khaksar M, Vasilev K, De Mastro G and Lombi E, Water Res, 2015, 77, 72–84. [DOI] [PubMed] [Google Scholar]
- 7.Levard C, Hotze EM, Lowry GV and Brown GE, Environ. Sci. Technol, 2012, 46, 6900–6914. [DOI] [PubMed] [Google Scholar]
- 8.Gottschalk F, Sonderer T, Scholz RW and Nowack B, Environ. Sci. Technol, 2009, 43, 9216–9222. [DOI] [PubMed] [Google Scholar]
- 9.Batley GE and McLaughlin MJ, Fate of manufactured nanomaterials in the Australian Environment, CSIRO Niche Manufacturing Flagship, 2010. [Google Scholar]
- 10.Wang P, Menzies NW, Dennis PG, Guo J, Forstner C, Sekine R, Lombi E, Kappen P, Bertsch PM and Kopittke PM, Environ. Sci. Technol, 2016, 50, 8274–8281. [DOI] [PubMed] [Google Scholar]
- 11.Pradas del Real AE, Castillo-Michel H, Kaegi R, Sinnet B, Magnin V, Findling N, Villanova J, Carrière M, Santaella C, Fernández-Martıínez A, Levard C and Sarret G, Environ. Sci. Technol, 2016, 50, 1759–1768. [DOI] [PubMed] [Google Scholar]
- 12.Donner E, Scheckel K, Sekine R, Popelka-Filcoff RS, Bennett JW, Brunetti G, Naidu R, McGrath SP and Lombi E, Environ. Pollut, 2015, 205, 78–86. [DOI] [PubMed] [Google Scholar]
- 13.Unrine JM, Shoults-Wilson WA, Zhurbich O, Bertsch PM and Tsyusko O, Environ. Sci. Technol, 2012, 46, 9753–9760. [DOI] [PubMed] [Google Scholar]
- 14.Dimkpa CO, McLean JE, Martineau N, Britt DW, Haverkamp R and Anderson AJ, Environ. Sci. Technol, 2012, 47, 1082–1090. [DOI] [PubMed] [Google Scholar]
- 15.Wang P, Menzies NW, Lombi E, Sekine R, Blamey FPC, Hernandez-Soriano MC, Cheng M, Kappen P, Peijnenburg WJGM, Tang C and Kopittke PM, Nanotoxicology, 2015, 9, 1041–1049. [DOI] [PubMed] [Google Scholar]
- 16.Kaveh R, Li Y-S, Ranjbar S, Tehrani R, Brueck CL and Van Aken B, Environ. Sci. Technol, 2013, 47, 10637–10644. [DOI] [PubMed] [Google Scholar]
- 17.Coskun D, Britto DT, Jean Y-K, Schulze LM, Becker A and Kronzucker HJ, J. Exp. Bot, 2011, 63, 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niemietz CM and Tyerman SD, FEBS Lett, 2002, 531, 443–447. [DOI] [PubMed] [Google Scholar]
- 19.Camacho-Cristóbal JJ, Martín-Rejano EM, Herrera-Rodríguez MB, Navarro-Gochicoa MT, Rexach J and González-Fontes A, J. Exp. Bot, 2015, 66, 3831–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukuda S, Gomi K, Yamamoto H and Akimitsu K, Plant Mol. Biol, 2006, 60, 125–136. [DOI] [PubMed] [Google Scholar]
- 21.Sekine R, Brunetti G, Donner E, Khaksar M, Vasilev K, Jämting ÅK, Scheckel KG, Kappen P, Zhang H and Lombi E, Environ. Sci. Technol, 2014, 49, 897–905. [DOI] [PubMed] [Google Scholar]
- 22.Kuppusamy P, Yusoff MM, Maniam GP and Govindan N, Saudi Pharm. J, 2015, 24, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravel B and Newville M, J. Synchrotron Radiat, 2005, 12, 537–541. [DOI] [PubMed] [Google Scholar]
- 24.Webb SM, Phys. Scr, 2005, T115, 1011–1014. [Google Scholar]
- 25.Dan Y, Zhang W, Xue R, Ma X, Stephan C and Shi H, Environ. Sci. Technol, 2015, 49, 3007–3014. [DOI] [PubMed] [Google Scholar]
- 26.Malysheva A, Lombi E and Voelcker NH, Nat. Nanotechnol, 2015, 10, 835–844. [DOI] [PubMed] [Google Scholar]
- 27.Pace HE, Rogers NJ, Jarolimek C, Coleman VA, Gray EP, Higgins CP and Ranville JF, Environ. Sci. Technol, 2012, 46, 12272–12280. [DOI] [PubMed] [Google Scholar]
- 28.Chawla V, Jayaganthan R, Chawla AK and Chandra R, J. Mater. Process. Technol, 2009, 209, 3444–3451. [Google Scholar]
