Abstract
Adult stature variation is commonly attributed to differential stress-levels during development. However, due to selective mortality and heterogeneous frailty, a population’s tall stature may be more indicative of high selective pressures than of positive life conditions. This article examines stature in a biocultural context and draws parallels between bioarchaeological and living populations to explore the multidimensionality of stature variation in the past. This study investigates: 1) stature differences between archaeological populations exposed to low or high stress (inferred from skeletal indicators); 2) similarities in growth retardation patterns between archaeological and living groups; and 3) the apportionment of variance in growth outcomes at the regional level in archaeological and living populations. Anatomical stature estimates were examined in relation to skeletal stress indicators (cribra orbitalia, porotic hyperostosis, linear enamel hypoplasia) in two medieval bioarchaeological populations. Stature and biocultural information were gathered for comparative living samples from South America. Results indicate 1) significant (P < 0.01) differences in stature between groups exposed to different levels of skeletal stress; 2) greater prevalence of stunting among living groups, with similar patterns in socially stratified archaeological and modern groups; and 3) a degree of regional variance in growth outcomes consistent with that observed for highly selected traits. The relationship between early stress and growth is confounded by several factors—including catch-up growth, cultural buffering, and social inequality. The interpretations of early life conditions based on the relationship between stress and stature should be advanced with caution.
Keywords: indicators of stress, stunting; middle ages, anatomical method, interdisciplinarity, biocultural
In recent years, genomic analyses have shown that hundreds of genes are likely involved in controlling the expression of stature (Lettre, 2009) and large-scale twin studies have provided high estimates of stature heritability (females: 0.68–0.84; males: 0.89–0.93) among affluent populations (Silventoinen et al., 1993). However, the large number of genes involved implies that growth is not a predefined, immutable process. Rather, skeletal growth outcomes result from complex interactions between an individual’s genetic potential and nutritional and health compromises experienced during growth and development (King and Ulijaszek, 1999). Consequently, while the potential for adult stature may be highly heritable, its expression is contingent upon environmental quality during growth and development.
Biological anthropologists have long observed a general association between a population’s stature and its overall life conditions. Because early life conditions in the past cannot be assessed directly, bioarchaeologists routinely rely on stature as an indicator of overall health status during growth (Saunders and Hoppa, 1993; Larsen, 1997; Hoppa and Fitzgerald, 1999). Exemplary cases of stature variation over time and in relation to major population events (e.g., subsistence shifts, population contact, climate change) have been reported and demonstrated the value of skeletal growth for reconstructing accounts of life in the past (Larsen, 1997). Nonetheless, the relationship between early life conditions and growth is often equivocal (Larsen, 1997; Temple, 2008). In particular, as more information on the biocultural environment experienced by past populations becomes available, the long-held assumption that stature is a clear indicator of early life conditions comes into question. This evidence does not imply that stature has little inferential value; rather, it underlines the need for an improved understanding of the multidimensionality of statural growth variation.
Research in human biology over the past few decades has shown that, except in the case of certain pigmy populations whose short stature has genetic bases and was arguably selected for being advantageous in certain environments (Shea and Bailey, 1996; Perry and Dominy, 2009), in most human groups across the globe reduced growth outcomes are associated with adverse conditions experienced early in life. Starting in the 1980s, many scholars dedicated their efforts to evaluating whether reduction in statural growth was associated with functional impairments or if it represented an adaptive response to limited resource availability. Under this latter view, height reduction would be a no-cost adaptation to suboptimal environmental conditions and individuals would be “small but healthy” (Seckler, 1980). Numerous authors have since demonstrated that a low height-for-age (i.e., stunting) is a major sign of poor health, and is associated with compromised immune competence, poor psychological performance, diminished productivity, reduced reproductive potential, and increased mortality risk (Martorell, 1989; Pelletier et al., 1995; Paajanen et al., 2010). Most importantly, the “small but healthy” debate propelled growth studies toward an improved understanding of the “process of becoming small” (Beaton, 1989) and its long-term consequences in relation to a variety of biocultural factors (Martorell and Habitch, 1986; Messer, 1989).
Today, growth retardation, or stunting, may still affect more than 50% of children younger than 5 years of age in some developing countries (WHO, 2014) and is relatively widespread even among the poorest segments of affluent populations in countries such as the United States (Lewit and Kerrebrock, 1997). Research efforts on this topic have underlined the complex nature of growth processes and their disruptions, and it is clear that stunting may be caused by a variety of factors, including food scarcity, malnutrition, micronutrient deficiencies, parasitic infestations and infectious disease (Martorell et al., 1975; Allen, 1994; Ulijaszek, 1994; Black et al., 2008). Additionally, regardless of their specific nature, the timing, duration and intensity of growth insults may have different long-term impacts on growth (Martorell et al., 1994).
Further complicating the understanding of the mechanisms affecting adult growth outcomes is the potential for catch-up growth, that is, the reversal of earlier growth retardation. Evidence indicates that the potential for catch-up growth depends on several factors, but mostly on age at onset of stunting and age at the time environmental conditions are improved. Catch-up growth has been documented in several populations, but its mechanisms are not entirely known (Martorell et al., 1994). In general, it is thought that in response to improved environmental conditions stunted children can—at least in part—recover from linear growth retardation through increased growth rates and/or delayed maturation (Golden, 1994; Martorell et al., 1994; Gafni and Baron, 2000). However, faster growth rates in an improved environment might actually anticipate maturation, hence reducing the potential for catch-up growth (Proos et al., 1991). Even though the mechanisms by which improved conditions mediate catch-up growth are complex, it appears that developmental plasticity throughout the life course may allow for growth recovery at different phases of growth. In contrast with a previously held notion that allowed for catch-up growth only during early childhood, growing literature on the topic indicates that growth recovery may take place also during pre-adolescent (Adair et al., 1999) and even adolescent, pre-adult (Prentice et al., 2013) years. Overall, the research on growth outcomes and growth retardation shows incontrovertibly that stature is very sensitive to perturbations. However, the complexity of growth and its disruptions implies that stature, as the terminal, measurable outcome of a prolonged and mutable process, may not always allow us to infer growth conditions experienced by individuals and populations. This is clearly problematic for evaluating growth and development in past human groups, which are typically characterized by limited relevant contextual information on nutrition and disease throughout the life course.
Nonetheless, even with a dearth of specific information on environmental quality, it may be argued that being a sensitive indicator of developmental stress, stature undergoes natural selection. It may be selected directly in relation to specific metabolic or climatic adaptations (Perry and Dominy, 2009), or indirectly because it is associated with other undesirable growth outcomes (Martorell, 1989). Under these latter circumstances, when short stature is a correlate of poor health, the expectation is that selection will eliminate short-statured individuals from the population. The implication of this selective pressure is that the survivor group’s stature distribution may not be representative of all variation within a population. In fact, the observed variation, as screened by natural selection, is contingent upon environmental conditions. This implies that, when overall stress is elevated, selection on stature should be more evident due to an overall increase in mortality. In contrast, when overall stress levels decrease, natural selection is lessened and mortality decreases, hence increasing the degree of observable variation in stature (Darwin, 1859). On theoretical grounds, it is possible to predict that under stricter selection the survivor group’s mean stature will be greater than in phases of milder selection. This counterintuitive conclusion was illustrated by Wood and colleagues (1992) in their formulation of the “Osteological Paradox.” When applied to stature, the paradox challenges us to refrain from simplistic conclusions based on absolute bone lengths. Regardless, the study of skeletal growth in past populations is fraught with complications intrinsic to the nature of skeletal assemblages drawn from archaeological contexts, such as small sample size, poor preservation, minimal knowledge of the factors contributing to environmental quality, and composition biases of mortuary samples. Subadult skeletal growth is particularly problematic, because these individuals represent the segment of the population that did not survive stressors experienced during growth and development.
Adult stature, on the other hand, may provide insights into early life conditions of the group who survived the juvenile stage in any death assemblage. However, even the study of adult individuals is fraught with methodological complications, which should be taken into account when making inferences regarding the relationship between stature and life conditions. Specifically, skeletal remains from archaeological contexts are often too fragmentary and incomplete to allow estimating stature using anatomical methods, that is, techniques that estimate living stature as the sum of all its skeletal components and corrective factors for soft tissues (Fully, 1956; Raxter et al., 2006). Instead, most stature estimates are obtained by applying regression formulae based on the relationship between long bones and stature in reference populations. These formulae are easy to use with incomplete remains, but can be quite inaccurate when the body proportions of the reference population and the population being examined differ (Vercellotti et al., 2009 and references therein). Clearly, the use of inadequate regression methods can produce unreliable estimates and any inferences based on them would inevitably be equally unreliable. To address these issues, in recent years there have been notable advancements aimed at improving accuracy in estimating stature from skeletal remains (Raxter et al., 2006, 2007; Auerbach and Ruff, 2010; Petersen, 2011; Ruff et al., 2012; Niskanen et al., 2013).
Conversely, limited theoretical efforts have been made to re-examine the conceptual contributions of stature analyses to reconstructing the human past. In particular, is there evidence for “paradox” in stature variation in the past and, if so, how does this affect our interpretation of skeletal growth outcomes? Specifically, does stature of archaeological populations provide evidence for selective mortality, which may then confound the interpretation of life conditions during growth and development? More importantly, even though the selective model proposed by Wood and colleagues (1992) is certainly possible, it is not clear whether it is probable, at least when stature is concerned. This is an issue that remains largely unanswered (Saunders and Hoppa, 1993).
