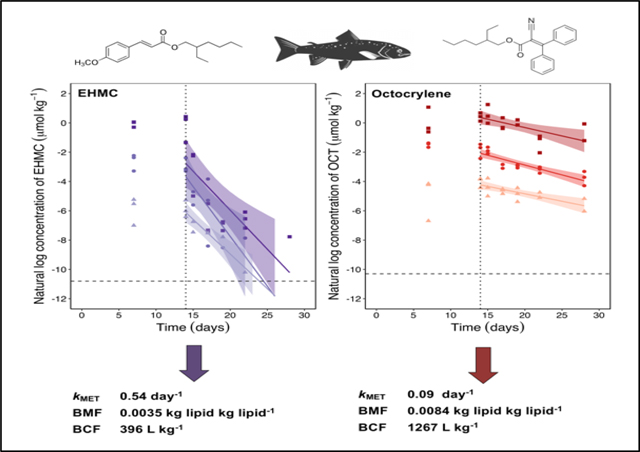
Abstract
This study investigated the dietary bioaccumulation and biotransformation of hydrophobic organic sunscreen agents, 2-ethylhexyM-methoxycinnamate (EHMC) and octocrylene (OCT), in rainbow trout using a modified OECD 305 dietary bioaccumulation test that incorporated non-biotransformed reference chemicals. Trout were exposed to three dietary concentrations of each chemical to investigate the relationship between dietary exposure concentration and observed accumulation and depuration. EHMC and OCT were significantly biotransformed, resulting in mean in vivo biotransformation rate constants (kMET) of 0.54 ± 0.06 and 0.09 ± 0.01 d−1, respectively. The kMET values generated for both chemicals did not differ between dietary exposure concentrations, indicating that chemical concentrations in the fish were not high enough to saturate biotransformation enzymes. Both somatic and luminal biotransformation substantially reduce EHMC and OCT bioaccumulation potential in trout. Biomagnification factors (BMF) and bioconcentration factors (BCF) of EHMC averaged 0.0035 kg lipid kg lipid−1 and 396 L kg−1, respectively, while those of OCT averaged 0.0084 kg lipid kg lipid−1 and 1267 L kg−1. These values are one to two orders of magnitude lower than the BMFs and BCFs generated for reference chemicals of similar log KOW. Additionally, for both chemicals, derived BMFs and BCFs fell below established bioaccumulation criteria (1.0 kg lipid kg lipid−1 and 2000 L kg−1, respectively), suggesting that EHMC ad OCT are unlikely to bioaccumulate to a high degree in aquatic biota.
Keywords: bioaccumulation, biotransformation, biomagnification, ultraviolet filters, toxicokinetics, sunscreens
Graphical Abstract
INTRODUCTION
Organic ultraviolet filters (UVFs) are used in personal care products, including sunscreens and cosmetics, to protect the skin from negative effects of UV exposure. In addition, UVFs are used in paints, plastics, and textiles to prevent UV-induced degradation (Tang et al. 2019). Depending on their usage, individual UVFs can enter the aquatic environment via wastewater treatment plant effluents or by loss from skin during swimming and other recreational activities. Measurable concentrations of UVFs have been reported in surface waters, sediments, sewage sludge, and aquatic biota (Nagtegaal et al. 1997; Balmer et al. 2005; Buser et al. 2006; Zenker et al. 2008; Fent et al. 2010; Bachelot et al. 2012; Gago-Ferrero et al. 2012; Gago-Ferrero et al. 2013; Picot Groz et al. 2014). Some UVFs are hydrophobic (log KOW > 4), which has lead to concern that they may bioaccumulate in aquatic organisms (Balmer et al. 2005).
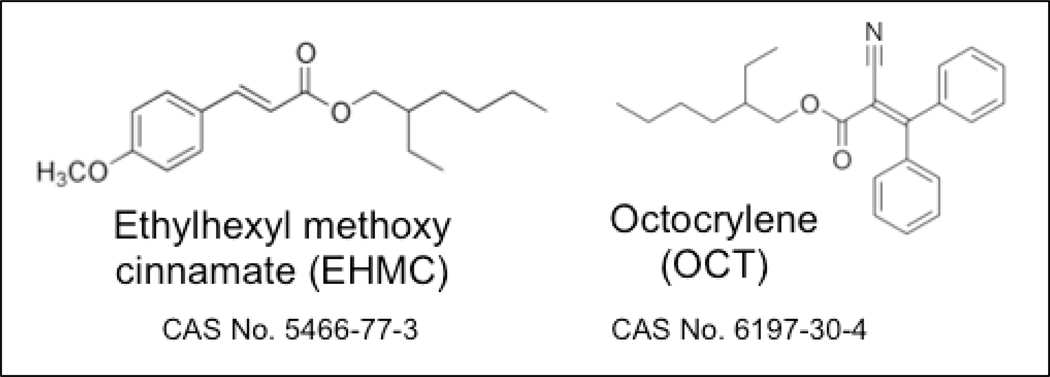
2-Ethylhexyl-4-methoxycinnamate (EHMC) and octocrylene (OCT; Figure 1), two of the most widely used UVFs, enter the environment primarily via their application in cosmetic products (Christen et al. 2011; Bluthgen et al. 2014). Both chemicals are very hydrophobic (log KOW of 5.80 and 6.88 for EHMC and OCT, respectively), and both have been detected in piscivorous birds (Fent et al. 2010) and marine mammals (Gago-Ferrero et al. 2013; Alonso et al., 2015). Field-derived biomagnification factors (BMF) for EHMC, obtained by comparing measured concentrations in freshwater fish and their invertebrate prey, ranged from 0.6 to 1.5 kg lipid kg lipid−1 (Fent et al. 2010). A field-derived BMF of 1.1 kg lipid kg lipid−1 was reported for OCT, based on the ratio of measured concentrations in two species of marine fish and a smaller prey fish species (Peng et al. 2017). In contrast, Pawlowski et al. (2019) reported a laboratory-derived BMF of 0.034 kg lipid kg lipid−1 for OCT (Pawlowski et al. 2019). Presently, there are no established regulatory criteria for chemical BMFs in fish; however, a BMF ≥ 1.0 is generally interpreted as evidence of probable bioaccumulation potential (Gobas et al. 2009).
Figure 1.
Structures, names, abbreviations, and chemical abstract service (CAS) registry numbers of 2-ethylhexyl-4-methoxycinnamate (EHMC), and octocrylene (OCT).
Although several field studies have reported concentrations of EHMC and OCT in fish and other higher trophic level organisms, empirical bioconcentration factors (BCF) (US National Library of Medicine 2006; Blüthgen et al. 2014; Sigma-Aldrich 2014; Pawlowski et al. 2019), bioaccumulation factors (BAF) (Fent et al. 2010), and biota-sediment accumulation factors (BSAF) (Gago-Ferrero et al. 2015; Tang et al. 2019) in fish tend to fall well below established bioaccumulation criteria (Table 1). These findings suggest that some fish species have the capacity to biotransform EHMC and OCT. Specifically, ester groups present on both chemicals (Figure 1) may provide a target for esterases and/or cytochrome P450 (CYP) enzymes. EHMC and OCT were shown to be metabolized in vitro by rainbow trout liver S9 fractions (Saunders et al. 2019). Incubations performed with and without added NADPH (a reduced form of nicotinamide adenine dinucleotide phosphate) suggested that hydrolysis by carboxylesterases and CYP-mediated biotransformation are important metabolic routes for both chemicals (Saunders et al. 2019).
Table 1.
Bioaccumulation metrics reported for 2-ethylhexyl-4-methoxycinnamate (EHMC) and octocrylene (OCT).
| Metric | EHMC | OCT | Bioaccumulation Criteria | Regulatory Program |
|---|---|---|---|---|
| log KOW | 5.80a | 6.88a | ≥ 4.50 | REACH |
| BCF | 175–433b | 41–972c,d,e | ≥2000 ‘B’ ≥ 5000 ‘Very B’ | REACH |
| BAF | 12–970f,g | 16–125f | ≥ 5000 | CEPA |
| Lab-derived BMF | N/A | 0.034e | ≥ 1.0 | NL |
| Field-derived BMF | 0.6–1.5g | 1.1h | ||
| BSAF | 0.04–0.3i | 0.04–0.3i | ≥ 1.0 | NL |
Log KOW = Logarithmic octanol water partition coefficient; BCF = Bioconcentration Factor (L kg−1); B = Bioaccumulative; BAF = Bioaccumulation Factor (L kg−1); BMF = Biomagnification Factor (kg lipid kg lipid−1); BSAF = Biota-Sediment Accumulation Factor (kg organic carbon kg lipid−1); CEPA = Canadian Environmental Protection Act; REACH = European Union Registration, Evaluation, Authorization, and Restriction of Chemicals; N/A = not available; NL = indicates that the B metric is not listed under a regulatory program
The rate of biotransformation is a key parameter in computational models used to predict chemical bioaccumulation in fish (Arnot and Gobas 2003; Arnot and Gobas 2004). Presently, information on UVF biotransformation in fish is limited. In vivo biotransformation rates for EHMC and OCT, expressed as apparent whole-body biotransformation rate constants (kMET; d−1), can be estimated by in vitro-in vivo extrapolation (IVIVE) of measured in vitro activity (Saunders et al. 2019). Alternatively, these rates may be predicted using existing quantitative structure activity relationship (QSAR) models (EPISuite; US Environmental Protection Agency 2012). In vivo data are needed, however, to evaluate the accuracy of biotransformation rates predicted using either approach.
