Abstract

Protein and peptide therapeutics tend to have a short blood circulation time mainly caused by rapid clearance in kidney, leading to a low therapeutic efficacy. Here, we demonstrate that the antitumor activity of a small-sized protein binder can be significantly enhanced by prolonged blood half-life through site-specific lipidation. An unnatural amino acid was genetically incorporated into a specific site with the highest accessibility in a human interleukin-6 (IL-6)-targeting protein binder with a size of 30.8 kDa, followed by conjugation with palmitic acid using cooper-free click chemistry. The resulting protein binder was shown to have a binding capacity for serum albumin, maintaining a comparable binding affinity for human IL-6 to the native protein binder. The terminal half-life of the lipidated protein binder was estimated to be 10.7 h, whereas the native one had a half-life of 20 min, resulting in a significantly enhanced tumor suppression effect. The present approach can be generally applied to small-sized therapeutic proteins for the elongation of circulation time and increase of bioavailability in blood, consequently enhancing their therapeutic efficacy.
1. Introduction
For decades, proteins and peptides with small size have been widely used in number of diseases.1−3 Despite high therapeutic potency, however, their small size was known to undergo a fast renal clearance and poor biodistribution profile, which often result in frequent dosing and low therapeutic efficacy.4,5 In an efforts to overcome poor pharmacokinetic profiles of small-sized therapeutic proteins, various strategies have been suggested.6,7 Either serum albumin or Fc of immunoglobulin was conjugated to therapeutic proteins through genetic fusion or chemical methods for the FcRn-mediated recycling.8−14 PEGylation, biotinylation, and multimerization are also widely used to increase the hydrodynamic radius of such proteins.15−22 A natural binder of serum albumin, fatty acid, was conjugated to a glucagon-like peptide-1 (GLP-1) analogue using a chemically synthesized short-length peptide, significantly extending its blood half-life.23−25 Some factors affecting the biological activity and half-life of a therapeutic protein in fatty acid conjugation were investigated.26−28 Even though such methods have been widely employed, some shortcomings still remain to be improved. Chemical conjugation or multimerization can lead to heterogeneous products, and the resulting proteins often undergo a steric hindrance for a binding to their target molecules.15,21 Genetic fusion with serum proteins or Fc is expensive and complicated because of its requirement of mammalian expression system.29,30 Furthermore, genetic fusion or chemical conjugation with exogenous proteins often causes unwanted immunogenicity.31−33 In particular, chemical conjugation methods tend to need complex chemical reaction steps, which result in high heterogeneity and a low yield.
Herein, we show that site-specific lipidation of a protein binder with low molecular weight through incorporation of an unnatural amino acid significantly enhances the blood circulation time and consequently the antitumor activity in vivo. As a proof-of concept, a previously developed human IL-6 targeting protein binder (∼31 kDa) was employed.34 A specific site of the protein binder with highest accessibility was selected and incorporated with an unnatural amino acid, 4-azido-l-phenylalanine, followed by conjugation with palmitic acid through copper-free click chemistry (Figure 1). The property of the lipidated protein binder was investigated in terms of binding ability for serum albumin and human IL-6. The utility of our approach was shown by a significantly prolonged half-life and enhanced tumor suppression in the xenograft mice model. Details are reported herein.
Figure 1.
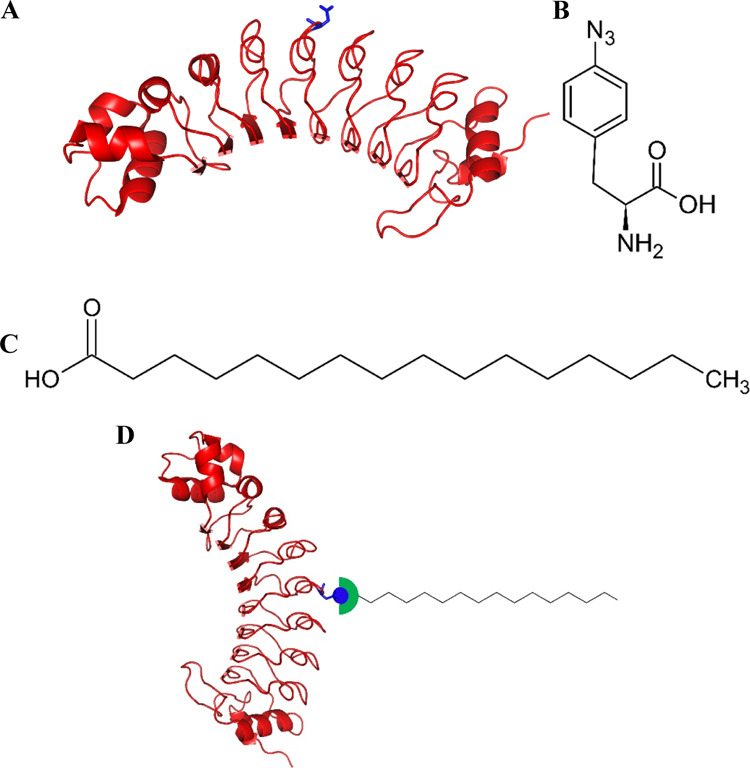
Site-specific lipidation of a human IL6-targeting repebody. (A) Structure of the repebody (PDB ID: 4J4L) and a lipidation site on its convex region indicated by a blue color. (B) Chemical structure of 4-azido-l-phenylalanine. (C) Chemical structure of palmitic acid. DBCO-palmitic acid was used for conjugation with 4-azido-l-phenylalanine through copper-free click chemistry. Chemical structures were drawn by using ChemSketch. (D) Schematic diagram of site-specifically lipidated repebody.
