Abstract
A carbon dot (CD)-intercalated NiFe2O4 (NFO)/graphitic carbon nitride (g-C3N4, g-CN) ternary Z-scheme heterojunction was synthesized by the facile wet chemical method and used for photo-Fenton degradation. The structural, optical, electrical, vibrational, and morphological properties of the photocatalysts were investigated through various analytical methods. The CD-intercalated heterojunction formation was analyzed by high-resolution transmission electron microscopy (HRTEM). The intercalated CD acted as an electron donor/acceptor, which converted a type-II heterojunction to a Z-scheme heterojunction. The formation of Z-scheme heterojunction was confirmed by the enormous production of radicals (hydroxyl (OH•) and superoxide (O2–)) and the elemental trapping experiment. In particular, the heterojunction photocatalyst NFO/5g-CN/7.5CD showed the highest photo-Fenton degradation efficiency of 99% for rhodamine B (Rh B) and 93% for tetracycline (TCN) in the presence of H2O2. The charge separation and electron transport behaviors of the photocatalyst were examined by photoluminescence (PL) and photocurrent measurements. In the Z-scheme photo-Fenton system, hydroxyl and superoxide radicals played a vital role in the visible-light-driven degradation process. Hence, the prepared Z-scheme ternary photocatalyst is well suitable for wastewater treatment in practical use.
1. Introduction
In recent years, the heterogeneous semiconductor-based advanced oxidation process (AOP) has attracted great attention in the field of wastewater treatment. AOP is considered as a most promising method for effective elimination of contaminants (organic, inorganic, and biological) compared to other conventional purification methods. The highly reactive hydroxyl (OH•) radicals are produced in AOP, which react with the pollutant and liberate water and carbon dioxide. Among various AOPs, the photo-Fenton-like catalysis is the effective method for elimination of contaminants. In this process, the Fenton reaction is accelerated by photon sources. The highly reactive OH• radicals are produced by the reaction between the oxidizing agents (H2O2, O3, KMnO4, etc.) and photon-induced semiconductor electrons. The photo-Fenton process does not consume the catalyst and does not produce any pollutant byproducts.1,2
The visible-light-driven catalyst developed for the photo-Fenton reaction plays a vital role in the degradation process. An iron (Fe)-containing spinel binary metal oxide has gained importance in photocatalytic degradation of organic pollutants in the presence of a chemical oxidant. The Fe-based spinel metal ferrite systems with the general molecular structure MFe2O4 (M = Ni, Zn, Co, Ca, Mn, Mg, and Cu) have gained technological importance in photocatalysis due to their highly visible light utilization, low band gap, high-temperature stability, and extraordinary chemical stability. Moreover, the soft magnetic nature (except CoFe2O4) of the spinel ferrites helps to recover the catalyst after the photocatalytic reaction.3,4 Among various metal ferrites, NiFe2O4 (NFO) has attracted researchers in the field of photocatalysis due to its narrow band gap of 2.19 eV, which helps to harvest the visible light. In addition to this, the Fe ion in the NiFe2O4 crystal system readily reacts with the chemical oxidant and produces reactive hydroxyl radicals. However, the practical usage of NiFe2O4 becomes the bottleneck, due to the high recombination rate of photoinduced charge carriers.5−9 To overcome this drawback, several strategies have been employed to improve the photocatalytic efficiency. In recent years, the formation of heterojunctions between NiFe2O4 and other semiconductors (ZnO, TiO2, Cu2O, etc.) has been widely studied in photocatalytic energy and environmental remediation applications.10−12
The polymeric metal-free graphitic-phase carbon nitride (g-C3N4, g-CN) is the most stable allotrope form at ambient conditions compared to other forms of carbon nitrides (α, β, cubic, and pseudo). The g-C3N4 is considered as the potential candidate in the photocatalysis field due to its cost-effective production, moderate band gap (2.7 eV), visible light utilization, and high chemical and thermal stability. Moreover, the coordinated nest surrounded by π–π bonds in carbon nitride provides active sites, and it can be easily peeled off into thin layers to provide more active sites for the photocatalytic process. However, the bare g-C3N4 is not suitable for practical implementation due to fast recombination of electron–hole pairs in it, sluggish exciton dissociation, low surface area, and insufficient light absorption ability.13−15 Hence, various strategies have been employed such as fabricating nanostructures, coupled with conducting materials, foreign atom doping, and creating vacancies to improve the photocatalytic efficiency of bare g-C3N4.16,1716,17 In addition to this, molecular engineering strategies have also been developed for g-C3N4 to extend the optical absorption ability and accelerate the charge transfer.18 Among various developed methods, the construction of heterojunctions and addition of photosensitizers can improve the photogenerated charge carrier separation and increase the quantum efficiency.15 In recent years, the heterojunction between NiFe2O4 and g-C3N4 has been widely studied in photocatalytic energy and environmental remediation fields due to its suitable band structure for effective separation of charge carriers.5−9,19
Further improvement in separation of charge carriers has been achieved by incorporating the noble metals such as Au, Pt, Ag, and Pd. The research on Au-deposited NiFe2O4/g-C3N4 ternary composites exhibits their higher photocatalytic activity compared to the binary system due to suppression of electron–hole recombination.9 However, the practical usage of noble metals has been limited due to their high cost. Therefore, the recent research studies focused on carbon dot (CD) as an electron mediator, to suppress the photoinduced charge carrier recombination.20 The carbon quantum dot is widely used in the photocatalytic process due to its low cost, zero dimension, biocompatibility, chemical stability, charge transport property, and excellent light absorption ability.21,22 Carbon dots act as electron acceptors and donors, which facilitate to harvest a wide range of visible light spectrum.23,24
Based on this literature, herein, we have reported the CD-decorated NiFe2O4/g-C3N4 ternary magnetic nanocomposite system for visible-light photocatalytic organic pollutant degradation. To the best of our knowledge, there are no reports on the NiFe2O4/g-C3N4/CD ternary composite for photo-Fenton-like catalytic degradation. The simple wet chemical route has been employed to prepare the ternary nanocomposite. The prepared ternary nanocomposite has been used to degrade the organic pollutants under visible light irradiation in the presence of a chemical oxidant. The addition of CD into a type-II heterojunction produces an effective Z-scheme photocatalysis system. In this Z-scheme photocatalysis system, the produced charge carriers have been effectively separated and transferred from one semiconductor to another semiconductor through solid-state electron mediators.2,25 This type of electron mediator made the Z-scheme photocatalytic system beneficial for enhancing charge separation and photocatalytic degradation processes. The optical, structural, morphological, and magnetic properties of the prepared ternary nanocomposites were well discussed. The ternary system exhibits the highest degradation efficiency compared to pure and binary composite systems. The enhanced photocatalytic behavior of the ternary magnetic nanocomposite revealed the synergistic effect and improved charge carrier separation. Besides, the simultaneous production of superoxide and OH• radicals also plays a crucial role in the effective degradation process. The superoxide radical and hydroxyl radical production was confirmed by the nitroblue tetrazolium (NBT) method and terephthalic acid (TA)-assisted fluorescence method, respectively. The effective Z-scheme photo-Fenton catalytic reaction and the active radicals for the degradation process have been investigated with the help of the elemental trapping experiment. Moreover, the stability of the composite photocatalysts has been examined by the recycling process.
2. Results and Discussion
2.1. X-ray Diffraction (XRD) Analysis
The crystal phase and crystalline nature of the prepared nanoparticles were investigated by the powder X-ray diffraction technique. The obtained X-ray diffraction patterns of pure and composite photocatalysts are displayed in Figure 1. The pure g-CN has two diffraction peaks positioned at 12.92 and 27.74°, indicating the diffraction planes (100) and (002), respectively. The diffraction plane (100) described the in-plane separation of the repeated tri-s-triazine unit in carbon nitride with a d-spacing value of 0.684 nm. The peak positioned at 27.74° has an interplanar distance of about 0.321 nm ascribed the distance between conjugated aromatic systems.2,4 Nickel ferrite shows the diffraction peaks positioned at 2θ values of 18.43, 30.26, 35.65, 37.28, 43.31, 54.09, 57.24, and 62.88° assigned to the (111), (220), (311), (223), (400), (422), (511), and (440) planes, respectively. The obtained NFO diffraction pattern was well consistent with the literature report and JCPDS card (89-4927).5 The XRD pattern of the CD is depicted in Figure 1c. The CD showed a diffraction peak at 26.27°, which was assigned to the (002) plane, and it well-matched with the literature.16 The binary and ternary nanocomposites showed all diffraction peaks related to g-CN and NFO. However, the CD peak was not observed in the ternary composite photocatalyst system due to the lower concentration.23 From the XRD patterns of the composite systems, it was noted that the diffraction intensity of g-C3N4 and NFO reduced in comparison with pure nanoparticles. This result demonstrated that the g-CN affected the crystalline nature of the NFO nanoparticles and vice versa. In addition to this, the peak shift was also observed in the (002) plane toward the lower angle (27.69°) side in the binary nanocomposite. The peak shift toward the lower angle side showed the increasing lattice plane distance between the conjugated aromatic systems in carbon nitride. The increasing lattice distance showed that the NFO nanoparticles are placed in between the g-CN aromatic layers, and it produced a strong guest–host interaction between the metal oxide and carbon nitride matrixes. Besides, the addition of CD also improved the d-spacing value of g-CN with a lower angle side peak shift. The observed peak positions of the (002) plane were 27.60, 27.58, 27.47, and 27.58° corresponding to 2.5, 5, 7.5, and 10 wt % CD addition to the binary NFO/5g-CN composite system. It is noteworthy that the addition of CD increased the aromatic layer distance in the g-CN matrix up to 7.5 wt %. From the XRD results, it was noted that the addition of CD increased the aromatic layer distance of g-CN up to a certain limit due to the intercalation of CD between the g-CN layer systems. A further increase in CD addition (10 wt %) reduced the lattice distance of the (002) plane. This result indicates that a higher amount of CD produces lattice distortion in carbon nitride, and it is well consistent with the previous report.26 On the other hand, the diffraction peaks of NFO in nanocomposites shifted to a higher angle side in comparison with pure nanoparticles, which suggests changes of lattice parameters in the NFO crystal system. Hence, an increase in the d-space value and peak position shift on the binary and ternary nanocomposites clearly suggested a strong chemical interaction between the nanoparticles. Furthermore, the XRD pattern of the ternary nanocomposite confirmed the addition of CD intercalated between the NFO and g-CN nanoparticles during the heterojunction formation.