The incorporation of concepts, hypotheses, and methods of allied social and natural sciences in bioarchaeology has promoted significant advances in the study of past life conditions (Larsen, 2006). Further integration and multidisciplinarity is not only desirable, but required to advance our understanding and interpretation of the human experience in the past. However, interdisciplinarity in physical anthropology is hindered by several factors. First, in spite of common interests in the human condition, specific research agendas differ and tend to pull apart, rather than bring together, colleagues working in different specialties. As a consequence of different perspectives, even on the same topics, it often appears that research endeavors have little appeal or application to subdisciplines that did not generate them. For instance, even though human biologists commonly evaluate growth in terms of deviation from the mean (i.e., through z-scores), similar methods are seldom applied to past populations, hence limiting the possibility of carrying out meaningful comparisons between living and archaeological human groups. Second, different research interests, combined with progressive methodological specialization, lead to parallel bodies of knowledge that, while centered on common themes (e.g., growth), do not allow for direct data comparisons. Third, the nature of academic advancement and funding agencies tend to limit the scope of research projects to more manageable, narrowly focused endeavors. At least insofar as stature is concerned, we argue that an improved understanding of growth in the past can only be achieved by crossing the boundaries between bioarchaeology and human biology. There is much to be gained by collaborating with colleagues working with living populations and by collaboratively framing research questions that might be addressed in both living and archaeological populations. Such integration may allow us to overcome, at least conceptually, some difficulties limiting the study of skeletal populations, namely small sample sizes, poor preservation, and limited availability of contextual information. Because these issues do not equally vex human biological investigations, studies of living populations can provide useful insights and allow us to draw instructive parallels between present and past human biological variation. At the same time, a closer collaboration may also benefit human biologists by providing their research on living populations with a deeper temporal perspective, on a scale that is unlikely to be achieved even through multigenerational longitudinal studies.
This study represents a first effort to bridge the divide between the study of past and present human populations by examining stature data in a broader biocultural context and drawing parallels between archaeological and living populations to explore the multidimensionality of stature variation in the past. Specifically, three research questions are addressed:
Is tall stature associated with low or high stress (inferred from skeletal indicators) in past populations? This question is explored by examining stature in two medieval populations, as well as between socioeconomic status groups in one medieval population.
What can we learn by applying human biology methods to the investigation of growth retardation in skeletal populations? How does growth retardation in archaeological populations compare to that observed in living groups? This question is addressed by comparing the observed prevalence of stunting in two bioarchaeological samples from Europe and three modern samples from South America.
Is the degree of variation in growth outcomes observed in the archaeological and living populations examined in this study the result of comparable selective pressures? Much of the theoretical framework concerning stature variation assumes that this trait is not selectively neutral. However, this assumption is rarely tested in practice, with the risk of reducing the study of growth to unfounded, adaptationist approaches. Therefore, we examine whether variation in growth outcomes provides evidence for selection by estimating FST, a measure of intergroup variation in quantitative traits that can be related to underlying genetic variation (Relethford and Blangero, 1990).
MATERIALS AND METHODS
Archaeological samples
The Middle Ages are known as a time when European populations were exposed to a variety of biological and social stressors including: climate change, population growth, socioeconomic stratification, and complex population dynamics that favored warfare, mobility, interregional trade, and the spread of infectious disease (White, 1962; Dyer, 1994). Thus, the Middle Ages represent an ideal period for exploring the relationship between overall biological stress (as inferred from skeletal indicators) and stature. Two skeletal populations, one from Giecz, Poland, and the other from Trino Vercellese, Italy, were selected for this study based on completeness of the skeletons, wealth of archaeological data, and the existence of known socio-economic variation.
Giecz
The sample from Giecz, Poland, includes 66 (20 female; 46 male) adult skeletons aged 20 to 55 years (mean-= 39.9; SD = 8.4) recovered at the medieval site Gz. 4 (Vercellotti et al., 2009). The settlement is located in the birthplace of the Polish state and has been the object of extensive archaeological investigations starting in the 1950s (Kostrzewski, 1952). Archaeological evidence of farming tools, scales, craft implements, and furnaces with associated metal slag suggests that the local population was mainly engaged in subsistence activities such as farming, smelting, handicraft, and trade (Koztrewski, 1964). Paleocarpological evidence attests to the cultivation of a variety of grains, in particular millet and, to a lesser extent, wheat, rye, and barley (Indycka, 2000). Extensive faunal remains document the presence of domestic animals and the abundance of fish scales and fishing implements suggests that aquatic resources were exploited as well. Paleodietary isotope analyses conducted by Reitsema et al. (2010) indicate that the medieval population inhabiting the settlement had a mainly terrestrial, omnivorous diet. The evidence of faunal remains at the site is consistent with regular meat consumption and the reliance on plants utilizing both C3 (wheat, rye) and C4 (millet) photosynthetic pathways is supported by archaeo-botanical findings (Indycka, 2000). Additionally, Reitsema et al. (2010) identified sex-related differences in diet in the population from Giecz: male diets featured more meat and millet-based foods than female diets. The cemetery followed conventional Christian funerary practices in the deposition and orientation of the bodies; spatial distribution and burial typology tend to exclude the existence of social stratification at the cemetery site and it has been suggested that individuals of higher status were buried elsewhere (Koztrewski, 1964). Preliminary paleopathological examinations revealed high frequencies of environmental stress indicators such as cribra orbitalia, porotic hyperostosis, and linear enamel hypoplasia (LEH). Over 80% of adults exhibited signs of porotic hyperostosis and 50% of sampled teeth were affected by LEH. This pattern suggests that the population was exposed to high levels of environmental stress during growth. No significant differences in the frequency of stress indicators were observed between males and females.
Trino Vercellese
The sample from Trino Vercellese, Italy includes a total of 52 adult individuals of high (6 female; 14 male) and low (14 female; 18 male) socioeconomic status (SES) excavated at the church of San Michele (for more details on status differences and sample composition, see Vercellotti et al., 2011). All individuals were between 20 and 60 years, and no significant differences (P = 0.354) in age distribution were detected between sex and status subsamples. Extensive archaeological studies carried out on the site indicate that the medieval settlement consisted of a fortified village, the economy of which was centered on farming and livestock breeding (Negro Ponzi Mancini, 1999). As the seat of local nobility and clergy, the settlement was also involved in active regional trade. Primary crops were represented by cereals (including wheat, rye, and millet), legumes, and aromatic plants (Negro Ponzi Mancini, 1999). Isotopic analyses revealed that the population had a mainly terrestrial diet, which included regular consumption of animal protein during childhood. Dietary quality differences between low status males and all other samples emerging after childhood were also observed (Reitsema and Vercellotti, 2012), suggesting the existence of cultural practices aimed at buffering children’s diets. The population experienced relatively favorable life conditions without major growth disruptions (Negro Ponzi Mancini, 1999). The prevalence of most stress indicators was found in low frequency; LEH, however, affected all segments of the population, albeit with notable variation by sex and socioeconomic status. Specifically, LEH affected between 31.1% (males) and 41.3% (females) of high SES individuals and more than half (males: 58.0%; females: 52.0%) of low SES skeletons (see Table 1). LEH frequencies show significant differences between high and low SES groups (sexes combined P < 0.01; males P = 0.0001; females P = 0.002).
TABLE 1.
Frequencies of skeletal stress indicators and average stature for males and females from Giecz and Trino Vercellese
| Males | |||||
|---|---|---|---|---|---|
| Giecz | Trino (all) | Trino (high SES) | Trino (low SES) | ||
| CO (%) | 25.4 | 0.0 | 0.0 | 0.0 | |
| PH (%) | 91.8 | 0.0 | 0.0 | 0.0 | |
| LEH (%) | 48.3 | 48.9 | 31.1 | 58.0 | |
| Stature (cm) | 172.4 | 167.3 | 171.1 | 164.4 | |
| S.D. | 7.1 | 5.6 | 4.2 | 4.9 | |
| Females | |||||
| Giecz | Trino (all) | Trino (high SES) | Trino (low SES) | ||
| CO (%) | 44.4 | 0.0 | 0.0 | 0.0 | |
| PH (%) | 87.1 | 0.0 | 0.0 | 0.0 | |
| LEH (%) | 51.9 | 48.8 | 41.3 | 52.0 | |
| Stature (cm) | 157.2 | 152.5 | 154.2 | 151.7 | |
| S.D. | 4.5 | 5.0 | 2.6 | 5.6 | |
The frequency of stress indicators in Giecz was determined from unpublished data collected by HM Justus and AM Agnew as part of the Global History of Health Project. The incidence of stress indicators in Trino Vercellese was derived from data published in Negro Ponzi Mancini, 1999. Analyses are limited to adults of known sex for whom a given trait could be scored. CO = cribra orbitalia; PH = porotic hyperostosis; LEH = linear enamel hypoplasia.
Comparative living samples
The comparative living samples for this study—Ribeirinhos, Makushi, and Cali from South America—were chosen because they experience relatively high levels of stress, conceptually more similar to those experienced by past populations than those found in modern industrialized countries. Additionally, the samples are from the same broad geographic region of South America and, while likely exposed to similar overall ecogeographic environmental factors, they differ in regard to cultural practices, settlement type, and subsistence strategies. These characteristics of the comparative samples allow interpreting patterns of variation in growth outcomes in relation to a variety of biocultural factors. The same anthropometric variables and similarly extensive biocultural information were available for all samples examined. Descriptive statistics for stature in the living comparative samples are reported in Table 2.
TABLE 2.
Descriptive statistics for stature in the comparative living samples
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| All | Stunted | Non-stunted | All | Stunted | Non-stunted | ||
| 160.4 | 153.6 | 164.6 | 146.5 | 143.1 | 150.9 | ||
| S.D. | 7.1 | 5.3 | 4.1 | 5.5 | 4.6 | 3.0 | |
| N | 84 | 32 | 52 | 88 | 50 | 38 | |
| Makushi | 160 | 156.2 | 163.9 | 149.3 | 144.5 | 151.9 | |
| S.D. | 5.5 | 4.7 | 3 | 4.6 | 3 | 2.9 | |
| N | 126 | 63 | 63 | 406 | 145 | 261 | |
| Cali low SES | 152.9 | 145.2 | 155 | ||||
| S.D. | - | - | - | 6 | 2.5 | 4.9 | |
| N | 517 | 110 | 407 | ||||
| Cali mid-low SES | 153.1 | 144.9 | 155.1 | ||||
| S.D. | - | - | - | 5.9 | 2.9 | 4.5 | |
| N | 592 | 118 | 474 | ||||
| Cali high SES | 158.4 | 145.9 | 158.9 | ||||
| S.D. | - | - | - | 5.6 | 1.9 | 5.1 | |
| N | 458 | 17 | 441 | ||||
All measurements are reported in centimeters.
