Abstract
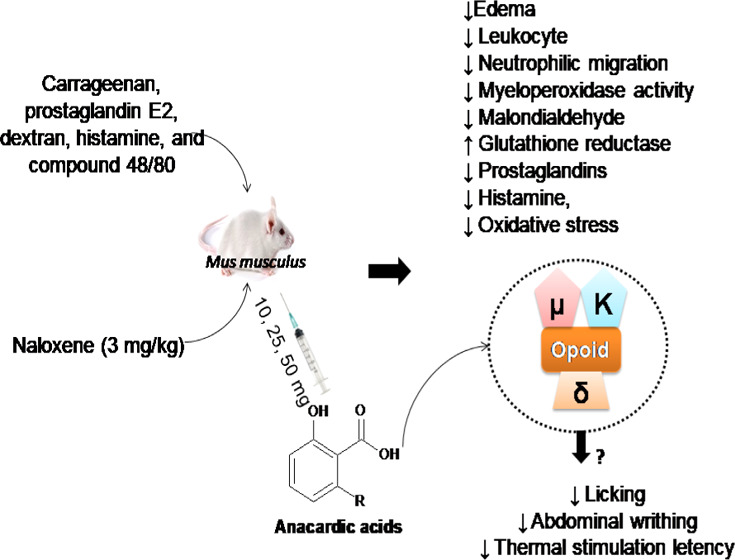
Anacardic acid (AA), a compound extracted from cashew nut liquid, exhibits numerous pharmacological activities. The aim of the current investigation was to assess the anti-inflammatory, antinociceptive, and antioxidant activities of AA in mouse models. For this, Swiss albino mice were pretreated with AA (10, 25, 50 mg/kg, intraperitoneally, ip) 30 min prior to the administration of carrageenan, as well as 25 mg/kg of prostaglandin E2, dextran, histamine, and compound 48/80. The antinociceptive activity was evaluated by formalin, abdominal, and hot plate tests, using antagonist of opioid receptors (naloxene, 3 mg/kg, ip) to identify antinociceptive mechanisms. Results from this study revealed that AA at 25 mg/kg inhibits carrageenan-induced edema. In addition, AA at 25 mg/kg reduced edema and leukocyte and neutrophilic migration to the intraperitoneal cavity, diminished myeloperoxidase activity and malondialdehyde concentration, and increased the levels of reduced glutathione. In nociceptive tests, it also decreased licking, abdominal writhing, and latency to thermal stimulation, possibly via interaction with opioid receptors. Taken together, these results indicate that AA exhibits anti-inflammatory and antinociceptive actions and also reduces oxidative stress in acute experimental models, suggesting AA as a promising compound in the pharmaceutical arena.
Introduction
Natural products have been used to treat various types of human illnesses and have dominated the pharmaceutical industry for many years. Plants produce several chemically diverse secondary metabolites, which may exhibit a myriad of biological activities.1,2 In addition, medicinal plants are commonly used as an alternative treatment to conventional therapy to treat various pathological conditions, including inflammation and pain.3 Inflammation is a stereotyped response to the body’s defense against cellular damage and vascular tissue.4 Pain, in turn, is the end result of the processing of sensory stimuli. Pain signaling pathways may become dysfunctional through inflammation and/or trauma to tissues and nerves.5 Both inflammation and pain are widely investigated in pharmacological studies with molecules from natural products.
Anacardium occidentale L. is a tree of the family Anacardiaceae, which produces a fruit known as “cashew”. This fruit has great agricultural and economic importance for Northeast Brazilian, owing to the high commercial value of its chestnut.6,7 In the course of processing the cashew nuts, a byproduct known as cashew nut shell liquid (CNSL) is obtained. It contains unsaturated long-chain phenols, with AA as the major component.8 Research findings indicated that AA possesses antioxidant,6 antitumoral,9 antibacterial,10 gastroprotective,11 and lipoxygenase inhibitory activities.12
Phytochemicals derived from pallets and/or synthetics, containing phenolic compounds and tannins with high antioxidant capacity, have analgesic effects and may be used as drug candidates.13 On the other hand, reactive oxygen species (ROS) play a role in inflammatory processes,14 and some anti-inflammatory drugs can cause various adverse effects, including gastrointestinal ulcers.15 Thus, studies are needed to discover new drugs that can modulate the side effects of anti-inflammatory drugs.16 Accordingly, the present investigation was conducted to evaluate the anti-inflammatory, antinociceptive, and antioxidant activities and/or mechanisms of AA in mouse models.
Results
Anti-Inflammatory Effect of AA on Carrageenan-Induced Paw Edema
Table 1 presents our results of the anti-inflammatory effect of AA. The results reveal that carrageenan (300 μg/paw) induced intense edema in mice (Table 1) at a maximum level after 3 h. Pretreatment with INDO (10 mg/kg) and AA at 10 mg/kg resulted in a considerable reduction (p < 0.05) in the development of paw edema after 4 h. On the other hand, the dose of 25 mg/kg of AA caused a significant (p < 0.05) reduction of edema after 1, 2, 3, and 4 h by 81.25, 66.66, 48.97, and 54.76%, respectively. AA at 50 mg/kg reduced edema only in the periods of 3 h (51.02% inhibition) and 4 h (45.23% inhibition). Finally, 25 mg/kg of AA was selected for further studies as it showed the best results related to protection against inflammation induced by carrageenan.
Table 1. Anti-Inflammatory Effect of AA on Carrageenan-Induced Paw Edemaa.
| paw
edema (mL) |
|||||
|---|---|---|---|---|---|
| treatments | dose (mg/kg, ip) | 1 h | 2 h | 3 h | 4 h |
| VEH | 0.032 ± 0.004 | 0.048 ± 0.003 | 0.098 ± 0.007 | 0.084 ± 0.005 | |
| INDO | 10 | 0.016 ± 0.004 | 0.038 ± 0.005 | 0.070 ± 0.007 | 0.048 ± 0.004* |
| (50.00) | (20.83) | (28.57) | (42.85) | ||
| AA | 10 | 0.026 ± 0.005 | 0.034 ± 0.006 | 0.078 ± 0.008 | 0.063 ± 0.011* |
| (18.74) | (29.16) | (20.40) | (25.00) | ||
| AA | 25 | 0.006 ± 0.004* | 0.016 ± 0.009* | 0.050 ± 0.006* | 0.038 ± 0.005* |
| (81.25) | (66.66) | (48.97) | (54.76) | ||
| AA | 50 | 0.020 ± 0.007 | 0.040 ± 0.006 | 0.048 ± 0.013* | 0.046 ± 0.132* |
| (37.50) | (14.89) | (51.02) | (45.23) | ||
Values are mean ± standard error of the mean (SEM) (n = 5). The percentage of inhibition of paw edema is indicated in parentheses. * p < 0.05 vs control (one-way analysis of variance (ANOVA) followed by the Newman–Keuls test with post hoc test). VEH: vehicle; INDO: indomethacin; AA: Anacardic acid.
Anti-Inflammatory Effect of AA on Paw Edema Induced by Different Proinflammatory Agents
Induction of mouse paw edema by different proinflammatory agents promoted the development of intense edema, causing a significant increase (p <0.05) in the paw volume, compared to the VEH (Figure 1A–D). Our results demonstrated that when prostaglandin E2 (0.064 ± 0.005 mL; Figure 1A) and dextran (0.052 ± 0.008 mL; Figure 1B) were used, peak edema was attained after 60 min of administration, while histamine (0.056 ± 0.005 mL; Figure 1C) and compound 48/80 (0.098 ± 0.008 mL, Figure 1D) after 30 and 120 min, respectively. Results also showed that INDO treatment inhibits (p < 0.05) edema induced by prostaglandin E2 (inhibition of 58.6%, 120 min: Figure 1A), dextran (inhibition of 73.9%, 120 min: Figure 1B), histamine (inhibition of 31.6%, 60 min: Figure 1C), and compound 48/80 (inhibition of 41.0%, 120 min: Figure 1D). Pretreatment with 25 mg/kg AA significantly inhibited both prostaglandin E2 (Figure 1A) and dextran-induced edema (Figure 1B) illustrating reductions of 59.4 and 73.1% after 60 min and 97.5 and 62.5% after 240 min, respectively. Similarly, AA inhibited (p < 0.05) 70.2 and 41.7% histamine-induced edema (Figure 1C) after 30 and 240 min, respectively. At 120 and 240 min, inhibition (p < 0.05) of 36.4 and 61.9% of the edema was verified by AA in the model induced by compound 48/80 (Figure 1D).