2. Results and Discussion
2.1. Construction of an Unnatural Amino Acid-Incorporated Repebody
As a model, a previously developed human IL-6-specific repebody (31 kDa) was employed. To select a site on the repebody for fatty acid conjugation, a relative surface accessibility was assessed for each amino acid residue based on the repebody structure (PDB ID: 4J4L) using a web server.35 As a result, aspartic acid at position 126 on the convex region was selected (Figure S1), and its codon was mutated to TAG (Figure S2). With an amber codon-recognizing tRNA synthetase, 4-azido-l-phenylalanine (AzF) was incorporated into the position 126 on the repebody. The resulting repebody (AzF-Rb) was expressed in Origami B (DE3) as a soluble form and further purified for high homogeneity (Figure S3).
For efficient conjugation of a fatty acid to the human IL-6-specific repebody, the azido group of 4-azido-l-phenylalanine on the repebody should be accessible and functionally active. We checked the functionality of azido group on the repebody by conjugation of various molecules. PEG (MW 5 kDa) was tested, and PEGylation was confirmed by a shift in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis (Figure S4). When DBCO-modified Cy3 dye was conjugated, a distinct fluorescence signal was detected by gel imaging (Figure S5). In addition, biotin was treated with the repebody, and biotinylated repebody was detected by HRP-conjugated streptavidin (Figure S6). These results indicate that the azido group of 4-azido-l-phenylalanine incorporated into the repebody is accessible and functionally active to be conjugated with PEG, fluorescent dye, and biotin.
2.2. Site-Specific Conjugation of a Fatty Acid
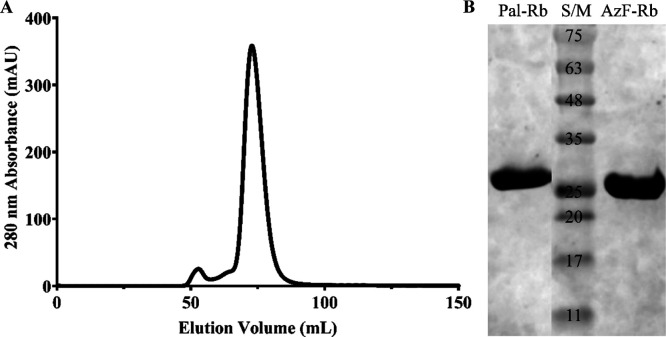
Fatty acid is known to bind to a serum albumin through hydrophobic interaction.36−40 To extend the blood circulation time of a repebody, we conjugated palmitic acid (14-carbon length) to 4-azido-l-phenylalanine on the repebody through copper-free click chemistry. The resulting repebody (Pal-Rb) showed a single large peak by size exclusion chromatography (Figure 2A) and high purity with expected molecular weight (31.5 kDa) by SDS-PAGE (Figure 2B). The Pal-Rb showed a distinct size shift in SDS-PAGE compared to the 4-azido-l-phenylalanine-incorporated repebody (AzF-Rb). These results indicate a site-specific conjugation of palmitic acid and homogeneity of the lipidated repebody.
Figure 2.
Purification and analysis of a palmitic acid-conjugated repebody. (A) Elution profile of a palmitic acid-conjugated repebody (Pal-Rb) by size exclusion chromatography. (B) SDS-PAGE analysis of Pal-Rb. The Pal-Rb band was shifted compared to the 4-azido-l-phenylalanine-incorporated repebody (AzF-Rb).
2.3. Binding Characteristics of a Lipidated Repebody
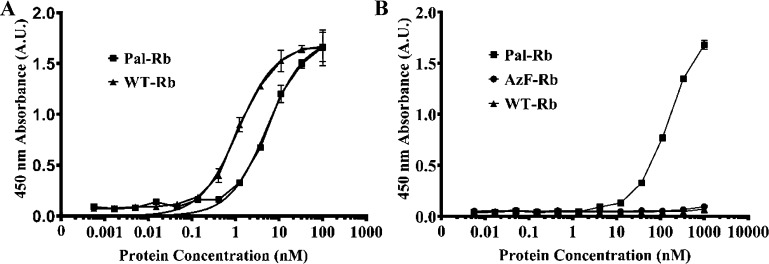
We checked the binding capacity of Pal-Rb for a target protein, human IL-6, using direct ELISA. It is desirable that conjugation of a fatty acid has no effect on the binding ability of the repebody. A 96-well plate was coated with human IL-6, followed by addition of a serially diluted Pal-Rb or wild-type repebody (WT-Rb). Amount of target-bound repebody was detected by biotin-conjugated antirepebody antibody and HRP-labeled streptavidin. As a result, Pal-Rb showed a 4-fold decreased binding affinity compared to WT-Rb (Figure 3A), and this seems to be because of a steric hindrance caused by a conjugated-palmitic acid.