Ribeirinhos
The Ribeirinho (“Amazon river people”) sample is represented by 172 (88 female; 84 male) adults between 18 and 77 years of age (Vercellotti and Piperata, 2012) inhabiting upper-land communities located in and around the Caxiuanã National Forest in the Brazilian state of Para (Piperata, 2007). At the time of data collection, the communities were rural, had no electricity or running water, and only a few households had pit toilets. The majority of households used the forest and river for waste disposal. Water for cooking and drinking was collected from the river or, in a few cases, from hand-dug wells, and trash was burned, buried, or dumped in the river. All the people practiced slash and burn agriculture. Bitter manioc (Manihot esculenta) served as the dietary staple, providing 50% of all energy and 70% of the carbohydrates in the diet (Piperata et al., 2011). Fish and hunted game were the most important sources of dietary protein (42% and 19%, respectively). Açaí (Euterpe oleracea), a local palm fruit, was an important source of calories when in season (30%) (Piperata et al., 2011). In terms of dietary fats, the most important sources were the aça ı fruit, purchased, processed soybean oil, and fish. While the people produced and collected the majority of their food, they were also actively involved in, and dependent upon, the regional market economy. In 2009, a survey carried out in 73 households (greater than 1/3 of households in the region) revealed high levels of perceived food insecurity (Piperata et al., 2013). Mothers’ perceptions of insufficient food were supported by detailed dietary data collected at the household-level (n = 51), which demonstrated that, on average, households only met 75% of their total energy needs. Household-level protein consumption, however, exceeded needs (154%). Data collected on the individual-level were similar. Among women and children, energy intakes proved insufficient (63% and 77%, respectively) to meet needs but protein intakes came close to meeting or exceeded individual requirements (94% and 183%, respectively). No differences in energy or protein adequacy between boys and girls were detected (Piperata et al., 2013).
Access to medical care was limited due to the remote location of the communities, and trips to town for care were limited to emergency situations. Respiratory infections, gastrointestinal problems, skin related issues, and general aches and pains related to heavy labor and/or arthritis were the most common causes of morbidity. Overall, Ribeirinhos are subject to chronic stress, which has been associated with nutritional stress during growth and development and poor access to healthcare (Silva and Crews, 2006).
Makushi
The Makushi sample is composed of 532 (126 male; 406 female) Makushi Amerindians inhabiting 11 communities in the North Rupununi region of interior British Guyana (Wilson et al., 2011). All individuals were between 19 and 82 years. The Makushi lived in villages located on the savannahs and maintained farms in nearby forests. Houses were built with wattle-and-daub or clay bricks and had thatched roofs (Forte, 1996). High infant (93/1000 live births) and child (169/1000) mortality suggests that living conditions during the early growth period are challenging (Wilson et al., 2006). Subsistence practices were relatively autarkic, and consisted of slash-and-burn agriculture used to cultivate high-cyanide varieties of manioc. Compared to other societies, the Makushi had low dietary diversity (Nagel et al., 2014), an indication of an inadequate diet (Kant, 1996; Hatløy et al., 1998). An examination of nutrient intake in diets of Makushi children and women in two of the 11 villages, showed that major energy sources were represented by manioc (47%), cereals/grains (12%), nuts (11%), (fish 9%), fruits and vegetables (8%), sugar (7%) and, to a lesser extent, meat and dairy products (5%) (Palmer, 2009). Fish was the major source (49%) of protein in the diet, followed by nuts (12%), domestic meat (11%), cereal and grains (10%), and manioc (7%). Overall, the children’s annual median percent of daily recommended intake of calories was 91.1%, while it was 99.2% for proteins, 30.8% for fats, and 126.9% for carbohydrates. Additionally, Palmer (2009) observed that children’s intake of certain micronutrients, such as zinc, iron and vitamin A, in these communities was low in relation to recommended intake. Although detailed data collection over a relatively long period of time is required to develop accurate estimates of micronutrient intake, micronutrient intake among Makushi children was interpreted to be inadequate to the point of being potentially harmful to growth and immunocompetence (Wilson et al., 2011). Women’s diets were on average similar to those of the children, but included a greater proportion of manioc and less fruit and vegetables. Drinking water was drawn from rivers, streams and wells and rarely boiled (Wilson et al., 2006). Water sources were frequently contaminated by organic pollutants and diarrhea and parasitic infestations were widespread (Wilson et al., 2011). Additionally, malaria and dengue fever were endemic in the villages located along rivers. Even though all villages had access to a health post and a community health worker trained in diagnosing and treating common ailments, the health posts were often without supplies.
Cali
The Cali comparative sample includes 1568 women (age range: 18–44 years) living in Cali, Colombia (Dufour et al., 1994). These women were assigned to three SES groups (Low = 517; Mid-Low = 592; High-= 458) based on barrio (neighborhood) of residence (Dufour et al., 1994). Rapid urbanization in the 1960s and 1970s led to concentric growth around the city center, accompanied by an increase in social stratification and inequality. At the time of data collection, low SES barrios were peripherally located, had unpaved streets, lacked access to public transportation, and some housing was precarious in nature. Indeed, some houses were either under construction or built with temporary, recycled materials. Many homes lacked access to water, electricity, telephone lines, and sewage and waste disposal services. However, in several cases the residents of the barrios had devised “unofficial” connections to water and electric sources (Dufour, 1994). Mid-low SES barrios were typically older settlements with some paved streets and access to public transportation. Houses in these neighborhoods were typically made of bricks or cement blocks and had water, electricity, and sewerage connections through the city; few homes had telephone access (Dufour et al., 1994, 1997). In contrast, high SES barrios were characterized by private homes or condominiums, benefitted from all city services including phones, and had access to city parks and green zones (Dufour et al., 1994). Striking differences among SES groups were also evident in terms of education and employment (Dufour et al., 1994), which resulted in variation in levels of food security with the lowest SES group being the most food insecure (Dufour et al., 1997).
In fact, while food availability and intake among high SES women was not restricted and likely more than adequate to meet their energetic needs, access to food among poorer groups was inconsistent and limited by financial circumstances (Dufour et al., 1997). Among Low and Mid-Low SES women included in this study, protein accounted for 11.6%, carbohydrate 70.1%, and fat 18.3% of total energy intake. Primary energy sources were white rice (23.0%), sugared beverages (14.3%), white bread (7.5%), beans (6.7%), beef (6.1%), and plantains (4.4%) (for detailed dietary information see Dufour et al., 1997).
Even though detailed information on migration in the region is not available, it is possible that a portion of the poorest segment of the population (Low SES) may have consisted of recent migrants, who arrived in Cali as adults. Most migrants were originally from Tumaco, a city on the Pacific coast that was afflicted by an earthquake in 1979. The population in Tumaco was primarily Afro-Colombian and the diets in that region are known to include more fish than is seen in the typical Cali diet. The staple carbohydrate in Tumaco was rice, which is similar to the rest of the country. Other migrants originated from rural areas in the central Magdalena River valley and the llanos, which they left to escape the violence in their home regions. Diets in these rural regions were likely not significantly different than those of the non-migrant urban poor (Swanberg and Shipley, 1975). The mid-low and high SES samples did not include many, if any, migrants. Overall, the presence of migrants in the poorest segments of Cali’s population is unlikely to have had a major impact on the representativeness of the Low SES sample.
Access to healthcare was available to the majority of the population through government-sponsored health posts located in the barrios, even though high SES individuals could take advantage of the services provided by private clinics. In general, Cali’s inhabitants suffered from a variety of gastrointestinal and respiratory ailments, which were found to be more severe among the poorer segments of the population (Koopman, 1978; Wilson et al., 1999). Specifically, Koopman (1978) reported prevalence rates among low SES school age children for diarrheal disease (18.9%), vomiting (14.3%), colds (50.6%), and head lice infestations (39.1%). Both diarrhea and vomiting were associated with unhygienic conditions in the schools and, to a lesser extent, crowding. In a study of parasitic burden among boys living in Cali and surrounding rural communities, Wilson and colleagues (1999) reported that 63% were affected by at least one species of gastrointestinal parasite. Significant differences were observed between SES groups in terms of overall infection prevalence (high SES: 54%; low SES: 68%; P < 0.001) and prevalence of polyparasitic infections (high SES: 19%; low SES: 34%; P < 0.001). These results are consistent with previous investigations carried out in Cali, which revealed the persistence of parasitic infestations into adulthood (Faust, 1958; Faust and Giraldo, 1960). No differences were observed between Low SES boys from urban and rural environments (Wilson et al., 1999). Even though the majority (80–95%) of infected boys had light parasite loads, Wilson and coworkers (1999) detected significant associations between parasite infections and reduced growth outcomes.
Methods
For the skeletal populations, sex and age-at-death of all individuals included in the study were estimated using standard osteological methods (Buikstra and Ubelaker, 1994).
In this study, only anatomical stature estimates were employed. To this end, all elements contributing to living stature (cranial height, vertebral heights between C2-S1, bicondylar femur length, tibia condylo-malleolar length and the height of talus and calcaneus in articulation) were measured according to the method devised by Fully (1956) and modified by Raxter and colleagues (2006, 2007). Skeletal height was calculated as the sum of all elements above and living stature estimates calculated from skeletal height (Raxter et al., 2007).
Frequencies of skeletal stress indicators (cribra orbitalia, porotic hyperostosis, LEH) in medieval Giecz and Trino Vercellese were obtained from the literature or calculated from data provided by the curators of the collections (Table 1). These stress indicators are commonly employed by bioarchaeologists to assess past populations’ stress levels during growth and development. Cribra orbitalia and porotic hyperostosis are porotic lesions of the orbital vault and outer cranial table considered to be indicators of anemic conditions of diverse etiologies suffered during childhood and adolescence (Walker et al., 2009). Linear enamel hypoplasias are linear grooves observed in the crown of teeth that suffered from interruptions in the formation of dental enamel (amelogenesis) (Goodman and Rose, 1990). Even though these indicators do not provide detailed information on the nature and severity of stress episodes at the individual level, they are suggestive of the overall stress levels experienced by the group during the earlier years of life. In general, the use of multiple indicators allows inferring different aspects of early life conditions of past populations (Goodman and Martin, 2002). In our analyses, the frequencies of these skeletal stress indicators were used to determine differences in overall stress during development in the two populations examined for the purposes of comparing anthropometric data between more or less stressed groups.