Figure 1.
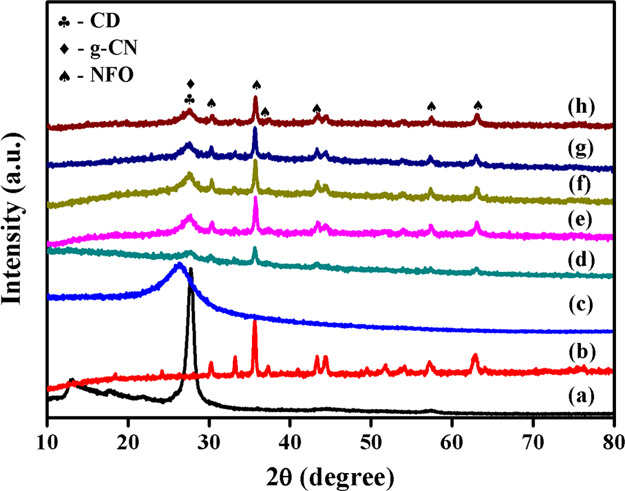
XRD patterns of pure and composite nanoparticles: (a) g-CN, (b) NFO, (c) CD, (d) NFO/5g-CN, (e) NFO/5g-CN/2.5CD, (f) NFO/5g-CN/5CD, (g) NFO/5g-CN/7.5CD, and (h) NFO/5g-CN/10CD.
2.2. Fourier Transform Infrared (FTIR) Analysis
The chemical interaction, chemical composition, and vibrational properties of the prepared photocatalysts were examined by FTIR spectroscopy, and the obtained spectra of the nanoparticles are portrayed in Figures S1 and 2. The FTIR spectrum of pure g-CN showed the transmittance peak related to the s-triazine ring, which was positioned at 805 cm–1. The peak observed at the 1200–1300 cm–1 position was assigned to the heterocycle C–N–C stretching vibration. The repeated tri-s-triazine unit was depicted at 1400–1600 cm–1. The IR peak of pure NFO located at 548 cm–1 was related to the metal–oxygen stretching vibration in the spinel crystal system. The peaks that were related to pure g-CN and NFO were observed in the binary (NFO/5g-CN) composite, which confirmed the strong chemical interaction between the two nanoparticles.4,5 The CD showed the FTIR peaks positioned at 1077, 1748, and 2910 cm–1 assigned to the stretching vibrations of C–O–C/O–H, C=O, and C–H groups, respectively.22,27,28 The FTIR spectra of ternary nanocomposites showed similar peaks related to NFO/5g-CN, which were due to the following reasons: (1) overlapping of CD and g-CN peaks, (2) low concentration of CD, and (3) the addition of CD that did not alter the structure of CD.19,20 From the FTIR spectrum, it was clearly observed that the CD, g-CN, and NFO nanoparticles are chemically attached, which well-matched with the obtained XRD results.
Figure 2.
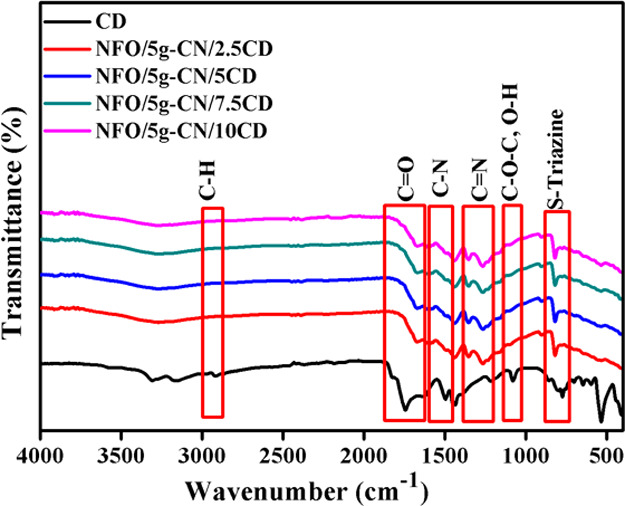
FTIR spectra of pure and composite photocatalysts.
2.3. UV–Vis Differential Reflectance Spectroscopy (DRS) Analysis
It is significant to study the optical absorption property of the prepared photocatalysts to know the effective utilization of the visible light for the photocatalytic degradation process. The UV–vis–diffused reflectance spectroscopy study has been performed for the nanoparticles to explore the band gap and optical absorption ability. The absorption spectra of pure and composite photocatalysts are illustrated in Figure 3. The band gap of the nanoparticles was calculated according to the equation29
From the absorption spectrum, it is noted that the bare g-CN has a band gap of 2.66 eV with an absorption wavelength of 466 nm. The NFO nanoparticle exhibits an absorption wavelength of 712 nm with a band gap of 1.74 eV, which described the Ni2+–O–Fe3+-to-Ni+–O–Fe4+ transition.8 The binary nanocomposite NFO/5g-CN shows an absorption wavelength of 582 nm, which is higher than that of the bare g-CN. Moreover, the absorption intensity has also been enhanced in comparison with those of pure g-CN and pure NFO. The UV–vis–DRS spectrum of the binary nanocomposite demonstrates that the electronic coupling between NFO and g-CN increases the surface electric charge due to the restraining of the contact barrier.2,8
Figure 3.
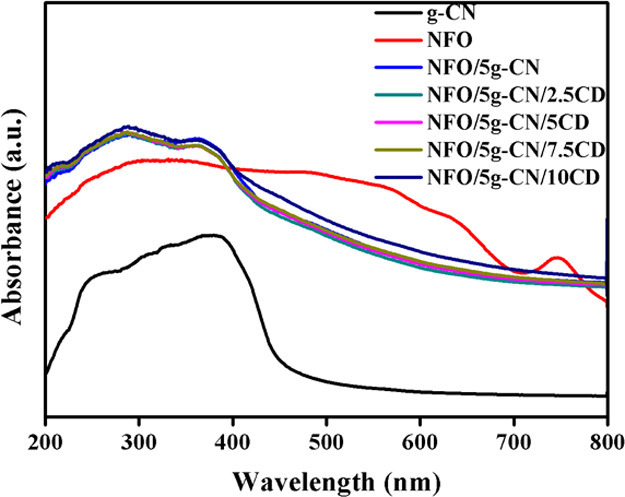
UV–vis–DRS absorption spectra of pure and composite photocatalysts.
Besides, a blue shift was observed in the ternary nanocomposite with up to 7.5 wt % CD addition. This blue shift was related to the quantum confinement effect of g-C3N4 due to increasing interlayer spacing. The band gap of the nanoparticles was related to the grain size, particle shape, and lattice parameters of the crystal. The electronic states of the nanoparticles were completely discrete, which hindered the electronic transition and generated electronic population. The produced electronic population in the discrete state led to a blue shift in the nanoparticles. This result showed that the optical absorption blue shift in the ternary nanocomposite was mainly due to the quantum confinement effect.19,30 The blue shift of the ternary nanocomposite revealed the slackening of the conjugated aromatic system of g-CN. The obtained UV–vis–DRS result for ternary photocatalysts well-matched with XRD patterns and the increasing d-space value of the g-CN plane (002). A further increase in the CD amount led to a red shift in the optical absorption compared to the ternary composite. The increase in the absorption wavelength was related to the d-space value reduction of the g-CN plane (002) in the ternary NFO/5g-CN/10CD composite. The observed red shift in the composite was evident for the interfacial contact between nanoparticles (g-CN, NFO, and CD).21 The measured optical absorption wavelengths of the ternary nanocomposites were 573, 575, 579, and 596 nm for 2.5, 5, 7.5, and 10 wt % CD addition, respectively. Hence, from the UV-DRS results, it is noted that all ternary nanocomposite photocatalysts exhibit a strong optical absorption ability compared to pure g-CN, which is suitable for the visible-light-active photocatalysis process.
2.4. Photoluminescence (PL) and Photocurrent Analysis
The light-harvesting property alone is not an important factor for enhancement of the photocatalytic degradation process. The charge carrier separation and transportation also play a major role in degradation efficiency. Therefore, it is necessary to perform PL and photocurrent analyses on prepared nanoparticles to ensure charge separation and transportation for an effective photocatalytic degradation process. The suppression of photoinduced charge carrier recombination in the photocatalysts was analyzed by room-temperature PL spectroscopy with an excitation wavelength of 395 nm. The PL emission spectra of bare g-CN and composite photocatalysts are shown in Figure 4. The bare g-CN exhibits a high-intensity PL emission peak positioned at 460 nm, which well-matches with the UV spectroscopy result. The intensity reduction of the emission spectrum reveals the heterojunction formation between the nanoparticles, reducing the electron–hole recombination. From the PL spectroscopy result, it was noted that the nanocomposite effectively inhibits the photoinduced electron–hole recombination. The addition of CD further decreases the emission peak intensity of the ternary composite (NFO/5g-CN/7.5CD) in comparison to the binary NFO/5g-CN nanocomposite. Therefore, one can conclude that the addition of CD into the binary composite system effectively separates the electron–hole pair, which is favorable for enhancing the photocatalysis process.5,23
Figure 4.