Figure 1.
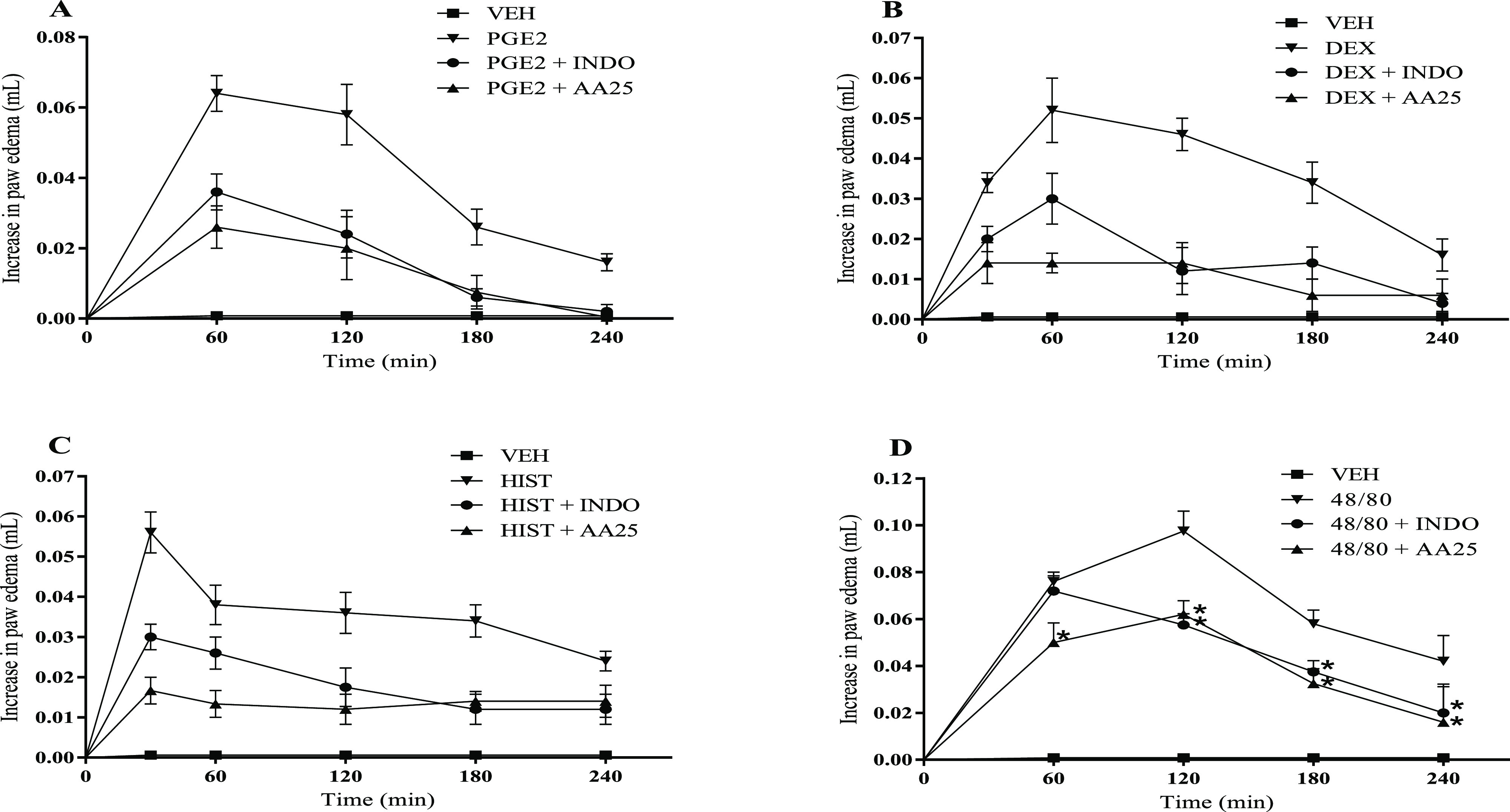
Effect of AA on paw edema induced by different proinflammatory agents. Edema was induced by: (A) prostaglandin E2 (PGE2), (B) dextran (Dex), (C) histamine (Hist), and (D) compound 48/80 (48/80). Animals were treated with AA (25 mg/kg), vehicle (VEH, control group), or INDO (10 mg/kg, intraperitoneally (ip)). The dots represent mean ± SEM (n = 5). *p < 0.05 vs VEH group (one-way ANOVA followed by the Newman–Keuls test with post hoc test).
Evaluation of the Effects of AA on Histological Alterations
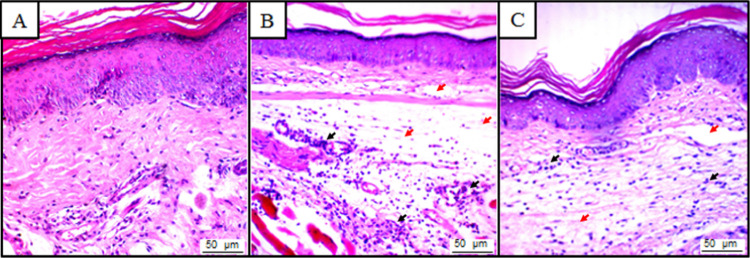
In the VEH-treated animals, no tissue changes were observed. The tissue displayed a normal histological appearance, with dispersed cellular infiltrate and integral membranes (Figure 2A). However, animals treated with carrageenan presented significant tissue changes such as increased cell infiltrate, mainly neutrophils and intense edema (Figure 2B). It was also observed that pretreatment by AA (25 mg/kg) reduced neutrophil infiltration and the intensity of the edema (Figure 2C).
Figure 2.
Photomicrographs of the paw of mice stained with hematoxylin and eosin (A–C; magnification: 400×). (A) Vehicle group (5% dimethylsulfoxide, DMSO), which exhibit normal histological characteristics, with intact paw and no evidence of inflammation. (B) Carrageenan group, which shows an inflammatory response pronounced with intense polymorphonuclear cells (black arrow) and plasma exudate (red arrow). (C) AA group + carrageenan, which shows that cell migration (black arrow) was decreased with AA treatment.
Effect of AA on Carrageenan-Induced Peritonitis
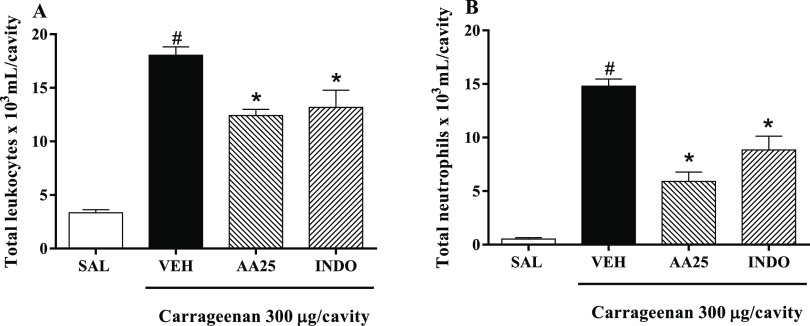
Through induction of peritonitis by carrageenan, it was possible to verify the significant increase (p < 0.05) in leukocyte migration from 18.05 × 103 ± 0.77 × 103 cells/mL to the peritoneal cavity, being mostly neutrophils, 14.79 × 103 ± 0.68 × 103 cells/mL (Figure 3A,B). However, pretreatment with AA (25 mg/kg) showed a considerable (p < 0.05) decrease in leukocyte migration (12.41 × 103 ± 0.58 × 103 cells/mL) and neutrophils (5.91 × 103 ± 0.86 × 103 cells/mL) to the peritoneal cavity, also evidenced by pretreatment with INDO (leukocytes: 13.18 × 103 ± 1.60 × 103 cells/mL; neutrophils: 8.85 × 103 ± 1.27 × 103 cells/mL) at 10 mg/kg (Figure 1A,B).
Figure 3.
Anti-inflammatory effect of AA in carrageenan-induced peritonitis model. (A) Number of leukocytes in the peritoneal cavity. (B) Total neutrophils in the peritoneal cavity. Animals were treated with 0.9% saline (SAL), vehicle (VEH, control group, 10 mL/kg, ip), anacardic acid (AA25, 25 mg/kg, ip), or indomethacin (INDO, 10 mg/kg, ip) after 30 min received 300 μL/well of carrageenan. Values are mean ± SEM (n = 5). #p < 0.05 vs SAL group; *p < 0.05 vs VEH group (one-way ANOVA followed by the Newman–Keuls test with post hoc test).