On the living populations, anthropometric measures included: total stature and sitting height measured to the nearest 0.1 cm following standard methods (Lohman et al., 1988). Subischial leg length was calculated as stature minus sitting height.
Data analysis
The existence of significant differences in stature between the bioarchaeological samples was tested using the Mann–Whitney or Kruskal–Wallis tests. Because males and females exhibit sexual dimorphism in stature, all comparisons were limited to same-sex samples.
The comparison of growth outcomes between bioarchaeological and living populations was achieved through the calculation of z-scores and the identification of individuals exhibiting growth retardation. The calculation and evaluation of height-for-age z-scores (HAZ) is common practice in human biology, as it allows the detection of adverse long-term consequences of stress during early life. Growth retardation, also referred to as stunting, was defined as a z-score falling below −2 (WHO, 1995), which characterizes individuals whose growth is outside the normal range of variation. Individual HAZ scores were calculated using the National Health and Nutrition Examination Surveys (NHANES III) reference values provided in Frisancho (2008).
To test whether growth outcomes in the groups examined were suggestive of selective pressures, we examined the degree of regional (Medieval Europe, Northern South America) variation in growth outcomes through the estimation of Wright’s FST statistic from quantitative traits using the Relethford and Blangero (1990) method. In its canonical definition, FST is a measure of genetic variance calculated as the ratio between intergroup variation and total variation expected under conditions of panmixia. Adopting an equal and additive effect model of polygenic inheritance and assuming total heritability, the Relethford–Blangero method allows for the calculation of a minimum estimate of FST from the phenotypic variance–covariance matrix. The statistic reflects the relative apportionment of variance at different hierarchical levels and ranges between 0% (no variation) and 100% (complete variation). This is due to the fact that selective processes tend to reduce a trait’s heterozygosity and consequently constrain phenotypic variance in populations exposed to specific pressures. Selectively neutral traits, such as cranial morphology and blood polymorphisms, typically show a much greater degree of variation at the local level (i.e., regional, within population) than at a larger scale, with values of FST around 85% (Relethford, 2002). In contrast, traits subjected to high selective pressures such as skin color, show an opposite pattern characterized by limited variability (9%) at a local scale (Relethford, 2002). The Relethford–Blangero model has been employed to explore the relative degree of selection of different quantitative traits, including craniometrics (Relethford, 1994), pelvic geometric morphometrics (Betti et al., 2013), and long bone lengths (Kudaka et al., 2013). In this study, the Relethford–Blangero method was applied to skeletal segment lengths (skeletal trunk height, femur, tibia) for archaeological populations, and to stature, sitting height, and leg length for living populations. Because actual heritability estimates were not available for all groups examined and population differences in stature heritability may exist (Luke et al., 2001), a conservative approach to estimating FST was adopted, and complete heritability and equal census size were assumed in the analyses. If growth outcomes are indeed subject to selective pressures, then it is expected that the apportionment of variance at the regional level will be relatively modest and similar to that observed for highly selected traits. Statistical analyses were performed using SPSS 19.0, and Microsoft Excel 2007. Statistical significance was defined as P ≤ 0.05.
RESULTS
Stress indicators and stature
Skeletal stress indicators suggested that individuals from different sites, as well as individuals of different socioeconomic status, were subject to different levels of stress. Overall, the individuals from Giecz represented the most stressed group, followed by low SES individuals from Trino Vercellese. Consistent with expectations, the high SES group from Trino Vercellese exhibited the lowest frequencies of skeletal stress indicators (Table 1).
The analysis of stature in the two bioarchaeological populations revealed a mixed pattern: tall stature appeared to be associated with both greater and lower stress levels. Indeed, individuals from Giecz exhibited the greatest average stature (males cm, SD = 7.1; females cm; SD = 4.5) and the greatest prevalence of stress indicators (90% of adults had porotic hyperostosis; 51% had LEH). Individuals from Trino Vercellese (SES groups combined) exhibited overall shorter stature (males cm, SD = 5.6; females cm, SD = 5.0) and lower prevalence of stress indicators (no porotic hyperostosis, 50% LEH). The difference in the stature distributions between Giecz and Trino males (status subsamples combined) was significant (P = 0.003). When comparisons were carried out on social status subsamples, no significant differences were found between males from Giecz and high status Trino males (P = 0.801), while significant differences were found between Giecz males and low status Trino males (P ≤ 0.001). Similar results were found in the female subsamples, where stature distributions were significantly different between Giecz females and all females from Trino (P = 0.015), as well as Giecz females and low status Trino females (P = 0.011). No differences (P = 0.921), however, were detected between Giecz females and high status Trino females. Interestingly, within the Trino population, significant (P = 0.001) differences in stature and LEH were found between high and low status males; no differences were found between female subsamples for stature, but there were differences in LEH (P = 0.002). In this case, greater stature was associated with lower stress (Table 3).
TABLE 3.
P values for comparisons of stature
| Comparison | Males | Females |
|---|---|---|
| Giecz vs. Trino all | 0.003 | 0.015 |
| Giecz vs. Trino high SES | 0.801 | 0.921 |
| Giecz vs. Trino low SES | 0.001 | 0.011 |
| Trino high SES vs. Trino low SES | 0.001 | 0.326 |
Highlighted P values are significant at the 0.05 level.
Growth retardation
Stunting prevalence and average HAZ for the archaeological and living samples are reported in Table 4 and Figure 1. These results revealed the presence of very few stunted individuals in the bioarchaeological populations. Only 4% of males and no females from Giecz were classified as stunted; average HAZ values were only slightly negative (males: −0.38; females: −0.62). In Trino Vercellese, differences between SES groups were striking: no high status individuals were classified as stunted, while 23% of low status individuals had z-scores falling below −2. Clear differences in average HAZ were also visible across SES groups: high status individuals had average scores (males: −0.52; females: −1.00) that were less negative than those observed among low status individuals (males: −1.40; females: −1.39). Neither prevalence of stunting nor average z-scores differed significantly between low status males and females, while high status males had average HAZ scores that were lower than their female counterparts.
TABLE 4.
Prevalence of stunting and average HAZ by sex and socioeconomic status
| Sample | Percent stunted | Average Z-score | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Giecz | 4 | 0 | −0.38 | −0.62 |
| Trino Vercellese, | 22 | 23 | −1.40 | −1.39 |
| low SES | ||||
| Trino Vercellese, | 0 | 0 | −0.52 | −1.00 |
| high SES | ||||
| Ribeirinhos | 38 | 57 | −1.88 | −2.17 |
| Makushi | 50 | 36 | −1.97 | −1.73 |
| Cali low SES | - | 21 | - | −1.24 |
| Cali mid-low SES | - | 20 | - | −1.21 |
| Cali high SES | - | 4 | - | −0.44 |
Fig. 1.
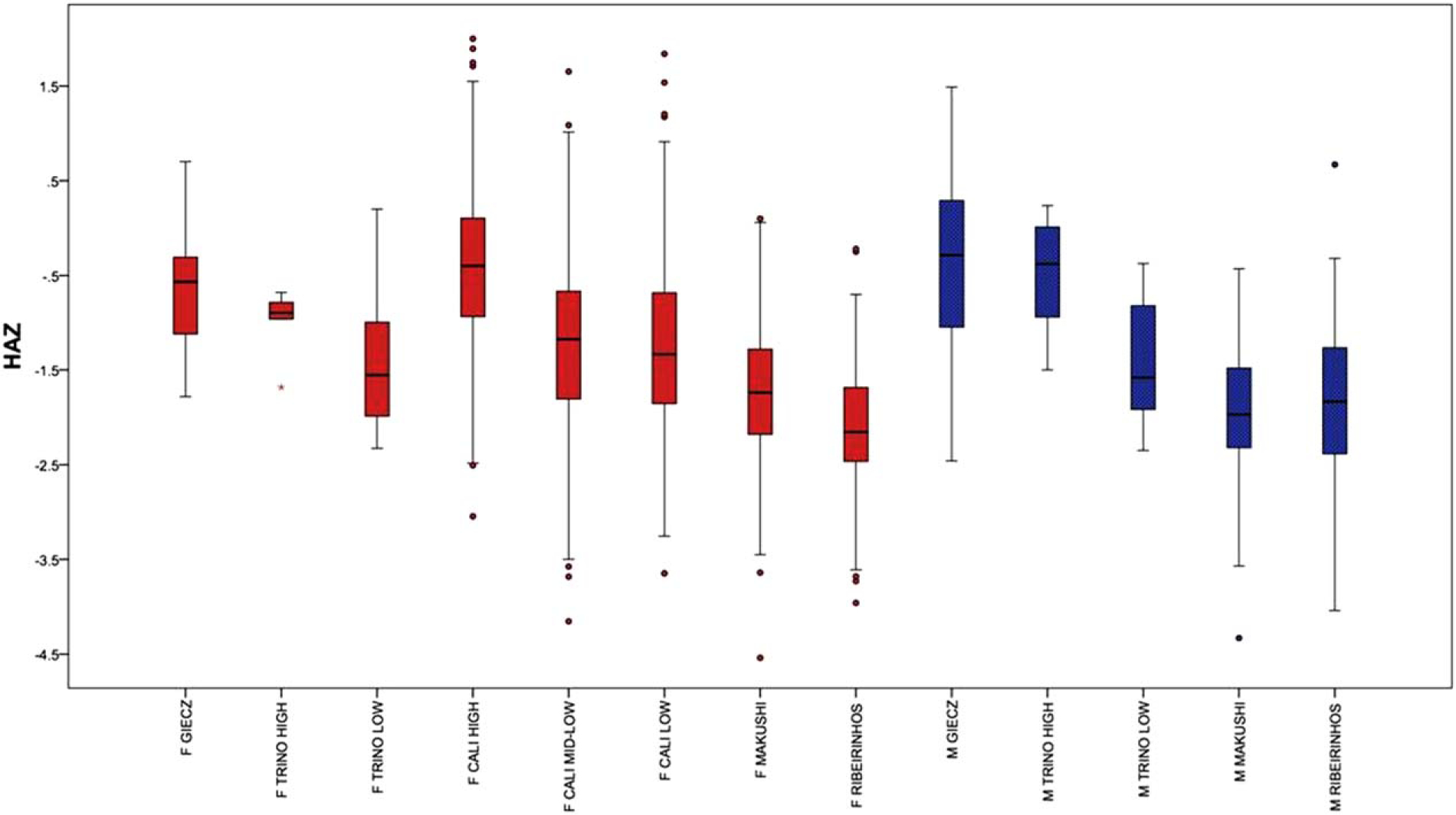
Distribution of height-for-age z-scores (HAZ) in archaeological and living samples (F = female; M = male).