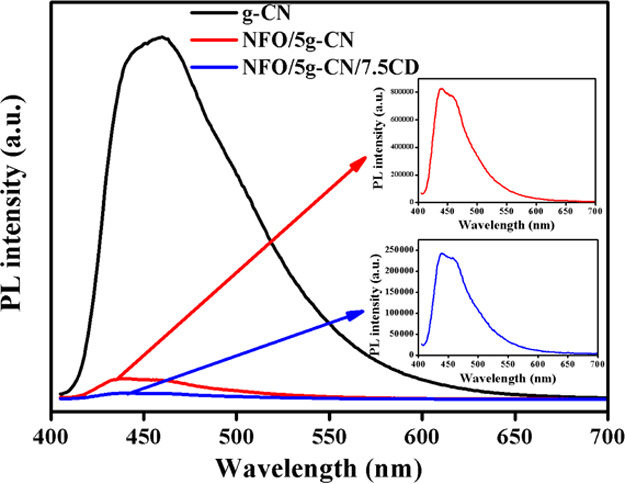
PL spectra of g-CN, NFO/5g-CN, and NFO/5g-CN/7.5CD.
To identify the photoresponse and electron transportation behavior of the CD-added ternary composite photocatalyst for the effective degradation process, the photocurrent measurement was performed, and the obtained results are portrayed in Figure S2. The photocurrent was observed in all photocatalysts when the light was switched on and reached the steady state. When the light was switched off, the photocurrent response immediately diminished. The calculated photocurrent density of the ternary (NFO/5g-CN/7.5CD) photocatalyst was 0.31 μA, which is higher than any other photocatalyst. The observed current densities of bare g-CN, pure NFO, and the binary photocatalyst were 0.03, 0.006, and 0.21 μA, respectively. The photocurrent increment for the binary and ternary nanocomposites clearly demonstrated that the formation of heterojunction greatly inhibited the electron–hole recombination and improved the electronic conductivity. The highest photocurrent response of the ternary nanocomposite was due to the fact that the added CD acted as an electron acceptor and the charge separation center.6,9,22 The obtained photocurrent result was well consistent with the UV–vis–DRS and PL results. Therefore, it can be concluded that the ternary composite photocatalyst system is favorable toward enhancing the photocatalytic degradation process in terms of visible-light absorption, charge separation, and electron transportation.
2.5. Morphological Analysis
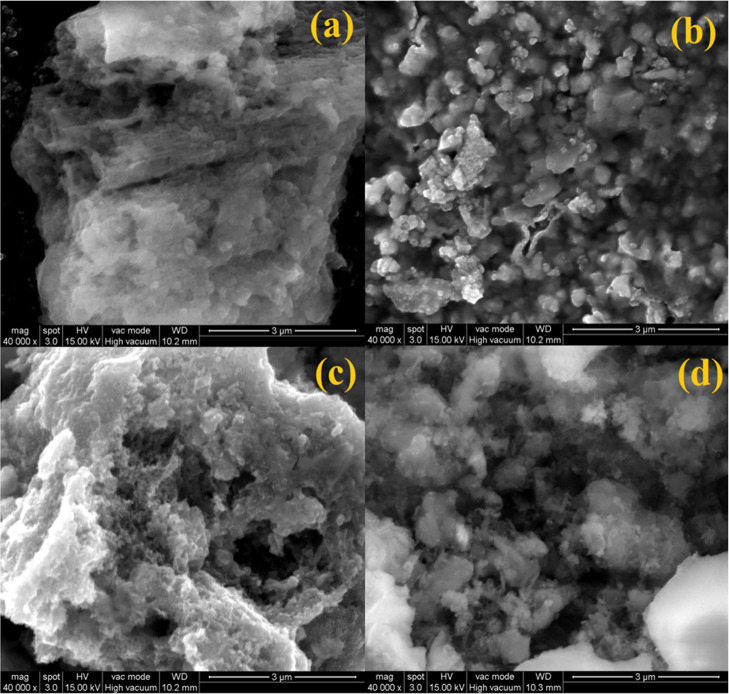
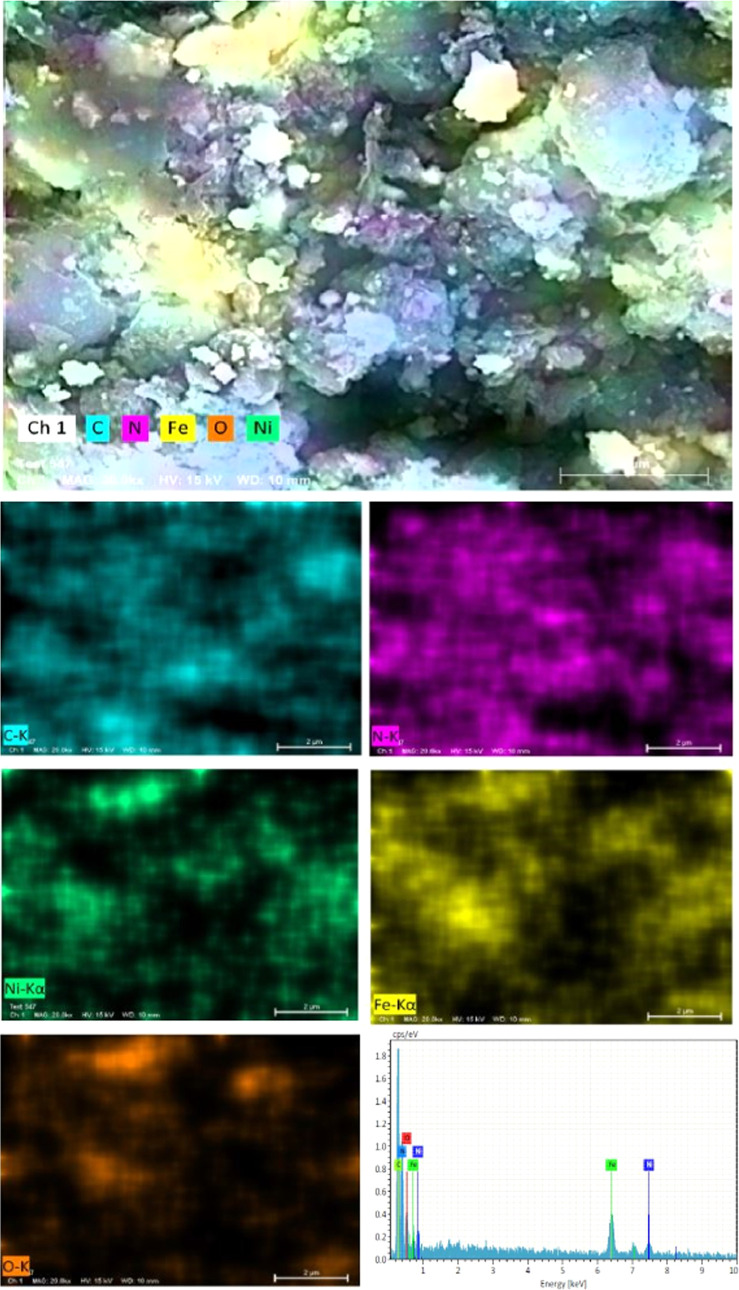
The field emission scanning electron microscopy (FESEM) and transmittance electron microscopy (TEM) analyses were used as a tool to investigate the surface morphological nature of the prepared photocatalysts. The surface morphologies of the prepared g-CN, NFO, and binary/ternary composites were analyzed by FESEM, and the obtained micrographs are displayed in Figure 5. The FESEM micrograph of g-CN shows a wrinkled lamellar sheetlike structure. NFO shows shapeless highly agglomerated particle-like morphology and is shown in Figure 5b. The heterojunction formation between NFO and g-CN nanoparticles is depicted in Figure 5c. It is clearly shown that the NFO nanoparticles bind with the g-CN nanosheet. The ternary NFO/5g-CN/7.5CD nanocomposite photocatalyst micrograph is shown in Figure 5d. The ternary composite photocatalysts show a similar morphological nature to the binary composite. It is very difficult to distinguish the heterojunction formation between the CD and the NFO/g-CN composite from FESEM analysis due to the stacking nature of the g-CN nanosheet.24 The elemental composition and surface elemental distribution of the NFO/g-CN/7.5CD ternary nanocomposite photocatalyst were analyzed by energy-dispersive spectrometry (EDS) analysis, and the results are portrayed in Figure 6. The elemental distribution and EDS spectrum results revealed the presence of carbon (C), nitrogen (N), nickel (Ni), iron (Fe), and oxygen (O) on the surface of the ternary photocatalyst. Hence, one can conclude the existence of a heterojunction among NFO, g-CN, and CD nanoparticles.
Figure 5.
FESEM images of (a) g-CN, (b) NFO, (c) NFO/5g-CN, and (d) NFO/5g-CN/7.5CD.
Figure 6.
EDS elemental mapping of NFO/g-CN/7.5CD.
