Abstract

For the construction of a chemical model of contemporary living cells, the so-called water-in-oil emulsion transfer (WOET) method has drawn much attention as one of the promising preparation protocols for cell-sized liposomes encapsulating macromolecules and even micrometer-sized colloidal particles in high yields. Combining the throughput and accuracy of the observation is the key to developing a synthetic approach based on the liposomes prepared by the WOET method. Recent advances in microfluidic technology can provide a solution. By means of surface modification of a poly(dimethylsiloxane)-type microfluidic device integrating size-sorting and trapping modules, here, we enabled a simultaneous direct observation of the liposomes with a narrow size distribution, which were prepared by the WOET method. As a demonstration, we evaluated the variance of encapsulation of polystyrene colloidal particles and water permeability of the cell-sized liposomes prepared by the WOET method in the device. Since the liposomes prepared by the WOET method are useful for constructing cell models with an easy protocol, the current system will lead to a critical development of not only supramolecular chemistry and soft matter physics but also synthetic biology.
Introduction
Liposomes have attracted increasing attention in the field of synthetic biology.1,2 Since liposomes have a structural commonality to the current living cells, they can be a scaffold to remodel cellular dynamics aiming at clarifying an unexplored chemical logic in the cells.2−4 Thus far, various cell models mimicking fundamental features of living cells (liposome-based cell models) have been proposed: growth and division,5−8 protein synthesis inside liposomes,9−12 communication with other liposomes,13 and energy synthesis.14 In addition, investigating liposome-based cell models in view of supramolecular chemistry and soft matter physics would be a promising approach to clarify the fundamental features of cellular membranes.15−18
As for the preparation methods of liposomes, the so-called water-in-oil (w/o) emulsion transfer method (WOET method) has drawn attention because of its highly efficient encapsulation of various substances including (bio)macromolecules and even micrometer-sized colloidal particles into the inner water phase of liposomes.19,20 Briefly, the w/o emulsion is deposited onto an aqueous phase, and the water droplets coated with a lipid monolayer in the emulsion are transferred from the upper oil phase to the lower aqueous phase using a centrifuge. Liposomes are formed by the transfer of the droplets because the lipid monolayer formed at the interface of the upper oil phase and the lower aqueous phases wraps the monolayer-coated droplets. Basically, the inner water phase of the formed liposome carries over the inner phase of droplets in the emulsion, and the encapsulation efficiency is much better than the traditional preparation methods of liposomes, by which macromolecules were hardly encapsulated or tend to be lost to the external solution.21−24 Because of no requirement of any specific apparatus but a benchtop centrifuge, the WOET method is available for the studies in versatile fields including synthetic biology.
For use of liposomes in biological studies, it is also important to establish precise and efficient technique handling the liposomes under controlled environmental conditions25 because of the difficulty in microscopic tracking of cell-sized liposomes as they freely move by an exchange of the outer solution. Recent developments of microfluidic devices made of poly(dimethylsiloxane) (PDMS) have solved the problem as the device allowed to array liposomes and to observe them in detail over time while repeatedly exchanging the outer solution.26−29
However, hydrophobicity of PDMS sometimes limits the applicability. Namely, hydrophobic molecules could adhere to the surface forming aggregates,30 which was also the case in using liposomes prepared by the WOET method. Hydrophilic surface modification can reduce such undesired adsorptions31−34 but in turn causes wetting of liposomes and membrane spreading.35,36 Thus, the hydrophilic modification would be problematic for a longtime observation.
In this study, we present that the surface modification of the microfluidic device using a perfluoroalkyl group was useful to reduce the undesired adhesion of lipid aggregates over the long-term liposome observations. Based on the microfluidic system that we recently developed,29 we validated the surface modification through sequential size-sorting and trapping of the cell-sized liposomes prepared by the WOET method. As a demonstration, we first encapsulated multiple polystyrene-made fluorescent microbeads (diameter = 1.0 μm) in the liposomes and performed a statistical evaluation of the encapsulation efficiency based on microscopic tracking. We compared the intrinsic variance in the distribution of the encapsulation yields in the device and the bulk measurement. As a second demonstration, we estimated water permeability of the liposomes prepared by the WOET method under precisely regulated perfusion of the hypertonic solution.
Perfusion Chamber Design
The schematic illustration of a microfluidic device integrating size-sorting and trapping of cell-sized liposomes is shown in Figure 1a. The device was designed according to the previous report.29 The device contains a size-sorting region and a trapping region. Size-sorting mechanics was implemented by the deterministic lateral displacement (DLD).37,38 By DLD, large particles are bumped up when the particle collided to periodically juxtaposed microposts because of its excluded volume (bump mode), while small particles swim straightly among the microposts (zigzag mode). In this paper, by combining two DLDs, we designed the device to select liposomes of 11.8–12.2 μm in diameter in theory. For the trapping region where the selected liposomes were introduced, we adopted a large circular microstructure (nest) to trap only one liposome, where the trapped liposome can be easily tracked even under a transient and stochastic backflow caused by the abrupt stop of the external flow. Owing to the nest structure, the relatively static condition of liposomes is easily realized with the microfluidic device for the purpose of comparing the previous studies on cell-sized liposomes with batch microscopic observation. We note that in our experimental setup, the frequency of such a transient backflow itself was suppressed owing to plumbing (Figures 1b and S1). The mechanism to trap only one liposome in one nest is as follows: the flow at the entrance of the nest bifurcates into the trap path and bypass path. The trap path is clogged by a first trapped liposome, resulting in an increase of the flow resistance of the trap path (Figure S2). Thus, the height of the bifurcation decreased after the first liposome is trapped. Since only liposomes of selected diameter were introduced into the trapping region, secondary liposomes cannot enter the nest.
Figure 1.