The overall prevalence of stunting among Ribeirinhos was 48% and the mean HAZ for the combined sex sample was −2.03. The mean HAZ in the male and female sub-samples were −1.88 and −2.17, respectively. A significantly higher rate (χ2 = 5.31; P = 0.02) of stunting was detected in females (57%) compared to males (38%). Among the Makushi, 39% of the sample were stunted. Sex differences were evident, with males exhibiting higher rates of stunting than females (males: 50%; females: 36%; χ2 = 7.65; P = 0.006). However, average z-scores did not differ significantly between the sexes (males: −1.97; females: −1.73). Overall, the rate of stunting in the Cali population was 16%, but there were clear differences in the prevalence of stunting between SES subsamples. Among the High SES group the rate of stunting was only 4% while it was 20 and 21% in the mid-low and low SES samples. Average HAZ scores were −0.44 for the High SES sample, −1.21 for the mid-low SES sample, and −1.24 for the Low SES group.
Apportionment of variance at the regional level
The minimum estimate of FST at the regional level for the medieval populations (distinguishing status subsamples) was 6.3%, while the minimum estimate for the living samples was 20.9%. As expected, these values suggested that the variables examined were not selectively neutral, and in fact subject to notable selective pressures.
DISCUSSION
Stature and stress in two medieval populations
Interpopulation comparisons.
The relationship between stature and stress indicators in the two bioarchaeological populations highlights that the interpretation of early life conditions based on stature alone should be advanced with caution. Indeed, the population characterized by greater frequencies of stress indicators (Giecz) is also the one exhibiting taller stature in both the male and female subsamples. These results are contrary to expectations based on the generally held association between stress and stature. This finding does not imply that stress has no impact on terminal growth outcomes, but rather points to the fact that the stature of the surviving group might be skewed due to high selective pressures that eliminated short-statured individuals. In support of this interpretation, Agnew and colleagues (2007) found evidence for early high mortality of short-statured individuals in this population. In examining the relationship between dental development and long bone length, they observed that in terms of height-forage, the Giecz subadults consistently fell below the modern reference standard 10th percentile. In the adults, a strong, significant positive correlation existed between age and stature in both males (r = 0.929; P < 0.000) and females (r = 0.837; P < 0.000). This result is consistent with results obtained from large bioarchaeological samples (Kemkes-Grottenthaler, 2005) and supports the notion that short stature may be reflective of systemic functional compromises related to poor environmental conditions experienced during growth and development. Based on this evidence, tall stature in Giecz would in fact be a “paradox,” indicative of high selection rather than positive life conditions. However, this interpretation alone does not explain all of the evidence. In particular, it is important to point out that the environment must have been able to support the survivor group’s catch-up growth that led, in the end, to tall adult stature. It is generally held that catch-up growth can only occur if environmental quality improves (Golden, 1994). Assuming that early stressors would not affect older individuals due to either immunological or cultural buffers, it is conceivable that survivors in Giecz experienced catch-up growth if provided adequate nutrition. Isotopic data for Giecz (Reitsema et al., 2010) suggest the consumption of abundant animal protein at least during adulthood. Even though isotopic data on subadults’ diets in Giecz are not available, if the same amount of animal source food were made available to children or adolescents then they would have been able to experience catch-up growth following earlier growth retardation.
Interestingly, while significant differences in stature were found between the two archaeological populations, such differences were mainly due to the inclusion of low status individuals from Trino Vercellese in the analyses. This result was unexpected, because the differences in stress indicators between high SES Trino males and Giecz males were the most pronounced. It would thus appear that tall stature might be at the same time the result of favorable life conditions (low selection) and greater environmental stress (high selection). As a consequence, it can be argued that stature alone is not a reliable indicator of environmental conditions during growth, at least when catch-up growth might play a role in determining terminal growth outcomes. An instructive example of how terminal stature may not reflect stress levels is provided by the work of Steckel (1987). Steckel (1987) used nutritional data and historical records to explain the anomalous growth patterns of American slaves, who were characterized by extremely short stature during childhood and almost average stature in adulthood. Had the analysis been limited to adults, positive nutritional status throughout development could mistakenly be inferred. These results point to the importance of complementing the interpretation of stature with information on the biocultural context of a population. Stature may not be a good indicator of positive or negative environmental quality per se, but may be used to improve the understanding of specific conditions and life histories of individuals and populations. In this regard, whenever possible it is instructive to examine stature variation between subsamples of a population, which—in spite of many possible socioeconomic differences—have many ecological and cultural factors in common.
Intrapopulation comparisons.
In Trino Vercellese, when social status was taken into consideration, stress indicators and stature were in agreement. High status was associated with lower LEH and taller stature among males; no stature differences were found among females, but significant differences in LEH suggest that low status females experienced worse environmental conditions. The lack of stature differences between the female subsamples may therefore be attributed to a number of other factors, including small sample size or biological buffering. For example, Reitsema and Vercellotti (2012) examined life history changes in diet in the population and observed that childhood diets did not vary significantly by sex and status, suggesting that children’s dietary intakes may have been buffered, that is, adults made conscious efforts to ensure that children would have adequate nutrition. In contrast, the isotopic data indicated status differences in diet among adults. Low-status adult males differed considerably from women and high-status males in that they consumed diets with more millet and less meat, a difference that developed after childhood. Overall, when the evidence from Trino Vercellese is considered in its entirety, it appears that under environmental conditions of relatively low stress, selective mortality may not have been as important. In support of this interpretation, no significant association was found between age at death and stature in any of the sex and status subsamples, suggesting that even shorter individuals did not necessarily suffer from functional impairment as a result of poorer early life conditions.
It should be noted that differences in stress indicators were taken into consideration at the aggregate level, which were employed simply to define overall stress levels during development. No analyses on associations between individual stature and stress indicators were carried out, as not all variables were equally available for each individual. Based on available information, it is unclear whether any significant associations of this kind would be found. For instance, Temple (2008—and see also references therein) did not find any association between LEH episodes and stature, suggesting that the acute stress episodes leading to hypoplastic defects might not have had an impact on adult stature, possibly due to the confounding effects of catch-up growth on terminal growth outcomes.
Growth retardation in bioarchaeological and living populations
The contextual examination of growth retardation in both bioarchaeological and living groups provides an opportunity to draw useful parallels between past and present groups’ variation, and to incorporate biocultural information in the interpretation of the patterns observed.
Sex-related differences
Our analyses showed clear differences and similarities in the pattern of growth retardation exhibited by different groups in relation to sex and socioeconomic status. The Amazonian groups exhibited significant differences in the prevalence of stunting between the sexes: fewer Ribeirinho males were stunted than their female peers, while the opposite pattern was found among the Makushi. These findings may be explained in terms of cultural norms and practices in these two populations. Indeed, even though the Ribeirinhos did not explicitly favor their sons over their daughters, customs related to food allocation, mobility, and sexual behaviors during adolescence tended to limit catch-up growth opportunities for females (Vercellotti and Piperata, 2012). Conversely, the Makushi traditionally practice matrilocal, cross-cousin unions, which places higher social value on female offspring (Wilson et al., 2011). It is therefore conceivable that girls were accorded preferential treatment and benefited more from improved conditions than their male siblings, ultimately lowering the number of stunted females in the population. In this regard, it is interesting to note that, while differences did not reach significance, Nagel et al. (2014) did observe greater dietary diversity among Makushi girls than boys during infancy and early childhood in the same communities examined in this study. It is possible that such sex-related differences were more pronounced when the individuals included in the study were growing up, at a time when the Makushi inhabiting the North Rupununi region experienced greater isolation (Baines, 2005). In contrast to what was observed among the living groups, sex differences in stunting were not detected in the bioarchaeological samples. This finding may suggest more egalitarian child-raising practices in these communities. While sex-related dietary differences have been detected among adult individuals from Giecz (Reitsema et al., 2010), skeletal and isotopic data may suggest that boys and girls experienced similar dietary conditions. Nonetheless, because of the limited number of stunted individuals in Giecz, it is impossible to exclude the existence of significant sex-related differences in growth retardation. However, the low prevalence of stunting in this sample is, in itself, noteworthy. The almost absolute absence of stunted individuals in Giecz, combined with contextual information indicative of high stress levels, suggests that growth retardation in this group might have been so severe as to be lethal for most stunted individuals, or that catch-up growth opportunities, possibly supported by improved post-childhood conditions, were common.
Skeletal growth outcomes would suggest that such catch-up growth opportunities were available to both males and females, even though lower female HAZ values may indicate that reproductive costs might have limited the extent of catch-up growth among women, which would be similar to observations made among Ribeirinhos (Vercellotti and Piperata, 2012). A similarly egalitarian pattern in the prevalence of stunting was also detected in the population from Trino Vercellese. Even though status-related differences existed, no differences were detected between the sexes. Nonetheless, it should be noted that high status males had the highest HAZ in this population, suggesting that they might have benefited from better life conditions during growth. This notion is consistent with expectations for stratified patriarchal societies and is supported by analyses of body proportions suggesting differential opportunities for catch-up growth during adolescence (Vercellotti et al., 2011).