Figure 3.
Binding profiles of a palmitic acid-conjugated repebody (Pal-Rb) against human IL-6 and MSA. (A) Binding profile of Pal-Rb against human IL-6. A serially diluted Pal-Rb was added to a human IL-6-coated 96-well plate, and the amount of bound Pal-Rb was measured by direct ELISA. As a control, wild-type human IL-6-specific repebody (WT-Rb) was used. (B) Binding profile of Pal-Rb against MSA. A serially diluted Pal-Rb was added to an MSA-coated 96-well plate, and the amount of bound Pal-Rb was measured by ELISA. WT-Rb and AzF-Rb were used as controls. Error bars describe mean ± standard deviation (n = 3).
We next tested whether a repebody-conjugated palmitic acid has a binding capacity for mouse serum albumin (MSA). Pal-Rb, AzF-Rb, or WT-Rb was serially diluted and added to a 96-well plate, which had been coated with MSA, followed by analysis of the bound repebody (Figure 3B). Pal-Rb was shown to have a distinct binding ability for MSA, whereas both WT-Rb and AzF-Rb displayed a negligible binding capacity. These results indicate that site-specifically conjugated palmitic acid can bind to a MSA and therefore might lead to longer circulation time in blood by reducing renal clearance and FcRn-mediated recycle. A slight decrease in binding affinity of a Pal-Rb is expected to be compensated by its prolonged blood circulation time in terms of therapeutic efficacy.
2.4. Pharmacokinetic Profile of a Lipidated Repebody
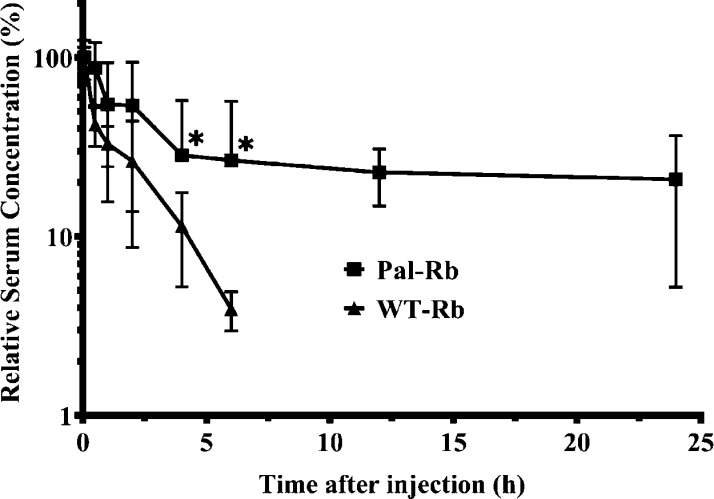
We investigated the pharmacokinetic profile of a palmitic acid-conjugated repebody (Pal-Rb) in mice (Figure 4). Each construct was injected intravenously, and the serum levels of repebody were monitored. The distribution half-life was 4.2 h, and elimination half-life was 10.7 h for Pal-Rb. In contrast, initial half-life was estimated to be 20 min for WT-Rb. Terminal half-life was not able to be calculated because of its fast clearance. The AUC of Pal-Rb was about 747%·h, which is 4.2-fold higher than WT-Rb. This result demonstrates that conjugation of palmitic acid significantly improved the pharmacokinetic property of the repebody (p < 0.05), leading to an increased bioavailability compared to WT-Rb. Therefore, our present approach can be widely applied to small-sized proteins and peptides that suffer from short blood circulation time.
Figure 4.
Pharmacokinetic profile of a palmitic acid-conjugated repebody in mice. A palmitic acid-conjugated repebody (Pal-Rb) was intravenously injected (n = 5 per each group). The levels of Pal-Rb inside blood plasma were determined by sandwich ELISA. WT-Rb was used as a control. Pharmacokinetic parameters for each constructs were estimated based on the profile. Error bars describe mean ± standard deviation (*p < 0.05).
2.5. Antitumor Activity of a Palmitic Acid-Conjugated Repebody In Vivo
Based on the significant enhancement in the pharmacokinetic profile of Pal-Rb, we tested its therapeutic efficacy in the tumor xenograft mice model. IL-6 is known to play a crucial role in cancer cell proliferation through stimulation of signal transducer and activator of transcription 3 (STAT3)-signaling pathway.41−43 Thus, the IL-6-specific repebody is expected to inhibit the STAT3-signaling pathway, suppressing the growth of tumor cells. H1650 cells, which are nonsmall cell lung cancer cell line, were used. Pal-Rb (10 mg/kg) was injected on every 3 days intravenously (Figure 5A). As the controls, WT-Rb and PBS were used. As a result, Pal-Rb suppressed the tumor growth level significantly when compared with controls (Figure 5B,C). The tumor size remained around 200 mm3 until 27 days when Pal-Rb was administered (p < 0.01), whereas tumor continued to grow over 600 mm3 when WT-Rb and PBS were injected. The body weight change was not significant for all three groups (Figure 5D). Furthermore, the factors of hepatotoxicity, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were insignificant between all three groups. In addition, nephrotoxicity factor creatine was very low for all three groups, and blood urea nitrogen (BUN) levels were estimated to be insignificant between the groups after 27 days (Figure 5E–G), implying negligible toxicity on both the liver and kidney. These results indicate that site-specific lipidation is effective for enhancing the antitumor activity of small-sized protein therapeutics through prolonged blood circulation time and increased bioavailability.