This study investigated the in vivo biotransformation and bioaccumulation of EHMC and OCT in rainbow trout using a modified OECD 305 dietary bioaccumulation test that incorporates non-biotransformed reference chemicals (Lo et al. 2015a). The main objective of the study was to generate whole-body biotransformation rate constants for This article is protected by copyright. All rights reserved.
EHMC and OCT at three different dosing levels. Three dosing levels were selected to evaluate the potential concentration dependence of in vivo biotransformation, as the concentration dependence of in vitro biotransformation for these two chemicals was recently demonstrated (Saunders et al. 2019). Measured BMFs for EHMC and OCT were obtained directly from the resulting datasets. A model-based approach was then employed to estimate BCFs (Gobas and Lo 2016) and rates of biotransformation in the lumen of the gastrointestinal tract (Lo et al. 2015a).
MATERIALS AND METHODS
Fish
Rainbow trout (Oncorhynchus mykiss, Erwin strain) were obtained as eggs from the US Geological Survey Upper Midwest Environmental Sciences Center in LaCrosse, WI, and reared to desired size (~35 g) at the US EPA laboratory in Duluth, MN. The study was performed in 40 gal fiberglass tanks (Dura-Tech Industrial and Marine) supplied with 0.5 L min−1 Lake Superior water (single-pass, sand-filtered, and ultraviolet treated). Fish were fed commercial fish chow (3.0 mm Skretting sinking chow) at a target rate of 1.3% body weight per day. Mean (± SD) water characteristics were: temperature 11 ± 0.5 °C; pH 7.4 ± 0.02; total organic carbon 1.60 ± 0.29 mg L−1; total ammonia 0.07 ± 0.01 mg L−1; dissolved oxygen 90 ± 0.02% of saturation. The study was performed under a 12:12 h light:dark schedule. Fish were acclimatized for 2 weeks to the experimental conditions before initiating chemical exposures.
Chemicals
The test chemicals 2-ethylhexyl-4-methoxycinnamate (EHMC; CAS No. 546677–3) and octocrylene (OCT; CAS No. 6197–30-4) were purchased from Sigma-Aldrich. This article is protected by copyright. All rights reserved. 1,3,5-Trichlorobenzene (3TCBz), 1,2,4,5-tetrachlorobenzene (4TCBz), pentachlorobenzene (PCBz), hexachlorobenzene (HCBz), d8-naphthalene, and d12-chrysene were obtained from Sigma-Aldrich. 2,2’,5,5’-Tetrachlorobiphenyl (PCB 52), 2,3,4,4’,6-pentachlorobiphenyl (PCB 115), and 2,2’,4,4’,6,6’-hexachlorobiphenyl (PCB 155) were purchased from AccuStandard. 13C-Hexachlorobenzene (13C-HCBz) was obtained from MSD Isotopes (now Cambridge Isotopes). Solvents were purchased from Fisher Chemical. Bondesil-C18 and PSA silica bulk sorbents were purchased from Agilent Technologies. All chemicals were reagent-grade or higher in quality, with purities > 97%.
Study design
Fish were fed a control diet or a contaminated diet containing EHMC or OCT and 6 reference chemicals (i.e., 3TCBz, 4TCBz, PCBz, HCBz, PCB 52, PCB 155) at a target daily feeding rate of 1.3% bodyweight per day. The reference chemicals were selected because of their recognized persistence and resistance to biotransformation. While biotransformation of some reference chemicals (e.g., PCB 52) has been observed in fish (Koenig et al. 2012), biotransformation rates were found to be too low to have a significant effect on the derivation of whole-body biotransformation rate constants in fish (Lo et al. 2015a).
Dietary concentrations of EHMC and OCT were varied by approximately two orders of magnitude to represent low, medium, and high treatment levels. Measured concentrations of EHMC averaged 0.004 mmol kg−1, 0.038 mmol kg−1, and 0.318 mmol kg−1 for the low, medium, and high treatment levels, respectively, while those of OCT averaged 0.003 mmol kg−1, 0.086 mmol kg−1, and 1.05 mmol kg−1 (Supplemental Data, Table S1). Dietary concentrations were below chronic toxicity thresholds (no and lowest observed effect levels) available through ECOTOX (US Environmental Protection Agency 2018). Measured concentrations of reference chemicals in food ranged from approximately 0.01 mmol kg−1 to 0.35 mmol kg−1 (Table S1).
Dietary exposures were conducted in 7 tanks (6 test and 1 control), each of which contained 21 fish to start. Fish in treatment tanks were exposed to a contaminated diet for 14 d, followed by a 14 d depuration period when fish were fed the control diet. All fish were fed daily at 3 PM. On sampling days, fish were collected by 9 AM. Three fish were collected from each treatment tank on days 7, 14, 15, 17, 19, 22, and 28 of the experiment, and analyzed independently. Four or 5 fish from the control tank were collected on days 7, 14, 17, 22, 28, and analyzed independently to test for sample background contamination and toxicity.
Fish were euthanized with an overdose of buffered ethyl 3-aminobenzoate methanesulfonate (Finquel, Argent Laboratories). Sampled fish were separated into liver, gastrointestinal tract (minus the pyloric ceca, stomach, and gut contents), and carcass. The anterior intestine was combined with the carcass samples for analysis. Samples were frozen at −80 °C until processing and extraction.
Food preparation
Test and reference chemicals were dissolved in 15 mL acetone containing 0.875 g corn oil. This spiking solution was slowly added to 175 g of fish food and left to mechanically stir in an open system overnight. The spiked diets were then stored at −20 °C in sealed containers. The control diet was prepared in a similar manner, but without test or reference chemicals. All fish received the control diet during a 2 wk acclimation period prior to the experiment. Measured concentrations of EHMC, OCT, and reference chemicals in the control diet were below their MDL (Supplemental Data, Table S2).
Sample preparation and extraction
Samples were extracted using a modified QuEChERS method. These procedures were based on those used to extract UVFs in marine mussels (Picot Groz et al. 2014) and are similar to methods for extracting PCBs from fish tissues (Chamkasem et al. 2016). Whole liver (283 to 966 mg) samples were processed in their entirety. Carcass samples (35.98 to 56.57 g) were homogenized with two volumes of MQ water and extracted in 6 g batches. Fish food was sub-sampled on days 0, 7, and 14 of the exposure and was extracted in 1 g batches. Each sample was placed in a 50 mL polypropylene centrifuge tube and spiked with 50 μL of a 10 ppm internal standard solution prepared in acetone. A volume of MQ water was added followed by 30 s of vortexing. Acetonitrile was added and the tube was vigorously shaken by hand for 2 min. A salt mixture containing 8 parts anhydrous Na2SO4, 2 parts NaCl, 2 parts sodium citrate dihydrate, and 1 part sodium citrate dibasic sesquihydrate was added. The tube was then shaken by hand for 1 min and centrifuged at 3500 g for 5 min. Following centrifugation, the upper ACN layer was transferred to a 15 mL polypropylene tube containing 1 g of bulk sorbents (3 parts Na2SO4, 1 part Bondesil-C18 and 1 part PSA silica) for dispersive solid-phase extraction (d-SPE). Formic acid was added, followed by 1 min of vortexing and 5 min centrifugation at 5000 g. The exact amounts of added water, ACN, salts, sorbents, and formic acid were adjusted to each type of sample and are given in the Supplemental Data, Table S3.
Following d-SPE, the ACN supernatant was transferred to a 4 mL amber glass vial, placed under a gentle stream of N2, and evaporated to ~ 0.5 mL at 35 °C. N-hexane (0.5 mL) was added to the vial and the sample was vortexed for 1 min to extract analytes and internal standards. The combined hexane-ACN extract was transferred quantitatively to a second 2 mL amber vial (2 rinses with 250 μL n-hexane). The vial was then centrifuged at 7000 g for 10 min. Finally, the extract was filtered through a Pasteur pipette containing 0.25 g of hexane-washed d-SPE sorbents to eliminate sample lipids. Filtered extracts that were below the method detection limit (MDL) were pooled and reanalyzed. Extraction recoveries and MDLs of UVFs and reference chemicals in fish tissues and food are presented in Supplemental Data, Table S2.
Gas chromatography mass spectrometry analysis
Sample extracts were analyzed using an Agilent 6890N gas chromatograph coupled to an Agilent 5975C mass spectrometer. Separations were performed on a DB−1HT 15 m x 320 μm, 0.25 μm film column (Agilent). The oven temperature was 45 °C for 1.5 min, increasing to 150 °C at 15 °C min−1, and finally increasing 10 °C min−1 to 285 °C, and held for 5 min. The injection port and ion source temperatures were 45 °C and 230 °C, respectively. The carrier gas was helium flowing at 1 mL min−1. The MS data was acquired in the selected ion monitoring mode (136 for d8-naphthalene, 180 for 3TCBz, 216 for 4TCBz, 240 for d12-chrysene, 250 for PCBz, 284 for HCBz, 290 for 13C-HCBz, 292 for PCB 52, 326 for PCB 115, and 360 for PCB 155). For EHMC, the quantification and identification ions were 178 and 161, respectively. For OCT, the quantification ion was 249 and the identification ions were 232 and 204. A 1 μL sample of extract was injected into the column using a 5 μL gas-tight glass syringe (Agilent). Peak areas were integrated and used to quantify test chemicals using Chemstation software (Hewlett Packard). Chemical concentrations were calculated using the relative response factor approach.