Schematic illustrations of the developed microfluidic device to array liposomes in juxtaposed trapping structures (nests) (a) and plumbing of the microfluidic system (b).
Results and Discussion
Surface Modification of PDMS and Microfluidic Trap of Cell-Sized Liposomes Prepared by the WOET Method
As shown in Figure 2a, a considerable amount of adhesion of lipid aggregates was observed in the size-sorting region when we used an unmodified device. Since the collision of particles and the microposts is the key to DLD mechanics, such adhesion collapsed the performance of the device.
Figure 2.

(a, b) Fluorescence microscopy images of the start point of the size-sorting region with no treatment (a) and with surface modification (b). Images were taken after 12 h of introduction of liposome dispersion with the WOET method. (c) Fluorescence image of arrayed liposomes after 2 h of introduction of liposome dispersion. Contours of the nests were superimposed by the gray lines. Scale bars, 100 μm.
Thus, we considered changing the surface of the device to be hydrophobic and lipophobic. During the bonding process of the PDMS channel with the glass slide, the silanol group was generated on the surface through O2 plasma treatment (Table 1, runs 1 and 2).39 However, after 30 min of baking (experimentally determined shortest bonding time, by which the PDMS channel and glass slide were bonded without any leakage), the contact angles of both water and hexadecane got remarkably recovered (Table 1, run 3), indicating that the reactive sites diminished. Thus, we chose the trifunctional silane coupling reagents expecting the polycondensation of trimethoxy(1H,1H,2H,2H-heptadecafluorodecyl)silane (PFS). We measured the contact angles for water and hexadecane according to the previous report40 with PDMS blocks baked for 30 min after the plasma treatment and following surface modification. Compared to the unmodified but plasma-treated PDMS block (water, 96.1 ± 1.6°; hexadecane, 42.3 ± 1.5°; Table 1, run 3), the modified PDMS block showed larger contact angles both for water and hexadecane (water, 107.7 ± 6.1°; hexadecane, 71.4 ± 2.2°; Table 1, run 4). The contact angle of the modified PDMS block for water was comparable to the initial value (107.7 ± 6.1 and 105.33 ± 2.3°, respectively) and that for hexadecane was clearly increased (43.9 ± 2.1 and 71.4 ± 2.2°, respectively). Since plasma-treated PDMS block did not recover the initial contact angle for water even after 20 h without further modification (83.2 ± 9.1°), we deduced that the modification increased both hydrophobicity and lipophobicity of the surface of PDMS. Note that the contact angles were unchanged even after 20 h from the modification (Table 1, run 5).
Table 1. Contact Angles of Water and Hexadecane on PDMS Blocks before and after the Modification.
| contact
angle/deg |
|||
|---|---|---|---|
| run | condition | water | hexadecane |
| 1 | untreated PDMS block | 105.3 ± 2.3 | 43.9 ± 2.1 |
| 2 | just after plasma treatment | 32.9 ± 3.7 | 32.2 ± 2.3 |
| 3 | 30 min after plasma treatment | 96.1 ± 1.6 | 42.3 ± 1.5 |
| 4 | 30 min after PFS treatment | 107.7 ± 6.1 | 71.4 ± 2.2 |
| 5 | 20 h after PFS treatment | 103.8 ± 2.0 | 68.4 ± 3.7 |
We then examined the performance of the surface-modified device for the liposomes prepared by the WOET method. The adhesion of the lipid aggregates, which collapse the size-sorting mechanics based on the DLD method, considerably reduced (Figure 2b). In addition, the liposomes were trapped in the trapping region within 2 h (Figure 2c). In the figure, 57% of nests (16/28) were occupied and only one liposome was trapped for 81% of the occupied nests (13/16). The trapping efficiency (57%) was relatively low compared to the previous report where liposomes prepared by a sugar-doped thin-film hydration method were used.29 The tendency of the time course was similar to the previous report:29 a fast increase in the early time range followed by a plateau (Figure S3a). In addition, more than 75% of the trapped liposomes trapped stably at least 2 h (Figure S3b). Thus, the low trapping efficiency could be assigned to the fraction of liposomes of a desired diameter in the liposome dispersion. On the other hand, although a slight amount of lipid aggregates was observed both for the size-sorting region and trapping region, the one-to-one rate (81%) was equivalent to the previous report,29 suggesting that the trapping strategy worked well. The size of trapped liposomes was 10.1 μm in average (coefficient of variation = 15%). Compared to the theoretical expectation for the average size calculated from the design of DLD (11.8–12.2 μm), the average diameter of the trapped liposomes was smaller. According to the previous report, owing to the deformability, DLD tends to select slightly larger particles when it was applied to liposomes (∼10% larger than the theoretical estimation).26 The obtained tendency in this research was the opposite. We consider the reason as follows. Small liposomes could be introduced into the trapping region when a liposome collides with microposts with the other adjacent liposomes because the diameter of the liposome is overestimated. Importantly, the trapping structure used in the device imposed an additional step of size selection at the entrance of the nest: only liposomes whose diameter was smaller than the bifurcation height was able to enter the nest. As we designed the bifurcation height for the empty nest to be low enough to exclude secondary liposomes after the first trap of the liposome, most of the liposomes (diameter 11.8–12.2 μm) bypassed the nest and the liposomes smaller than the DLD design (∼10 μm) were selectively trapped at the nests. Note that the coefficient of variation of the diameter of trapped liposomes (15%) was better than those obtained in the previous preparation protocols based on the WOET method.41,42 Taking the results together, we deduced that the modification was effective to change the surface property of PDMS and thus extended the applicability of the device to the liposomes prepared by the WOET method.