To confirm the heterojunction formation among CD, NFO, and g-CN, TEM analysis was performed, and the images of the obtained ternary composite (NFO/5g-CN/7.5CD) are shown in Figure 7. From the TEM micrograph, it was demonstrated that the NFO and CD nanoparticles are dispersed on the g-CN nanosheet. The high-resolution TEM (HRTEM) image of NFO/5g-CN/7.5CD is portrayed in Figure 7b. It was observed from the HRTEM image that the d-spacing values of 0.249 and 0.33 nm were related to the (311) and (002) planes of NFO and CD, respectively. The heterojunction formation between three nanoparticles was observed from the high-resolution TEM micrograph. However, the lattice fringes of g-CN were not observed in the composite due to the ultrathin layer structure.2 The thin-layered structure of the g-CN was observed due to the intercalation of NFO and CD nanoparticles in between the aromatic conjugated layers. However, the HRTEM image of bare g-CN (Figure S3b) shows the highly densely packed layered structure with an interplanar spacing of 0.32 nm due to bulk nature, which is in well accordance with the XRD result. The selected-area electron diffraction (SAED) pattern of the ternary nanocomposite was attributed to the polycrystalline nature of the prepared nanoparticle. The elemental analysis of the ternary nanocomposite was carried out by energy-dispersive spectrometry (EDS), and the result is displayed in Figure S6. The TEM, HRTEM, and SAED patterns of the pure g-CN and NFO nanoparticles and binary NFO/5g-CN are illustrated in the Supporting Information (SI). The strong interaction between the three nanoparticles was facilitated to improve the separation of charge carriers and enhance the visible light utilization for the photocatalytic degradation process. The HRTEM and XRD results of the ternary nanocomposite clearly showed that the prepared NFO and CD were intercalated between the carbon nitride nanosheets. This intercalated formation helps to improve the charge transport behavior of the nanocomposite, and it is well-matched with the obtained PL and photocurrent experiment results.
Figure 7.
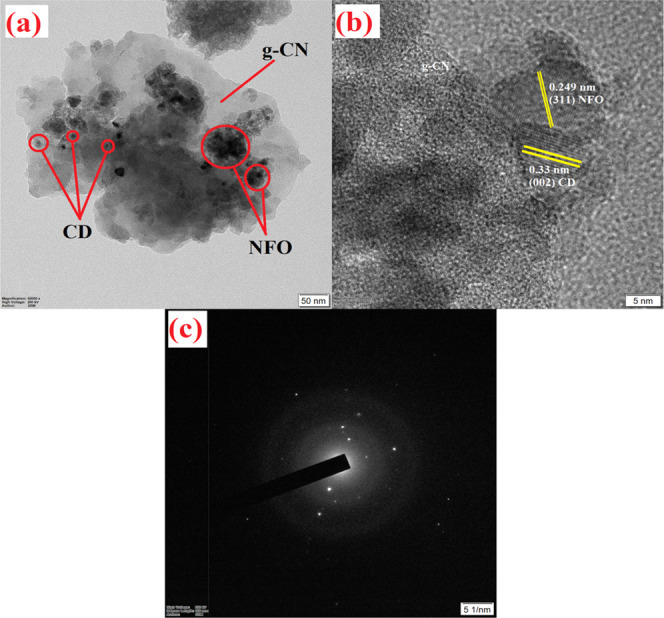
NFO/5g-CN/7.5CD (a) TEM, (b) HRTEM, and (c) SAED.
2.6. X-ray Photoelectron Spectroscopy (XPS) Analysis
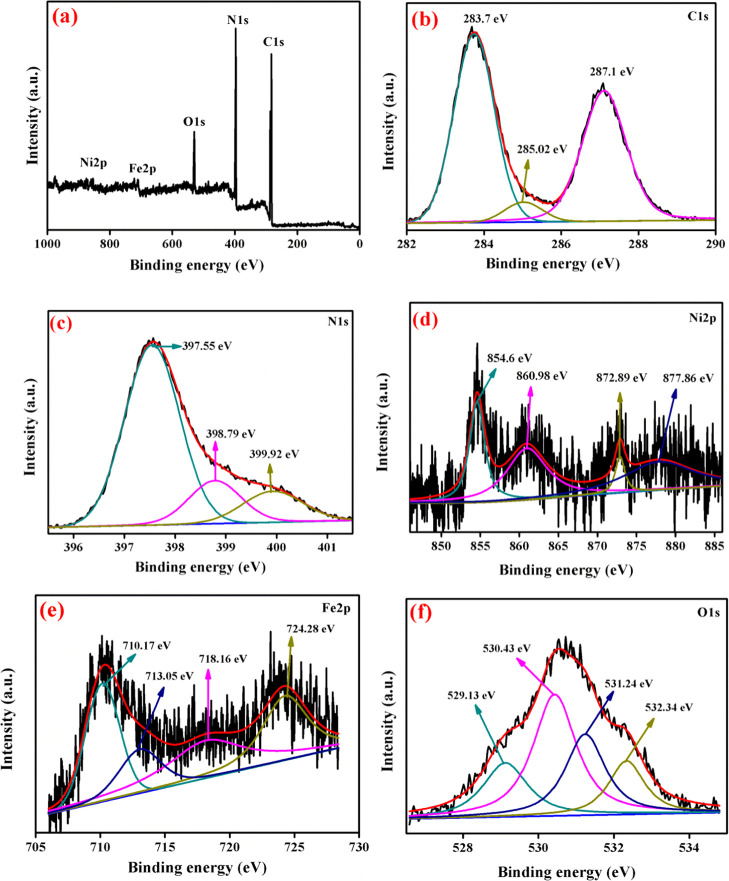
The chemical composition of the photocatalyst surface and the oxidation state of the elements were explored by XPS analysis, and the obtained results are displayed in Figure 8. The survey spectrum of the ternary nanocomposite (NFO/5g-CN/7.5CD) system consists of carbon, nitrogen, oxygen, nickel, and iron, which confirms the presence of all elements in the surface of the photocatalyst. The high-resolution spectrum of carbon 1s is shown in Figure 8b. The deconvoluted peaks presented in C 1s positioned at a binding energy of 283.7 and 287.1 eV attributed to the sp2 hybridized carbon and N–C=N in g-CN matrix. In addition to this, the peak positioned at 285.02 eV related to the C–O/C–OH group, which indicates the metal–carbon bonding and the presence of CD in the composite system.5,22,23 The deconvoluted peaks in the N 1s high-resolution spectrum present at the binding energies of 395.77, 398.79, and 399.92 eV are assigned to the nitrogen in the repeated tri-s-triazine ring (pyridinic N), pyrrolic nitrogen (C–N–H), and graphitic nitrogen.4,26 The high-resolution Ni 2p XPS spectrum is shown in Figure 8d. The two deconvoluted peaks at 854.6 and 872.29 eV are ascribed to Ni 2p3/2 and Ni 2p1/2 with an oxidation state of Ni2+. The satellite peak of the Ni 2p spectrum was observed at the binding energies of 860.98 and 877.76 eV.5 The oxidation state of Fe3+ was confirmed by the presence of deconvolution spectrum at the binding energies of 710.17 and 713.05 eV present in Fe 2p3/2. In addition to this, the satellite peak associated with Fe 2p3/2 is observed at the binding energy of 718.16 eV. The satellite peak was positioned at 8 eV higher than the Fe 2p3/2 spectrum. The presence of Fe 2p3/2 and the associated satellite peak confirms the presence of Fe3+ in the octahedral site in the NFO crystal system. The iron 2p1/2 peak was observed at the binding energy of 724.28 eV.4,5 The O 1s peak deconvoluted into four peaks, which were positioned at 529.13, 530.43, 531.24, and 532.34 eV. The Ni–O–Fe bonding of the NFO nanoparticles was associated with the binding energies of 529.13 and 530.43 eV. The peak at 531.24 eV was attributed to the surface-adsorbed OH molecule or C–O–C bonding.4,23 The peak of the HO–C=O bond was observed at 532.34 eV. Hence, the XPS result revealed that the CD, g-CN, and NFO nanoparticles chemically interact with each other and result in the formation of a heterojunction.
Figure 8.
XPS analysis of NFO/5g-CN/7.5CD: (a) survey, (b) C 1s, (c) N 1s, (d) Ni 2p, (e) Fe 2p, and (f) O 1s.
2.7. Vibrating Sample Magnetometer (VSM) Analysis
The magnetic nature of the pure NFO and ternary NFO/5g-CN/7.5CD photocatalysts was examined by a vibrating sample magnetometer. The recorded room-temperature magnetic hysteresis loop of pure and composite photocatalysts is shown in Figure 9. From the hysteresis loop analysis, it was confirmed that both the nanoparticles displayed the ferromagnetic nature at room temperature. The calculated magnetic saturation value of the ternary nanocomposite (10.16 emu/g) was lower than that of the pure NFO (37.14 emu/g). The reduced magnetization value of the composite was attributed to the addition of nonmagnetic g-C3N4 and CD. The observed coercivity values of NFO and NFO/5g-CN/7.5CD were 200 and 183 Oe, respectively. However, the ternary nanocomposite has been easily recovered by the external magnetic field. From the magnetic hysteresis loop analysis, one can conclude that the prepared photocatalysts were suitable for the recycling process.
Figure 9.
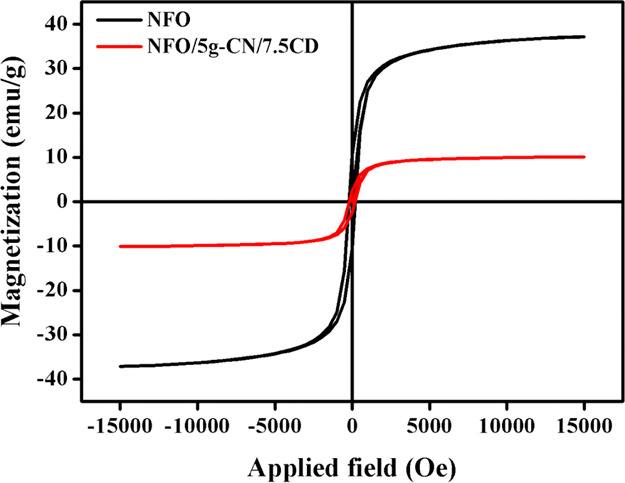
VSM magnetic hysteresis loop.