Lipid content determination
Total lipid content (Bligh and Dyer 1959) was determined for livers from sampled control fish that had not been selected for chemical extraction (days 7, 14, 17, 22, 28). Additional measurements were made for homogenized carcass samples from all control and exposed animals on days 7, 14, 22, and 28. The carcass lipid content averaged across these sampling days provided an overall mean value for each tank. Then to determine the fractional lipid content of whole fish (ϕBL) from treatment and control tanks, the mean mass of lipid in the carcass samples was added to the mean mass of lipid determined for livers from control fish, and divided by the combined wet weights of the 2 samples. This approach assumes there were no treatment-related effects on the lipid content of the liver. Fish food containing 0.5% corn oil was also analyzed for total lipids to determine the fractional dietary lipid content (ϕDL).
Chemical concentrations in the fish
Chemical concentrations in the liver (CL) were determined by dividing the chemical masses measured in the liver by the wet weights of the liver. Chemical concentrations in the fish soma (CB) were determined by summing chemical masses measured in liver and carcass samples, and then dividing by the combined wet weight of the 2 compartments.
Whole-body depuration rate constants
Whole-body depuration rate constants (kBT; d−1) were derived for EHMC, OCT, and reference chemicals by linear regression of the natural logarithm of chemical concentrations in the fish soma against time during the depuration phase:
| (1) |
where CB is the chemical concentration (μmol kg−1) at the beginning of the depuration period and Ct is the concentration at time t (d).
Whole-body biotransformation rate constants
To determine whole-body biotransformation rate constants, a linear least squares weighted regression of the measured depuration rate constants of the reference chemicals (kBT,R) and the reciprocal of each chemical’s KOW (1/KOW) was performed:
| (2) |
where 1/ω and β are regression coefficients in units of d−1 (Gobas and Lo 2016). The slope term (1/ω) describes the depuration of hydrophobic organic chemicals to water predominantly via the respiratory route (k2; d−1) while the intercept (β) describes the contribution of other depuration processes, limited in this instance to the growth dilution and fecal egestion. For EHMC and OCT, the kBT,R was derived using Equation 2 and represents the depuration rate constant of EHMC and OCT in absence of biotransformation.
Whole-body biotransformation rate constant (kMET; d−1) for EHMC and OCT were then calculated as the difference between the measured whole-body depuration rate constants (kBT) of EHMC and OCT and the corresponding kBT,R values as (Lo et al. 2015a):
| (3) |
The standard error of kMET (SEkMET) was propagated from the standard errors of kBT (SEkBT) and kBT,R (SEkBT,R) using the equation (Gobas and Lo 2016):
| (4) |
Calculation of dietary uptake efficiency
Dietary uptake efficiency (ED,M) was determined for the reference chemicals, EHMC, and OCT by fitting chemical concentrations measured in the soma to the integrated form of the kinetic rate equation for constant dietary exposure (OECD 2012):
| (5) |
where I is the feeding rate, CB is the concentration in the fish (soma) at the beginning of the depuration period (μmol kg−1), CD is the concentration in the diet (μmol kg−1), and t is time (d).
Bioaccumulation Potential of EHMC and OCT
Kinetic lipid normalized biomagnification factors (BMF) were generated for EHMC, OCT, and reference chemicals according to:
| (6) |
where ED,M is the dietary uptake efficiency; I is the proportional feeding rate; kBT is the somatic depuration rate constant (d−1); ϕDL is the measured fractional dietary lipid content (0.079 ± 0.003 [SD] kg lipid kg food−1; this study), and ϕBL is the fractional lipid content of the fish (kg lipid kg fish−1).
Respiratory uptake rate constants (k1) and BCFs were generated from the dietary bioaccumulation tests according to Gobas and Lo (2016).
| (7) |
where 1/ω is the slope term derived from Equation 2, dL is the density of the fish lipids (assumed to be 0.90 kg L−1), and ϕBL is the measured lipid content of fish (kg lipid kg fish−1). A detailed derivation of Equation 7 is provided in Gobas and Lo (2016) and is based on the assumptions that it applies to test chemicals with log KOW ≥ 3, and that chemical partitioning between the fish and water (i.e., k1/k2) is represented by KOW × ΦBL. Bioconcentration factors (L kg−1) expressed on a free chemical basis were determined as:
| (8) |
where COC is the measured total concentration of organic carbon in water (1.60 × 10−6 kg L−1; this study) and KOC is the octanol-carbon partition coefficient, calculated as log KOC = 0.97 × log KOW - 1.27 (Burkhard 2000).
Chemical transformation in the gastrointestinal tract
The dietary uptake efficiency for a non-biotransformed chemical (ED,R) was related to KOW by the relationship (Lo et al. 2015a):
| (9) |
where α and β are fitted coefficients determined by a weighted non-linear regression of empirical ED observations (ED,M) of the reference chemicals. The parameters α and β characterize organic (i.e., octanol or lipid-like) and aqueous phase resistances, respectively.
Intestinal (luminal) biotransformation rate constants (klumen) for EHMC and OCT were calculated from ED,M and from ED,R determined for a non-biotransformed reference chemical of equivalent KOW (Equation 9; Lo et al. 2015a):
| (10) |
where GGE (kg digesta d−1) is the fecal egestion rate and WG (kg) is the steady-state amount of digesta in the entire intestinal tract (including stomach, pyloric ceca, and anterior intestine). The GGE was estimated from the administered food ingestion rate (GI; kg food d−1 [or I × WB]) and the food assimilation efficiency (γGI; unitless) as GI × γGI. The γGI was estimated from the diet composition and the assimilation efficiencies of the diet constituents using values for the assimilation efficiencies of various food constituents (Supplemental Data, Table S5). The γGI was approximately 0.59 and is similar to the value of 0.52 measured in rainbow trout using chromic oxide (Gobas et al. 1999). The WG was estimated as the ratio of GI to δ, where δ is the digesta evacuation rate constant (2.07 d−1), which is approximated by the 95% digesta evacuation time (tE,95; 1.45 d) as 3/tE,95 (Lo et al. 2015a). Parameters and equations used in this analysis are provided in Table S5.
Contribution of luminal and somatic biotransformation
To describe the contribution of somatic (whole-body) and luminal biotransformation, the fish is divided into two compartments: the soma (B) and the gastrointestinal content or digesta in the lumen (G). A detailed derivation of the model is described elsewhere (Lo et al. 2015a; 2016). The relative contributions of somatic (Φsoma) and luminal (Φlumen) biotransformation to total chemical biotransformation in fish can be calculated as:
| (11) |
| (12) |
where MB and MG are the masses of chemical in the fish soma and lumen, respectively. The subscript ‘X’ denotes whether the exposure was through a dietary (D) or aqueous (AQ) route.
For the dietary exposure, the MB,D is the mass of chemical (CB × WB) in the soma measured in the present study on day 14, where as the MG,D was estimated as (Lo et al. 2015a):
| (13) |
where kBG is the rate constant for chemical transfer from fish soma to lumen (d−1), kGB is the rate constant for chemical transfer from lumen to fish soma (d−1), and kGE is the rate constant for fecal egestion (d−1).
Using rate constants generated here for respiratory uptake (k1) and whole-body depuration (kBT), the steady-state mass of chemical in the fish soma following an aqueous exposure (MB,AQ) can be estimated as:
| (14) |
where CWT is the total concentration of chemical in the water (μmol L−1) and Φ is the bioavailable solute fraction (unitless) which is equal to 1/(1+ COC × KOC) (Equation 8).
Using Equation 13, the MG,AQ is calculated by replacing MB,D with MB,AQ and setting CD equal to 0 μmol kg−1. Parameters and equations used in this analysis are provided in Supplemental Data, Table S5.
Statistical Analyses
All statistical analyses were performed in R (Version 3.3.3). An analysis of variance (ANOVA) followed by a Tukey’s Honestly Significant Difference (HSD) test was used to evaluate differences in the hepatosomatic index (HSI; liver mass/body mass × 100) among the treatment and control tanks. A multiple regression model was used to test for differences in the slopes (i.e., kBT) of the depuration curves (Equation 1) to evaluate whether EHMC or OCT depuration rate constants differed with respect to dietary exposure concentration. Differences kMET and ED,M with respect to dietary exposure concentration were evaluated by linear regression. Standard errors of ED,M, BMF, k1, BCF, and klumen were propagated according to Gobas et al. (2019). For all analyses, a p-value < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Fish
Three of 126 fish exposed to UVFs died: one each in the OCT low, EHMC low, and EHMC high tanks on days 23, 25, and 28, respectively. No fish died in the control population. Growth rate constants (kG; d−1) determined for each tank did not differ statistically from zero, indicating negligible growth throughout the 28-day study period (Table 2 and Figure S1). Mean HSI values (Table 2) and carcass lipid contents (Table S4) did not differ between control and treatment tanks (p=0.4609 and p=0.3606, respectively). The lipid contents of livers sampled from control fish averaged 3.5 ± 0.2 %. The soma lipid content (ϕBL), determined from the mass of lipid in the liver and carcass samples (Table S4), ranged between 3.5% and 4.6% across control and treatment tanks (Table 2).