Distribution of Encapsulated Microbeads in Liposomes Prepared by the WOET Method
One of the most striking advantages of the WOET method is to encapsulate not only macromolecules but also the colloidal particles of the micrometer scale at high volume fractions in liposomes.43,44 To prove that the device is appropriate for the investigation of liposomes prepared by the WOET method, we prepared liposomes with fluorescent polystyrene microbeads and observed them with the microfluidic device. As a result, the streamline of fluorescent microbeads was visualized, which indicated the bipolar convection inside the liposomes (Figure 3a and Movie S1; see Figure S4 for the monochronic images decomposed from the original image for each color channel corresponding to the liposomal membrane and fluorescent microbeads). Since the stroboscopic lines shown in cyan were the trajectory of the microbeads during the exposure time, we can see the convection in the trapped liposome, which was parallel to the cross section of the liposome. The tendency was common to all of the observed liposomes. When the flow was abruptly stopped after the trapping, the convection was suspended in accordance with the stoppage of the flow of the outside of the liposomes (Figure 3b–d and Movie S2). Recently, it was theoretically and experimentally reported that shear can generate bipolar convections inside liposomes under the fluidic conditions45 and hemispherical liposomes adhered to the solid surface.46,47 Although the details of the fluidic property and state of liposomes were different, we consider that the convection observed in our system is analogous to the previous reports. Note that, in any case, the bipolar convection visualized by the fluorescent microbeads can be regarded as a clear proof that the device was able to trap the liposomes encapsulating multiple micrometer-sized microbeads inside, which was one of the characteristic advantages of liposomes prepared by the WOET method.
Figure 3.

Composite fluorescence microscopy images of liposomes trapped in the microfluidic device under a constant flow (40 μL/h) (a) and under a stopped flow (b–d). Images were taken 5 s (b), 9 s (c), and 13 s (d) after the manipulation of the valve. Lipid is depicted in red (emission, 600–690 nm), and fluorescent microbeads are shown in cyan (emission, 515–545 nm). The optical exposure time was 500 ms. Scale bars, 10 μm.
The liposomes captured and observed in one experiment are only a part of liposomes in the original liposome dispersion as the smaller coefficient of variation of the diameters indicated (approximately 15% in the device and 32% in the bulk measurement) (Figure 4a). Therefore, it is important to validate whether the distribution of the trapped liposomes is not biased with that obtained in the batch observation. Since the encapsulation yield is the core competence of the WOET method,48 we counted the number of fluorescent microbeads encapsulated in the trapped liposomes after abruptly stopping the external flow and compared the result to that obtained in the batch observation. Note that we used a far diluted microbead dispersion compared to the previous reports44 to count the number of microbeads inside correctly. To avoid the effect of the difference of the volume, we calculated the volume fraction of the encapsulated microbeads to the liposome. As shown in Figure 4b, the shapes of the frequency plots of the volume fraction obtained from the microfluidic measurement (n = 113) and that obtained from the batch measurement (n = 173) showed similar tendency (p = 0.23). On the contrary, when the 2-fold diluted microbead dispersion was used for the inner aqueous phase of the liposome dispersion, frequency distribution slightly left-shifted (n = 64; p = 0.058). The difference in the distribution was also demonstrated by the average number of microbeads encapsulated in liposomes: 1.5 for the twofold diluted dispersion and 2.1 for the original dispersion. Overall results were consistent with the hypothesis that the liposomes arrayed in the microfluidic device were not biased at least in terms of the distribution of the encapsulation yield.
Figure 4.

(a, b) Histograms of diameters of trapped liposomes (a) and volume fractions of fluorescent beads encapsulated in the liposomes (b). Liposomes prepared with original density (1×) or two times diluted (2×) bead dispersion were observed under a microfluidic device (MFD) or bulk specimen.
Water Permeability of Liposomes Prepared by the WOET Method
As stated above, one of the advantages of the microfluidic observation is the sequential and simultaneous tracking of liposomes under a controlled outer environment. To demonstrate this point, we observed the liposomes exposed to a hypertonic solution under a microfluidic environment to evaluate the water permeability. Namely, after the trap of the liposomes, the external solution was exchanged with the hypertonic solution, and the following dynamics was monitored by microscopic observation. Figure 5a shows the representative time-lapsed fluorescence images. The diameter of each liposome calculated from the area of the cross-sectional image linearly decreased during 480–690 s (Figure 5b,c), indicating the consistency with the previous works49 and the uniformity of the concentration of the external solution during the time range. The averaged water permeability of liposomes was calculated as 10.2 ± 5.0 μm/s (n = 10), and even in maxima, it was 18.1 μm/s (see the Methods section for details). The estimated value was remarkably smaller than the previously reported value for the pure POPC membrane (∼102 μm/s).50,51 Rather, the value was comparable to that of the POPC membrane containing ca. 40% of cholesterol.51 According to the previous report,26 the measurement of water permeability in the device afforded comparable average water permeability to the other bulk measurements52 as for liposomes prepared by the film swelling method. Moreover, in our experimental system, continuous shear, which is known to enhance the permeability across the membrane,53,54 was imposed on the trapped liposomes. Therefore, the obtained low water permeability should not be attributed to the methodology using microfluidics. Since the previous report elsewhere demonstrated that the residual oil compound (n-decane) used in the WOET method contaminated the liposomal membrane during the emulsification process,55 we suspect that the liquid paraffin used in the current study afforded low water permeability.
Figure 5.

(a) Representative fluorescence images (top) and their binary images (bottom) of a liposome exposed to the hypertonic solution. Scale bar, 10 μm. (b) Time course of the relative diameter normalized by the initial diameter of each liposome. The diameter was estimated from the binary image in (a). The average value is plotted in tense blue, and all data are plotted in pale blue. (c) Absolute diameter of liposomes plotted for 480–690 s (indicated as the red rectangle in (b)). Each color represents data of each liposome.