Effect of AA on MPO Activity and Oxidative Stress
The MPO activity (Table 2) was measured in the peritoneal exudate, which was significantly increased (p < 0.05) in the carrageenan group (18.20 ± 1.28 U/mL) compared to the saline group (3.30 ± 0.92 U/mL). A significant inhibition (p < 0.05) of 61.6% of the MPO enzyme was observed in the group treated with 25 mg/kg of AA (6.98 ± 0.87 U/mL) compared to the carrageenan treatment group only. A significant decrease (p < 0.05) in glutathione (GSH) levels (20.62 ± 1.48 μg/mL) and an increase in malondialdehyde (MDA) (44.33 ± 4.71 nM/mL) were observed after administration of carrageenan (35.77 ± 2.51 μg/mL and 2.19 ± 0.91 nM/mL, respectively). Pretreatment with AA caused a significant (p < 0.05) increase in GSH (32.32 ± 1.13 μg/mL) and a decrease in MDA (17.16 ± 3.32 nM/mL) levels compared to carrageenan treatment.
Table 2. Effect of AA on MPO Activity, Reduced Levels of GSH, and Concentration of MDA in the Peritoneal Exudatea.
| |
parameters |
|||
|---|---|---|---|---|
| treatments | dose (mg/kg) | MPO (U/mL) | GSH (μg/mL) | MDA (nmol/mL) |
| VEH | 3.30 ± 0.92 | 35.77 ± 2.51 | 2.19 ± 0.91 | |
| carrageenan | 18.20 ± 1.28* | 20.62 ± 1.48* | 44.33 ± 4.71* | |
| AA | 25 | 6.98 ± 0.87# | 32.32 ± 1.13# | 17.16 ± 3.32# |
MPO: myeloperoxidase; GSH: reduced glutathione; MDA: malondialdehyde. Values are mean ± SEM (n = 5) *p < 0.05 vs VEH group, #p < 0.05 vs carragenine (one-way ANOVA followed by Newman–Keuls test). VEH: vehicle; AA: anacardic acid.
Antinociceptive Effects of AA in Experimental Models of Nociception
Test of Abdominal Writhing Induced by Acetic Acid
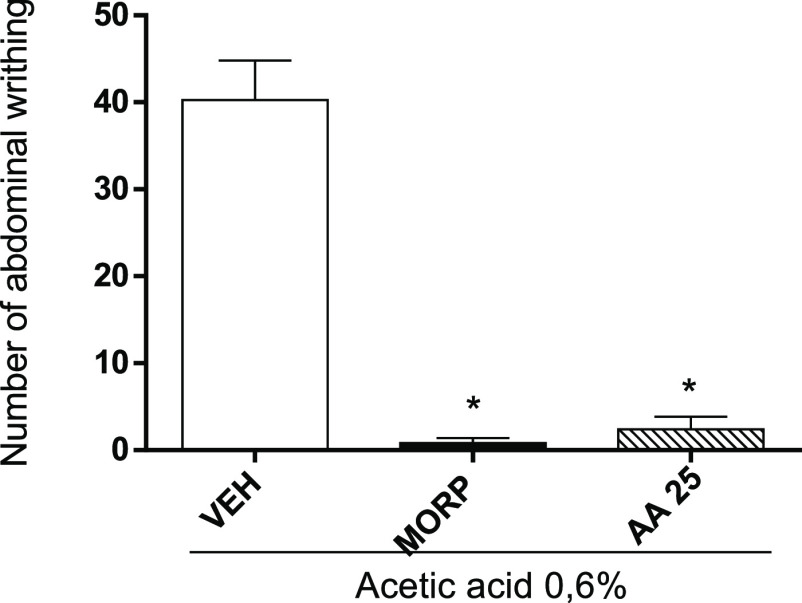
The antinociceptive activity of AA was verified by the acetic acid-induced abdominal writhing test (Figure 4). In the abdominal writhing test, reduction of writhing (94.0%) after pretreatment with AA (2.4 ± 1.47) at a dose of 25 mg/kg can be comparable to that of morphine (0.8 ± 0.58).
Figure 4.
Antinociceptive effect of AA through the acetic acid-induced abdominal writhing test. Animals were treated with vehicle (VEH, control group, 10 mL/kg, ip), MORP (5 mg/kg, ip), or AA (25 mg/kg, ip). The dots represent mean ± SEM (n = 5). *p < 0.05 vs VEH group; #p < 0.05 vs MORP group (one-way ANOVA followed by Newman–Keuls test with post hoc test).
Hot Plate Test
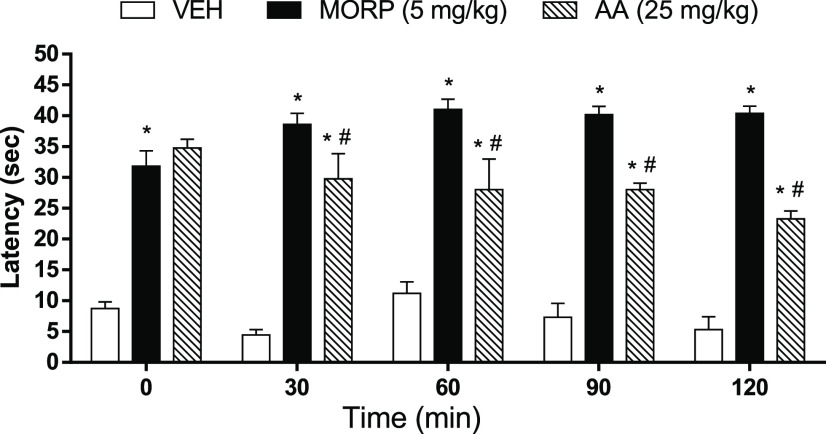
Results from this test showed that animals pretreated with AA (25 mg/kg) displayed a considerable increase (p < 0.05) in the reaction time latency to thermal stimulus throughout the test, compared to the VEH group (Figure 5). However, the effects were lower than the MORP (5 mg/kg) group.
Figure 5.
Antinociceptive effect of AA through the acetic acid-induced abdominal writhing test. Animals were treated with vehicle (VEH, control group, 10 mL/kg), morphine (MORP, 5 mg/kg), or anacardic acid (AA, 25 mg/kg). The dots represent mean ± SEM (n = 5). *p < 0.05 vs VEH group; #p < 0.05 vs MORP group (one-way ANOVA followed by Newman–Keuls test with post hoc test).
Formalin Test
The formalin-induced nociception model revealed that the group pretreated with VEH shows a significant increase (p < 0.05) in lick time in the first phase (72.40 ± 16.07 s) and in the second phase (57.20 ± 7.70 s). Pretreatment with AA (25 mg/kg) presented an antinociceptic action due to the significant reduction (p < 0.05) to the lick time in the first phase (2.0 ± 1.30 s) and the second phase (2.6 ± 1.12 s), similar to results observed in the group pretreated with MORP (4.6 ± 0.75 and 1.4 ± 0.24 s, respectively) at 5 mg/kg. To verify the possible involvement of the interaction of AA with opioid receptors, two groups of mice (n = 5) were pretreated with naloxene (3 mg/kg, opioid receptor antagonist) 30 min before administration of AA (25 mg/kg) or MORP (5 mg/kg) followed by administration of 2.5% formalin (20 μL in the subplantar region of the right animal paw). Results show that there was a reversal (increase) of the lick time in the AA and MORP groups pretreated with the nonselective opioid receptor antagonist (naloxene), both in the first phase (78.0 ± 14.79 and 73.40 ± 17.65 s, respectively) and the second phase (56.0 ± 20.404 and 47.4 ± 13.63 s, respectively) of the test, indicating that the antinociceptive action of AA occurs through interaction with this receptor (Figure 6).
Figure 6.