Figure 5.
Antitumor activity of a palmitic acid-conjugated repebody in H1650 tumor xenograft mice. (A) Experimental schedule for the assessment of tumor growth level when injected with Pal-Rb. (B,C) Effect of Pal-Rb on the tumor growth in xenograft mice. Pal-Rb was injected intravenously (10 mg/kg, n = 5 for each group), and tumor growth was monitored in every 3 days for 27 days. WT-Rb and PBS were used as controls. Pal-Rb resulted in a significant suppression of tumor growth compared to WT-Rb (**p < 0.01). Error bars describe mean ± standard deviation. (D) Body weight changes of mice injected with Pal-Rb. The body weight change was monitored for 27 days. (E) Hepatotoxicity of Pal-Rb. Blood was analyzed for aspartate aminotransferase (ALT) and alanine aminotransferase (AST) levels at day 27. (F,G) Nephrotoxicity of Pal-Rb. Blood was analyzed for creatine and BUN levels at day 27. Error bars describe mean ± standard deviation.
3. Conclusions
We have showed that site-specific lipidation is effective for elongating the blood circulation time and consequently enhancing the antitumor activity of the human IL-6-targeting repebody. A palmitic acid conjugated in repebody showed a binding capacity for a MSA, extending the blood circulation time up to 10.7 h, whereas WT-Rb had a shorter half-life of 20 min. The prolonged blood half-life and enhanced bioavailability of the lipidated repebody led to a significant enhancement in tumor suppression in the xenograft mice model, even though its binding affinity for human IL-6 was decreased by around 4-fold. It is interesting to note that conjugation of a palmitic acid had negligible side effects on mice. These results show that the current method can be effective to elongate the circulation time in blood and increase the bioavailability of proteins with low molecular weight and therefore lead to enhancement of their therapeutic effect.
4. Experimental Section
4.1. Materials
For cloning, the protein expression vector pET21a of Novagen (Madison, WI, USA), NdeI, XhoI restriction enzymes, and T4 DNA Ligase of Takara Bio (Shiga, Japan) was used. For incorporation of 4-azido-l-phenylalanine, the pEVOL-pAzF plasmid was purchased from Addgene (Watertown, MA, USA). 4-Azido-l-phenylalanine was from Bachem (Bubendorf, Switzerland) and Chem-Impex International (Wood Dale, IL, USA). DBCO-amine and NHS–palmitic acid were manufactured by Sigma-Aldrich (St. Louis, MO, USA). DBCO-mPEG (5 kDa), DBCO-Cy3, and Dde-Biotin-DBCO were purchased from Click Chemistry Tools (Scottsdale, AZ, USA). LB media, ampicillin, and kanamycin were supplied by Duchefa Biochemie (Haarlem, The Netherlands). Tetracycline and streptomycin were purchased from Sigma-Aldrich. Origami B (DE3) competent cells were provided by EMD Millipore (Burlington, MA, USA). Isopropyl β-d-1-thiogalactopyranoside (IPTG) used for induction of repebody, and human IL-6 expression was supplied by LPS Solution (Daejeon, Korea). Nickel–nitrilotriacetic acid (Ni–NTA) agarose resin for the purification of his-tagged proteins was supplied by Qiagen (Hilden, Germany), and Sephadex G-25 in PD-10 Desalting column, HiLoad Superdex 75 16/600 column, and Superdex 200 Increase 10/300 column were purchased from GE Healthcare (Uppsala, Sweden). For binding property assessments, Maxibinding 96-well immunoplates were purchased from SPL Life Sciences (Seoul, Korea). MSA and bovine serum albumin (BSA) were purchased from Equitech-Bio (Kerrville, TX, USA) and GenDEPOT (Katy, TX, USA), respectively. All other reagents purchased were of analytical grade and used as received.