We should also note that the obtained variance of the water permeability was not negligible. Although the estimation process based on the cross-sectional image associated the considerable intrinsic variance (up to 30% in the coefficient of variation (CV)),52 the obtained variance (CV ∼ 50%) could suggest another factor of variation, the amount of residual oils. However, it is technically difficult to estimate the amount of oil contaminants for the observed individual liposomes in the device, and further elucidation is desired. In any case, the molecular transportation across the lipid membrane is of great interest in view of, for instance, future application of liposomes as a therapeutic evaluation platform for drugs,56,57 mechanistic investigation of cell-penetrating peptides,58 and a synthetic approach toward the cell model.14 Therefore, it should be remembered that even the maximum of the water permeability of the liposomes prepared by the current WOET method was much smaller than that of the liposomes prepared by the film swelling method50,51 as a potential nature of the preparation protocol affecting the membrane property.
Conclusions
In this paper, we showed that the modification of the surface of the device by the perfluoroalkyl group enabled the steady trapping and parallel observation of liposomes prepared by the WOET method. Simultaneous direct observation of trapped liposomes prepared by the WOET method is advantageous for the easy and precise tracking of the time course of liposomes with a statistical data set, which demands time-consuming repetition of the experimental protocol in batch measurements and is impossible for flow cytometry. We demonstrated the potential of the device as a perfusion chamber by the following three measurements: visualization of the bipolar convection in the liposomes under microfluidic environment, evaluation of the encapsulation yield, and long-term tracking of liposomes upon the osmotic stress. Significantly, the water permeability of the liposomes was much smaller than the previous reports. It should be noted as the potential artifact on the future development of liposome-based cell models using the current WOET method. However, the WOET method can afford liposomes containing even large colloidal particles and hence biofunctional polymers without special apparatus. In the support of capillary devices or PDMS-based microfluidic devices, some organic solvents other than liquid paraffin can suppress the contamination of liposomal membrane.59−61 This is advantageous for not only synthetic biology but also pharmaceutical applications.62−64 Thus, the combination of the microfluidic experiment and the WOET method can encourage the further development of a wide range of research fields related to liposomes.
Methods
Materials
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was purchased from NOF Corporation (Tokyo, Japan). Cholesterol was provided by Avanti Polar Lipids. Texas Red 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (triethylammonium salt) (Texas Red DHPE) was supplied by Thermo Fisher Scientific, Inc. (Waltham, MA). Glucose, sucrose, tris(hydroxymethyl)aminomethane (Tris), hydrochloric acid, methanol, chloroform, hexadecane, liquid paraffin, and pH standard solutions (phthalate pH standard solution, phosphate pH standard equimolal solution, and tetraborate pH standard solution) were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Trimethoxy(1H,1H,2H,2H-heptadecafluorodecyl)silane (PFS) and fluorescent microbeads (Fluoresbrite YG Microspheres, Calibration Grade 1.00 μm) were provided by Tokyo Chemical Industry Corporation (Tokyo, Japan) and Polysciences, Inc. (Warrington, PA), respectively. For PDMS fabrication, SILPOT 184 W/C purchased from Dow Toray Co., Ltd. was used. All materials were used without further purification.
Measurement of the Contact Angle of the PDMS Block
PDMS blocks used for the measurement of the contact angle were fabricated as follows. The base of SILPOT 184 W/C and its curing agent were mixed in a 10:1 weight ratio (Thinky, Inc., AR-100). The mixture was heated at 75 °C for more than 2 h. For the surface modification of the PDMS block, we further adopted the following procedures. We exposed the PDMS block to a single-step oxygen plasma treatment: 25 W, 20 cc/min, 5 s (SAMCO, FA-1). After being heated at 75 °C for 30 min, the PDMS block was dipped into an approximately 10 wt % PFS/methanol solution containing an aliquot of 6 M hydrochloric acid for 60 min. Then, the PDMS block was washed with an excess amount of methanol and dried at room temperature (approximately 25 °C). The fabricated PDMS block was placed on a horizontal table, and 10 μL of Milli-Q water or hexadecane was placed on the PDMS block. The image of the droplet was taken by a commercially available contact angle meter (Excimer Inc., SImage Entry 5). The measurement proceeded independently five times for each experimental condition. The results were presented as mean values ± standard deviations.
Preparation of Microbead Dispersion
Fluorescent microbead dispersion (ca. 100 μL) was diluted by 600 μL of Milli-Q water. The bead dispersion was centrifuged (1900 g, 5 min, 25 °C) by a high-speed microcentrifuge (HITACHI, Ltd., Himac CT15RE), and the supernatant was removed. Milli-Q water (600 μL) was added, and the dispersion was vigorously vortexed. The procedures were repeated five times. After the fifth centrifugation, 350 mM glucose/150 mM sucrose/50 mM Tris–HCl buffer (measured pH, 8.05; inner phase solution) was added instead of Milli-Q water. The bead dispersion was stored under 4 °C (at most 3 days) and used after further dilutions (typically diluted threefold).
Preparation of Cell-Sized Liposome Dispersion
Stock solutions of POPC (40 mM in chloroform, 96 μL) and Texas Red DHPE (14.5 μM in chloroform, 160 μL) were mixed in liquid paraffin (1 mL). The oil dispersion was heated at 80 °C by a dry bath (Rocker Scientific Co., Ltd., Sahara 310) for 6 h. After cooling of the oil dispersion, the bead dispersion (100 μL) was poured into the lipid/paraffin solution and vigorously vortexed for 20 s. The resulting emulsion (200 μL) was gently put onto 1 mL of 500 mM glucose/50 mM Tris–HCl buffer (measured pH, 8.06; outer phase solution) in a microtube. The microtube was centrifuged (18 800g, 30 min, 25 °C), and the supernatant was removed. The residual (∼50 μL) was redispersed by gentle pipetting and put to a fresh microtube. The outer phase solution (1 mL) was added to the microtube and gently pipetted to disperse. The liposome dispersion was centrifuged again (18 800g, 30 min, 25 °C). Almost all of the supernatant was carefully removed. Finally, to eliminate the density difference, 100 μL of inner phase solution was added to the precipitate and gently pipetted to redisperse. The prepared liposome dispersion was used within 1 day, although it remained stable at least 2 days after preparation.