2.8. Photocatalytic Activity
The photocatalytic performance of the prepared pure and composite heterojunction photocatalysts was examined by degradation of rhodamine B (Rh B) and tetracycline (TCN) organic pollutants in the presence of a chemical oxidant at ambient conditions. Before light-emitting diode (LED) irradiation, the pollutant solution was stirred under dark conditions along with photocatalysts for 30 min to attain the adsorption–desorption equilibrium, and the adsorption efficiency is displayed in Figure S7. The adsorption experiment revealed that degradation happened mainly due to the presence of LED light irradiation. The photolysis process (Figure 10) of the organic pollutants in the presence of H2O2 and LED irradiation showed the negligible amount of degradation, which demonstrated that the pollutants are more stable in environmental conditions. The adsorption and photolysis processes clearly described that the degradation of organic pollutants happened due to the presence of light and photocatalysts.
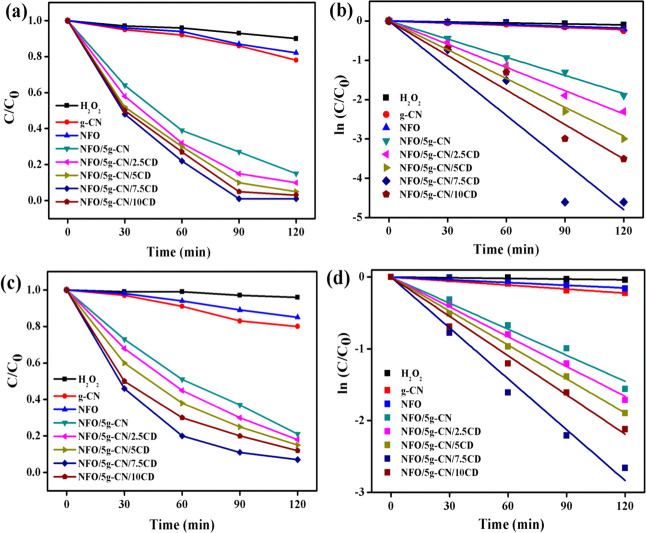
Figure 10.
Degradation plots of (a) Rh B and (c) TCN; first-order kinetic plots of (b) Rh B and (d) TCN (Rh B concentration, 20 mg/L; TCN concentration, 20 mg/L; catalyst dosage, 500 mg/L; H2O2, 1 mL; pH, 6.3).
From Figure 10, it can be observed that the binary and ternary composite photocatalysts exhibit better degradation efficiency in comparison with bare g-CN and pure NFO nanoparticles under LED light irradiation. The Rh B dye concentration plot concerning irradiation time and the kinetics of degradation plot as illustrated in Figure 10a,b. From the Rh B degradation plot, it was confirmed that the composite catalysts show the maximum degradation efficiency. Among all composite photocatalysts, the ternary NFO/5g-CN/7.5CD composite showed the highest photo-Fenton-like degradation efficiency of about 99% over 90 min of light irradiation. The binary composite exhibited the maximum degradation efficiency of 85% after the irradiation of 120 min, which was higher than that of the pure g-CN (22%) and NFO (18%) nanoparticles. The enhanced degradation efficiency of the NFO/5g-CN photocatalyst attributed to the synergistic effect between the nanoparticles helps to harvest more amount of light radiation and promote charge carrier separation. It was noted that the addition of CD further enhanced the degradation efficiency, and the maximum efficiency was reached at 7.5 wt % CD addition. However, the low visible-light absorption of NFO/5g-CN/7.5CD shows the superior degradation efficiency due to the effective photoinduced electron–hole separation and electron transport behavior. The recorded Rh B dye degradation values after 120 min of LED light irradiation in the presence of H2O2 were 90, 95, and 97% attributed to 2.5, 5, and 10 wt % CD addition to the NFO/5g-CN composite. The decreases in the degradation efficiency observed at 10 wt % CD addition described that the higher amount of CD may act as the electron–hole recombination center.31 The first-order pseudokinetic plot is displayed in Figure 10d, and the rate constant, “k”, value was calculated using the relation2
where C is the initial concentration of the dye solution and C0 refers to the dye concentration at time “t”. The term k denotes the rate constant of dye degradation. It is concluded that the rate constant of binary and ternary composites has a higher value when compared to that of pure nanoparticles. The calculated highest rate constant of about 0.04 min–1 was assigned to the ternary nanocomposite NFO/5g-CN/7.5CD photocatalyst. This value is 21, 26, and 2.6 times higher than those of the bare g-CN, pure NFO, and binary NFO/5g-CN photocatalysts. The calculated rate constants for Rh B dye degradation by 2.5, 5, and 10 wt % CD-added ternary photocatalysts were 0.0197, 0.0244, and 0.0293 min–1, respectively.
The extended photocatalytic activity of the prepared nanoparticles was examined by colorless tetracycline organic pollutant degradation, and the obtained results are displayed in Figure 10c,d. From the degradation plot, it was confirmed that the ternary composite system exhibited a higher degradation efficiency compared to the pure and binary photocatalysts. The maximum degradation efficiency of 93% was observed for the 7.5 wt % CD-added ternary photocatalyst after the 120 min of visible light irradiation in the presence of H2O2. The photolysis process showed the very low degradation efficiency of about 4% in the presence of a chemical oxidant, proving the stability of the TCN molecule under light irradiation. The TCN degradation efficiencies of 20 and 16% were observed for pure g-CN and NFO nanoparticles. The binary NFO/5g-CN photocatalyst showed a degradation efficiency of 79% after visible light irradiation. The TCN degradation efficiencies of 2.5, 5, and 10 wt % CD-added photocatalyst were 82, 85, and 88% respectively. The calculated rate constant for the NFO/5g-CN/7.5CD photocatalyst was 0.0236 min–1, which was 2, 1.8, 1.5, and 1.3 times higher than those of the binary NFO/5g-CN and ternary NFO/5g-CN/2.5CD, NFO/5g-CN/5CD, and NFO/5g-CN/10CD nanocomposites correspondingly. The enhanced degradation efficiency of the nanocomposite suggested that the synergistic effect among the NFO, g-CN, and CD nanoparticles greatly reduced the charge carrier recombination and improved charge transportation behavior.
To confirm the mineralization ability of the ternary NFO/5g-CN/7.5CD nanocomposite, the total organic carbon (TOC) analysis was performed on Rh B and TCN photo-Fenton degradation processes. During the degradation of organic pollutants, intermediate products could be formed, which could be more toxic than the original organic pollutants. Therefore, it is necessary to perform the TOC analysis for the degradation process.32 The TOC removal efficiency of the ternary nanocomposite is shown in Figure S8. The TOC mineralization efficiencies for Rh B and TCN degradation under LED light irradiation were 71 and 63% in the presence of H2O2, respectively. Hence, this TOC analysis result implies that the prepared ternary NFO/5g-CN/7.5CD nanocomposite is a highly efficient photocatalyst for organic pollutant mineralization.
The solution pH also influences the degradation efficiency of the heterogeneous photo-Fenton reaction. The degradation plot of the organic pollutant under different pH conditions using the ternary NFO/5g-CN/7.5CD nanocomposite is shown in Figure S9. The degradation efficiency of the photocatalyst decreased on increasing the pH of the pollutant solution. The initial pH of the organic pollutant solution was adjusted by the diluted HCL acid solution and diluted NaOH solution. The pH of the solution was varied from ∼3 to ∼12, and the degradation efficiency was recorded. Under acidic conditions, the photocatalyst exhibited a higher degradation efficiency compared to that under basic conditions. Under lower pH conditions, the H3O2+ produced, and it enhanced the stability of H2O2. This formation helped to extremely scavenge the OH• radicals by H+. Under high pH conditions, the formation of the OH• radical reduced due to hydrolysis of Fe2+.3232
The degradation of TCN and Rh B occurred at a wide range of pH values, which indicates that the prepared ternary nanocomposite NFO/5g-CN/7.5CD photocatalyst is a promising candidate for organic pollutant degradation for practical use.
To confirm the active species for the dye degradation process and conversion of heterojunction from type-II to Z-scheme, the elemental trapping experiment was conducted for binary and ternary composite photocatalysts on Rh B dye degradation, and the results are shown in Figure 11. The different radical scavengers such as benzoquinone (BQ), ethylenediaminetetraacetic acid (EDTA), and isopropyl alcohol (IPA) were used to detect the production of superoxide radicals, holes, and hydroxyl radicals produced during the photocatalysis process. It was concluded from the elemental trapping experiment for ternary NFO/5g-CN/7.5CD photocatalysts that the hydroxyl and superoxide radicals are the main active species for the dye degradation process. The recorded degradation efficiency of about 95% was achieved with the addition of EDTA, which demonstrated that holes did not involve in the degradation process. The addition of BQ and IPA reduced the Rh B dye degradation efficiency to 52 and 40%, respectively. The elemental trapping experiment indicates that the hydroxyl radicals and superoxide radicals both contribute to the dye degradation process. However, this is controversial with the binary photocatalyst elemental trapping experiment result, which indicates that OH• radicals are the main active species for the dye degradation process. The superoxide radical does not contribute to the degradation process in binary NFO/5g-CN photocatalysts. The recorded binary photocatalyst degradation efficiencies after 180 min of LED irradiation were 92, 99, and 37% corresponding to the addition of BQ, EDTA, and IPA scavengers. It was noted for the binary system that the addition of EDTA slightly improved the dye degradation efficiency when compared to that without the scavenger. During the photocatalysis process, electron–hole pairs generated at the photocatalysts. The addition of EDTA trapped the holes produced in the photocatalyst, which led to high electron migration at the conduction band (CB) of the photocatalysts. The electrons at the conduction band of photocatalysts produced a higher amount of superoxide radicals, which led to higher catalytic degradation.5 However, the superoxide radical scavenger did not alter the photocatalytic degradation efficiency while using the NFO/5g-CN binary photocatalyst. However, the addition of BQ reduced the dye degradation efficiency up to 52% while using the NFO/5g-CN/CD ternary photocatalyst. The elemental trapping experiment clearly described that the ternary composite exhibited the Z-scheme photocatalysts, but the binary system showed the type-II heterojunction.