Table 2.
General parameters for the treatment and control tanks including mean weights of fish (WB; g), mean hepatosomatic indices (HSI; g liver g fish−1 × 100), mean lipid content of the fish soma (ϕBL; g lipid g fish−1), and growth rate constants (kG; d−1).
| Treatment | WBa,b | HSIa,b | ϕBLb | kGc |
|---|---|---|---|---|
| Control | 37.24 ± 7.64 | 1.23 ± 0.24 | 0.035 ± 0.004 | 0.0099 ± 0.0053 |
| OCT Low | 32.94 ± 5.75 | 1.18 ± 0.13 | 0.036 ± 0.007 | 0.0028 ± 0.0057 |
| OCT Med | 37.04 ± 7.38 | 1.27 ± 0.20 | 0.038 ± 0.006 | 0.0004 ± 0.0063 |
| OCT High | 36.58 ± 9.11 | 1.21 ± 0.23 | 0.038 ± 0.006 | 0.0080 ± 0.0068 |
| EHMC Low | 38.56 ± 5.81 | 1.17 ± 0.33 | 0.042 ± 0.006 | 0.0035 ± 0.0047 |
| EHMC Med | 36.62 ± 5.73 | 1.24 ± 0.18 | 0.046 ± 0.007 | 0.0018 ± 0.0052 |
| EHMC High | 39.92 ± 9.20 | 1.13 ± 0.22 | 0.038 ± 0.010 | 0.0024 ± 0.0073 |
Mean value for all sampled animals during the 28 d exposure.
Error values represent the standard deviation of the mean.
Error values represent the standard error of the estimate.
Whole-body depuration rate constants of EHMC, OCT, and reference chemicals
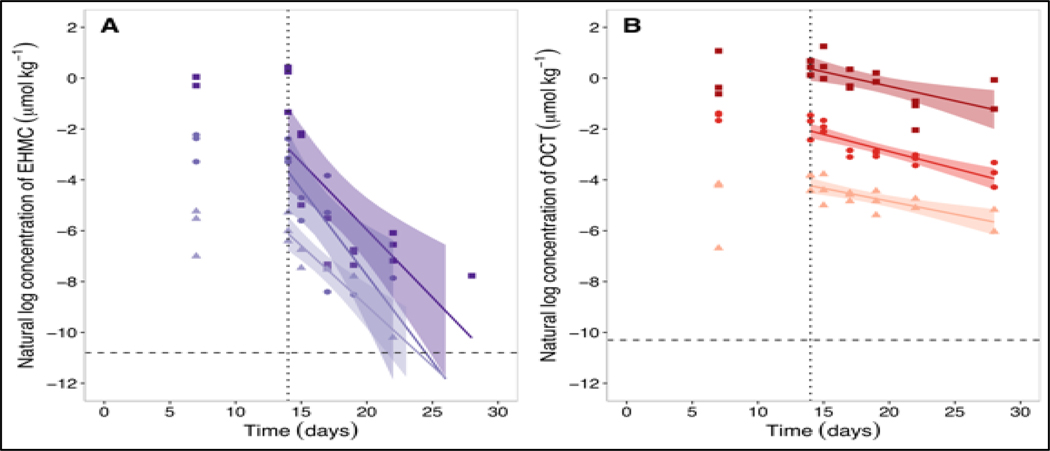
Measured concentrations of EHMC and OCT in fish on day 14 were similar to those on day 7, suggesting that the fish were approaching steady state (Figure 2). After day 14, upon initiation of the depuration phase, concentrations of EHMC and OCT in the This article is protected by copyright. All rights reserved. fish declined in a log-linear manner. Measured concentrations of EHMC and OCT in the soma of control fish were below their MDL. The whole-body depuration rate constants (kBT; Equation 1) calculated for fish exposed to EHMC were (mean ± SE) 0.473 ± 0.078 d−1 (low), 0.680 ± 0.195 d−1 (medium), and 0.532 ± 0.138 d−1 (high). The kBT values for fish exposed to OCT were 0.102 ± 0.023 d−1 (low), 0.134 ± 0.020 d−1 (medium), and 0.114 ± 0.035 d−1 (high). These fitted rate constants did not differ significantly among treatment groups, indicating that for both chemicals the kinetics of depuration were independent of dietary exposure concentration (p = 0.7872 [EHMC] and 0.6700 [OCT]).
Figure 2.
Natural logarithm transformed concentrations of EHMC (A) and OCT (B) in the fish soma throughout the dietary bioaccumulation experiment following exposure to “high” (filled squares), “medium” (filled circles), and “low” (filled triangles) doses. The vertical dotted line represents the beginning of the depuration phase of the experiment. The horizontal dashed line represents the method detection limit (MDL; Table S1).
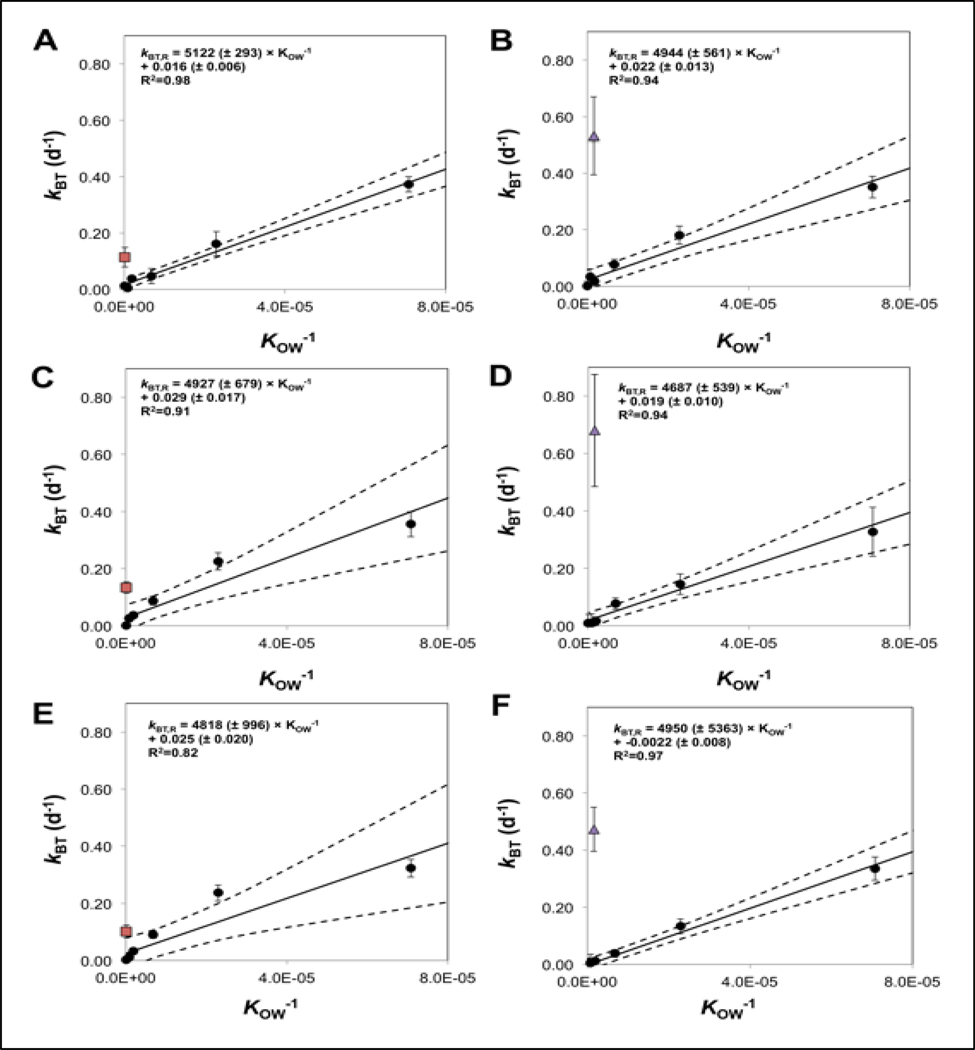
Measured concentrations of the 6 reference chemicals in fish soma increased throughout the 14 d exposure, declining thereafter during the depuration phase (Figure S2). For the most hydrophobic reference chemicals (HCBz, PCB52, and PCB 155) concentrations measured at 14 d were substantially higher than those measured at 7 d, suggesting that fish were far from steady state. By comparison, concentrations measured at 7 and 14 d for 3TCBz, 4TCBz, and PCBz were relatively similar. Measured concentrations of reference chemicals in the soma of control fish were below their MDL. Calculated whole-body depuration rate constants (kBT) for the 6 reference chemicals exhibited an inverse relationship with chemical log KOW (Table S6). Averaged across all 6 treatment tanks, the kBT values were (mean ± SE) 0.344 ± 0.019 d−1, 0.181 ± 0.042 d−1, 0.069 ± 0.022 d−1, 0.026 ± 0.012 d−1, 0.015 ± 0.012 d−1, and 0.0004 ± 0.013 d−1 for 3TCBz, 4TCBz, PCBz, HCBz, PCB 52, and PCB 155, respectively. Measured kBT values for the reference chemicals determined each tank were plotted against the reciprocal of chemical KOW to obtain a set of tank-specific linear relationships that describe the KOW-dependence of chemical depuration that occurs by all non-metabolic pathways (kBT,R; Figure 3).
Figure 3.