Surface Modification of the PDMS-Based Microfluidic Device
The PDMS-based microfluidic device was designed and fabricated according to the previous report.26,29 To bond the glass slide and the PDMS channel, we adopted a two-step oxygen plasma treatment: 25 W, 20 cc/min, 2 min for glass slide only and then 25 W, 20 cc/min, 5 s for glass slide and the PDMS channel (SAMCO, FA-1). After the plasma treatment, the glass slide and the PDMS channel were quickly contacted together and then heated on a hot plate at 75 °C for 30 min. The microfluidic device was cooled for 5 min at room temperature (ca. 20 °C), and approximately 10 wt % PFS/methanol solution mixed with an aliquot of 6 M hydrochloric acid was introduced into the device. After 60 min of incubation at room temperature (approximately 20 °C), the device was washed with an excess amount of methanol (>5 mL) and then with Milli-Q water (>5 mL).
Trapping of Liposomes
The liposome dispersion and the outer solution were introduced into the microfluidic device by two syringe pumps (YMC, YSP-202; Harvard Apparatus, Pump 11 Pico Plus Elite) for typically 30 min. Flow rates were set to 6.5 and 40 μL/h, respectively. The schematic description of the plumbing of the whole experimental system is described in Figure 1b. After a moderate number of liposomes (10–14) were trapped in the nests, the flow of the liposome dispersion was shut out by switching valve A. For the osmotic stress experiments, the outer solution was exchanged by connecting the sample loop to the microfluidic device through switching valve B. Another pump (Harvard Apparatus, Pump 11 Pico Plus Elite) was used to fill the sample loop. For the abrupt stop of the flow of the outer solution, valve C was switched.
Evaluation of the Trapping Process and Stability of the Trapped Liposomes
The liposome dispersion was introduced as described in the former section. After the flow rate was changed to 40 μL/h, fluorescence images were taken for every 60 s for 2 h. Then, valve A was switched to shut out the liposome dispersion, and again, fluorescence images were taken for every 60 s for 2 h to evaluate the stability of the trapped liposomes. The obtained images were analyzed by ImageJ.
Microscopic Observations
In the case of microfluidic experiments, liposomes were arrayed in the device via the procedures explained above. On the other hand, in the case of batch observation, the liposome dispersion was confined in a frame-sealed chamber with two glass slips and a spacer (thickness ∼280 μm; Bio-Rad, SLF-0201). The specimen was observed under an inverted microscope (Olympus, IX71) equipped with a cooled charge-coupled device camera (Olympus, DP72). A dual-band excitation filter (excitation, 490–505, 560–580 nm; emission, 515–545, 600–650 nm) and a single-band excitation filter (excitation, 565–585 nm; emission, 600–690 nm) were used. For counting green fluorescent microbeads in cell-sized liposomes stained by Texas Red DHPE, image processing was performed as follows. Once green and red fluorescence from those liposomes were captured in one snapshot or movie, the original snapshot or movie was decomposed into each color channel (blue, green, and red). Since we did not use blue fluorescence, we omitted the blue channel image and reassembled red and green channel images by assigning pseudocolors of red and cyan, respectively.
Counting of Encapsulated Microbeads inside Liposomes
The microscopic observation was performed as explained above. Especially for the microfluidic measurements, the movie was captured after the flow of the liposome dispersion and the external solution was shut off to visualize individual fluorescent microbeads. On the other hand, for the bulk measurement, we selected liposomes larger than approximately 5 μm for counting encapsulated microbeads. During the capture of the movie, the focal plane was carefully scanned by hand not to overlook the microbeads out of the focal plane. The captured movie was analyzed with ImageJ. To compare the results, we adopted Mann–Whitney’s U test. The nth data in the group k were ranked (rkn) accordingly. The statistic index (Uk) was calculated as follows
where Nk is the number of data in the group k. We calculated the p-value assuming the normal distribution of Z value defined as follows
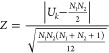 |
Note that for the comparison of MFD-1× and Bulk-1×, we evaluated a two-sided test because a large/small relationship was unknown. On the contrary, for the comparison of MFD-1× and MFD-2×, we adopted a one-sided test since it was obvious from the shape of the obtained distribution that when the two groups had some statistically detectable difference, data of MFD-1× was larger than that of MFD-2×.
Estimation of Water Permeability
Liposomes were arrayed in one observation field as described above. The initial outer solution (350 mM glucose/150 mM sucrose/50 mM Tris–HCl buffer (pH: 8.05)) was then substituted to a hypertonic solution (385 mM glucose/165 mM sucrose/55 mM Tris–HCl buffer (pH: 8.08)). After 5 min, fluorescence images were taken every 30 s. Taken images were trimmed for each trapped liposome. The images were binarized, and their cross-sectional areas were measured by ImageJ. The diameter of each liposome was calculated from the value of their area. In the hypertonic solution, the diameter of the liposome decreased linearly along the time, which was described as follows52
where R is the diameter, t is the time, P is the water permeability, α is the molar volume of water (18 mL/mol), and ΔC is the difference in the concentrations. We calculated the water permeability by the linear regression of the time course of the diameter taken during 480–690 s. Only liposomes having at least four data points were measured during the time range. The calculated permeabilities were presented as mean values ± standard deviations.