Figure 11.
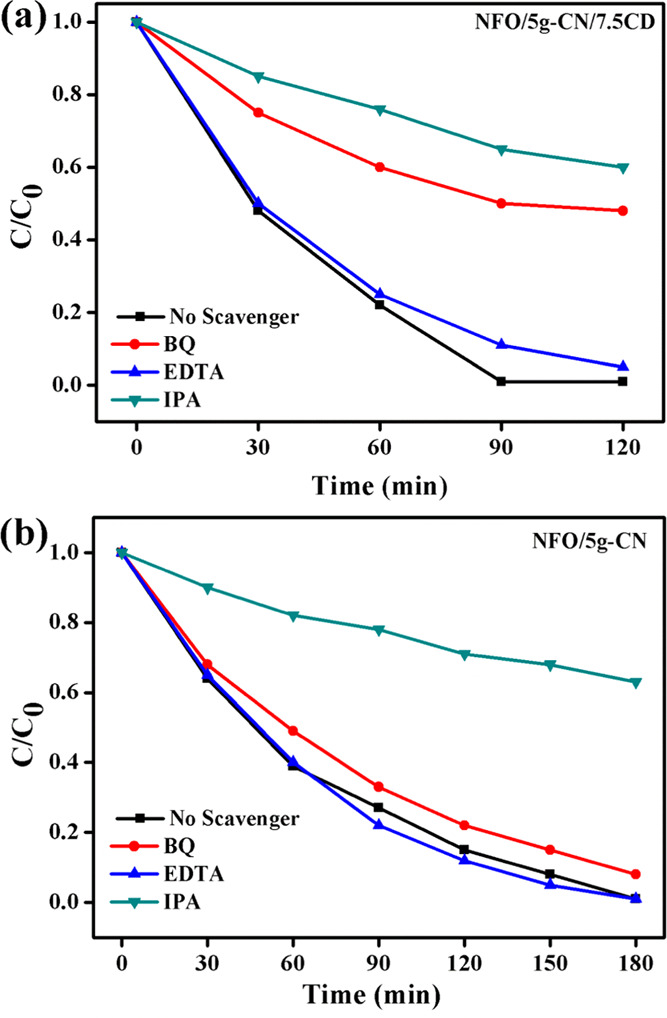
Elemental trapping experiment on Rh B dye degradation: (a) NFO/5g-CN/7.5CD and (b) NFO/5g-CN (Rh B concentration, 20 mg/L; catalyst dosage, 500 mg/L; H2O2, 1 mL; pH, 6.3; BQ, 0.1 mmol; EDTA, 0.1 mmol; IPA, 3 mL; pH, 6.3).
To confirm the production of O2– and OH• radicals, the NBT and TA tests were conducted on pure g-CN and NFO, binary NFO/5g-CN, and ternary NFO/g-CN/7.5CD photocatalysts, and the results are displayed in Figure 12a,b. The reaction between TA and OH• radicals produced fluorescent 2-hydroxy terephthalic acid (HTA). The production of HTA was observed by the PL emission peak at 425 nm. From Figure 12a, it was observed that the peak intensity gets increased on increasing the irradiation time. As evident from Figure 12, the highest peak intensity was observed for the NFO/5g-CN/7.5CD photocatalyst compared to other pure and binary photocatalysts, which described the higher amount of OH• radical production. The higher production of hydroxyl radicals confirms the suppression of photoinduced electron–hole pair recombination.33 The TA-assisted PL analysis result was well consistent with that of the elemental trapping experiment, in which OH• radicals were the main active species for the dye degradation process on both binary and ternary composite photocatalysts.
Figure 12.
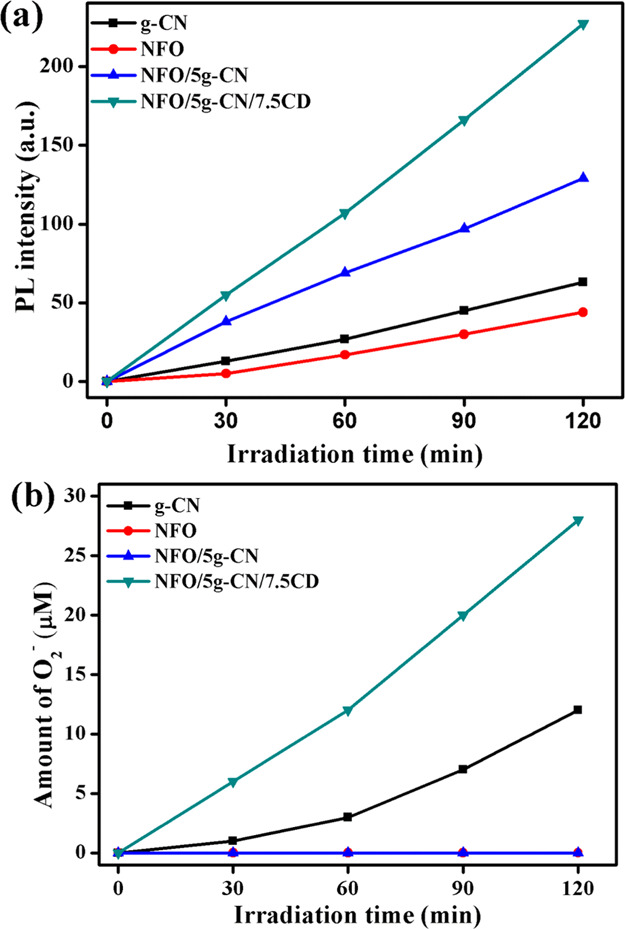
(a) Terephthalic acid (TA) test and (b) nitroblue tetrazolium (NBT) test.
The production of superoxide radicals was confirmed by the nitroblue tetrazolium method. During the photocatalysis process, the NBT absorbance intensity gets reduced at 260 nm. The NBT concentration vs time plot is illustrated in Figure S10. The degradation of NBT was used to detect the production of O2– radicals. According to the relation between nitroblue tetrazolium and superoxide radicals (in 1:4 molar ratio), the production of O2– was evaluated and is shown in Figure 12b.34 The ternary NFO/5g-CN/7.5CD photocatalyst produces 28 μM superoxide radicals after 120 min of LED light irradiation. The pure carbon nitride produces 12 μM superoxide radicals. Surprisingly, the pure NFO and NFO/5g-CN binary photocatalysts do not produce the superoxide radicals after 120 min of light irradiation. The obtained result well-matched with those of the elemental trapping experiment on binary NFO/5g-CN and ternary NFO/5g-CN/7.5CD photocatalysts.
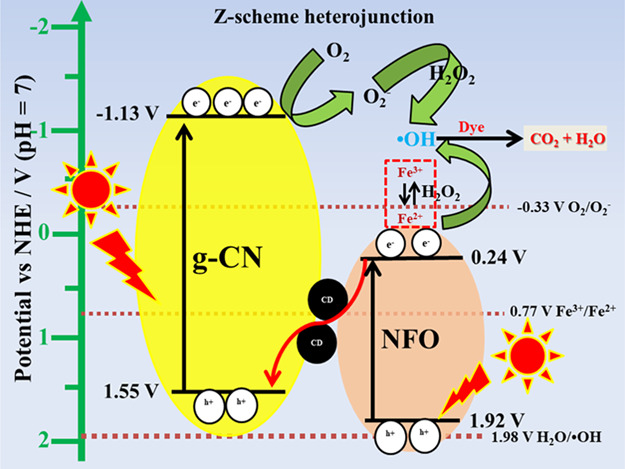
Furthermore, the possible reaction mechanism for the photocatalytic dye degradation process was proposed based on the elemental trapping experiment, and the mechanism is illustrated in Figures 13 and S11. It is important to know the band potential of NFO and g-CN nanoparticles to propose the reaction mechanism. The conduction and valance band (VB) potentials of the NFO and g-CN nanoparticles were taken from the previous report.5 The conduction band and valance band potential energies (vs normal hydrogen electrode (NHE)) of g-C3N4 were −1.13 and 1.55 V, respectively. However, valance and conduction band energies of pure NiFe2O4 were 0.24 and 1.92 V, respectively. When light was irradiated on the surface of the photocatalysts, the electrons were excited from the valance band and transferred to the conduction band (CB), leaving the holes at valance band (VB) on both catalysts. The electrons at the CB of g-CN transferred to CB of NFO, and holes at VB of NFO were transferred to VB of g-CN in binary photocatalysts. The CB electrons at the NFO nanoparticle have a low reduction potential (0.24 V) compared to the standard reduction potential (−0.33 V vs NHE). On the other hand, the g-CN VB has a low oxidation potential (1.55 V) in comparison with the standard oxidation potential. In the photo-Fenton reaction, the electrons at CB of NFO react with H2O2 and produce the active OH• radicals, which further degrade the organic pollutant due to the type-II heterojunction. This possible reaction mechanism for the NFO/5g-CN photocatalyst well-matched with the elemental trapping and TA experiments. Moreover, the NBT experiment demonstrated that the superoxide radicals did not produce in the binary photocatalyst due to the low reduction potential of NFO.
Figure 13.
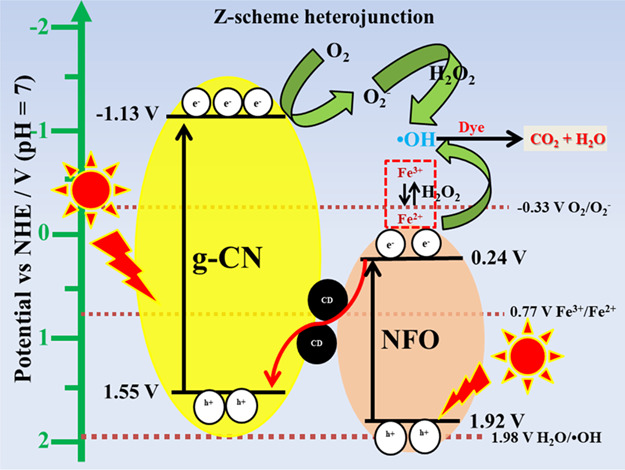
Possible reaction mechanism of NFO/5g-CN/7.5CDF.