Status-related differences
The incorporation of stratified groups in this study provides insights on the effects of differential access to resources on skeletal growth outcomes in past and present societies. In Trino Vercellese, no high SES individuals of either sex were stunted, while about 23% of low SES males and females were. The fact that this group experienced less severe levels of chronic stress (as suggested by lack of anemic and metabolic bone diseases), combined with stature data, suggests that while some segments of the population may have experienced suboptimal conditions, they might not have been so severe as to be lethal. Additionally, analyses of dietary life histories in all segments of this population indicate that people enacted practices to improve the perceived quality of children’s and women’s diet, regardless of their status (Reitsema and Vercellotti, 2012). In this regard, it is interesting to observe a parallel between Trino Vercellese and Cali. Dufour and colleagues (1997) demonstrated that low SES women in Cali adopted a variety of coping strategies to reduce the negative impacts of household food insecurity. While such strategies did not completely buffer individuals from low energy intakes on a daily basis, they appear, over the long-term, to have allowed women to meet their energy needs. One example is where women’s intakes exceeded their needs in times of plenty (feast) and then were reduced when availability was low (fast) (Dufour et al., 1997). A similar pattern of consumption, characterized by alternate states of “feasting and fasting,” was noted in the European medieval populace (Montanari, 1994). Similar rates of stunting among low SES groups in both populations (~20%) may attest to similar stressors during childhood. In a way, the conditions of poor sanitation, crowding, and precarious access to food experienced by individuals living in the low SES barrios in Cali were likely similar to those that characterized the conditions experienced by medieval peasants, even though lower HAZ values in Trino may indicate somewhat higher levels of stress in that group. In stark contrast, a clear buffer of high SES individuals’ growth is evident in both Trino and Cali, in agreement with preferential access to resources by more affluent groups.
Setting-related differences
The striking intergroup differences in growth retardation observed in this study highlight the importance of setting-specific conditions in determining terminal growth outcomes. By complementing growth data with contextual information, it is possible to highlight some major differences between the settings examined here.
The prevalence of stunting in Trino Vercellese and Cali departs from the very high levels of stunting observed among rural Amazonians (Ribeirinhos, Makushi), suggesting overall better access to resources in agglomerated settings. For example, in terms of dietary energy, Cali women, despite their low SES, had adequate intakes (Dufour et al., 1997). Furthermore, isotopic data for Trino Vercellese and dietary information for Cali indicate consistent access to high-quality, animal-source protein. In addition to being high-quality sources of protein, these foods also provide other key nutrients important for growth and development such as vitamin A, vitamin B12, folate, iron, and iodine (Black et al., 2008). The importance of animal-source foods in children’s diets is supported by the strong association between their consumption and statural growth in several rural groups in developing countries (Allen et al., 1992; Marquis et al., 1997; Leonard et al., 2000). In contrast, the diets of the rural Amazonian groups appear less suited to meet growing children’s nutritional demands. In terms of energy, Piperata and colleagues (2013) observed that only 12% of Ribeirinho households were able to meet or exceed their daily energy needs. Individual-level data on Amazonian women and children also revealed inadequate energy intakes (63% and 77%, respectively). Access to high-quality protein (fish, game meat) in these Ribeirinho communities was, however, sufficient. At the household-level, protein availability exceeded need (154%). Children’s daily protein intakes also well exceeded their needs (183%). Mothers’ intakes, while less adequate, came close to meeting their daily protein needs (94%). The dietary data indicate that the high rate of stunting in these Ribeirinho communities is likely due to chronically inadequate energy intakes and a diet that is both bulky (high fiber) and low in fat. Piperata (2007) has argued that the bulky nature of farinha (toasted manioc meal) may lead to children feeling satiated before consuming enough food to meet their energy and micronutrient needs. In addition, lack of access to medical care often meant that chronic, low-grade infections and parasitic burdens went untreated which, due to the energetic costs of immune activation, may be further undermining growth in these rural com munities. Similarly, Wilson and coworkers (2011) noted that Makushi children’s diets did not meet nutritional requirements for fat and several important micronutrients, due primarily to the consumption of low-energy-density foods. Even though mothers in both rural communities enact feeding practices aimed at buffering child food intake (Wilson et al., 2011; Piperata et al., 2013), growth outcomes suggest that mild-to-moderate malnutrition may be more prevalent in these settings than in Trino Vercellese and Cali.
Based on the evidence reviewed in this study, the life conditions experienced in medieval Giecz appear very different from those of all other groups examined. In particular, even though isotopic data (Reitsema et al., 2010) indicate that animal-source foods were regularly consumed by adults in Giecz, it is not known to what extent and/or at what time in life such foods were introduced into children’s diets. The high prevalence of cribra orbitalia and porotic hyperostosis in this sample suggests that during growth the majority of the population suffered from anemia or other metabolic disorders caused by micronutrient deficiencies (Walker et al., 2009). In a way, the much higher rates of stunting observed among the other groups suggest that while stressors exist and may be important, selective pressures on these populations may be more relaxed than those experienced in medieval Giecz. Alternatively, medieval populations might have had greater opportunities for catch-up growth than the living groups examined. In this regard, comparisons of body proportions in these populations (Vercellotti, 2012) suggest that lower levels of stunting observed among medieval groups may be related to different stressors experienced throughout life, selective mortality, and differential opportunities for catch-up growth. For instance, it is possible that infectious disease epidemics, well documented as a major cause of mortality during the Middle Ages, were a predominant cause of growth retardation in the past, while chronic malnutrition may be the primary growth limiting factor in modern populations. Clearly such a dichotomous distinction between stressors in unlikely, but it serves this discussion well.
Even though both disease and malnutrition may have similar (and additive) effects on the growing body, it is possible to imagine important differences in their long-term effects on growth. First, infectious diseases may be contracted in spite of overall good nutrition. Second, infectious diseases (with the exception of parasitic infestations) tend to be acute episodic stressors with an impact on survival rather than long-term growth outcomes (obviously multiple acute episodes could have effects similar to those of prolonged chronic stress). Third, infectious diseases are probabilistically more likely to affect the growing body in earlier phases of life, when the immune system is still immature and na€ıve. Therefore, provided that an individual can survive earlier disease episodes, as he/she grows his/her likelihood of contracting the same diseases decreases. Consequently, if other environmental conditions, nutrition in particular, are favorable, upon surviving diseases individuals may experience catch-up growth. The net result of this is that the most susceptible individuals die off and do not grow up to become stunted individuals.
In contrast, moderate malnutrition has the tendency to be a chronic stressor that exerts its action on the growing body for a prolonged time. The causes of malnutrition may be multiple and may involve not only dietary nutrient restriction, but also parasitic infestations. Except for the most extreme cases, malnutrition is not lethal, but can also compromise growth to a varied extent (Chen et al., 1980; Wachs, 1995; Black et al., 2008). Additionally, unless its causes are eliminated, which is rarely the case, malnutrition is a long-term stressor experienced throughout the entire period of growth. Under these circumstances, individuals may not die or experience catch-up growth and thus grow up to be stunted adults. Obviously, infectious disease and malnutrition are likely to co-occur to various extents in most human populations, but their relative prevalence may lead to specific interactions between the growing body and the environment, hence altering growth patterns in predictable ways. It is possible that the importance of infectious disease and malnutrition varies in relation to ecological and climatic factors, as expected based on different ecosystems’ productivity and infectious diseases ecogeographic distribution (see Tanner, this issue).
Apportionment of variance at the regional level
The FST values among archaeological and living populations highlight a fairly limited degree of variation at the regional level, in agreement with expectations for traits subject to selective pressures (Relethford, 2002). These results are consistent with the findings reported by Kudaka and colleagues (2013), who observed a relatively high degree of interregional measures of variance of long bone lengths in Asian populations. It should be noted that the estimates of FST provided herein are conservative and reflect the minimum amount of regional variation observed. Using lower estimates of heritability would have produced larger estimates of regional variation. Nonetheless, as noted by Relethford (2002), even though different heritability values would affect the estimates of the single components of variance, the pattern of variation is the same regardless of the heritability estimates used. It is interesting to note that the analyses revealed greater phenotypic variance among living groups than in the archaeological samples. Combined with the paleopathological data and contextual information, this finding suggests that the medieval populations examined here might have been exposed to relatively higher selective pressures—and possibly greater selective mortality—than groups from modern developing countries. However, this result may be due to other factors. For instance, it is possible that greater variance in the living samples may be due to greater differences in settlement type, subsistence practices, and access to healthcare. Alternatively, differences may be due to the fact that the variables used in the analyses are analogous but not identical, due to the presence of soft tissues in the measurements of living individuals. Additionally, even though there is no evidence pointing to preservation bias in the bioarchaeological populations examined, it is conceivably possible that the skeletal samples examined may not be representative of the entire survivor groups at these sites.
CONCLUSIONS
This study represents a first attempt at crossing boundaries between present and past human biology by comparing stature variation in living and archaeological populations. The observations and parallels drawn in this study underline the multidimensionality of human growth retardation and warn from uniformitarian assumptions about life conditions even in similar time periods or geographical areas. Understanding the specific eco-socio-biocultural factors that mold a population’s specific environment is essential to attempt reconstructing life conditions in the past. Unfortunately, rich contextual information is not always available for archaeological populations; in its absence it may not be possible to reach definite conclusions. Two complementary research strategies may allow bioarchaeologists to transcend such limitations: a greater commitment to first-hand acquisition of contextual information through archaeological excavation, and increased interdisciplinarity and collaboration with colleagues working in different areas of human biology.
Regardless of the options adopted, researchers should be mindful that the interpretation of stature in the past is complex. To be meaningful, it requires an understanding of the specific environmental conditions experienced by a population, the examination of stress indicators and diet, the analysis of growth and its retardation in both survivors and non-survivors, and the incorporation of biocultural information, whenever possible. Future studies should explore the impact of different stressors (chronic vs. acute; malnutrition vs. infectious diseases) on human growth to develop a more comprehensive model of biological variation in response to specific environmental conditions. In bioarchaeology, researchers should address stress and growth at the individual level, in order to test the association between stress and stature data, keeping in mind that catch-up growth may confound the evidence.