Mean (± SE) depuration rate constants for the reference chemicals (filled round circles), OCT (red squares), and EHMC (purple triangles) in the fish soma versus KOW−1. Data generated for the OCT are presented in Panels A (high), C (medium), and E (low) whereas the data generated for EHMC are in Panels B (high), D (medium) and F (low). The solid line represents the model used to fit the depuration rate constant data for the non-biotransformed reference chemicals. The dashed lines represent the 95% confidence intervals for the predicted model values.
Measured kBT values for EHMC and OCT were greater than their corresponding kBT,R values, suggesting a significant contribution of biotransformation to chemical depuration from the fish soma (Figure 3). The kBT,R values determined for a hypothetical reference chemical with a KOW equivalent to that of EHMC were (mean ± SE) 0.006 ± 0.009 d−1 (low), 0.027 ± 0.013 d−1 (medium), and 0.030 ± 0.013 d−1 (high). Calculated in the same manner, the kBT,R values for OCT were 0.025 ± 0.020 d−1 (low), 0.030 ± 0.016 d−1 (medium), and 0.017 ± 0.006 d−1 (high). For EHMC, measured kBT values were approximately 17 to 80-fold greater than corresponding kBT,R values (depending on the tank). The measured kBT values for OCT were approximately 4 to 6-fold greater than corresponding kBT,R values.
Whole-body biotransformation rate constants of EHMC and OCT
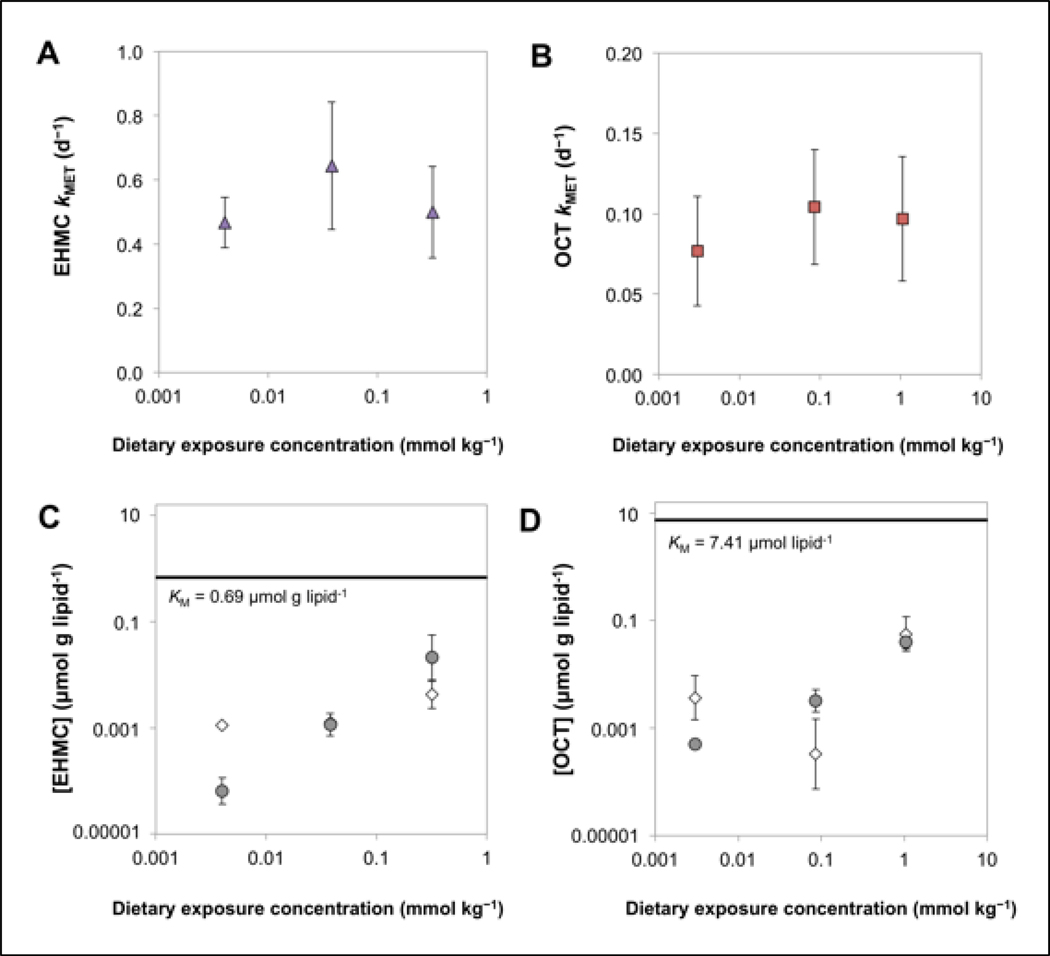
The whole-body biotransformation rate constants (kMET) for EHMC, calculated as the difference between kBT,R and kBT, were (mean ± SE) 0.467 ± 0.078 d−1 (low), 0.653 ± 0.197 d−1 (medium), and 0.502 ± 0.141 d−1 (high; Figure 4A). For OCT, the calculated kMET values were 0.077 ± 0.034 d−1 (low), 0.104 ± 0.036 d−1 (medium), and 0.097 ± 0.039 d−1 (high; Figure 4B). Overall mean (± SE) whole body biotransformation half-lives (i.e., ln(2)/kMET) for EHMC and OCT were 1.31 ± 0.13 d and 7.60 ± 0.71 d, respectively. There was no significant relationship between kMET and dietary exposure concentrations of EHMC (p = 0.8429) or OCT (p = 0.7820), suggesting that UVF concentrations in the fish were not high enough to saturate biotransformation enzymes. Previously, Saunders et al. (2019) determined KM values for EHMC and OCT using a trout liver S9 system and expressed these values on a g lipid−1 basis. For EHMC, the lipid-normalized KM was 0.69 μmol g lipid−1, while that determined for OCT was 7.41 μmol g lipid−1. In either case, these lipid-normalized KM values are substantially higher than lipid-normalized chemical concentrations measured in the present study in fish soma or liver (Figure 4C and D). Taken together, these findings suggest that biotransformation of EHMC and OCT at all dietary dosing levels was occurring under near first-order conditions (i.e., CB or CL << KM).
Figure 4.
Whole-body biotransformation rate constants (kMET) for EHMC (A) and OCT (B) as a function of dietary exposure concentration. Mean (± standard deviation) lipid-normalized concentrations of EHMC (C) and OCT (D) that accumulated in the fish soma (circles) and liver (diamonds) following dietary exposure. The horizontal solid lines represents the Michaelis-Menten constant (KM) previously measured in liver S9 fractions by Saunders et al. 2019.
The assumption that chemical uptake and elimination processes exhibit first order kinetics is thought to be appropriate to describe accumulation of neutral organic chemicals in animals exposed to the relatively low concentrations in most field scenarios (Kim et al. 2016). The highest dietary concentrations of EHMC and OCT evaluated in the present study (95 and 380 mg kg food−1, respectively) are substantially higher than measured concentrations in field-collected aquatic biota (Gago-Ferrero et al. 2012), but within the range of spiking concentrations recommended in the OECD 305 protocol (1 to 1000 mg kg food−1; OECD 2012). For EHMC and OCT, current OECD guidelines appear to provide recommended test concentrations that avoid saturation of biotransformation enzymes in vivo.
In other cases, however, saturation of biotransformation enzymes in laboratory exposures and field settings remains a possibility. For example, previously reported KM values for several polycyclic aromatic hydrocarbons are up to an order of magnitude lower (Lo et al. 2015b; Nichols et al. 2018) than the KM values generated for EHMC and OCT (Saunders et al. 2019). Nichols et al. (2018) compared the measured KM for pyrene, expressed on a free chemical basis, to aqueous chemical concentrations commonly employed in standardized BCF testing efforts (i.e., 1/100th lethal levels). The results of this analysis suggested that pyrene concentrations in fish during in vivo testing may approach levels associated with enzyme saturation, potentially resulting in concentration-dependent accumulation. For such chemicals, it may be necessary to perform in vivo exposures at concentrations close to environmental concentrations so that the laboratory data can be extrapolated to field scenarios with greater confidence (Oliver and Niimi 1985).