Acknowledgments
We thank Hirofumi Yoshida (Institute of Industrial Science, The University of Tokyo) for his advice and support for the fabrication of the microfluidic device. Part of this study was supported by Grant-in-Aid for JSPS Research Fellow (to H.S.; Grant number JP18J22004), the ANRI fellowship (to H.S.), Scientific Research (B) (to T.T.; Grant number JP16H04032), and Platform for Dynamic Approaches to Living System from The Ministry of Education, Culture, Sports, Science and Technology, Japan.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01371.
Stroboscopic fluorescent streamline of microbeads swimming in the size-sorting region; schematic illustration of the nest structure and its terminology and design parameters; performance of the trapping device and the stability of the trapped liposomes; monochronic images for each color channel corresponding to the liposomal membrane and fluorescent microbeads (PDF)
Composite movie of liposome-encapsulated microbeads under a constant flow (MP4)
Composite movie of liposome-encapsulated microbeads after the flow was abruptly stopped (MP4)
Author Contributions
H.S., T.O., S.T., and T.T. conceived and designed the experiments. H.S. performed the experiments, analyzed data, and wrote the original manuscript. All authors discussed the results, edited the manuscript, and gave approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Hartwell L. H.; Hopfield J. J.; Leibler S.; Murray A. W. From molecular to modular cell biology. Nature 1999, 402, C47–C52. 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- Szostak J. W.; Bartel D. P.; Luisi P. L. Synthesizing life. Nature 2001, 409, 387–390. 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- Benner S. A. Defining Life. Astrobiology 2010, 10, 1021–1030. 10.1089/ast.2010.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H.; Toyota T. Toward Experimental Evolution with Giant Vesicles. Life 2018, 8, 53 10.3390/life8040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick R.; Walde P.; Luisi P. L. Light-microscopic investigations of the autocatalytic self-reproduction of giant vesicles. J. Am. Chem. Soc. 1995, 117, 1435–1436. 10.1021/ja00109a031. [DOI] [Google Scholar]
- Kurihara K.; Tamura M.; Shohda K.; Toyota T.; Suzuki K.; Sugawara T. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat. Chem. 2011, 3, 775–781. 10.1038/nchem.1127. [DOI] [PubMed] [Google Scholar]
- Hardy M. D.; Yang J.; Selimkhanov J.; Cole C. M.; Tsimring L. S.; Devaraj N. K. Self-reproducing catalyst drives repeated phospholipid synthesis and membrane growth. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 8187–8192. 10.1073/pnas.1506704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J. M.; Sugiyama H.; Toyota T. Budding and division of giant vesicles linked to phospholipid production. Sci. Rep. 2019, 9, 165 10.1038/s41598-018-36183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noireaux V.; Maeda Y. T.; Libchaber A. Development of an artificial cell, from self-organization to computation and self-reproduction. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 3473–3480. 10.1073/pnas.1017075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.; Noireaux V. An E. coli Cell-Free Expression Toolbox: Application to Synthetic Gene Circuits and Artificial Cells. ACS Synth. Biol. 2012, 1, 29–41. 10.1021/sb200016s. [DOI] [PubMed] [Google Scholar]
- Furusato T.; Horie F.; Matsubayashi H. T.; Amikura K.; Kuruma Y.; Ueda T. De Novo Synthesis of Basal Bacterial Cell Division Proteins FtsZ, FtsA, and ZipA Inside Giant Vesicles. ACS Synth. Biol. 2018, 7, 953–961. 10.1021/acssynbio.7b00350. [DOI] [PubMed] [Google Scholar]
- Murtas G.; Kuruma Y.; Bianchini P.; Diaspro A.; Luisi P. L. Protein synthesis in liposomes with a minimal set of enzymes. Biochem. Biophys. Res. Commun. 2007, 363, 12–17. 10.1016/j.bbrc.2007.07.201. [DOI] [PubMed] [Google Scholar]
- Adamala K. P.; Martin-Alarcon D. A.; Guthrie-Honea K. R.; Boyden E. S. Engineering genetic circuit interactions within and between synthetic minimal cells. Nat. Chem. 2017, 9, 431–439. 10.1038/nchem.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhanu S.; Ueda T.; Kuruma Y. Artificial photosynthetic cell producing energy for protein synthesis. Nat. Commun. 2019, 10, 1325 10.1038/s41467-019-09147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T.; Inoue K.; Koseki K.; Suzuki H. Deformation Modes of Giant Unilamellar Vesicles Encapsulating Biopolymers. ACS Synth. Biol. 2018, 7, 739–747. 10.1021/acssynbio.7b00460. [DOI] [PubMed] [Google Scholar]
- Sakuma Y.; Imai M.; Yanagisawa M.; Komura S. Adhesion of binary giant vesicles containing negative spontaneous curvature lipids induced by phase separation. Eur. Phys. J. E 2008, 25, 403 10.1140/epje/i2007-10307-0. [DOI] [PubMed] [Google Scholar]
- Hotani H. Transformation pathways of liposomes. J. Mol. Biol. 1984, 178, 113–120. 10.1016/0022-2836(84)90234-1. [DOI] [PubMed] [Google Scholar]
- Urakami N.; Jimbo T.; Sakuma Y.; Imai M. Molecular mechanism of vesicle division induced by coupling between lipid geometry and membrane curvatures. Soft Matter 2018, 14, 3018–3027. 10.1039/C7SM02188G. [DOI] [PubMed] [Google Scholar]