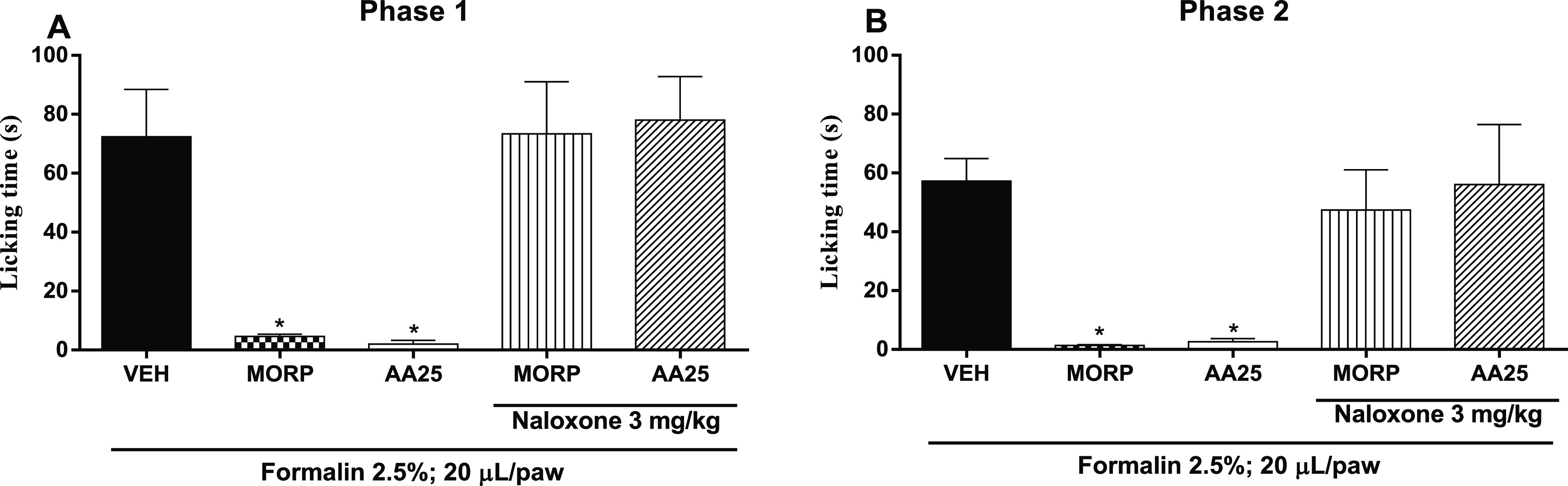
Evaluation of the antinociceptive activity of AA and association with naloxone through the formalin test. Lick time was determined in the first 5 min (A, first stage, neurogenic pain), and the second phase (B, inflammatory pain) was assessed 20 min after the start of the test (20–25 min). The animals were treated with vehicle (VEH, control group), anacardic acid (AA: 25 mg/kg, ip), or morphine (MORP; 5 mg/kg, ip) after 30 min receiving 500 μL/well of carrageenan. The dots represent mean ± SEM (n = 5). *p < 0.05 vs VEH group. (one-way ANOVA followed by the Newman–Keuls test with post hoc test).
Discussion
A. occidentale, grows in Brazil, and is very common in the Northeast region. Several parts of the cashew tree such as leaves, stem bark, chestnut, and cashew juice have been evident for important biological functions17 including antioxidant, anti-inflammatory,18 antimicrobial,19 antidiabetic,20 antihypertensive,21 antigenotoxic,22 nonmutagenic,23 and larvicidal activities.24 Cashew nuts release a liquid, internationally called CNSL, which is a source of phenolic compounds that are responsible for its antioxidant, antimicrobial,17,19 anti-inflammatory,25 cytotoxic, and nonmutagenic activities.23
The inflammatory process is a biological reaction triggered by tissue injury leading to an imbalance of homeostasis,26 causing redness, edema, pain, temperature increase, and loss of function.27 Edema and pain (nociception when evaluated in animals) are the main targets for novel anti-inflammatory compounds and analgesics.28 In the present work, we have explored the anti-inflammatory activities of AA in classic experimental models of inflammation and nociception and found that AA reduces the inflammatory process and increases nociception latency. AA, through immunological mechanisms and a balance between ROS and antioxidant defenses, can contribute to its anti-inflammatory activity. In this context, it should be emphasized that phenolic compounds exhibit antioxidant activities29 and that numerous plant-based compounds, including phenolic phytochemicals and tannins, exhibit anti-inflammatory potential.30
Carrageenan-induced edema was initially used to evaluate the anti-inflammatory effect of AA. This method is well established and widely used to evaluate the effect of agonist drugs against acute inflammatory responses. Carrageenan administration results in an inflammatory response. This process is biphasic in nature, since initially edema generation occurs due to the increase of hypersensitivity mediators (e.g., histamine, serotonin, and bradykinin) in the vascular endothelium.31 This increases vascular permeability and infiltration of polymorphonuclear cells at the site of damage,32 followed by a late response (after 72 h) and release of tumor necrosis factor α (TNF-α),33 prostaglandins, and nitric oxide,34 as well as increased monocyte migration.35 Studies on mice treated with cashew shell and docaule extracts showed anti-inflammatory effects owing to the presence of antibodies.34
Pretreatment with AA reduced carrageenan-induced edema, most effectively at 25 mg/kg, since it reduced edema at all evaluated times. This suggests that the anti-inflammatory effect is associated with the suppression of vascular permeability and cell migration, reduction of the increase, and production of inflammatory mediators and oxidative stress. According to some etinopharmacological studies, A. occidentale has been used by the population as an alternative treatment to conventional therapy, using it to cure numerous diseases, including inflammation at all.36,37 It is worth mentioning that AA, the major compound present in cashew nut liquid, is an important therapeutic candidate for the treatment of inflammation.38−40
To examine these proposed anti-inflammatory mechanisms of action for AA, different proinflammatory agents, including prostaglandin E2, dextran, histamine, and compound 48/80 were used to induce paw edema. Prostaglandin E2 product of the cyclooxygenase pathway (COX) is one of the most important inflammatory mediators, produced through the COX oxidation of AA released from the membrane phospholipids during the inflammatory process.41 The anti-inflammatory response of AA to PGE2-induced edema was similar to the effect observed for INDO, indicating that AA inhibits the acute inflammatory response caused by PGE2. On the other hand, dextran can activate Haggemann’s factor XII, which then activates the tissue, and tissue kallikrein responsible for the production of kinin.42 Dextran has been used as an edematogenic agent to induce partial degeneration and to produce edema with low protein and neutrophil levels through the osmotic increase,43 characterized by a rapid increase in edema, with the main mediators being histamine and serotonin.44
Similarly, compound 48/80 promotes edema through degranulation of mast cells, with the release of bioactive amines, such as histamine and serotonin.45 AA exhibited reduction of dextran- and compound 48/80-induced edema, indicating that the antidematogenic activity observed in this model might be associated with inhibition of the release of histamine and serotonin. These results were confirmed by the reduction of paw edema induced by histamine in mice pretreated with AA, establishing that AA has antagonistic effects on histamine receptors, since histamine receptors H1 and H2 when activated by histamine cause an increase in vascular permeability.46 Chemotactic mediators, lipid mediators, chemokines, and cytokines are released during inflammation. These mediators are responsible for migration of neutrophils to the focus of inflammation.47 Neutrophils constitute the first line of defense against the inflammatory process48 and are responsible for the release of proteases contained in their granules, such as MPO, elastase, and proteinase-3.49
Evaluation of the effect of AA on the reduction of neutrophilic migration was investigated using the carrageenan-induced peritonitis model. AA reduced the migration of total leukocytes and neutrophils into the peritoneal cavity and decreased the MPO activity. In neutrophils, MPO is responsible for the activation of signal transduction pathways (p38 MAPK and NF-kB), tyrosine phosphorylation, superoxide (O2•) production, degranulation, and prolonged survival, as well as may increase neutrophil–endothelial interaction. MPO can also trigger production of cytokines (IL-6 and IL-8) and oxygen-derived reactive species (ROS) by endothelial cells. On the other hand, hypochlorous acid (HOCl), derived from MPO, can induce the activation of necrosis factor kappa B (NF-κB), thiorosine phosphorylation, and TNF-α production.50
The inflammatory process can be potentiated by the increase of oxidative stress causing tissue injury and direct degradation of important cellular components, through neutrophil ROS and vascular endothelial cells.51 MDA is an important marker of oxidative stress, generated due to oxidation of lipids from biological membranes.52 Similarly, GSH is responsible for neutralization of ROS.53 Our results showed an increase in GSH concentration and decrease of MDA levels in the peritoneal fluid of mice pretreated with AA in carrageenan-induced peritonitis model.