Luminal biotransformation rate constants of EHMC and OCT
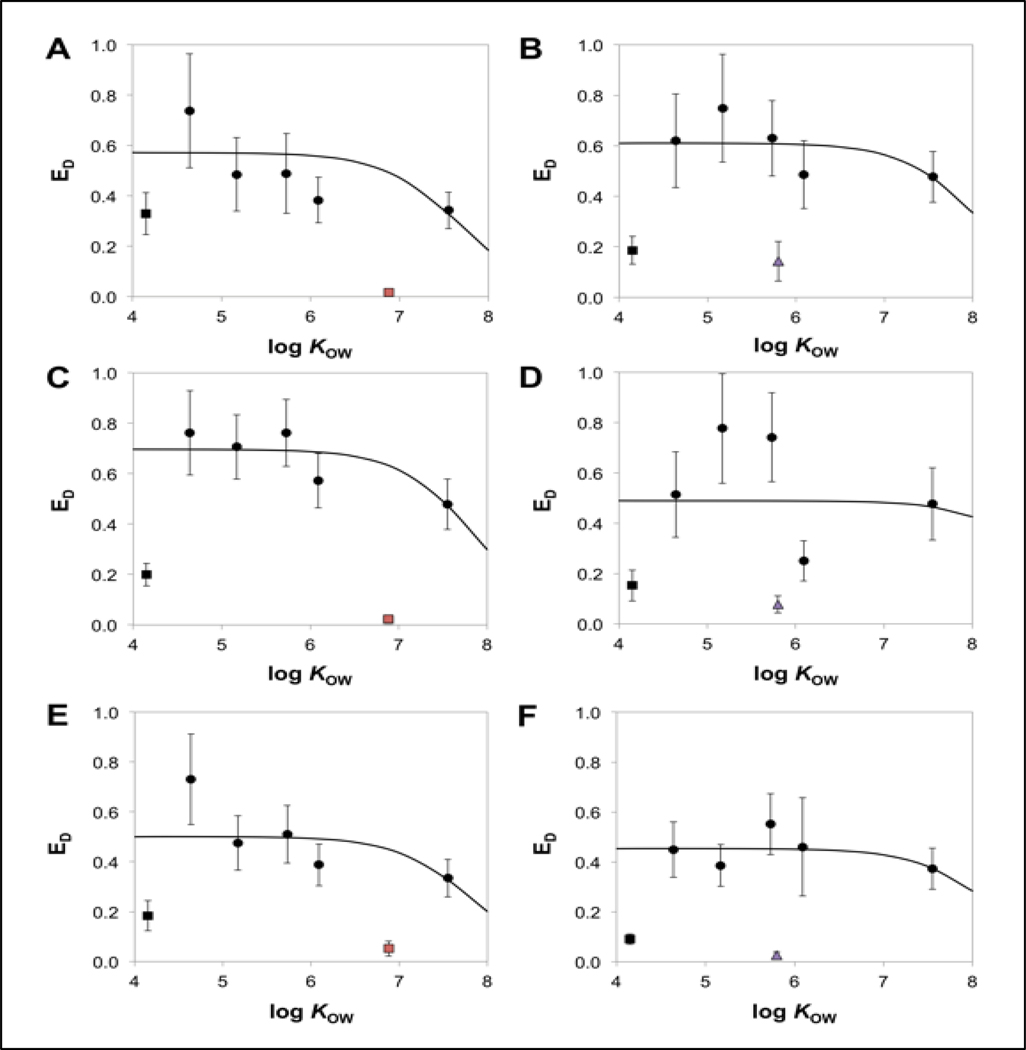
Measured dietary uptake efficiencies for 5 of the 6 reference chemicals were used to develop a set of weighted non-linear regressions that relate estimated dietary uptake efficiencies for non-biotransformed reference chemicals (ED,R) to chemical log KOW (Figure 5 and Table S7). The reference chemical 3TCBz was excluded from this analysis because the mean (± SE) ED,M for all tanks (19 ± 3.2%; n=6) was substantially lower than that determined for the other reference chemicals. This lower-than-expected ED,M may have been due to biotransformation of 3TCBz in the intestinal lumen of fish. If this was the case, 3TCBz may be a poor reference chemical to include in future investigations. The resulting non-linear regressions plateaued at maximal ED,R values ranging from approximately 44% to 69% for non-biotransformed reference chemicals with log KOW values between 4 and 7 (Figure 5).
Figure 5.
Mean (± SE) dietary uptake efficiencies of the reference chemicals (black data points), OCT (red squares), and EHMC (purple triangles) versus log KOW (error bars represent the standard error of the estimate). Data generated for the OCT are presented in Panels A (high), C (medium), and E (low) whereas the data generated for EHMC are in Panels B (high), D (medium) and F (low). The solid line represents nonlinear regression fit (Equation 9) to the dietary uptake efficiency data for 5 of 6 reference chemicals (black circles) and excludes 3TCBz (black square).
For all of the treatment tanks, the mean ED,R for PCB 155 (log KOW = 7.55) was lower than that determined for the other 4 reference chemicals (Figure 5). This finding is consistent with previous data indicating that ED,R values for fish decline with increasing log KOW at log KOW values greater than 7 (Gobas et al. 1988; Lo et al. 2015a; Arnot and Mackay 2018). However, the extent of this decline varied among the treatment tanks. It is possible that variability in the measurement of CD and CB could contribute to a higher estimate of ED,R for PCB 155. Also, the method used here to calculate ED,R requires an estimate of kBT (Equation 5). For very hydrophobic chemicals such as PCB 155, kBT is difficult to estimate because the rate of elimination is very slow. Extending the depuration period beyond 14 d would have addressed this issue, but there is a limit to which this can be done given the need to simultaneously measure kBT values for lower log KOW reference chemicals and for test chemicals that undergo biotransformation.
The ED,M values generated for EHMC and OCT fell well below the non-linear regression fit of ED,R and ranged between 2.7% and 14% for EHMC and between 2.4% and 5.2% for OCT (Figure 5 and Table 3). These low ED,M values may reflect significant biotransformation of EHMC and OCT in the lumen of the gastrointestinal tract (Lo et al. 2015a; Lo et al. 2016). There was no significant relationship between ED,M and dietary exposure concentration for EHMC (p = 0.2261) or OCT (p = 0.4856). Modeled luminal biotransformation rate constants (klumen) ranged between 10 d−1 and 35 d−1 for EHMC and between 17 d−1 and 86 d−1 for OCT (Table 3). For EHMC and OCT, the rates of biotransformation expressed in units of μmol d−1 in the lumen (i.e., klumen × MG) were up to 110-fold greater than biotransformation rates determined in the fish soma (i.e., kMET × MB; Table S8). Following dietary exposure, the relative contribution of luminal biotransformation (Φlumen) to total biotransformation was as high as 97% and 99% for EHMC and OCT, respectively (Equation 12; Table 3). One possible explanation for this apparent high level of biotransformation in the lumen is that gut microflora hydrolyze ester groups present on EHMC and OCT.
Table 3.
Empirical dietary uptake efficiencies (ED,M; unitless), estimated dietary uptake efficiencies of a non-biotransformed chemical of equivalent Kow (ED,R; unitless), luminal biotransformation rate constants (klumen; d−1) and proportions of total mass transformed in the lumen (Φlumen; unitless), and the fish soma (Φsoma; unitless) for EHMC and OCT following dietary exposure to low, medium, and high dose treatments. Error values represent the standard error.
| Treatment | Ed,m | Ed,r | klumen | Φlumen | Φsoma |
|---|---|---|---|---|---|
| OCT | |||||
| Low | 0.052 ± 0.030 | 0.450 ± 0.057 | 17 ± 12 | 0.97 | 0.02 |
| Medium | 0.024 ± 0.008 | 0.633 ± 0.041 | 86 ± 33 | 0.99 | 0.01 |
| High | 0.016 ± 0.007 | 0.446 ± 0.049 | 59 ± 30 | 0.99 | 0.01 |
| EHMC | |||||
| Low | 0.027 ± 0.011 | 0.452 ± 0.072 | 35 ± 19 | 0.97 | 0.03 |
| Medium | 0.078 ± 0.034 | 0.489 ± 0.130 | 13 ± 10 | 0.91 | 0.09 |
| High | 0.143 ± 0.078 | 0.608 ± 0.052 | 10 ± 8 | 0.85 | 0.15 |
Chemical biotransformation processes in the lumen and epithelial tissues of the gastrointestinal tract of fish have been shown to reduce chemical uptake from the diet (Van Veld et al. 1988; Kleinow et al. 1998) and may substantially reduce chemical bioaccumulation in fish (Lo et al. 2015a; Lo et al. 2016; Arnot and Mackay 2018). Luminal biotransformation rate constants derived here and elsewhere (Lo et al. 2015a; Lo et al. 2016) suggest that biotransformation in the gut lumen may contribute more to the overall biotransformation of some dietary contaminants than does somatic biotransformation. In combination with hepatic in vitro bioassays for estimating whole-body biotransformation rate constants, the development of in vitro assays for estimating intestinal biotransformation rates may provide additional screening tools needed to improve chemical bioaccumulation assessments. This could include in vitro assays performed using collected gut contents and/or cultured gut microflora, as well as assays that employ cultured epithelial cells and/or epithelial subcellular fractions.