- Pautot S.; Frisken B. J.; Weitz D. A. Production of unilamellar vesicles using an inverted emulsion. Langmuir 2003, 19, 2870–2879. 10.1021/la026100v. [DOI] [Google Scholar]
- Pautot S.; Frisken B. J.; Weitz D. A. Engineering asymmetric vesicles. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 10718–10721. 10.1073/pnas.1931005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham A. D.; Horne R. W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660. 10.1016/S0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- Angelova M. I.; Dimitrov D. S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. 10.1039/dc9868100303. [DOI] [Google Scholar]
- Tsumoto K.; Matsuo H.; Tomita M.; Yoshimura T. Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Colloids Surf., B 2009, 68, 98–105. 10.1016/j.colsurfb.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Dominak L. M.; Keating C. D. Polymer encapsulation within giant lipid vesicles. Langmuir 2007, 23, 7148–7154. 10.1021/la063687v. [DOI] [PubMed] [Google Scholar]
- Göpfrich K.; Platzman I.; Spatz J. P. Mastering Complexity: Towards Bottom-up Construction of Multifunctional Eukaryotic Synthetic Cells. Trends Biotechnol. 2018, 36, 938–951. 10.1016/j.tibtech.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazayama Y.; Teshima T.; Osaki T.; Takeuchi S.; Toyota T. Integrated microfluidic system for size-based selection and trapping of giant vesicles. Anal. Chem. 2016, 88, 1111–1116. 10.1021/acs.analchem.5b03772. [DOI] [PubMed] [Google Scholar]
- Paterson D. J.; Reboud J.; Wilson R.; Tassieri M.; Cooper J. M. Integrating microfluidic generation, handling and analysis of biomimetic giant unilamellar vesicles. Lab Chip 2014, 14, 1806–1810. 10.1039/C4LC00199K. [DOI] [PubMed] [Google Scholar]
- Robinson T.; Kuhn P.; Eyer K.; Dittrich P. S. Microfluidic trapping of giant unilamellar vesicles to study transport through a membrane pore. Biomicrofluidics 2013, 7, 44105 10.1063/1.4816712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H.; Osaki T.; Takeuchi S.; Toyota T. Hydrodynamic accumulation of small molecules and ions into cell-sized liposomes against a concentration gradient. Commun. Chem. 2020, 3, 32 10.1038/s42004-020-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocvirk G.; Munroe M.; Tang T.; Oleschuk R.; Westra K.; Harrison D. J. Electrokinetic control of fluid flow in native poly(dimethylsiloxane) capillary electrophoresis devices. Electrophoresis 2000, 21, 107–115. . [DOI] [PubMed] [Google Scholar]
- Gokaltun A.; Yarmush M. L.; Asatekin A.; Usta O. B. Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology. Technology 2017, 5, 1–12. 10.1142/S2339547817300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. W.; Ren X. Q.; Bachman M.; Sims C. E.; Li G. P.; Allbritton N. Surface modification of poly(dimethylsiloxane) microfluidic devices by ultraviolet polymer grafting. Anal. Chem. 2002, 74, 4117–4123. 10.1021/ac025700w. [DOI] [PubMed] [Google Scholar]
- Schneider M. H.; Willaime H.; Tran Y.; Rezgui F.; Tabeling P. Wettability Patterning by UV-Initiated Graft Polymerization of Poly(acrylic acid) in Closed Microfluidic Systems of Complex Geometry. Anal. Chem. 2010, 82, 8848–8855. 10.1021/ac101345m. [DOI] [PubMed] [Google Scholar]
- Hwang S.; Choi C.-H.; Lee C.-S. Regioselective surface modification of pdms microfluidic device for the generation of monodisperse double emulsions. Macromol. Res. 2012, 20, 422–428. 10.1007/s13233-012-0048-8. [DOI] [Google Scholar]
- Jass J.; Tjarnhage T.; Puu G. From liposomes to supported, planar bilayer structures on hydrophilic and hydrophobic surfaces: An atomic force microscopy study. Biophys. J. 2000, 79, 3153–3163. 10.1016/S0006-3495(00)76549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrova L.; Karakashev S. I.; Grigorov L.; Phan C. M.; Smoukov S. K. Wetting properties of phospholipid dispersion on tunable hydrophobic SiO2-glass plates. Adv. Colloid Interface Sci. 2015, 220, 1–7. 10.1016/j.cis.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Huang L. R.; Cox E. C.; Austin R. H.; Sturm J. C. Continuous particle separation through deterministic lateral displacement. Science 2004, 304, 987–990. 10.1126/science.1094567. [DOI] [PubMed] [Google Scholar]
- Inglis D. W.; Davis J. A.; Austin R. H.; Sturm J. C. Critical particle size for fractionation by deterministic lateral displacement. Lab Chip 2006, 6, 655–658. 10.1039/b515371a. [DOI] [PubMed] [Google Scholar]
- Bodas D.; Khan-Malek C. Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment - An SEM investigation. Sens. Actuators, B 2007, 123, 368–373. 10.1016/j.snb.2006.08.037. [DOI] [Google Scholar]
- Lamour G.; Hamraoui A.; Buvailo A.; Xing Y.; Keuleyan S.; Prakash V.; Eftekhari-Bafrooei A.; Borguet E. Contact Angle Measurements Using a Simplified Experimental Setup. J. Chem. Educ. 2010, 87, 1403–1407. 10.1021/ed100468u. [DOI] [Google Scholar]
- Nishimura K.; Suzuki H.; Toyota T.; Yomo T. Size control of giant unilamellar vesicles prepared from inverted emulsion droplets. J. Colloid. Interface Sci. 2012, 376, 119–125. 10.1016/j.jcis.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Morita M.; Onoe H.; Yanagisawa M.; Ito H.; Ichikawa M.; Fujiwara K.; Saito H.; Takinoue M. Droplet-Shooting and Size-Filtration (DSSF) Method for Synthesis of Cell-Sized Liposomes with Controlled Lipid Compositions. ChemBioChem 2015, 16, 2029–2035. 10.1002/cbic.201500354. [DOI] [PubMed] [Google Scholar]