When the CD was added to the system, it was intercalated between NFO and g-CN nanoparticles and acted as an electron acceptor/donor. This intercalated carbon dot led to a Z-scheme heterojunction for effective dye degradation. In the ternary nanocomposite system, the superoxide radicals also played a vital role to degrade the organic pollutant. In the conventional type-II heterojunction, the electrons migrated to the CB of NFO. The CB potential of NFO nanoparticles had insufficient energy to participate in the oxygen reduction reaction to generate the O2– radicals. The type-II heterojunction mechanism could not explain the obtained elemental trapping experiment results. Therefore, we have proposed the Z-scheme mechanism for the charge carrier transfer path.35
In the ternary photocatalyst system, the excited CB electrons at NFO were transferred to the surface of the CD. Then, the electron was transferred to VB of the g-CN photocatalyst and the migrated electrons at the VB of g-CN got excited by the photon source. The CB electrons at g-CN reduced the atmospheric oxygen to superoxide radicals due to its more negative energy than the standard reduction potential. Then, the oxidizing agent reacted with the O2– radicals and produced a high amount of OH• radicals by the Haber–Weiss reaction. This reaction resulted in a higher amount of OH• radical production, which was confirmed by the TA and NBT experiments. Moreover, the elemental trapping experiment also demonstrated that the superoxide and hydroxyl radicals are the main active species for the dye degradation process. On the other hand, the photo-Fenton reaction takes place at CB of NFO nanoparticles. The potential of CB of NFO is more negative than the standard potential of Fe3+/Fe2+ (0.77 V vs NHE). Therefore, the Fe3+ ions at the NFO nanoparticles reduced to Fe2+ ions under LED irradiation.32 Consequently, the generated Fe2+ ions produced OH• radicals by reacting with H2O2. Then, they were oxidized by H2O2 and converted into Fe3+ again to complete the Fe3+/Fe2+ cycle. Further, the generated OH• radicals attacked the organic pollutant adsorbed on the surface of the photocatalyst, giving rise to reaction intermediates. Moreover, the reaction intermediates were degraded to CO2 and H2O.36 The holes at the valance band of the NFO nanoparticle have low energy than the standard oxidation potential (1.98 V vs NHE). Therefore, the VB holes at NFO nanoparticles cannot contribute to the organic pollutant degradation process.35 In this way, the photoinduced electron–hole pairs separated in the opposite direction and led to higher degradation efficiency by the Z-scheme mechanism. The possible photo-Fenton and Haber–Weiss reactions are as follows2,4,37
2.9. Recycle Test
For practical implementation, it is necessary to perform the recycle test to ensure the stability of the prepared photocatalysts. The stability of the NFO/5g-CN/7.5CD ternary photocatalyst was examined by five successive cycles for Rh B dye degradation under LED light irradiation in the presence of H2O2. The recycle stability of the photocatalysts is displayed in Figure 14. The recycle test proves that the prepared ternary photocatalyst has high stability with a degradation efficiency of 90% even at the fifth cycle. Hence, it was concluded that the prepared NFO/5g-CN/7.5CD ternary photocatalysts are suitable for practical use.
Figure 14.
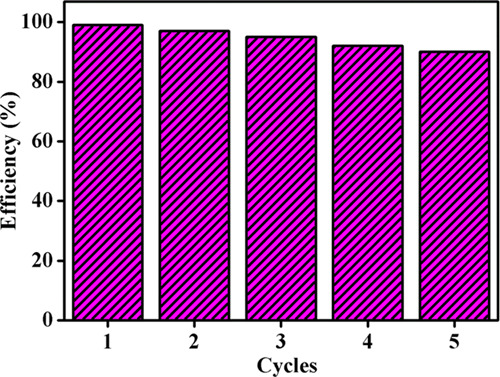
Recycle test for NFO/5g-CN/7.5CD.
3. Conclusions
In this report, the CD-intercalated NiFe2O4/g-C3N4 ternary heterojunction has been synthesized by the facile sol–gel method followed by calcination and wet chemical impregnation method. The strong interaction between the nanoparticles and the heterojunction formation are investigated through TEM and HRTEM analyses. The heterojunction formation helps to harvest high-intensity visible light for the photo-Fenton degradation process. The CD effectively separates the photoinduced electron–hole pairs. The highest degradation efficiency of the NFO/5g-CN/7.5CD ternary photocatalyst well-matches with the OH• radical and O2– radical production experiments. The electron transport pathway for the Z-scheme photo-Fenton process and the active radicals for dye degradation are analyzed by the elemental trapping experiment. Moreover, the recycle test confirms the stability of the photocatalyst. Hence, the magnetic NFO/5g-CN/7.5CD ternary heterojunction photocatalyst is a promising photocatalyst for visible-light-driven wastewater treatment.
4. Experimental Methods
4.1. Synthesis of NiFe2O4/g-C3N4/CD
The simple wet chemical impregnation method has been used to prepare the ternary nanocomposite photocatalysts. In this process, 100 mg of NFO/5g-CN and various amounts (2.5, 5, 7.5, and 10 wt %) of CD were dispersed in 5 mL of ethanol. The solution was subjected to ultrasonication for 45 min. Then, the obtained powder was dried at 80 °C for 2 h. The prepared nanocomposites were named as NFO/5g-CN/XCD (X = 2.5, 5, 7.5, and 10 wt %).
The detailed description of synthesis methods for pure nanoparticles, characterization techniques, and their photocatalytic activity test procedure has been given in the Supporting Information.
Acknowledgments
B.P. acknowledges the SRMIST for providing financial support to carry out the research. We acknowledge the HRTEM Facility at SRMIST setup with support from MNRE (Project no. 31/03/20143-15/PVSE-R&D), Government of India. We acknowledge the Nanotechnology Research Centre (NRC), SRMIST, for providing the research facilities such as XRD, UV–vis–DRS, VSM, XPS, FTIR, and FESEM.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02477.
Experimental methods; FTIR spectra; photocurrent measurement; TEM, HRTEM, and SAED images for pure and composite materials; EDS spectrum; TOC analysis; adsorption efficiency; NBT test; and reaction mechanism (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mousavi M.; Habibi-Yangjeh A.; Pouran S. R. Review on magnetically separable graphitic carbon nitride-based nanocomposites as promising visible-light-driven photocatalysts. J. Mater. Sci.: Mater. Electron. 2018, 29, 1719–1747. 10.1007/s10854-017-8166-x. [DOI] [Google Scholar]
- Palanivel B.; Jayaraman V.; Ayyappan C.; Alagiri M. Magnetic binary metal oxide intercalated g-C3N4: Energy band tuned p-n heterojunction towards Z-scheme photo-Fenton phenol reduction and mixed dye degradation. J. Water Process. Eng. 2019, 32, 100968 10.1016/j.jwpe.2019.100968. [DOI] [Google Scholar]
- Shekofteh-Gohari M.; Habibi-Yangjeh A.; Abitorabi M.; Rouhi A. Magnetically separable nanocomposites based on ZnO and their applications in photocatalytic processes: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 806–857. 10.1080/10643389.2018.1487227. [DOI] [Google Scholar]
- Palanivel B.; devi Mudisoodum perumal S.; Maiyalagan T.; Jayarman V.; Ayyappan C.; Alagiri M. Rational design of ZnFe2O4/g-C3N4 nanocomposite for enhanced photo-Fenton reaction and supercapacitor performance. Appl. Surf. Sci. 2019, 498, 143807 10.1016/j.apsusc.2019.143807. [DOI] [Google Scholar]
- Palanivel B.; Ayappan C.; Jayaraman V.; Chidambaram S.; Maheswaran R.; Mani A. Inverse spinel NiFe2O4 deposited g-C3N4 nanosheet for enhanced visible light photocatalytic activity. Mater. Sci. Semicond. Process. 2019, 100, 87–97. 10.1016/j.mssp.2019.04.040. [DOI] [Google Scholar]
- Ji H.; Jing X.; Xu Y.; Yan J.; Li H.; Li H.; Huang L.; Zhang Q.; Xu H.; Li H. Magnetic g-C3N4/NiFe2O4 hybrids with enhanced photocatalytic activity. RSC Adv. 2015, 5, 57960–57967. 10.1039/C5RA07148H. [DOI] [Google Scholar]
- Kamal S.; Balu S.; Palanisamy S.; Uma K.; Velusamy V.; Yang T. C. K. Synthesis of boron doped C3N4/NiFe2O4 nanocomposite: An enhanced visible light photocatalyst for the degradation of methylene blue. Results Phys. 2019, 12, 1238–1244. 10.1016/j.rinp.2019.01.004. [DOI] [Google Scholar]