Bioaccumulation potential of EHMC and OCT
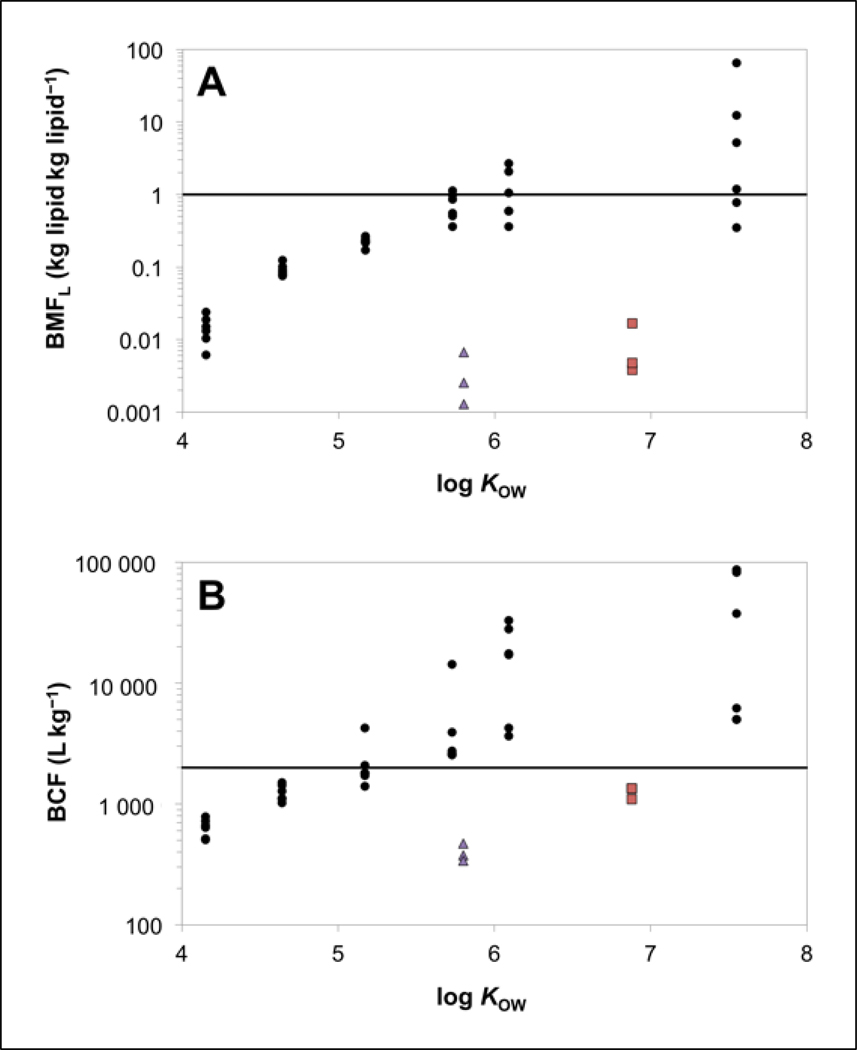
Calculated respiratory uptake rate constants (k1), BCF values, and lipid-normalized biomagnification factors (BMF) for the reference chemicals, EHMC, and OCT are provided in Supplemental Data, Table S7. The BMF values obtained for the 6 reference chemicals increased with increasing log KOW. A BMF exceeding 1.0 kg lipid kg lipid−1 is indicative of probable bioaccumulation potential (Gobas et al. 2009). The average BMF for PCB155, calculated across all tanks was (mean ± SE, n=6) 14 ± 10 kg lipid kg lipid−1. Calculated BMF values exceeding 1.0 kg lipid kg lipid−1 were noted for HCBz and PCB 52 in at least one of the six treatment tanks.
The calculated BMF values for EHMC were (mean ± SE) 0.0013 ± 0.0041 kg lipid kg lipid−1 (low), 0.0026 ± 0.040 kg lipid kg lipid−1 (medium), and 0.0067 ± 0.0275 kg lipid kg lipid−1 (high), while those determined for OCT were 0.0167 ± 0.004 kg lipid kg lipid−1 (low), 0.0048 ± 0.001 kg lipid kg lipid−1 (medium), and 0.0038 ± 0.001 kg lipid kg lipid−1 (high). All BMF values generated for EHMC and OCT were approximately two orders of magnitude lower than those obtained for reference chemicals with similar log KOW values (Figure 6A), and in each case were far below 1.0. This result illustrate how somatic biotransformation and luminal biotransformation can act to prevent biomagnification of chemicals taken up from the diet.
Figure 6.
Lipid normalized biomagnification factors (BMF; Panel A) and bioconcentration factors (BCF; Panel B) for reference chemicals (black circles), EHMC (purple triangles), and OCT (red squares) compared to log KOW. The horizontal solid lines represent bioaccumulation criteria of BMF of 1.0 kg lipid kg lipid −1 (Panel A) and BCF of 2000 L kg−1 (Panel B).
Few experimental BMF data for EHMC and OCT are available in the literature. For OCT, the BMF values calculated in the present study were consistent with a previously reported laboratory-derived BMF of 0.034 kg lipid kg lipid−1 (Pawlowski et al. 2019). However, BMF values calculated in the present study for EHMC and OCT were substantially lower than those determined in field-collected fish (BMF ≥ 1.0; Table 1). The higher lipid-normalized BMFs determined in field-collected fish may be due to a lower biotransformation capacity in the selected fish species. Other factors such as inadequate characterization of fish prey items or fish migration patterns can influence BMF values determined in field studies (Kidd et al. 2018). Additionally, spatial and temporal heterogeneity of EHMC and OCT concentrations in water could contribute to overestimation of true BMF values if fish were collected from areas where concentration gradients and/or seasonal fluctuations exist (Kim et al. 2016; Pawlowski et al. 2019). Based on laboratory-collected BMF data presented here and elsewhere (Pawlowski et al. 2019), we conclude that EHMC and OCT have a low potential to biomagnify in fish.
The respiratory uptake constants (k1; Table S7) determined for EHMC were (mean ± SE) 231 ± 17 L kg−1 d−1 (low), 240 ± 19 L kg−1 d−1 (medium), and 209 ± 24 L kg−1 d−1 (high), while those calculated for OCT were 217 ± 45 L kg−1 d−1 (low), 208 ± 29 L kg−1 d−1 (medium), and 242 ± 14 L kg−1 d−1 (high).
Bioconcentration factors (BCF) for the reference chemicals increased with increasing log KOW (Figure 6B). When the BCFs were averaged across treatment tanks, mean BCFs ranged from 641 ± 45 (SE, n=6) for 3TCBz to 37,359 ± 15,965 (SE, n=6) for PCB 155. Bioconcentration factors calculated for EHMC were (mean ± SE) 471 ± 77 L kg−1 (low), 340 ± 97 L kg−1 (medium), and 379 ± 98 L kg−1 (high), while those determined for OCT were 1345 ± 298 L kg−1 (low), 1105 ± 163 L kg−1 (medium), and 1345 ± 298 L kg−1 (high). In each case, BCFs for EHMC and OCT are approximately one to two orders of magnitude lower than the BCFs generated for reference chemicals with similar log KOW values. The comparatively lower BCF values generated for EHMC and OCT illustrates the influence of somatic biotransformation in reducing bioconcentration. When fish are exposed via the respiratory route, somatic contributes up to 99% and 93% of total biotransformation for EHMC and OCT, respectively (Equation 11; Table S8). The results also suggest that whole-body biotransformation rates are sufficient to reduce BCFs for EHMC and OCT below the European Union Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH) regulation criterion for bioaccumulative substances (2000 L kg−1; Figure 6B). Bioconcentration factors determined for EHMC were in good agreement with the empirical range of 175–433 L kg−1 measured in rainbow trout (Table 1), while those determined for OCT were only marginally higher than the upper range of empirical BCFs reported in zebrafish (41–972 L kg−1; Table 1).
SUMMARY AND CONCLUSIONS
The hydrophobic organic ultraviolet filters EHMC and OCT were biotransformed by rainbow trout following dietary exposure. Estimated whole-body biotransformation rate constants were independent of dietary exposure concentration. Lipid-normalized chemical concentrations in fish soma or liver were also much lower than previously generated KM values (i.e., CB or CL << KM). Collectively, these observations suggest that somatic biotransformation of EHMC and OCT was occurring under near first-order conditions. In addition to being biotransformed in the soma, a model-based evaluation of dietary uptake data suggested that metabolic activity in the gut lumen contributes substantially to biotransformation of EHMC and OCT. Somatic and luminal biotransformation greatly reduce the potential for bioaccumulation of EHMC and OCT in trout. Modeled BMFs and BCFs generated for both chemicals were one to two orders of magnitude lower than BMFs and BCFs generated for reference chemicals of similar log KOW. Additionally, for both chemicals, BMFs and BCFs fell below established bioaccumulation criteria (1.0 kg lipid kg lipid −1 and 2000 L kg−1, respectively), suggesting that EHMC and OCT are unlikely to pose a bioaccumulation hazard in rainbow trout.
Supplementary Material
Footnotes
Data availability—Data, associated metadata, and calculation tools are available from the corresponding author (gobas@sfu).
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/etc.4638.