These data support the fact that AA interacts with free radicals, neutralizing them through the formation of phenolic and allylic radicals during the inflammatory response. In addition, these data demonstrate that AA is able to decrease oxidative stress generated during the inflammatory process, possibly by its excellent antioxidant activities, because AA has the ability to eliminate free radicals, which are extremely pathogenic. Thus, AA has the ability to combat oxidative stress, which is a pathophysiological factor for various pathologies. AA lessens the production of O2• by inhibition of xanthine oxidase but does not sequester ROS because the length of the unsaturated 15-carbon chain was more associated with this activity compared to the activity of salicylic acid,54 which may prevent the transition-metal ions from initiating oxidation or inhibiting pro-oxidant enzymes. AA may also inhibit the generation of O2• by sequestration of the hydroxyl radical (HO•).55 According to our data, the anti-inflammatory and antioxidant actions of AA are tightly linked with one another. Published data show that AA exerts important antioxidant activity in some experimental models.56 Additionally, AA has anti-inflammatory properties similar to other known NSAIDs such as aspirin.57
Inflammatory mediators that cause sensitization of nociceptors are produced and released through the induction of abdominal controls by acetic acid, visceral pain model. This method is very sensitive for peripheral antinociceptive evaluation since the formation of inflammatory mediators, such as histamine, serotonin, prostaglandins, bradykinin, and substance P causes sensitization of the nociceptors. In this context, experimental models of inflammatory pain have been widely used in the search of new anti-inflammatory and analgesic drugs.58−60
To assess the antinociceptive effect of AA, the hot plate test was employed to verify the analgesic effect through possible interaction of AA with supraspinal and spinal receptors. AA showed an antinociceptive effect throughout the test, indicating that the analgesic effect arises through central mechanisms61 with a possible involvement of the GABAergic system.62 Our findings showed that, in animals pretreated with AA, a substantial decrease in the number of abdominal writhes induced by acetic acid was observed, similar to the results observed for morphine, suggesting inhibition of inflammatory pain, which activates peripheral nociceptors on the terminals of sensory nerve fibers.63,64
These results suggest that the possible antinociceptive effect of AA occurs through inhibition of the synthesis of inflammatory mediators or as antagonists of its receptors, a fact similar to that seen in the model of paw edema induced by PGE2, dextran, compound 48/80, and histamine, where AA significantly reduced the induction of edema by these proinflammatory agents. This is in agreement with previous studies which indicate that AA exerts an analgesic effect because it can lessen the number of contortions induced by acetic acid.65
We employed the formalin-induced paw-licking test to verify the antinociceptive effect of AA and to elucidate a possible mechanism of its action. Through this model, we can evaluate two distinctive stages of nociception: First stage: neurogenic pain, an acute response that occurs within the first 5 min after administration of formalin. This causes a chemical stimulation in type C nociceptors and part afferent Aδ fibers, in addition to the release of excitatory amino acids such as aspartate, glutamate, glycine, and taurine that take part in the transmission of peripheral nociception. Second stage: inflammatory pain, occurs between 15 and 60 min after administration of formalin. It is a tonic response related to the release of mediators, including bradykinin, histamine, serotonin, and prostaglandins, along with excitatory amino acids. Anacardic acid in the evaluated doses does not affect the locomodal activity of animals according to previous studies66 carried out by our group, a fact that is important because CNS depression or unspecified muscle relaxation can invalidate the behavioral tests for nociceptive assessment.67
Thus, results obtained from the present investigation demonstrated that AA reduces formalin-induced nociception in the two phases. These results are in agreement with those found in the acetic acid-induced abdominal writhing test since they confirm the performance of AA in the interaction with peripheral nociceptors due to the inhibition of the inflammatory stage, besides acting at the central level, inhibiting the neurogenic phase, confirming the results of the test hot plate. To show the involvement of the opioid pathway in the antinociceptive effect, it was shown that naloxone (opioid receptor nonselective antagonist) reversed the antinociceptive effect of AA, suggesting an involvement of the opioid pathway.
Conclusions
In summary, results from this study indicate that AA exhibits anti-inflammatory and antinociceptive effects. These findings also indicate that the anti-inflammatory effect of AA is mediated through mechanisms that involve inhibition of inflammatory mediators (histamine, serotonia, and prostaglandin), reduction of cranial degranulation, neutrophil migration, and oxidative stress. In addition, results show that AA produces an antinociceptive and a peripheral effect through the opioid pathway and a decrease in inflammatory mediators due to its antioxidant activities.
Experimental Section
Chemicals and Reagents
Chemicals, reagents, and drugs used throughout this study were obtained from commercial sources and used as received. We purchased λ-carrageenan, prostaglandin E2 (PGE2), dextran, histamine, compound 48/80 (4-methoxy-3,5-bis[[2-methoxy-5-[2-(methylamino)ethyl]phenyl]methyl]-N-methyl-benzeneethanamine, trihydrochloride (C32H45N3O3·3HCl)), dimethylsulfoxide (DMSO), acetic acid, and formaldehyde from Sigma-Aldrich (St Louis, MO). Morphine, naloxene, and heparin were acquired from Merck (São Paulo, SP, Brazil). All drugs were dissolved in sterile saline solution (0.9%), except AA, which was solubilized in 5% DMSO, and subsequently diluted in 0.9% NaCl (Vehicle). AA was extracted, isolated, and identified as described by Gomes Júnior and co-workers.66
Experimental Animals
Swiss albino mice (25–30 g) (Mus musculus) obtained from the animal house of the Federal University of Piauí (UFPI), Brazil, were used throughout this investigation. These animals were housed at a constant room temperature (25 ± 2 °C) and under standard conditions of light/dark cycle of 12 h, with free access to water and food (Purina, Campinas, Brazil). All experimental protocols were in accordance with the guidelines of the National Council for Control of Animal Experimentation and approved by the Animal Research Ethics Committee/UFPI (CEEA/UFPI 030/13). The mice were randomly divided into groups with five animals in each group (n = 5/group). Euthanasia was carried out by an intraperitoneal (ip) administration of (50:50) ketamine/xylazine, followed by the cervical dislocation.
Anti-Inflammatory Effect of AA
Carragenin-Induced Edema
Paw edema was produced according to previously published procedures.58 The animals were divided into six groups with five mice in each group. Edema was induced by injecting each mouse, in the right plantar aponeurosis, with 50 μL of a 0.3% (w/v) suspension of carrageenan (300 μg/paw, diluted in sterile saline at 0.9%). Drugs were given to animals intraperitoneally (ip) 30 min before carrageenan injection. The animals in the different groups received the following treatments: group I, vehicle (10 mL/kg, ip); group II, indomethacin, 10 mg/kg (reference drug); groups III, VI, and V, 10, 25, and 50 mg/kg of AA, respectively. The contralateral paw that was used as the control received 50 μL of sterile saline. Then, with the aid of a plethysmometer (Panlab, Barcelona, Spain), the volume of the right hind paw was measured before and after 1, 2, 3, and 4 h of carrageenan treatment. The effects of pretreatments were calculated using the following formula
where V0 and Vt are the volumes of the paw before and after induction of inflammation, respectively. The results are expressed as percent inhibition of edema relative to the paw volume of the saline-treated controls.
Induction of Paw Edema by Different Proinflammatory Agents
To induce edema using different inflammatory agents, the mice (n = 5) were injected with 50 μL of each of the following: dextran (300 μg/paw), compound 48/80 (12 μg/paw), and histamine (50.0 nM/paw or PGE2 3.0 nM/paw) in the right plantar aponeurosis, as outlined by Lo et al.59 and Feitosa et al.60 Thirty minutes before injection of the proinflammatory agents, the mice were pretreated with vehicle, indomethacin (INDO: 10 mg/kg), or AA (25 mg/kg); the control, which is the contralateral paw, received 50 μL of sterile saline (0.9%). The volume of the right hind paw was measured as described in the methodology of carrageenan-induced paw edema model.
Histological Evaluation
After completion of the carrageenan-induced edema, the paw tissue was removed and fixed in formalin (10%), embedded in paraffin, sectioned (5 μm), deparaffinized, and stained with hematoxylin and eosin (H&E). Slides were analyzed by means of optical microscopy by a qualified pathologist with no prior knowledge of the groups.
Carrageenan-Induced Leukocyte Migration in the Peritoneal Cavity (Peritonitis)
This model of edema induction was previously described by Feitosa et al.60 Initially, the animals were pretreated with 250 μL of vehicle (VEH: 10 mL/kg, ip), INDO (10 mg/kg, ip), or AA (25 mg/kg, ip). After 1 h, 250 μL of carrageenan (500 μg/well) was injected into the peritoneal cavity of each mouse. The mice (n = 5) were euthanized after 4 h, and the peritoneal cavity was washed with 1.5 mL of phosphate-buffered saline (PBS) for removal of the cells contained in the peritoneal fluid. The total cell count was performed using Neubauer’s chamber, and differential cell counts (100 cells in total) were accomplished after cytocentrifuging of slides, subsequently stained with hematoxylin and eosin. Results were presented as number of total leukocytes and neutrophils per milliliter of peritoneal exudate. The peritoneal exudate was also collected for the evaluation of myeloperoxidase (MPO), reduced glutathione (GSH), and malondialdehyde (MDA) levels.