- Mishra P.; Behera A.; Kandi D.; Parida K. Facile construction of a novel NiFe2O4@P-doped g-C3N4 nanocomposite with enhanced visible light- driven photocatalytic activity. Nanoscale Adv. 2019, 1, 1864–1879. 10.1039/C9NA00018F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J.; Song T.; Lv M.; Wang T.; Qin J.; Zeng H. Plasmonic photocatalysts Au/g-C3N4/NiFe2O4 nanocomposites for enhanced visible-light-driven photocatalytic hydrogen evolution. RSC Adv. 2016, 6, 54964–54975. 10.1039/C6RA08356K. [DOI] [Google Scholar]
- Zhu H.-Y.; Ru J.; Yong-Qian F.; Rong-Rong L.; Jun Y.; Sheng-Tao J. Novel multifunctional NiFe2O4/ZnO hybrids for dye removal by adsorption, photocatalysis and magnetic separation. Appl. Surf. Sci. 2016, 369, 1–10. 10.1016/j.apsusc.2016.02.025. [DOI] [Google Scholar]
- Šutka A.; Käämbre T.; Pärna R.; Döbelin N.; Vanags M.; Smits K.; Kisand V. Ag sensitized TiO2 and NiFe2O4 three-component nanoheterostructures: synthesis, electronic structure and strongly enhanced visible light photocatalytic activity. RSC Adv. 2016, 6, 18834–18842. 10.1039/C6RA00728G. [DOI] [Google Scholar]
- He Z.; Xia Z.; Tang B.; Su J. Fabrication and photocatalytic property of magnetic NiFe2O4/Cu2O composites. Mater. Res. Express 2017, 4, 095501 10.1088/2053-1591/aa7cb8. [DOI] [Google Scholar]
- Gebreslassie G.; Bharali P.; Chandra U.; Sergawie A.; Baruah P. K.; Das M. R.; Alemayehu E. Hydrothermal Synthesis of g-C3N4/NiFe2O4 Nanocomposite and Its Enhanced Photocatalytic Activity. Appl. Organomet. Chem. 2019, 33, e5002 10.1002/aoc.5002. [DOI] [Google Scholar]
- Akhundi A.; Habibi-Yangjeh A.; Abitorabi M.; Pouran S. R. Review on photocatalytic conversion of carbon dioxide to value-added compounds and renewable fuels by graphitic carbon nitride-based photocatalysts. Catal. Rev. 2019, 61, 595–628. 10.1080/01614940.2019.1654224. [DOI] [Google Scholar]
- Donghui H.; Chen Z.; Guangming Z.; Yang Y.; Danlian H.; Longlu W.; Hou W. A multifunctional platform by controlling of carbon nitride in the core-shell structure: From design to construction, and catalysis applications. Appl. Catal., B 2019, 258, 117957 10.1016/j.apcatb.2019.117957. [DOI] [Google Scholar]
- Wang W.; Zeng Z.; Zeng G.; Zhang C.; Xiao R.; Zhou C.; Xiong W.; Yang Y.; Lei L.; Liu X.; Huang D.; Cheng M.; Yang Y.; Fu Y.; Luo F.; Zhou Y. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light. Chem. Eng. J. 2019, 378, 122132 10.1016/j.cej.2019.122132. [DOI] [Google Scholar]
- Yang Y.; Chen Z.; Danlian H.; Guangming Z.; Jinhui H.; Cui L.; Hengyun Z.; Wenjun W.; Hai G.; Wenjing X.; Rui D.; Min Ch.; Weiping X. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal., B 2019, 245, 87–99. 10.1016/j.apcatb.2018.12.049. [DOI] [Google Scholar]
- Yang Y.; Zeng G.; Huang D.; Zhang C.; He D.; Zhou C.; Wang W.; Xiong W.; Li X.; Li B.; Dong Y.; Zhou Y. Molecular engineering of polymeric carbon nitride for highly efficient photocatalytic oxytetracycline degradation and H2O2 production. Appl. Catal., B 2020, 272, 118970 10.1016/j.apcatb.2020.118970. [DOI] [Google Scholar]
- Liu Y.; Song Y.; You Y.; Fu X.; Wen J.; Zheng X. NiFe2O4/g-C3N4 heterojunction composite with enhanced visible-light photocatalytic activity. J. Saudi Chem. Soc. 2017, 22, 439–448. 10.1016/j.jscs.2017.08.002. [DOI] [Google Scholar]
- Guo Y.; Zhang J.; Zhou D.; Dong S. Fabrication of Ag/CDots/BiOBr ternary photocatalyst with enhanced visible-light driven photocatalytic activity for 4-chlorophenol degradation. J. Mol. Liq. 2018, 262, 194–203. 10.1016/j.molliq.2018.04.091. [DOI] [Google Scholar]
- Liu E.; Xu C.; Jin C.; Fan J.; Hu X. Carbon quantum dots bridged TiO2 and Cd0.5Zn0.5S film as solid-state Z-scheme photocatalyst with enhanced H2 evolution activity. J. Taiwan Inst. Chem. Eng. 2019, 97, 316–325. 10.1016/j.jtice.2019.02.027. [DOI] [Google Scholar]
- Wu X.; Zhao J.; Wang L.; Han M.; Zhang M.; Wang H.; Hui H.; Liu Y.; Kang Z. Carbon dots as solid-state electron mediator for BiVO4/CDs/CdS Z-scheme photocatalyst working under visible light. Appl. Catal., B 2017, 206, 501–509. 10.1016/j.apcatb.2017.01.049. [DOI] [Google Scholar]
- Asadzadeh-Khaneghah S.; Habibi-Yangjeh A.; Abedi M. Decoration of carbon dots and AgCl over g-C3N4 nanosheets: Novel photocatalysts with substantially improved activity under visible light. Sep. Purif. Technol. 2018, 199, 64–77. 10.1016/j.seppur.2018.01.023. [DOI] [Google Scholar]
- Matheswaran P.; Thangavelu P.; Palanivel B. Carbon dot sensitized integrative g-C3N4/AgCl Hybrids: An synergetic interaction for enhanced visible light driven photocatalytic process. Adv. Powder Technol. 2019, 30, 1715–1723. 10.1016/j.apt.2019.05.024. [DOI] [Google Scholar]
- Natarajan T. S.; Thampi K. R.; Tayade R. J. Visible Light Driven Redox-mediator-free Dual Semiconductor Photocatalytic Systems for Pollutant Degradation and the Ambiguity in Applying Z-scheme Concept. Appl. Catal., B 2018, 227, 296–311. 10.1016/j.apcatb.2018.01.015. [DOI] [Google Scholar]
- Fang S.; Xia Y.; Lv K.; Li Q.; Sun J.; Li M. Effect of Carbon-dots Modification on the Structure and Photocatalytic Activity of g-C3N4. Appl. Catal., B 2016, 185, 225–232. 10.1016/j.apcatb.2015.12.025. [DOI] [Google Scholar]
- Zhou L.; Liu J.; Zhang X.; Liu R.; Huang H.; Liu Y.; Kang Z. Template-free fabrication of mesoporous carbons from carbon quantum dots and their catalytic application to the selective oxidation of Hydrocarbons. Nanoscale 2014, 6, 5831–5837. 10.1039/c4nr00716f. [DOI] [PubMed] [Google Scholar]
- Xu H.; Yang X.; Li G.; Zhao C.; Liao X. Green Synthesis of Fluorescent Carbon Dots for Selective Detection of Tartrazine in Food Samples. J. Agric. Food Chem. 2015, 63, 6707–6714. 10.1021/acs.jafc.5b02319. [DOI] [PubMed] [Google Scholar]
- Yan Y.; Yang H.; Yi Z.; Wang X.; Li R.; Xian T. Evolution of Bi Nanowires from BiOBr Nanoplates Through a NaBH4 Reduction Method with Enhanced Photodegradation Performance. Environ. Eng. Sci. 2020, 37, 64–77. 10.1089/ees.2019.0284. [DOI] [Google Scholar]
- Selvaraj S.; Mohan M. K.; Navaneethan M.; Ponnusamy S.; Muthamizhchelvan C. Synthesis and photocatalytic activity of Gd doped ZnO nanoparticles for enhanced degradation of methylene blue under visible light. Mater. Sci. Semicond. Process. 2019, 103, 104622 10.1016/j.mssp.2019.104622. [DOI] [Google Scholar]
- Zarezadeh S.; Habibi-Yangjeh A.; Mousavi M. Fabrication of novel ZnO/BiOBr/C-Dots nanocomposites with considerable photocatalytic performances in removal of organic pollutants under visible light. Adv. Powder Technol. 2019, 30, 1197–1209. 10.1016/j.apt.2019.03.016. [DOI] [Google Scholar]
- Guo S.; Zhang G.; Wang J. Photo-Fenton degradation of rhodamine B using Fe2O3–Kaolin as heterogeneous catalyst: Characterization, process optimization and mechanism. J. Colloid Interface Sci. 2014, 433, 1–8. 10.1016/j.jcis.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Sarkar A. K.; Saha A.; Tarafder A.; Panda A. B.; Pal S. Efficient Removal of Toxic Dyes via Simultaneous Adsorption and Solar Light Driven Photodegradation Using Recyclable Functionalized Amylopectin-TiO2-Au Nanocomposite. ACS Sustainable Chem. Eng. 2016, 4, 1679–1688. 10.1021/acssuschemeng.5b01614. [DOI] [Google Scholar]
- Yang Y.; Zhuotong Z.; Guangming Z.; Danlian H.; Rong X.; Chen Z.; Chengyun Z.; Weiping X.; Wenjun W.; Min C.; Wenjing X.; Hai G.; Xiang T.; Donghui H. Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production. Appl. Catal., B 2019, 258, 117956 10.1016/j.apcatb.2019.117956. [DOI] [Google Scholar]
- Guo T.; Wang K.; Zhang G.; Wu X. A novel α-Fe2O3@g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance. Appl. Surf. Sci. 2019, 469, 331–339. 10.1016/j.apsusc.2018.10.183. [DOI] [Google Scholar]
- Li J.; Xiao C.; Wang K.; Li Y.; Zhang G. Enhanced Generation of Reactive Oxygen Species under Visible Light Irradiation by Adjusting the Exposed Facet of FeWO4 Nanosheets To Activate Oxalic Acid for Organic Pollutant Removal and Cr(VI) Reduction. Environ. Sci. Technol. 2019, 53, 11023–11030. 10.1021/acs.est.9b00641. [DOI] [PubMed] [Google Scholar]
- Collin F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407 10.3390/ijms20102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.