4.2. Incorporation of an Unnatural Amino Acid and Protein Expression
For a site-specific conjugate of a fatty acid, an amino acid residue on the convex region of the human IL-6-specific repebody with highest surface accessibility was selected by analyzing the amino acid sequence using a web server,35 and its codon was changed to an amber codon (TAG). The mutated repebody gene was cloned into pET21a vector, and electrocompetent cells were generated using Origami (DE3) Escherichia coli. Additionally, the pEVOL-pAzF plasmid was inserted by electro-transformation as described elsewhere.44 A single colony was seeded in a 10 mL of LB medium containing antibiotics (ampicillin, kanamycin, tetracycline, and streptomycin) and grown overnight at 37 °C. Next day, cells were grown in 1 L of LB medium containing the antibiotics as above and grown at 37 °C until OD600 reached 0.8. Isopropyl-b-d-thiogalactopyranoside (IPTG) and 4-azido-l-phenylalanine (AzF) were added at 1 mM for induction of protein expression and incorporation of AzF, respectively, and incubated for additional 2 days at 30 °C. Cells were harvested by centrifugation at 8000 rpm for 20 min, and 20 mM Tris-HCl (pH 8.0) buffer containing 150 mM NaCl and 10 mM imidazole was added to resuspend cell pellets. Cells were sonicated, and debris was separated by centrifugation at 16,000 rpm for 1 h at 4 °C. Supernatants were purified with the Ni–NTA agarose resin column. Unbound proteins were removed with 20 mM Tris (pH 8.0), 150 mM NaCl, and 20 mM imidazole. Bound proteins were detached from NI–NTA agarose resin using 20 mM Tris (pH 8.0), 150 mM NaCl, and 250 mM imidazole. Eluted proteins were subjected to size exclusion chromatography (HiLoad Superdex 75 16/600). The human IL-6-specific repebody and human IL-6 protein were expressed and purified using the same method in our previous work.34
4.3. Confirmation of 4-Azido-l-phenylalanine Incorporation
Incorporation of 4-azido-l-phenylalanine into the human IL-6-specific repebody was confirmed by PEGylation, Cy3 labeling, and biotinylation. The purified human IL-6-specific repebody with incorporated 4-azido-l-phenylalanine was reacted with DBCO-mPEG (5 kDa), DBCO-Cy3, and Dde-Biotin-DBCO, respectively, at the molar ratio of 1:10 at room temperature for 24 h. Purification was conducted using the Superdex 200 Increase 10/300 column. PEGylation was checked by a size shift using SDS-PAGE analysis. The Cy3-labeled repebody was subjected to SDS-PAGE, and the fluorescence was detected by a gel image analyzer (Azure C600, Azure Biosystems, Dublin, CA, USA). The biotinylated repebody was analyzed by ELISA using the human IL-6-coated plate and HRP Streptavidin (BioLegend, San Diego, CA, USA).
4.4. Conjugation of a Fatty Acid
Site-specific conjugation of palmitic acid with 4-azido-l-phenylalanine on the repebody was carried out by copper-free click chemistry as described elsewhere.27 Briefly, DBCO-amine and NHS–palmitic acid were dissolved in dimethyl sulfoxide and mixed at the molar ratio of 1:5 with 0.1% deoxycholic acid (Sigma-Aldrich) in 1 mL of 1× DPBS (Welgene, Seoul, Korea), and amine reaction was conducted for 24 h at 37 °C. On the following day, remaining NHS–palmitic acid was quenched by adding 200 μL of 1 M Tris (pH 9.0). Following incubation at 37 °C for 2 h, 1 mL of DBCO-functionalized palmitic acid was added to 4-azido-l-phenylalanine incorporated human IL-6-specific repebody purified in 1× DPBS and incubated at room temperature for 12 h without exposure to light for cooper-free click chemistry reaction. Residual DBCO-palmitic acid was removed by the PD-10 desalting column using Sephadex G-25 and subjected to size exclusion chromatography with the Superdex 75 column. The NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the concentration of all the expressed proteins with appropriate extinction coefficients (Table S1). SDS-PAGE results were analyzed by the Gel Doc EZ Imager (Bio-Rad, Hercules, CA, USA).
4.5. Binding Ability Assessment
Binding affinity of a repebody against human IL-6 and MSA was tested using ELISA as in our previous work.16 Briefly, a 96-well plate coated with human IL-6 or MSA (10 μg/mL) was prepared at 4 °C overnight. Wells were blocked by blocking buffer for 1 h at room temperature. PBST-BSA (PBS pH 7.4, 0.05% Tween 20, 1% BSA) was used as the blocking buffer for human IL-6 binding test. A chemical blocker SuperBlock (T20) PBS (Thermo Scientific, Waltham, MA, USA) was used in case of binding test for MSA. Palmitic acid-conjugated repebody (Pal-Rb), 4-azido-l-phenylalanine-incorporated repebody (AzF-Rb), and WT-Rb were serially diluted by 3-fold with a blocking buffer starting from 1 μM. Each protein (100 μL) was added into well. Target-bound repebody was detected using biotinylated antirepebody antibody (Abclon, Seoul, Korea) with HRP-conjugated streptavidin diluted in blocking buffer. The signal was generated by adding 3,3′,5,5′-tetramethylbenzidien (TMB, Sigma-Aldrich). Absorbance (450 nm) was analyzed after the addition of 1 N H2SO4 by using the microplate reader (Infinite M200, Tecan GmbH, Crailsheim, Germany). PBST (PBS pH 7.4, 0.05% Tween 20) was used to wash the wells five times between each step, and experiment was conducted in triplicate.