Effect of AA against Oxidative Stress
Determination of Reduced Glutathione Levels
This test was performed according to the following general procedure: Aliquots of the peritoneal exudates were subjected to centrifugation at 3000 rpm for 15 min at 4 °C. This was followed by mixing 400 μL of each supernatant with 800 μL of Tris buffer (0.4 M, pH 8.9) and 20 μL of 0.01 M 5,5-dithio-bis(2-nitrobenzoic acid). Afterward, samples were stirred for 3 min and absorbance was spectrophotometerically measured at 412 nm.68 Results are presented as micrograms of GSH per milliliter (μg/mL) of exudates.
Determination of Malodialdehyde (MDA)
To determine the concentration of MDA in the peritoneal exudates, centrifugation of each aliquot was carried out at 3000 rpm for 15 min at 4 °C. This was followed by mixing 250 μL of each supernatant with 1.5 mL of 1% phosphoric acid (H3PO4) and 0.5 mL of 0.6% thiobarbituric acid (TBA). This mixture was heated in a water bath with stirring for 45 min. After cooling, 4 mL of n-butanol was added. The mixture was centrifuged for 10 min at 1200g to separate the n-butanol layer. Absorbance was then measured at 535 and 520 nm by means of a spectrophotometer.69 MDA concentrations are given as nmol/mL of exudates.
Determination of Myeloperoxidase (MPO) Activity
The enzyme myeloperoxidase (MPO) is primarily present in azurophil granules of neutrophils. This enzyme has been widely employed as a marker for the infiltration of granulocytes into peritoneal exudates. In this study, we measured the activity of MPO to assess the accumulation of neutrophils in peritoneal exudates. Foot tissue (50–100 mg) was homogenized at 50 mg/mL in potassium buffer containing 0.5% hexadecyltrimethylammonium bromide (HTAB). This was followed by centrifugation of the homogenate for 7 min at 40 000g. Pellet was resuspended, and the MPO activity was determined by measuring the absorbance at 450 nm using o-dianisidine dihydrochloride and 1% hydrogen peroxide (H2O2). Activity of MPO is expressed in units/mg of tissue, where one unit of MPO activity is defined as the conversion of 1 μM H2O2 into water in 1 min at 22 °C.70
Antinociceptive Effect of AA
Acetic Acid-Induced Abdominal Writhing Test
We employed the acetic acid-induced abdominal writhing test to assess the analgesic activity of AA. In this test, the mice (n = 5) were pretreated with AA (25 mg/kg, ip), vehicle (10 mL/kg, ip), or morphine (5 mg/kg, subcutaneously sc). After 30 min, the mice received 0.6% acetic acid (10 mL/kg of body weight, ip) and nociception intensity was measured by counting the total number of abdominal contortions including abdominal muscle contraction and hind paw extends over a period of 20 min.71
Hot Plate Test
To evaluate the analgesic activity of AA, we employed the hot plate test.72 In this test, each animal was placed twice on the heated plate (51 ± 1 °C) at a 30 min interval. First, the animals were familiarized with the test procedure, and a second evaluation served as a control for the reaction time (paw lick time or jump). The mice with a reaction time longer than 20 s were excluded. After the second test (control reaction time), the animals (n = 5) received vehicle (10 mL/kg, ip), AA (25 mg/kg, ip), or morphine (5 mg/kg, sc). Reaction time was measured at 0 (time 0) and at 30, 60, 90, and 120 min after administration of the compounds, with a cutoff time of 45 s to avoid paw injury.
Formalin Test
This test has been employed as a model for nociception related to tonic pain and localized inflammatory pain.73 According to this test, 20 μL of 2.5% formalin was administered (sc) in the right hind paw. The licking time was recorded from 0 to 5 min (stage 1, neurogenic pain) and from 20 to 25 min (stage 2, inflammatory pain). The mice (n = 5) were then treated with AA (25 mg/kg, ip), vehicle (10 mL/kg, ip), or morphine (5 mg/kg, sc) 30 min before formalin injection. To assess the effect of viaopioid-mediated AA, two groups (n = 5) were pretreated with naloxene (3 mg/kg, ip) 30 min before treatment with AA (25 mg/kg, ip) or morphine (5 mg/kg, sc), in which 20 mL of 2.5% formalin was subsequently administered in the subplantar region, as described previously.
Statistical Analysis
One-way analysis of variance (ANOVA) followed by the Neuman–Keuls test as a post hoc test were applied to the collected data. Results are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed with the aid of GraphPad Prism 6 (GraphPad Software, San Diego, California; http://www.graphpad.com); differences were significant at p ≤ 0.05.
Acknowledgments
The authors acknowledge the help and guidance provided by Prof. Dr. Rivelilson Mendes de Freitas (In memoriam) throughout this study. They are also grateful to the National Counsel of Technological and Scientific Development-CNPq, Brazil [grant number 232683/2014-0] for funding.
The authors declare no competing financial interest.
References
- Atanasov A. G.; Waltenberger B.; Pferschy-Wenzig E.-M.; Linder T.; Wawrosch C.; Uhrin P.; Temml V.; Wang L.; Schwaiger S.; Heiss E. H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy C. R.; Scully S. S. Analgesic substances derived from natural products (natureceuticals). Life Sci. 2005, 78, 476–484. 10.1016/j.lfs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Ekpenyong C. E.; Akpan E.; Nyoh A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin. J. Nat. Med. 2015, 13, 321–337. 10.1016/S1875-5364(15)30023-6. [DOI] [PubMed] [Google Scholar]
- Min S.-W.; Kim M.-J.; Baek N.-I.; Kim D.-H. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J. Ethnopharmacol. 2009, 125, 497–500. 10.1016/j.jep.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Babos M. B.; Grady B.; Wisnoff W.; McGhee C. Pathophysiology of pain. Dis.-Mon. 2013, 59, 330–358. 10.1016/j.disamonth.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Kubo I.; Masuoka N.; Ha T. J.; Tsujimoto K. Antioxidant activity of anacardic acids. Food Chem. 2006, 99, 555–562. 10.1016/j.foodchem.2005.08.023. [DOI] [Google Scholar]
- Oliveira M. S. C.; Morais S. M.; Magalhães D. V.; Batista W. P.; Vieira I. G. P.; Craveiro A. A.; de Manezes J. E. S. A.; Carvalho A. F. U.; de Lima G. P. G. Antioxidant, larvicidal and antiacetylcholinesterase activities of cashew nut shell liquid constituents. Acta Trop. 2011, 117, 165–170. 10.1016/j.actatropica.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Yuliana M.; Nguyen-Thi B. T.; Faika S.; Huynh L. H.; Soetaredjo F. E.; Ju Y. S. Separation and purification of cardol, cardanol and anacardic acid from cashew (Anacardium occidentale L.) nut-shell liquid using a simple two-step column chromatography. J. Taiwan Inst. Chem. Eng. 2014, 45, 2187–2193. 10.1016/j.jtice.2014.07.012. [DOI] [Google Scholar]
- Huang H.; Hua X.; Liu N.; Li X.; Liu S.; Chen X.; Zhao C.; Lan X.; Yang C.; Dou Q. P.; et al. Anacardic acid induces cell apoptosis associated with induction of ATF4-dependent endoplasmic reticulum stress. Toxicol. Lett. 2014, 228, 170–178. 10.1016/j.toxlet.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Kubo I.; Nihei K.; Tsujimoto K. Antibacterial action of anacardic acids against methicillin resistant Staphylococcus aureus (MRSA). J. Agric. Food Chem. 2003, 51, 7624–7628. 10.1021/jf034674f. [DOI] [PubMed] [Google Scholar]
- Morais T. C.; Pinto N. B.; Carvalho K. M. M. B.; Rios J. B.; Ricardo N. M. P. S.; Trevisan M. T. S.; Rao V. S.; Santos F. A. Protective effect of anacardic acids from cashew (Anacardiumoccidentale) on ethanol-induced gastric damage in mice. Chem.-Biol. Interact. 2010, 183, 264–269. 10.1016/j.cbi.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Ha T. J.; Kubo I. Lipoxygenase Inhibitory Activity of Anacardic Acids. J. Agric. Food Chem. 2005, 53, 4350–4354. 10.1021/jf048184e. [DOI] [PubMed] [Google Scholar]