ACKNOWLEDGMENTS
The authors are grateful to the Ribeirinhos, Makushi, and Cali people, who made anthropometric data collection possible. They thank Rui Murrieta, Walter Neves, Peter Mann de Toledo, and Ima Guimaraes for institutional support in Brazil. They thank the Slavia Foundation, Marek Polcyn, and Teresa Krysztofiak for granting permission to study the Giecz collection; the Global History of Health Project for sharing paleopathological data on Giecz; Emma Rabino-Massa, Director of the Museum of Anthropology and Ethnography in Torino, for granting permission to study the materials from Trino Vercellese. They are also grateful to Britney McIlvaine and Laurie Reitsema for inviting this contribution and for constructive feedback throughout the preparation of the manuscript. Lastly, they thank the Associate Editor and two anonymous reviewers for their valuable insights, which helped them improve the quality of the manuscript.
Grant sponsor: Wenner-Gren Foundation; Grant number: 6861; Grant sponsor: National Science Foundation; Grant number: BCS 0201936; Grant sponsor: National Institute of Health; Grant number: R21-HD47943; Grant sponsor: Universidad del Valle for Institutional Support in Colombia and NIH; Grant number: 5-R22-DK39714; Grant sponsor: Wenner-Gren Foundation, University of Calgary; Grant number: 75 4278; Grant sponsor: Iwokrama International Centre for Rainforest Conservation and Development.
LITERATURE CITED
- Adair LS. 1999. Filipino children exhibit catch-up growth from age 2 to 12 years. J Nutr 129:1140–1148. [DOI] [PubMed] [Google Scholar]
- Agnew AM, Streeter M, Stout SD. 2007. Histomorphological aging of subadults: a test of Streeter’s method on a medieval archaeological population. Am J Phys Anthropol 44:61. [Google Scholar]
- Allen LH. 1994. Nutritional influences on linear growth: a general review. Eur J Clin Nutr 48:S75–S89. [PubMed] [Google Scholar]
- Allen LH, Backstrand JR, Stanek E, Pelto GH, Chávez A, Molina E, Castillo JB, Mata A. 1992. The interactive effect of dietary quality on the growth and attained size of young Mexican children. Am J Clin Nutr 56:353–364. [DOI] [PubMed] [Google Scholar]
- Auerbach BM, Ruff CB. 2010. Stature estimation formulae for indigenous populations from North America. Am J Phys Anthropol 141:190–207. [DOI] [PubMed] [Google Scholar]
- Baines SG. 2005. Indigenous autonomies and rights on the Brazil-Guyana border: Makushi and Wapishana on an international border. S er Antropol 386:2–13. [Google Scholar]
- Beaton G 1989. Small but healthy? Are we asking the right question? Hum Organ 48:30–39. [PubMed] [Google Scholar]
- Betti L, von Cramon-Taubadel N, Manica A, Lycett SJ. 2013. Global geometric morphometric analyses of the human pelvis reveal substantial neutral population history effects, even across sexes. PLoS One 8:e55909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J, Maternal and Child Undernutrition Study Group. 2008. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371:243–260. [DOI] [PubMed] [Google Scholar]
- Buikstra JE, Ubelaker DH. 1994. Standards for data collection from human skeletal remains. Arkansas Archeological Survey Research Series No. 44. [Google Scholar]
- Chen LC, Chowdhury AKMA, Huffman SL. 1980. Anthropometric assessment of energy-protein malnutrition and subsequent risk of mortality among preschool aged children. Am J Clin Nutr 33:1836–1845. [DOI] [PubMed] [Google Scholar]
- Darwin C 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: J. Murray. [PMC free article] [PubMed] [Google Scholar]
- Dufour DL, Staten LK, Reina JC, Spurr GB. 1994. Anthropometry and secular changes in stature of urban Colombian women of differing socioeconomic status. Am J Hum Biol 6: 749–760. [DOI] [PubMed] [Google Scholar]
- Dufour DL, Staten LK, Reina JC, Spurr GB. 1997. Living on the edge: dietary strategies of economically impoverished women in Cali, Colombia. Am J Phys Anthropol 102:5–15. [DOI] [PubMed] [Google Scholar]
- Dyer C 1994. Everyday life in medieval England. Cambridge: Cambridge University Press. [Google Scholar]
- Faust EC. 1958. Parasitologic surveys in Cali, Departamento del Valle, Colombia: I. Incidence and morphologic characteristics of strains of Entamoeba histolytica. Am J Trop Med Hyg 7:4–15. [DOI] [PubMed] [Google Scholar]
- Faust EC, Giraldo LE. 1960. Parasitological surveys in Cali, Departamento del Valle, Colombia VI. Strongyloidiasis in Barrio Siloé, Cali, Colombia. Trans R Soc Trop Med Hyg 54:556–563. [DOI] [PubMed] [Google Scholar]
- Forte J 1996. Makusipe Komanto Iseru: sustaining Makushi way of life. Annai: North Rupununi District Development Board. [Google Scholar]
- Frisancho RA. 2008. Anthropometric standards: an interactive nutritional reference of body size. Ann Arbor: University of Michigan Press. [Google Scholar]
- Fully G 1956. Une nouvelle méthode de détermination de la taille. Ann Med Leg 36:266–273. [PubMed] [Google Scholar]
- Gafni RI, Baron J. 2000. Catch-up growth: possible mechanisms. Pediatr Nephrol 14:616–619. [DOI] [PubMed] [Google Scholar]
- Golden JSR. 1994. Is complete catch-up possible for stunted malnourished children? Eur J Clin Nutr 48:S58–S70. [PubMed] [Google Scholar]
- Goodman AH, Rose JC. 1990. Assessment of systemic physiological perturbations from dental enamel hypoplasias and associated histological structures. Am J Phys Anthropol 65:259–266. [Google Scholar]
- Goodman AH, Martin DL. 2002. Reconstructing health profiles from skeletal remains In: Steckel RH, Rose JC, editors. The backbone of history: health and nutrition in the western hemisphere. New York: Cambridge University Press; p 11–60. [Google Scholar]
- Hatløy A, Torheim LE, Oshaug A. 1998. Food variety—a good indicator of nutritional adequacy of the diet? A case study from an urban area in Mali, West Africa. Eur J Clin Nutr 52: 891–898. [DOI] [PubMed] [Google Scholar]
- Hoppa RD, Fitzgerald CM. 1999. Human growth in the past Studies from bones and teeth. Cambridge: Cambridge University Press, Cambridge Studies in Biological and Evolutionary Anthropology. 315p. [Google Scholar]
- Indycka E 2000. Z badań nad cmentarzyskami gieckiego kompleksu osadniczego. Stud Lednickie 6:69–90. [Google Scholar]
- Kant AK. 1996. Indexes of overall diet quality: a review. J Am Diet Assoc 96:785–791. [DOI] [PubMed] [Google Scholar]
- Kemkes-Grottenthaler A 2005. The short die young: the interrelationship between stature and longevity—evidence from skeletal remains. Am J Phys Anthropol 128:340–347. [DOI] [PubMed] [Google Scholar]
- King SE, Ulijaszek SJ. 1999. Invisible insults during growth and development: contemporary theories and past populations In: Hoppa RD, FitzGerald CM, editors. Human growth in the past: studies from bones and teeth. Cambridge: Cambridge University Press; p 161–182. [Google Scholar]
- Koopman JS. 1978. Diarrhea and school toilet hygiene in Cali, Colombia. Am J Epidemiol 107:412–420. [DOI] [PubMed] [Google Scholar]
- Kostrzewski B 1952. Sprawozdanie z badań na grodzisku w Gieczu w pow średzkim za rok 1949. Stud Wczesnośredniowieczne 1:149–170. [Google Scholar]
- Kostrzewski B 1964. The Duke’s borough in Giecz. Archaeol Polona 6:234–245. [Google Scholar]
- Kudaka M, Fukase H, Kimura R, Hanihara T, Matsumura H, Saso A, Fukumine T, Ishida H. 2013. Metric characteristics of human limb bones in Asian and Japanese populations. Anthropol Sci 121:49–62. [Google Scholar]
- Larsen CS. 1997. Bioarchaeology: interpreting behavior from the human skeleton. Cambridge: Cambridge University Press. [Google Scholar]
- Larsen CS. 2006. The changing face of bioarchaeology: an interdisciplinary science In: Buikstra JE, Beck LA, editors. Bioarchaeology: the contextual analysis of human remains. Amsterdam: Academic Press; p 359–374. [Google Scholar]
- Leonard WR, Dewalt KM, Stansbury JP, McCaston MK. 2000. Influence of dietary quality on the growth of highland and coastal Ecuadorian children. Am J Hum Biol 12:825–837. [DOI] [PubMed] [Google Scholar]
- Lettre G 2009. Genetic regulation of adult stature. Curr Opin Pediatr 21:515–522. [DOI] [PubMed] [Google Scholar]
- Lewit E, Kerrebrock N. 1997. Population-based growth stunting. Future Child 7:149–156. [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. 1988. Anthropometric standardization reference manual. Champaign: Human Kinetics. [Google Scholar]
- Luke A, Guo X, Adeyemo AA, Wilks R, Forrester T, Lowe W, Comuzzie AG, Martin LJ, Zhu X, Rotimi CN, Cooper RS. 2001. Heritability of obesity-related traits among Nigerians, Jamaicans, and US blacks. Int J Obes Relat Metab Disord 25: 1034–1041. [DOI] [PubMed] [Google Scholar]
- Marquis GS, Habicht JP, Lanata CF, Black RE, Rasmussen KM. 1997. Breast milk or animal-product foods improve linear growth of Peruvian toddlers consuming marginal diets. Am J Clin Nutr 66:1102–1109. [DOI] [PubMed] [Google Scholar]
- Martorell R 1989. Body size, adaptation and function. Hum Organ 48:15–20. [Google Scholar]
- Martorell R, Habicht J-P. 1986. Growth in early childhood in developing countries In: Falkner F, Tanner JM, editors. Human growth, Vol. 3. Methodology: ecological, genetic, and nutritional effects on growth, 2nd ed. New York: Plenum; p 241–262. [Google Scholar]