4.6. Pharmacokinetics
The pharmacokinetic profile of a palmitic acid-conjugated repebody in mice was analyzed as in our previous work.45 Briefly, Balb/c male mice of 4 weeks old (Orient Bio, Inc., Gwangju, Korea) were intravenously injected either with a Pal-Rb or WT-Rb (10 mg/kg, 100 μL). Whole blood was collected in predetermined time points postinjection (n = 5; 0.05, 0.5, 1, 2, 4, 6, 12 and 24 h). After separation of blood plasma by centrifugation, each sample was subjected to sandwich ELISA. Repebody in blood plasma was captured using antirepebody antibody, detected with biotinylated antirepebody antibody, and streptavidin–HRP conjugate prepared in SuperBlock T20 (PBS) blocking buffer. The signal was generated for 1 min after adding the TMB substrate, and 1 N H2SO4 was added. PBST (PBS pH 7.4, 0.05% Tween 20) was used to wash the wells five times between each step. The value at 3 min was set to 100% for relative comparison of serum concentration. The experiment was reviewed and approved by the IACUC of KAIST (approval no. KA2017-29)
4.7. Antitumor Activity in the Xenograft Mouse Model
4.7.1. Animal and Tumor Cell for Xenograft
The H1650 cell (ATCC, Manassas, VA, USA), which is a nonsmall cell lung cancer cell line, was cultured with RPMI 1640 media containing 10% fetal bovine serum and 100 U/mL penicillin/streptomycin (Capricorn Scientific, Ebsdorfergrund, Germany) and incubated in 37 °C, 5% CO2 chamber. Female BALB/c nude mice of five-week-old were provided by Orient Bio, Inc. H1650 cancer cells were injected into mice subcutaneously through the right lateral flank. Total 5 × 106 cells were subcutaneously injected into mice in a mixture with 100 μL of Matrigel Matrix (Corning Inc., Corning, NY, USA) and incubated until approximately 100 mm3 of tumor volume was reached. All animals were caged in standard and controlled conditions. The experiment was reviewed and approved by the IACUC of KAERI (approval no. KAERI-IACUC-2019-015).
4.7.2. Antitumor Activity
Pal-Rb, WT-Rb, or PBS (10 mg/kg, 100 μL, n = 5 per group) was intravenously injected into xenograft mice with an interval of 3 days when the volume of the tumor grew until approximately 100 mm3. The growth of tumor and body weight were monitored once every 3 days for 27 days. On each day, width and length of the tumor were measured using a caliper. The following formula was used to evaluate the tumor volume: width2 × length × 0.5. At 27 days postinjection, all mice were euthanized, and tissues were analyzed.
4.7.3. In Vivo Hepatic and Renal Toxicity
The blood plasma was separated by centrifugation at 3000 rpm for 20 min at 4 °C. Blood levels of alanine transaminases (ALTs) and aspartate transaminases (ASTs) were analyzed for hepatic toxicity. In case of renal toxicity, blood was analyzed for creatine and BUN levels. The toxicity parameters were detected by Fuji Dri-Chem NX 7000i (Fuji photo Film Co., Ltd., Tokyo, Japan).
4.8. Statistical Analysis
Error bars describe mean ± standard deviation. One-way analysis of variance was used to compare individual samples. Tukey’s multiple comparison test was used for intergroup comparisons. When the p value was less than 0.05 (p < 0.05), the results were described as statistically significant. Statistical analysis was conducted with Prism 8 (GraphPad Software, San Diego, CA, USA).
Acknowledgments
This research was supported by grants from National Research Foundation (NRF), funded by Ministry of Science and ICT of Korea: Bio & Medical Technology Development Program (NRF-2017M3A9F5031419), Mid-Career Researcher Program (NRF-2017R1A2A1A05001091), and Global Research Laboratory (NRF-2015K1A1A2033346). This work was supported in part by the Nuclear R&D program of the Ministry of Science and ICT (MSIT) of Korea and internal R&D project of KAERI (523260-20).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02555.
Analysis of repebody residues for site-specific lipidation, purification of 4-azido-l-phenylalanine incorporated repebody, confirmation of 4-azido-l-phenylalanine incorporation in repebody, and parameters for measurement of protein concentrations (PDF)
Author Present Address
§ Department of Hematology & Hematopoietic Cell Transplantation, Beckman Research Institute, City of Hope, Duarte 91010, CA, USA.
Author Present Address
∥ Department of Food and Nutrition, Chungnam National University, Daejeon 34134, Korea.