- 29.Bao D, Oh ZG and Chen Z, Front. Plant Sci, 2016, 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain H, Yi Z, Rookes J, Kong L and Cahill D, J. Nanopart. Res, 2013, 15, 1–15. [Google Scholar]
- 31.Zhu Z-J, Wang H, Yan B, Zheng H, Jiang Y, Miranda OR, Rotello VM, Xing B and Vachet RW, Environ. Sci. Technol, 2012, 46, 12391–12398. [DOI] [PubMed] [Google Scholar]
- 32.Zhai G, Walters KS, Peate DW, Alvarez PJJ and Schnoor JL, Environ. Sci. Technol. Lett, 2014, 1, 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larue C, Laurette J, Herlin-Boime N, Khodja H, Fayard B, Flank A-M, Brisset F and Carriere M, Sci. Total Environ, 2012, 431, 197–208. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Han J, Xiao JQ and Jin Y, J. Environ. Monit, 2008, 10, 713–717. [DOI] [PubMed] [Google Scholar]
- 35.Corredor E, Testillano PS, Coronado M-J, González-Melendi P, Fernández-Pacheco R, Marquina C, Ibarra MR, de la Fuente JM, Rubiales D, Pérez-de-Luque A and Risueño M-C, BMC Plant Biol, 2009, 9, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC and Xing B, Environ. Sci. Technol, 2012, 46, 4434–4441. [DOI] [PubMed] [Google Scholar]
- 37.Dimkpa CO, Latta DE, McLean JE, Britt DW, Boyanov MI and Anderson AJ, Environ. Sci. Technol, 2013, 47, 4734–4742. [DOI] [PubMed] [Google Scholar]
- 38.Lin DH and Xing BS, Environ. Sci. Technol, 2008, 42, 5580–5585. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Viezcas JA, Castillo-Michel H, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Ge Y, Priester JH, Holden PA and Gardea-Torresdey JL, ACS Nano, 2013, 7, 1415–1423. [DOI] [PubMed] [Google Scholar]
- 40.Hong J, Peralta-Videa JR, Rico C, Sahi S, Viveros MN, Bartonjo J, Zhao L and Gardea-Torresdey JL, Environ. Sci. Technol, 2014, 48, 4376–4385. [DOI] [PubMed] [Google Scholar]
- 41.Ma Y, Zhang P, Zhang Z, He X, Zhang J, Ding Y, Zhang J, Zheng L, Guo Z, Zhang L, Chai Z and Zhao Y, Environ. Sci. Technol, 2015, 49, 10667–10674. [DOI] [PubMed] [Google Scholar]
- 42.Zhang P, Ma YH, Zhang ZY, He X, Guo Z, Tai RZ, Ding YY, Zhao YL and Chai ZF, Environ. Sci. Technol, 2012, 46, 1834–1841. [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Menzies NW, Dennis PG, Guo J, Forstner C, Sekine R, Lombi E, Kappen P, Bertsch PM and Kopittke PM, Environ. Sci. Technol, 2016, 50, 8274–8281. [DOI] [PubMed] [Google Scholar]
- 44.Eichert T and Goldbach HE, Physiol. Plant, 2008, 132, 491–502. [DOI] [PubMed] [Google Scholar]
- 45.Ma X, Geiser-Lee J, Deng Y and Kolmakov A, Sci. Total Environ, 2010, 408, 3053–3061. [DOI] [PubMed] [Google Scholar]
- 46.Dietz K-J and Herth S, Trends Plant Sci, 2011, 16, 582–589. [DOI] [PubMed] [Google Scholar]
- 47.Aubert T, Burel A, Esnault MA, Cordier S, Grasset F and Cabello-Hurtado F, J. Hazard. Mater, 2012, 219.–, 111–118. [DOI] [PubMed] [Google Scholar]
- 48.Lucas WJ and Lee JY, Nat. Rev. Mol. Cell Biol, 2004, 5, 712–726. [DOI] [PubMed] [Google Scholar]
- 49.Koo Y, Wang J, Zhang Q, Zhu H, Chehab EW, Colvin VL, Alvarez PJJ and Braam J, Environ. Sci. Technol, 2015, 49, 626–632. [DOI] [PubMed] [Google Scholar]
- 50.Schwab F, Zhai G, Kern M, Turner A, Schnoor JL and Wiesner MR, Nanotoxicology, 2015, 1–22. [DOI] [PubMed] [Google Scholar]
- 51.Larue C, Laurette J, Herlin-Boime N, Khodja H, Fayard B, Flank A-M, Brisset F and Carriere M, Sci. Total Environ, 2012, 431, 197–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