- Shah S. M.; Sadiq A.; Shah S. M.; Ullah F. Antioxidant, total phenolic contents and antinociceptive potential of Teucrium stocksianum methanolic extract in different animal models. BMC Complementary Altern. Med. 2014, 14, 181. 10.1186/1472-6882-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualillo O.; Eiras S.; Lago F.; Dieguez C.; Casanueva F. F. Evaluated serum leptin concentrations induced by experimental acute inflammation. J. Ethnopharmacol. 2001, 75, 213–218. 10.1016/S0378-8741(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Qin H. Y.; Wu J. C.; Tong X. D.; Sung J. J.; Xu H. X.; Bian Z. X. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J. Gastroenterol. 2011, 46, 164–174. 10.1007/s00535-010-0321-6. [DOI] [PubMed] [Google Scholar]
- Cai C.; Chen Y.; Zhong S.; Ji B.; Wang J.; Bai X.; Shi G. Anti-Inflammatory Activity of N-Butanol Extract from Ipomoea stoloniferaIn Vivo and In Vitro. PLoS One 2014, 2, e95931 10.1371/journal.pone.0095931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B.; Gültekin-Özgüven M.; Kırkın C.; Özçelik B.; Morais-Braga M. F. B.; Bezerra C. F.; da Silva T. G.; Coutinho H. D. M.; Amina B.; Armstrong L.; Selamoglu Z.; et al. Anacardium Plants: Chemical, Nutritional Composition and Biotechnological Applications. Biomolecules 2019, 9, 465 10.3390/biom9090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza N. C.; de Oliveira J. M.; da Silva Morrone M.; D’Oliveira Albanus R.; Amarante M. D. S. M.; et al. Antioxidant and anti-Inflammatory properties of Anacardium occidentale leaf extract. Evidence-Based Complementary Altern. Med. 2017, 2017, 2787308 10.1155/2017/2787308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista A.; Gonçalves R. V.; Bressan J.; Pelúzio M. D. C. G. andies of crude extracts and fractions of cashew (Anacardium occidentale L.), Cajui (Anacardium microcarpum), and Pequi (Caryocar brasiliense C.): A Systematic Review. Oxid. Med. Cell. Longevity 2018, 2018, 3753562 10.1155/2018/3753562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal Y. S.; Tatke P. A.; Gabhe S. Y.; Vaidya A. B. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J. Tradit., Complementary Med. 2017, 7, 421–427. 10.1016/j.jtcme.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchikaya F. O.; Bantsielé G. B.; Kouakou-Siransy G.; Datté J. Y.; Yapo P. A.; Zirihi N. G.; Offoumou M. A. Anacardium occidentale Linn. (Anacardiaceae) Stem Bark Extract Induces Hypotensive and Cardio-Inhibitory Effects in Experimental Animal Models. Afr. J. Tradit., Complementary Altern. Med. 2011, 8, 452–461. 10.4314/ajtcam.v8i4.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Melo-Cavalcante A. A.; de Moura Dantas S. M. M.; de Sousa Leite A.; Matos L. A.; de Castro e Sousa J. M.; Picada J. N.; da Silva J. In vivo antigenotoxic and anticlastogenic effects of fresh and processed cashew (Anacardium occidentale) apple juices. J. Med. Food 2011, 14, 792–798. 10.1089/jmf.2010.0153. [DOI] [PubMed] [Google Scholar]
- Leite A. S.; Dantas A. F.; Oliveira G. L. S.; Júnior A. L. G.; de Lima S. G.; Citó A. M. G. L.; de Freitas R. M.; Melo-Cavalcante A. A. C.; Lopes J. A. D. Evaluation of Toxic, Cytotoxic, Mutagenic, and Antimutagenic Activities of Natural and Technical Cashew Nut Shell Liquids Using the Allium cepa and Artemia salina Bioassays. Biomed. Res. Int. 2015, 2015, 626835 10.1155/2015/626835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matutino Bastoss T.; Russo H. M.; Moretti N. S.; Schenkman S.; Marcourt L.; Gupta M. P.; Wolfender J. L.; Queiroz E. F.; Soares M. B. P. Chemical Constituents of Anacardium occidentale as Inhibitors of Trypanosoma cruzi Sirtuins. Molecules 2019, 24, 1299 10.3390/molecules24071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza M. Q.; Teotônio I. M. S. N.; de Almeida F. C.; Heyn G. S.; Alves P. S.; Romeiro L. A. S.; Pratesi R.; Nóbrega Y. K. M.; Pratesi C. B. Molecular evaluation of anti-inflammatory activity of phenolic lipid extracted from cashew nut shell liquid (CNSL). BMC Complementary Altern. Med. 2018, 18, 181 10.1186/s12906-018-2247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Bano S.; Javed K.; Ahmad S.; Rathish I. G.; Singh S.; Chaitanya M.; Arunasree K. M.; Alam M. S. Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur. J. Med. Chem. 2013, 65, 51–59. 10.1016/j.ejmech.2013.04.056. [DOI] [PubMed] [Google Scholar]
- Damasceno S. R.; Oliveira F. R.; Carvalho N. S.; Brito C. F.; Silva I. S.; Sousa F. B. M.; Silva R. O.; Sousa D. P.; Barbosa A. lR.; Freitas R. M.; et al. Carvacryl acetate, a derivative of carvacrol, reduces nociceptive and inflammatory response in mice. Life Sci. 2014, 94, 58–66. 10.1016/j.lfs.2013.11.001. [DOI] [PubMed] [Google Scholar]
- da Silveira Vasconcelos M.; Gomes-Rochette N. F.; de Oliveira M. L.; Nunes-Pinheiro D. C.; Tomé A. R.; de Sousa F. Y. M.; Pinheiro F. G. M.; Moura C. F. H.; Miranda M. R. A.; et al. Anti-inflammatory and wound healing potential of cashew apple juice (Anacardium occidentale L.) in mice. Exp. Biol. Med. 2015, 240, 1648–1655. 10.1177/1535370215576299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini M.; Peluso I.; Raguzzini A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–832. 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- Ren K.; Dubner R. Inflammatory models of pain and hyperalgesia. ILAR J. 1999, 40, 111–118. 10.1093/ilar.40.3.111. [DOI] [PubMed] [Google Scholar]
- Morris C. J.Carrageenan-Induced Paw Edema in the Rat and Mouse. In Methods in Molecular Biology; Humana Press, 2003; Vol. 225, pp 115–121. [DOI] [PubMed] [Google Scholar]
- Posadas I.; Bucci M.; Roviezzo F.; Rossi A.; Parente L.; Sautebin L.; Cirino G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004, 142, 331–338. 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami S. S.; Hohmann M. S. N.; Staurengo-Ferrari L.; Carvalho T. T.; Zarpelon A. C.; Possebon M. I.; de Souza A. R.; Veneziani R. C. S.; Arakawa N. S.; Casagrande R.; Verri W. A. Jr. Pimaradienoic Acid Inhibits Carrageenan-Induced Inflammatory Leukocyte Recruitment and Edema in Mice: Inhibition of Oxidative Stress, Nitric Oxide and Cytokine Production. PLoS One 2016, 11, e0149656 10.1371/journal.pone.0149656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiara P.; Bartalini M.; Tagliabue A.; Boraschi D. Anti-inflammatory activity of IFN-beta in carrageenan-induced pleurisy in the mouse. Clin. Exp. Immunol. 1986, 66, 606–614. [PMC free article] [PubMed] [Google Scholar]
- Cartaxo S. L.; Souza M. M.; de Albuquerque U. P. Medicinal plants with bioprospecting potential used in semi-arid northeastern Brazil. J. Ethnopharmacol. 2010, 131, 326–342. 10.1016/j.jep.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Ribeiro D. A.; Oliveira L. G.; Macêdo D. G.; Menezes I. R.; Costa J. G. M.; da Silva M. A. P.; Lacerda S. R.; Souza M. M. A. Promising medicinal plants for bioprospection in a Cerrado area of Chapada do Araripe, Northeastern Brazil. J. Ethnopharmacol. 2014, 155, 1522–1533. 10.1016/j.jep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- Kubo I.; Ha T. J.; Tsujimoto K.; Tocoli F. E.; Green I. R. Evaluation of lipoxygenase inhibitory activity of anacardic acids. Z. Naturforsch C 2008, 63, 539–546. 10.1515/znc-2008-7-812. [DOI] [PubMed] [Google Scholar]
- Sung B.; Pandey M. K.; Ahn K. S.; Yi T.; Chaturvedi M. M.; Liu M.; Aggarwal B. B. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyl-transferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood 2008, 111, 4880–4891. 10.1182/blood-2007-10-117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T. J.; Kubo I. Lipoxygenase inhibitory activity of anacardic acids. J. Agric. Food Chem. 2005, 53, 4350–4354. 10.1021/jf048184e. [DOI] [PubMed] [Google Scholar]