Author Contributions
⊥ T.Y.K. and Y.R.N. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Walsh G. Biopharmaceutical benchmarks 2010. Nat. Biotechnol. 2010, 28, 917–924. 10.1038/nbt0910-917. [DOI] [PubMed] [Google Scholar]
- Aggarwal S. R. What’s fueling the biotech engine-2012 to 2013. Nat. Biotechnol. 2014, 32, 32–39. 10.1038/nbt.2794. [DOI] [PubMed] [Google Scholar]
- Daniel Lagassé H. A.; Alexaki A.; Simhadri V. L.; Katagiri N. H.; Jankowski W.; Sauna Z. E.; Kimchi-Sarfaty C. Recent advances in (therapeutic protein) drug development. F1000Research 2017, 6, 113. 10.12688/f1000research.9970.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L.; Persky A. M.; Hochhaus G.; Meibohm B. Pharmacokinetic aspects of biotechnology products. J. Pharm. Sci. 2004, 93, 2184–2204. 10.1002/jps.20125. [DOI] [PubMed] [Google Scholar]
- Tryggvason K.; Wartiovaara J. How does the kidney filter plasma?. Physiology 2005, 20, 96–101. 10.1152/physiol.00045.2004. [DOI] [PubMed] [Google Scholar]
- Mitragotri S.; Burke P. A.; Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov 2014, 13, 655–672. 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontermann R. E. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011, 22, 868–876. 10.1016/j.copbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Benjamin W. U.; Sun Y. N. Pharmacokinetics of Peptide-Fc fusion proteins. J. Pharm. Sci. 2014, 103, 53–64. 10.1002/jps.23783. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Chen F.; Wan D.; Liu Y.; Yang L.; Feng H.; Cui X.; Gao X.; Song H. Expression and Characterization of a Potent Long-Acting GLP-1 Receptor Agonist, GLP-1-IgG2σ-Fc. PLoS One 2016, 11, e0156449 10.1371/journal.pone.0156449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinski J. T.; Sønderby P.; Antunes F.; Andersen B.; Schmidt E. G. W.; Peters G. H. J.; Harris P. Glucagon-like Peptide 1 Conjugated to Recombinant Human Serum Albumin Variants with Modified Neonatal Fc Receptor Binding Properties. Impact on Molecular Structure and Half-Life. Biochemistry 2017, 56, 4860–4870. 10.1021/acs.biochem.7b00492. [DOI] [PubMed] [Google Scholar]
- Andersen J. T.; Cameron J.; Plumridge A.; Evans L.; Sleep D.; Sandlie I. Single-chain Variable Fragment Albumin Fusions Bind the Neonatal Fc Receptor (FcRn) in a Species-dependent Manner. J. Biol. Chem. 2013, 288, 24277–24285. 10.1074/jbc.m113.463000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopenian D. C.; Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- Merlot A. M.; Kalinowski D. S.; Richardson D. R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol 2014, 5, 299. 10.3389/fphys.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D.; Karle A.; Meissburger B.; Höfig I.; Stork R.; Kontermann R. E. Improved pharmacokinetics of recombinant bispecific antibody molecules by fusion to human serum albumin. J. Biol. Chem. 2007, 282, 12650–12660. 10.1074/jbc.m700820200. [DOI] [PubMed] [Google Scholar]
- Deyev S. M.; Waibel R.; Lebedenko E. N.; Schubiger A. P.; Plückthun A. Design of multivalent complexes using the barnase·barstar module. Nat. Biotechnol. 2003, 21, 1486–1492. 10.1038/nbt916. [DOI] [PubMed] [Google Scholar]
- Kim T. Y.; Seo H.-D.; Lee J.-j.; Kang J. A.; Kim W. S.; Kim H.-M.; Song H.-Y.; Park J. M.; Lee D.-E.; Kim H.-S. A dimeric form of a small-sized protein binder exhibits enhanced anti-tumor activity through prolonged blood circulation. J. Controlled Release 2018, 279, 282–291. 10.1016/j.jconrel.2018.04.039. [DOI] [PubMed] [Google Scholar]
- Jin C.-H.; Chae S. Y.; Son S.; Kim T. H.; Um K. A.; Youn Y. S.; Lee S.; Lee K. C. A new orally available glucagon-like peptide-1 receptor agonist, biotinylated exendin-4, displays improved hypoglycemic effects in db/db mice. J. Controlled Release 2009, 133, 172–177. 10.1016/j.jconrel.2008.09.091. [DOI] [PubMed] [Google Scholar]
- Fishburn C. S. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 2008, 97, 4167–4183. 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- Caliceti P.; Veronese F. M. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv. Drug Delivery Rev. 2003, 55, 1261–1277. 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- Strohl W. R. Fusion Proteins for Half-Life Extension of Biologics as a Strategy to Make Biobetters. BioDrugs 2015, 29, 215–239. 10.1007/s40259-015-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen K. R.; Gong R.; Bramhill D.; Wiersma D. A.; Kirkpatrick S. A.; Wang Y.; Feng Y.; Dimitrov D. S. Pharmacokinetics of engineered human monomeric and dimeric CH2 domains. mAbs 2012, 4, 466–474. 10.4161/mabs.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S. M.; Mantovani G.; Wang X.; Haddleton D. M.; Brayden D. J. Advances in PEGylation of important biotech molecules: delivery aspects. Expert Opin. Drug Delivery 2008, 5, 371–383. 10.1517/17425247.5.4.371. [DOI] [PubMed] [Google Scholar]