- Natsume Y.; Pravaz O.; Yoshida H.; Imai M. Shape deformation of giant vesicles encapsulating charged colloidal particles. Soft Matter 2010, 6, 5359–5366. 10.1039/c0sm00396d. [DOI] [Google Scholar]
- Natsume Y.; Toyota T. Giant Vesicles Containing Microspheres with High Volume Fraction Prepared by Water-in-oil Emulsion Centrifugation. Chem. Lett. 2013, 42, 295–297. 10.1246/cl.2013.295. [DOI] [Google Scholar]
- Sebastian B.; Favero T.; Dittrich P. S. The Effects of Shear Force Transmission Across Vesicle Membranes. J. Phys. Chem. Lett. 2017, 8, 6128–6134. 10.1021/acs.jpclett.7b02676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honerkamp-Smith A. R.; Woodhouse F. G.; Kantsler V.; Goldstein R. E. Membrane Viscosity Determined from Shear-Driven Flow in Giant Vesicles. Phys. Rev. Lett. 2013, 111, 038103 10.1103/PhysRevLett.111.038103. [DOI] [PubMed] [Google Scholar]
- Woodhouse F. G.; Goldstein R. E. Shear-driven circulation patterns in lipid membrane vesicles. J. Fluid Mech. 2012, 705, 165–175. 10.1017/jfm.2012.118. [DOI] [Google Scholar]
- Chiba M.; Miyazaki M.; Ishiwata S. Quantitative Analysis of the Lamellarity of Giant Liposomes Prepared by the Inverted Emulsion Method. Biophys. J. 2014, 107, 346–354. 10.1016/j.bpj.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher P.; Fischer A.; Luisi P. L.; Oberholzer T.; Walde P. Giant vesicles as biochemical compartments: The use of microinjection techniques. Langmuir 1998, 14, 2712–2721. 10.1021/la971318g. [DOI] [Google Scholar]
- Lande M. B. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J. Gen. Physiol. 1995, 106, 67–84. 10.1085/jgp.106.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensure R. H.; Zeidel M. L.; Hill W. G. Lipid raft components cholesterol and sphingomyelin increase H+/OH- permeability of phosphatidylcholine membranes. Biochem. J. 2006, 398, 485–495. 10.1042/BJ20051620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroske E.; Elwenspoek M.; Helfrich W. Osmotic shrinkage of giant egg-lecithin vesicles. Biophys. J. 1981, 34, 95–109. 10.1016/S0006-3495(81)84839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A. L.; Guedeau-Boudeville M. A.; Marchi-Artzner V.; Gulik-Krzywicki T.; di Meglio J. M.; Jullien L. Shear-induced permeation and fusion of lipid vesicles. J. Colloid Interface Sci. 2005, 287, 298–306. 10.1016/j.jcis.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Chakravarthy S. R.; Giorgio T. D. Shear stress-facilitated calcium-ion transport across lipid bilayers. Biochim. Biophys. Acta 1992, 1112, 197–204. 10.1016/0005-2736(92)90392-Y. [DOI] [PubMed] [Google Scholar]
- Kamiya K.; Kawano R.; Osaki T.; Akiyoshi K.; Takeuchi S. Cell-sized asymmetric lipid vesicles facilitate the investigation of asymmetric membranes. Nat. Chem. 2016, 8, 881–889. 10.1038/nchem.2537. [DOI] [PubMed] [Google Scholar]
- Eyer K.; Paech F.; Schuler F.; Kuhn P.; Kissner R.; Belli S.; Dittrich P. S.; Kramer S. D. A liposomal fluorescence assay to study permeation kinetics of drug-like weak bases across the lipid bilayer. J. Controlled Release 2014, 173, 102–109. 10.1016/j.jconrel.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Runas K. A.; Malmstadt N. Low levels of lipid oxidation radically increase the passive permeability of lipid bilayers. Soft Matter 2015, 11, 499–505. 10.1039/C4SM01478B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba-Bon A.; Pan Y. C.; Biedermann F.; Guo D. S.; Nau W. M.; Hennig A. Fluorescence Monitoring of Peptide Transport Pathways into Large and Giant Vesicles by Supramolecular Host-Dye Reporter Pairs. J. Am. Chem. Soc. 2019, 141, 20137–20145. 10.1021/jacs.9b09563. [DOI] [PubMed] [Google Scholar]
- Shum H. C.; Kim J. W.; Weitz D. A. Microfluidic fabrication of monodisperse biocompatible and biodegradable polymersomes with controlled permeability. J. Am. Chem. Soc. 2008, 130, 9543–9549. 10.1021/ja802157y. [DOI] [PubMed] [Google Scholar]
- Shum H. C.; Lee D.; Yoon I.; Kodger T.; Weitz D. A. Double emulsion templated monodisperse phospholipid vesicles. Langmuir 2008, 24, 7651–7653. 10.1021/la801833a. [DOI] [PubMed] [Google Scholar]
- Deshpande S.; Caspi Y.; Meijering A. E. C.; Dekker C. Octanol-assisted liposome assembly on chip. Nat. Commun. 2016, 7, 10447 10.1038/ncomms10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky N.; Kaduri M.; Zinger A.; Shainsky-Roitman J.; Goldfeder M.; Benhar I.; Hershkovitz D.; Schroeder A. Synthetic Cells Synthesize Therapeutic Proteins inside Tumors. Adv. Healthcare Mater. 2018, 7, 1701163 10.1002/adhm.201701163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K.; Adachi T.; Doi N. Artificial Cell Fermentation as a Platform for Highly Efficient Cascade Conversion. ACS Synth. Biol. 2018, 7, 363–370. 10.1021/acssynbio.7b00365. [DOI] [PubMed] [Google Scholar]
- Luo C.; Hu X.; Peng R.; Huang H.; Liu Q.; Tan W. Biomimetic Carriers Based on Giant Membrane Vesicles for Targeted Drug Delivery and Photodynamic/Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 43811–43819. 10.1021/acsami.9b11223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