REFERENCES
- Alonso MB, Feo ML, Corcellas C, Gago-Ferrero P, Bertozzi CP, Marigo J, Flach L, Meirelles ACO, Carvalho VL, Azevedo AF, et al. 2015. Toxic heritage: Maternal transfer of pyrethroid insecticides and sunscreen agents in dolphins from Brazil. Environ Pollut 207:391–402. [DOI] [PubMed] [Google Scholar]
- Arnot JA, Gobas F. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22:337–345. [Google Scholar]
- Arnot JA, Gobas FAPC 2004. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environ Toxicol Chem 23:2343–2355. [DOI] [PubMed] [Google Scholar]
- Arnot JA, Mackay D. 2018. The influence of chemical degradation during dietary exposures to fish on biomagnification factors and bioaccumulation factors. Environ Sci Process Impacts 20:86–97. [DOI] [PubMed] [Google Scholar]
- Bachelot M, Li Z, Munaron D, Le Gall P, Casellas C, Fenet H, Gomez E. 2012. Organic UV filter concentrations in marine mussels from French coastal regions. Sci Total Environ 420:273–279. [DOI] [PubMed] [Google Scholar]
- Balmer ME, Buser H-R, Müller MD, Poiger T. 2005. Occurrence of some organic UV Filters in wastewater, in surface waters, and in fish from Swiss lakes. Environ Sci Technol 39:953–962. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can JBiochem Phys 37:911–917. [DOI] [PubMed] [Google Scholar]
- Blüthgen N, Meili N, Chew G, Odermatt A, Fent K. 2014. Accumulation and effects of the UV-filter octocrylene in adult and embryonic zebrafish (Danio rerio). Sci Total Environ 476-477:207–217. [DOI] [PubMed] [Google Scholar]
- Burkhard LP. 2000. Estimating dissolved organic carbon partition coefficients for nonionic organic chemicals. Environ Sci Technol 34:4663–4668. [Google Scholar]
- Buser H-R, Balmer ME, Schmid P, Kohler M. 2006. Occurrence of UV Filters 4-methylbenzylidene camphor and octocrylene in fish from various Swiss rivers with inputs from wastewater treatment plants. Environ Sci Technol 40:1427–1431. [DOI] [PubMed] [Google Scholar]
- Chamkasem N, Lee S, Harmon T. 2016. Analysis of 19 PCB congeners in catfish tissue using a modified QuEChERS method with GC-MS/MS. Food Chem 192:900–906. [DOI] [PubMed] [Google Scholar]
- Christen V, Zucchi S, Fent K. 2011. Effects of the UV-filter 2-ethyl-hexyl-4-trimethoxycinnamate (EHMC) on expression of genes involved in hormonal pathways in fathead minnows (Pimephalespromelas) and link to vitellogenin induction and histology. Aquat Toxicol 102:167–176. [DOI] [PubMed] [Google Scholar]
- Fent K, Zenker A, Rapp M. 2010. Widespread occurrence of estrogenic UV-filters in aquatic ecosystems in Switzerland. Environ Pollut 158:1817–1824. [DOI] [PubMed] [Google Scholar]
- Gago-Ferrero P, Alonso MB, Bertozzi CP, Marigo J, Barbosa L, Cremer M, Secchi ER, Domit C, Azevedo A, Lailson-Brito J, et al. 2013. First determination of UV filters in marine mammals. Octocrylene levels in Franciscana dolphins. Environ Sci Technol 47:5619–5625. [DOI] [PubMed] [Google Scholar]
- Gago-Ferrero P, Díaz-Cruz MS, Barceló D. 2012. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. AnalBioanal Chem 404:2597–2610. [DOI] [PubMed] [Google Scholar]
- Gago-Ferrero P, Díaz-Cruz MS, Barcelo D. 2015. UV filters bioaccumulation in fish from Iberian river basins. Sci Total Environ 518-519:518–525. [DOI] [PubMed] [Google Scholar]
- Gobas FAPC, Muir DCG, Mackay D 1988. Dynamics of dietary bioaccumulation and faecal elimination of hydrophobic organic chemicals in fish. Chemosphere 17:943–962. [Google Scholar]
- Gobas FAPC, Wilcockson JWB, Russell RW, Haffner GD 1999. Mechanism of biomagnification in fish under laboratory and field conditions. Environ Sci Technol 33:133–141. [Google Scholar]
- Gobas FAPC, de Wolf W, Burkhard LP, Verbruggen E, Plotzke K 2009. Revisiting bioaccumulation criteria for POPs and PBT assessments. Integr Environ Assess Manag 5:624–637. [DOI] [PubMed] [Google Scholar]
- Gobas FAPC, Lo JC 2016. Deriving bioconcentration factors and somatic biotransformation rates from dietary bioaccumulation and depuration tests. Environ Toxicol Chem 35:2968–2976. [DOI] [PubMed] [Google Scholar]
- Gobas FAPC, Lee YS, Lo JC, Parkerton TF, Letinski DJ 2019. A toxicokinetic framework and analysis tool for interpreting 0ECD-305 dietary bioaccumulation tests. Environ Toxicol Chem. DOI: 10.1002/etc.4599. [DOI] [PubMed] [Google Scholar]
- Kidd KA, Burkhard LP, Babut M, Borgå L, Muir DCG, Perceval O, Ruedel H, Woodburn K, Embry MR. 2018. Practical advice for selecting or determining trophic magnification factors for application under the European Union Water Framework Directive. Integr Environ Assess Manag 15:266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Gobas FAPC, Arnot JA, Powell DE, Seston RM, Woodburn KB 2016. Evaluating the roles of biotransformation, spatial concentration differences, organism home range, and field sampling design on trophic magnification factors. Sci Total Environ 551-552:438–451. [DOI] [PubMed] [Google Scholar]
- Kleinow KM, James MO, Tong Z, Venugopalan CS. 1998. Bioavailability and biotransformation of benzo(a)pyrene in an isolated perfused in situ catfish intestinal preparation. Environ Health Perspect 106:155–166.9449680 [Google Scholar]
- Koenig S, Fernández P, Solé M. 2012. Differences in cytochrome P450 enzyme activities between fish and crustacea: relationship with the bioaccumulation patterns of polychlorobiphenyls (PCBs). Aquat Toxicol. 108:11–17. [DOI] [PubMed] [Google Scholar]
- Lo JC, Campbell DA, Kennedy CJ, Gobas FAPC 2015a. Somatic and gastrointestinal in vivo biotransformation rates of hydrophobic chemicals in fish. Environ Toxicol Chem 34:2282–2294. [DOI] [PubMed] [Google Scholar]
- Lo JC, Allard GN, Otton SV, Campbell DA, Gobas FAPC 2015b. Concentration dependence of biotransformation in fish liver S9: Optimizing substrate concentrations to estimate hepatic clearance for bioaccumulation assessment. Environ Toxicol Chem 34:2782–2790. [DOI] [PubMed] [Google Scholar]
- Lo JC, Letinski DJ, Parkerton TF, Campbell DA, Gobas FAPC 2016. In vivo biotransformation rates of organic chemicals in fish: Relationship with bioconcentration and biomagnification factors. Environ Sci Technol 50:13299–13308. [DOI] [PubMed] [Google Scholar]
- Nagtegaal M, Ternes TA, Baumann W, Nagel R. 1997. UV-filtersubstanzen in wasser und fischen. UWSF 9:79–86. [Google Scholar]
- Nichols JW, Ladd MA, Fitzsimmons PN. 2018. Measurement of kinetic parameters for biotransformation of polycyclic aromatic hydrocarbons by trout liver S9 fractions: Implications for bioaccumulation assessment. ApplIn Vitro Toxicol. 4:365–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver BG, Niimi AJ. 1985. Bioconcentration factors of some halogenated organics for rainbow trout: Limitations in their use for prediction of environmental resides. Environ Sci Technol 19:842–849. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation (OECD). 2012. Test No. 305: Bioaccumulation in fish: Aqueous and dietary exposure, OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris. [Google Scholar]
- Pawlowski S, Lanzinger AC, Dolich T, Füpi S, Salinas ER, Zok S, Weiss B, Hefner N, Sloun PV, Hombeck H, Klingelmann, Petersen-Thiery M 2019. Evaluation of the bioaccumulation of octocrylene after dietary and aqueous exposure. Sci Total Environ 672:669–679. [DOI] [PubMed] [Google Scholar]
- Peng X, Fan Y, Jin J, Xiong S, Liu J, Tang C 2017. Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea. Environ Pollut 225: 55–66. [DOI] [PubMed] [Google Scholar]
- Picot Groz M, Martinez Bueno MJ, Rosain D, Fenet H, Casellas C, Pereira C, Maria V, Bebianno MJ, Gomez E. 2014. Detection of emerging contaminants (UV filters, UV stabilizers and musks) in marine mussels from Portuguese coast by QuEChERS extraction and GC-MS/MS. Sci Total Environ 493:162–169. [DOI] [PubMed] [Google Scholar]
- Saunders LJ, Fontanay S, Nichols JW, Gobas FAPC 2019. Concentration dependence of in vitro biotransformation rates of hydrophobic organic sunscreen agents in rainbow trout S9 fractions: Implications for bioaccumulation assessment. Environ Toxicol Chem 38:548–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigma-Aldrich 2014. Safety Data Sheet: 2-Ethylhexyl 2-cyano-3,3-diphenylacrylate [Cited 2017 Sept 23] Available from: https://www.sigmaaldrich.com/catalog/product/aldrich/415820
- Tang Z, Zhong F, Cheng J, Nie Z, Han X, Han Y, Yang Y. 2019. Concentrations and tissue-specific distributions of organic ultraviolet absorbents in wild fish from a large subtropical lake in China. Sci Total Environ 647:1305–1313 [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency. 2012. Estimation Programs Interface Suite for Microsoft Windows, v. 4.11. Washington, DC [cited 2019 March 7]. Available from: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface
- US Environmental Protection Agency. 2018. ECOTOX User Guide: ECOTOXicology Knowledgebase System. Version 5.0 [cited 2019 March 15]. Available from: http:/www.epa.gov/ecotox
- US National Library of Medicine. 2006. TOXNET Hazardous Substances Data Bank. Washington, DC. [cited 2018 February 30]. Available from: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- Van Veld PA, Stegeman JJ, Woodin BR, Patton JS, Lee RF 1988. Induction of monooxygenase activity in the intestine of spot (Leiostomus xanthurus), a marine teleost, by dietary polycyclic aromatic hydrocarbons. DrugMetab Dispos 16:659–665. [PubMed] [Google Scholar]
- Zenker A, Schmutz H, Fent K. 2008. Simultaneous trace determination of nine organic UV-absorbing compounds (UV filters) in environmental samples. J Chromatogr A 1202:64–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.