- Lau J.; Bloch P.; Schäffer L.; Pettersson I.; Spetzler J.; Kofoed J.; Madsen K.; Knudsen L. B.; McGuire J.; Steensgaard D. B.; Strauss H. M.; Gram D. X.; Knudsen S. M.; Nielsen F. S.; Thygesen P.; Reedtz-Runge S.; Kruse T. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 2015, 58, 7370–7380. 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- Menacho-Melgar R.; Decker J. S.; Hennigan J. N.; Lynch M. D. A review of lipidation in the development of advanced protein and peptide therapeutics. J. Controlled Release 2019, 295, 1–12. 10.1016/j.jconrel.2018.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech E. M.; Pedersen S. L.; Jensen K. J. Chemical Strategies for Half-Life Extension of Biopharmaceuticals: Lipidation and Its Alternatives. ACS Med. Chem. Lett. 2018, 9, 577–580. 10.1021/acsmedchemlett.8b00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. I.; Mizuta Y.; Takasu A.; Hahn Y. S.; Kim Y. H.; Kwon I. Site-specific fatty acid-conjugation to prolong protein half-life in vivo. J. Controlled Release 2013, 170, 219–225. 10.1016/j.jconrel.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.; Lim S. I.; Yang B. S.; Hahn Y. S.; Kwon I. Generation of therapeutic protein variants with the human serum albumin binding capacity via site-specific fatty acid conjugation. Sci. Rep. 2017, 7, 18041. 10.1038/s41598-017-18029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.; Park J.; Kim S.; Kim J. C.; Tae G.; Jin M. S.; Kwon I. Intramolecular distance in the conjugate of urate oxidase and fatty acid governs FcRn binding and serum half-life in vivo. J. Controlled Release 2020, 321, 49–58. 10.1016/j.jconrel.2020.01.034. [DOI] [PubMed] [Google Scholar]
- Unverdorben F.; Richter F.; Hutt M.; Seifert O.; Malinge P.; Fischer N.; Kontermann R. E. Pharmacokinetic properties of IgG and various Fc fusion proteins in mice. mAbs 2016, 8, 120–128. 10.1080/19420862.2015.1113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M. T.; Rawsthorne H.; Schelde K. K.; Dagnæs-Hansen F.; Cameron J.; Howard K. A. Cellular recycling-driven in vivo half-life extension using recombinant albumin fusions tuned for neonatal Fc receptor (FcRn) engagement. J. Controlled Release 2018, 287, 132–141. 10.1016/j.jconrel.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Levin D.; Golding B.; Strome S. E.; Sauna Z. E. Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol. 2015, 33, 27–34. 10.1016/j.tibtech.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Sperinde G.; Montgomery D.; Mytych D. T. Clinical Immunogenicity Risk Assessment for a Fusion Protein. AAPS J. 2020, 22, 64. 10.1208/s12248-020-00447-y. [DOI] [PubMed] [Google Scholar]
- Chirino A. J.; Ary M. L.; Marshall S. A. Minimizing the immunogenicity of protein therapeutics. Drug Discovery Today 2004, 9, 82–90. 10.1016/s1359-6446(03)02953-2. [DOI] [PubMed] [Google Scholar]
- Lee J.-j.; Kim H. J.; Yang C.-S.; Kyeong H.-H.; Choi J.-M.; Hwang D.-E.; Yuk J.-M.; Park K.; Kim Y. J.; Lee S.-G.; Kim D.; Jo E.-K.; Cheong H.-K.; Kim H.-S. A high-affinity protein binder that blocks the IL-6/STAT3 signaling pathway effectively suppresses non-small cell lung cancer. Mol. Ther. 2014, 22, 1254–1265. 10.1038/mt.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen M. S.; Jespersen M. C.; Nielsen H.; Jensen K. K.; Jurtz V. I.; Sønderby C. K.; Sommer M. O. A.; Winther O.; Nielsen M.; Petersen B.; Marcatili P. NetSurfP-2.0: Improved prediction of protein structural features by integrated deep learning. Proteins 2019, 87, 520–527. 10.1002/prot.25674. [DOI] [PubMed] [Google Scholar]
- Ashbrook J. D.; Spector A. A.; Santos E. C.; Fletcher J. E. Long chain fatty acid binding to human plasma albumin. J. Biol. Chem. 1975, 250, 2333–2338. [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J. Lipid Res. 1975, 16, 165–179. [PubMed] [Google Scholar]
- van der Vusse G. J. Albumin as Fatty Acid Transporter. Drug Metab. Pharmacokinet. 2009, 24, 300–307. 10.2133/dmpk.24.300. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A. A.; Grüne T.; Curry S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin 1 1Edited by R. Huber. J. Mol. Biol. 2000, 303, 721–732. 10.1006/jmbi.2000.4158. [DOI] [PubMed] [Google Scholar]
- Fujiwara S.-i.; Amisaki T. Identification of high affinity fatty acid binding sites on human serum albumin by MM-PBSA method. Biophys. J. 2008, 94, 95–103. 10.1529/biophysj.107.111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge D. R.; Hurt E. M.; Farrar W. L. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer 2005, 41, 2502–2512. 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Bromberg J.; Wang T. C. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 2009, 15, 79–80. 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.; Pardoll D.; Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D.; Jessee J.; Bloom F. R. [4] Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 1991, 204, 63–113. 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- Kim T. Y.; Park J. H.; Shim H. E.; Choi D. S.; Lee D.-E.; Song J.-J.; Kim H.-S. Prolonged half-life of small-sized therapeutic protein using serum albumin-specific protein binder. J. Controlled Release 2019, 315, 31–39. 10.1016/j.jconrel.2019.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.