- Vane J. R.; Botting R. M. New insights into the mode of action of anti-inflammatory drugs. Inflammation Res. 1995, 44, 1–10. 10.1007/BF01630479. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P.; Joseph K.; Shibayama Y.; Nakazawa Y.; Ghebrehiwet B.; Reddigari S.; Silverberg M. Bradykinin formation. Plasma and tissue pathways and cellular interactions. Clin. Rev. Allergy Immunol. 1998, 16, 403–429. 10.1007/BF02737659. [DOI] [PubMed] [Google Scholar]
- Coura C. O.; Souza R. B.; Rodrigues J. A.; Vanderlei Ede S.; de Araújo I. W. F.; Ribeiro N. A.; Frota A. F.; Ribeiro K. A.; Chaves H. V.; Pereira K. M. A.; et al. Mechanisms involved in the anti-inflammatory action of a polysulfated fraction from Gracilaria cornea in rats. PLoS One 2015, 10, e0119319 10.1371/journal.pone.0119319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto J. B. Twenty-five years of research on medicinal plants in Latin America: a personal view. J. Ethnopharmacol. 2005, 100, 131–134. 10.1016/j.jep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chatterjea D.; Wetzel A.; Mack M.; Engblom C.; Allen J.; Mora-Solano C.; Paredes L.; Balsells E.; Martinov T. Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem. Biophys. Res. Commun. 2012, 425, 237–243. 10.1016/j.bbrc.2012.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkema L. E.; Bemis K. G.; Fleisch J. H. Production and antagonism of cutaneous vascular permeability in the guinea pig in response to histamine, leukotrienes and A23187. J. Pharmacol. Exp. Ther. 1984, 230, 550–557. [PubMed] [Google Scholar]
- Sadik C. D.; Luster A. D. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J. Leukocyte Biol. 2012, 91, 207–215. 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers C.; Rankin S. M.; Condliffe A. M.; Singh N.; Peters A. M.; Chilvers E. R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz B.; Horwitz M. S.; Jenne D. E.; Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen B. S.; de Winther M. P.; Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid. Redox Signaling 2009, 11, 2899–2937. 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- Li S.; Hong M.; Tan H. Y.; Wang N.; Feng Y. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxid. Med. Cell. Longevity 2016, 2016, 4234061 10.1155/2016/4234061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli M. L.; Novelli F.; Costa F.; Malagrinò L.; Melosini L.; Bacci E.; Cianchetti S.; Dente F. L.; Di Franco A.; Vagaggini B.; et al. Malondialdehyde in Exhaled Breath Condensate as a Marker of Oxidative Stress in Different Pulmonary Diseases. Mediators Inflammation 2011, 2011, 891752 10.1155/2011/891752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu M.; Courtney S. C.; Brinton M. A. Arsenite-induced stress granule formation is inhibited by elevated levels of reduced glutathione in West Nile virus-infected cells. PLoS Pathog. 2017, 13, e1006240 10.1371/journal.ppat.1006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omanakuttan A.; Nambiar J.; Harris R. M.; Bose C.; Pandurangan N.; Varghese R. K.; Kumar G. B.; Tainer J. A.; Banerji A.; et al. Anacardic Acid Inhibits the Catalytic Activity of Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9. Mol. Pharmacol. 2012, 82, 614–622. 10.1124/mol.112.079020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olakanmi O.; McGowan S. E.; Hayek M. B.; Britigan B. E. Iron sequestration by macrophages decreases the potential for extracellular hydroxyl radical formation. J. Clin. Invest. 1993, 91, 889–899. 10.1172/JCI116310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad F. B.; Mubofu E. B. Potential biological applications of bio-based anacardic acids and their derivatives. Int. J. Mol. Sci. 2015, 16, 8569–8590. 10.3390/ijms16048569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung B.; Pandey M. K.; Ahn K. S.; Yi T.; Chaturvedi M. M.; Liu M.; Aggarwal B. B. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood 2008, 111, 4880–4891. 10.1182/blood-2007-10-117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R. O.; Sousa F. B.; Damasceno S. R.; Carvalho N. S.; Silva V. G.; Oliveira F. R. M. A.; Sousa D. P.; Aragão K. S.; Barbosa A. L. R.; Freitas R. M.; Medeiros J. V. R. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. 10.1111/fcp.12049. [DOI] [PubMed] [Google Scholar]
- Lo T. N.; Almeida A. P.; Beaven M. A. Dextran and carrageenin evoke different inflammatory response in rat with respect to composition of infiltrates and effect of indomethacin. J. Pharmacol. Exp. Ther. 1982, 221, 261–267. [PubMed] [Google Scholar]
- Feitosa R. F.; Melciades G. B.; Assreuy A. M. S.; Rocha M. F.; Ribeiro R. A.; Lima A. A. The pharmacological profile of ovoalbumin induced paw oedema in rats. Mediators Inflammation 2002, 11, 155–163. 10.1080/09622935020138000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehkordi F. M.; Kaboutari J.; Zendehdel M.; Javdani M. The antinociceptive effect of artemisinin on the inflammatory pain and role of GABAergic and opioidergic systems. Korean J. Pain 2019, 32, 160–167. 10.3344/kjp.2019.32.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Júnior A. L. G.; Tchekalarova J. D.; Atanasova M.; Machado K. M.; Rios M. A. S.; Paz M. F. C. J.; Găman M.-A.; Găman A. M.; Yele S.; Shill M. C.; et al. Anticonvulsant effect of anacardic acid in murine models: Putative role of GABAergic and antioxidant mechanisms. Biomed. Pharmacother. 2018, 106, 1686–1695. 10.1016/j.biopha.2018.07.121. [DOI] [PubMed] [Google Scholar]
- Satyanarayana P. S.; Jain N. K.; Singh A.; Kulkarni S. K. Isobolographic analysis of interaction between cyclooxygenase inhibitors and tramadol in acetic acid-induced writhing in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 641–649. 10.1016/j.pnpbp.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Yin Z. Y.; Li L.; Chu S. S.; Sun Q.; Ma Z. L.; Gu X. P. Antinociceptive effects of dehydrocorydaline in mouse models of inflammatory pain involve the opioid receptor and inflammatory cytokines. Sci. Rep. 2016, 6, 27129 10.1038/srep27129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onasanwo S. A.; Fabiyi T. D.; Oluwole F. S.; Olaleye S. B. Analgesic and anti-inflammatory properties of the leaf extracts of Anacardium occidentale in the laboratory rodents. Niger. J. Physiol. Sci. 2012, 27, 65–71. [PubMed] [Google Scholar]
- Gomes Júnior A. L.; Tchekalarova J. D.; Machado K. C.; Moura; et al. Anxiolytic effect of anacardic acids from cashew (Anacardium occidentale) nut shell in mice. IUBMB Life 2018, 70, 420–431. 10.1002/iub.1738. [DOI] [PubMed] [Google Scholar]
- de Sá P. G. S.; Nunes X. P.; Lima J. T.; Siqueira-Filho J. A.; et al. Antinociceptive effect of ethanolic extract of Selaginella convoluta in mice. BMC Complementary Altern. Med. 2012, 12, 187 10.1186/1472-6882-12-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B. L.; Baquer N. Z. Hexokinase, glucose-6-phosphate dehydrogenase and antioxidant enzymes in diabetic reticulocytes: effects of insulin and vanadate. IUBMB Life 1998, 46, 1145–1152. 10.1080/15216549800204702. [DOI] [PubMed] [Google Scholar]
- Sedlak J.; Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 24, 192–205. 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Mullane K. M.; Kraemer R.; Smith B. Myeloperoxidase Activity as a Quantitative Assessment of Neutrophil Infiltration Into Ischemic Myocardium. J. Pharmacol. Methods 1985, 14, 157–167. 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Koster R.; Anderson M.; De Beer J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–417. [Google Scholar]
- Łuszczki J. J. Dose-response relationship analysis of pregabalin doses and their antinociceptive effects in hot-plate test in mice. Pharmacol. Rep. 2010, 62, 942–948. 10.1016/S1734-1140(10)70355-8. [DOI] [PubMed] [Google Scholar]
- Choi S.-S.; Lee J.-K.; Suh H.-W. Antinociceptive profiles of aspirin and acetaminophen in formalin, substance P and glutamate pain models. Brain Res. 2001, 921, 233–239. 10.1016/S0006-8993(01)03126-2. [DOI] [PubMed] [Google Scholar]