- Martorell R, Khan LK, Schroeder DG. 1994. Reversibility of stunting: epidemiologic findings in children from developing countries. Eur J Clin Nutr 48:S45–S57. [PubMed] [Google Scholar]
- Martorell R, Yarbrough C, Lechtig A, Habicht J-P, Klein RE. 1975. Diarrheal diseases and growth retardation in preschool Guatemalan children. Am J Phys Anthrop 43:341–346. [DOI] [PubMed] [Google Scholar]
- Messer E 1989. Small but healthy? Some cultural considerations. Hum Organ 48:39–52. [Google Scholar]
- Montanari M 1994. The culture of food. Cambridge: Blackwell Publishers. [Google Scholar]
- Nagel A, Moffat T, Bulkan J, Wilson WM. 2014. Dietary diversity scores to assess dietary quality among Guyanese Makushi Amerindians. Am J Hum Biol 26:274. [Google Scholar]
- Negro Ponzi Mancini MM. 1999. San Michele di Trino (VC) Dal villaggio romano al castello medievale. Florence: All’insegna del Giglio. [Google Scholar]
- Niskanen M, Maijanen H, McCarthy D, Junno J-A. 2013. Application of the anatomical method to estimate the maximum adult stature and the age-at-death stature. Am J Phys Anthropol 152:96–106. [DOI] [PubMed] [Google Scholar]
- Paajanen TA, Oksala NKJ, Kuukasjarvi P, Karhunen PJ. 2010. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur Heart J 31:1802–1809. [DOI] [PubMed] [Google Scholar]
- Palmer P 2009. Dietary intake and growth as an indicator of health among Guyanese Makushi Amerindian children: a biocultural approach. Master’s Thesis: University of Calgary, Alberta, Canada. [Google Scholar]
- Pelletier DL, Frongillo EA Jr, Schroeder DG, Habicht JP. 1995. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ 73:443–448. [PMC free article] [PubMed] [Google Scholar]
- Perry GH, Dominy NJ. 2009. Evolution of the human pygmy phenotype. Trends Ecol Evol 24:218–225. [DOI] [PubMed] [Google Scholar]
- Petersen HC. 2011. Technical note: a re-evaluation of stature estimation from skeletal length in the grave. Am J Phys Anthropol 144:327–330. [DOI] [PubMed] [Google Scholar]
- Piperata BA. 2007. Nutritional status of Ribeirinhos in Brazil and the nutrition transition. Am J Phys Anthropol 133:868–878. [DOI] [PubMed] [Google Scholar]
- Piperata BA, Ivanova SA, DaGloria P, Veiga G, Polsky A, Spence JE, Murrieta RSS. 2011. Nutrition in transition: dietary patterns of Amazonian women during a period of economic change. Am J Hum Biol 23:458–469. [DOI] [PubMed] [Google Scholar]
- Piperata BA, Schmeer KK, Hadley C, Ritchie-Ewing G. 2013. Dietary inequalities of mother-child pairs in the rural Amazon: evidence of maternal-child buffering? Soc Sci Med 96:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice AM, Ward KA, Goldberg GR, Jarjou LM, Moore SE, Fulford AJ, Prentice A. 2013. Critical windows for nutritional interventions against stunting. Am J Clin Nutr 97:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proos LA, Hofvander Y, Tuvemo T. 1991. Menarcheal age and growth pattern of Indian girls adopted in Sweden. II. Catch-up growth and final height. Indian J Pediatr 58:105–114. [DOI] [PubMed] [Google Scholar]
- Raxter MH, Auerbach BM, Ruff CB. 2006. Revision of the fully technique for estimating statures. Am J Phys Anthropol 130: 374–384. [DOI] [PubMed] [Google Scholar]
- Raxter MH, Ruff CB, Auerbach BM. 2007. Technical note: revised fully stature estimation technique. Am J Phys Anthropol 133:817–818. [DOI] [PubMed] [Google Scholar]
- Reitsema LJ, Vercellotti G. 2012. Stable isotope evidence for sexand status-based variations in life-history and diet at medieval Trino Vercellese, Italy. Am J Phys Anthropol 148:589–600. [DOI] [PubMed] [Google Scholar]
- Reitsema LJ, Crews DE, Polcyn M. 2010. Preliminary evidence for medieval Polish diet from carbon and nitrogen stable isotopes. J Archaeol Sci 37:1413–1423. [Google Scholar]
- Relethford JH. 1994. Craniometric variation among modern human populations. Am J Phys Anthropol 95:53–62. [DOI] [PubMed] [Google Scholar]
- Relethford JH. 2002. Apportionment of global human genetic diversity based on craniometrics and skin color. Am J Phys Anthropol 118:393–398. [DOI] [PubMed] [Google Scholar]
- Relethford JH, Blangero J. 1990. Detection of differential gene flow from patterns of quantitative variation. Hum Biol 62:5–25. [PubMed] [Google Scholar]
- Ruff CB, Holt BM, Niskanen M, Sladek V, Berner M, Garofalo E, Garvin HM, Hora M, Maijanen H, Niinimaki S, Salo K, Schuplerova E , Tompkins D. 2012. Stature and body mass estimation from skeletal remains in the European Holocene. Am J Phys Anthropol 148:601–617. [DOI] [PubMed] [Google Scholar]
- Saunders SR, Hoppa RD. 1993. Growth deficit in survivors and non-survivors: biological mortality bias in subadult skeletal samples. Yrbk Phys Anthropol 36:127–151. [Google Scholar]
- Seckler D 1980. Malnutrition: an intellectual odyssey. Western J Agr Econ 5:219–227. [Google Scholar]
- Shea BT, Bailey RC. 1996. Allometry and adaptation of body proportions and stature in African Pygmies. Am J Phys Anthropol 100:311–340. [DOI] [PubMed] [Google Scholar]
- Silva HP, Crews DE. 2006. Ecology of children’s growth: an example from transitional populations of the Brazilian Amazon. Int J Anthropol 21:97–109. [Google Scholar]
- Silventoinen K, Sammalisto S, Perola M, Boomsma DI, Corne BK, Davis C, Dunkel L, De Lange M, Harris JR, Hjelmborg JV, Luciano M, Martin NG, Mortensen J, Nistic o L, Pedersen NL, Skytthe A, Spector TD, Stazi MA, Willemsen G, Kaprio J. 2003. Heritability of adult body height: a comparative study of twin cohorts in eight countries. Twin Res 6:399–408. [DOI] [PubMed] [Google Scholar]
- Steckel RH. 1987. Growth depression and recovery: the remarkable case of American slaves. Ann Hum Biol 14:111–132. [DOI] [PubMed] [Google Scholar]
- Swanberg KG, Shipley E. 1975. The nutritional status of the rural family in east Cundinamarca, Colombia. Food Res Inst Stud 14:111–125. [Google Scholar]
- Tanner S, TAPS Bolivia Study Team. 2014. Health and disease: exploring the consequences of infection on nutritional status. Am J Phys Anthropol 155:221–228. [DOI] [PubMed] [Google Scholar]
- Temple DH. 2008. What can variation in stature reveal about environmental differences between prehistoric Jomon foragers? Understanding the impact of systemic stress on developmental stability. Am J Hum Biol 20:431–439. [DOI] [PubMed] [Google Scholar]
- Ulijaszek SJ. 1994. Between-population variation in pre-adolescent growth. Eur J Clin Nutr 4:S5–S13. [PubMed] [Google Scholar]
- Vercellotti G 2012. Assessment of inter and intra-population variation in stature and body proportions: a comparative study between living and bioarchaeological populations. Dissertation, Columbus (OH): The Ohio State University. p 368. [Google Scholar]
- Vercellotti G, Agnew AM, Justus HM, Sciulli PW. 2009. Stature estimation in an early medieval (XI-XIIc.) Polish population: testing the accuracy of regression equations in a bioarcheological sample. Am J Phys Anthropol 140:135–142. [DOI] [PubMed] [Google Scholar]
- Vercellotti G, Piperata B. 2012. The use of biocultural data in interpreting sex differences in body proportions among rural Amazonians. Am J Phys Anthropol 147:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G, Stout SD, Boano R, Sciulli PW. 2011. Intrapopulation variation in stature and body proportions: social status and sex differences in an Italian medieval population (Trino Vercellese, VC). Am J Phys Anthropol 145:203–214. [DOI] [PubMed] [Google Scholar]
- Wachs T 1995. Relation of mild-to-moderate malnutrition to human development: correlational studies. J Nutr 125:2245S–2254S. [DOI] [PubMed] [Google Scholar]
- Walker PL, Bathurst RR, Richman R, Gjerdrum T, Andrushko VA. 2009. The causes of porotic hyperostosis and cribra orbitalia: a reappraisal of the iron-defiency anaemia hypothesis. Am J Phys Anthropol 139:109–125. [DOI] [PubMed] [Google Scholar]
- White L 1962. Medieval technology and social change. Oxford: Oxford University Press. [Google Scholar]
- Wilson WM, Bulkan J, Piperata BA, Hicks K, Ehlers P. 2011. Nutritional status of Makushi Amerindian children and adolescents of Guyana. Ann Hum Biol 38:615–629. [DOI] [PubMed] [Google Scholar]
- Wilson WM, Dufour DL, Staten LK, Barac-Nieto M, Reina JC, Spurr GB. 1999. Gastrointestinal parasitic infection, anthropometrics, nutritional status, and physical work capacity in Colombian boys. Am J Hum Biol 11:763–771. [DOI] [PubMed] [Google Scholar]
- Wilson WM, Milner J, Bulkan J, Ehlers P. 2006. Weaning practices of the Makushi of Guyana and their relationship to infant and child mortality: a preliminary assessment of international recommendations. Am J Hum Biol 18:312–324. [DOI] [PubMed] [Google Scholar]
- Wood JW, Milner GR, Harpending HC, Weiss KM. 1992. The osteological paradox: problems of inferring prehistoric health from skeletal samples. Curr Anthropol 33:343–370. [Google Scholar]
- World Health Organization (WHO). 1995. Physical status: the use and interpretation of Anthropometry. Geneva: World Health Organization; p 452. [Google Scholar]
- World Health Organization (WHO). 2014. World health statistics. Geneva: World Health Organization; p 177. [Google Scholar]


