Abstract
Tobacco mosaic virus (TMV) has caused huge economic losses to tobacco, pepper, cucumber, and ornamental crops all over the world. However, few effective antiviral agents were developed and applied to control such plant disease. It is challenging to find an anti-TMV agent which is highly effective, less toxic, and environmentally friendly. In this work, a series of ferulic acid ester-containing sulfonamide moieties were designed and synthesized, and the antiviral activities of these compounds against TMV were evaluated. The anti-TMV biological activity test showed that the target compounds showed excellent anti-TMV activity in vitro and in vivo. In particular, compound 2 has excellent anti-TMV activity at 500 μg/mL, which is higher than that of the control drug ribavirin. The preliminary mechanism research results showed that compound 2 can obviously destroy the morphology of the virions to show excellent activity. The results show that the ferulic acid ester-containing sulfonamide moiety deserves further research and development.
Introduction
Plant viruses can cause severe damage to plants, thereby seriously threatening the development of the world economy. One of the most dangerous plant viruses is tobacco mosaic virus (TMV), and it is considered as the most ancient virus in plant virology as it was discovered in 1898.1,2 It has caused huge economic losses to tobacco, pepper, cucumber, and other ornamentals and flowers all over the world.3 Moreover, they can use the internal mechanism of the host and you can first spread it, or they can spread it through biological means, such as spreading through aphids.4 Although traditional chemicals can inhibit TMV within a certain range, they cannot effectively eliminate the virus and are not environmentally friendly. Therefore, the use of traditional chemicals is limited.
To find green and efficient antiplant virus drugs, researchers have conducted screening from natural product sources and chemical synthesis to obtain some effective antiplant virus drugs, such as ningnanmycin and ribavirin (Figure 1).5−7 However, the ribavirin activity is poor, whereas the field control efficacy of ningnanmycin is not ideal, and the cost is high.8−10 Therefore, finding new and highly effective environmentally friendly antiplant virus agents remains a major challenge for pesticide researchers.
Figure 1.
Commercially available antiviral agents of ferulic acid, ribavirin, and ningnanmycin.
Natural products have attracted widespread attention from biologists for their specific targets, special modes of action, and environmental protection.11−13 However, natural products have some drawbacks, such as complex structures, difficult synthesis, and easy decomposition of the active ingredients.14,15 These drawbacks limit the widespread use of natural products in pesticides. Ferulic acid (Figure 1) is a phenolic acid commonly found in plants and one of the active ingredients in Chinese medicine, such as Angelica sinensis, Ligusticum chuanxiong Hort, and Ferula sinkiangensis K. M. Shen. In addition, ferulic acid is high in wheat and rice brans. Studies have shown that ferulic acid and its derivatives have a wide range of biological activities,16,17 including bacteriostatic,18 antiviral,19−21 anticancer,22,23 anti-inflammatory,24 and other activities. A series of ferulic acid derivatives have been designed and synthesized by the research group in the early stage, and the results have shown that the ferulic acid derivatives exhibit good antiplant virus activity. At the same time, sulfonamides have attracted widespread attention from pesticide scientists because of their broad biological properties, such as antibacterial25 and antiviral activities.26−29
This project intends to use the principle of active splicing to introduce a wide range of biologically active sulfonamide structural units into the natural product ferulic acid with good antiplant virus activity to design (Figure 2) and synthesize a series of ferulic acid ester-containing sulfonamide moieties. The anti-TMV activities are tested using the half-leaf method to find compounds with high activity and study the preliminary mechanism of action of highly active compounds and viruses.
Figure 2.
Design ideas of the target compounds. The pictures in the figure, except the picture of ferulic, were taken by the author Xiaoli Ren. Copyright 2020. The picture of ferulic was freely available online, and the image of ferulic is a free domain.
Results and Discussion
Chemistry
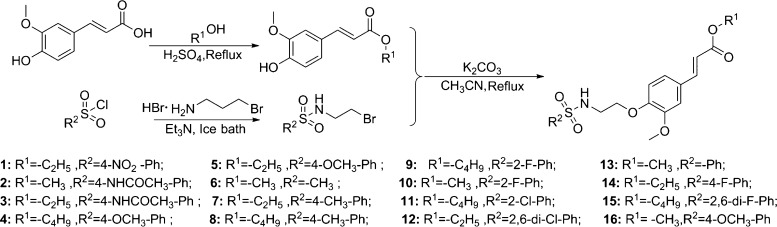
As shown in Figure 3, ferulic acid and alcohol are used as raw materials, concentrated sulfuric acid is used as a catalyst, and alcohol is used as a solvent, and stirring was performed under reflux for 6–8 h to obtain intermediate A. Dichloromethane was used as the solvent, and triethylamine was used as the acid-binding agent using sulfonyl chloride and bromoethylamine hydrobromide as raw materials; the reaction was performed in an ice bath for 4–6 h to obtain intermediate B. Intermediate A (1.0 mmol) was then dissolved in 15 mL of acetonitrile and potassium carbonate (2 mmol) and stirred. Next, different sulfonamides (1.2 mmol) were added and stirred at 80 °C for 8 h to obtain the target compound. Their structures have been identified by 1H NMR, 13C NMR, and high-resolution mass spectrometry (HRMS) (Supporting Information).
Figure 3.
Synthetic routes of target compounds 1–16.
Anti-TMV Activity in Vivo
The anti-TMV biological activity test showed that the target compound showed excellent anti-TMV activity at 500 μg/mL, and the result is as shown in Table 1. The curative effect activities of compounds 6, 7, 12, and 14 on TMV were 50.3, 57.9, 55.3, and 57.4%, which were significantly better than that of ribavirin (49.3%). The protective activity of compound 2 on TMV was 59.7%, which was significantly better than that of ribavirin (48.6%). The inactivation activity of compound 2 on TMV was 87.3%, which was better than that of ribavirin (72.7%). To further understand the antiviral activity of synthesized compounds, we calculated and summarized the concentration for 50% of maximal effect (EC50) values of them. The EC50 value of compound 2 (Table 2) was 84.8 μg/mL, which was better than that of ribavirin (138.3 μg/mL). Finally, the results of the above activity tests indicate that the activity of compound 2 is superior to that of the commercially available drug ribavirin.
Table 1. Antiviral Activities of Target Compounds 1–16 against TMV in Vivo at 500 mg/La,d.
| compound | curative effect activitya (%) | protection effect activitya (%) | inactivation effect activitya (%) |
|---|---|---|---|
| 1 | 34.0 ± 4.4 | 31.7 ± 1.4 | 64.8 ± 8.5 |
| 2 | 39.8 ± 0.8 | 59.7 ± 6.2 | 87.3 ± 2.5 |
| 3 | 36.8 ± 8.0 | 40.4 ± 9.7 | 68.4 ± 7.0 |
| 4 | 26.9 ± 4.1 | 31.2 ± 9.2 | 56.6 ± 10.2 |
| 5 | 30.2 ± 2.6 | 38.5 ± 7.7 | 45.1 ± 7.4 |
| 6 | 50.3 ± 1.9 | 32.7 ± 5.7 | 59.8 ± 7.4 |
| 7 | 57.9 ± 10.3 | 53.3 ± 1.3 | 77.7 ± 8.1 |
| 8 | 31.9 ± 8.6 | 38.3 ± 7.9 | 54.6 ± 6.8 |
| 9 | 37.3 ± 2.0 | 41.0 ± 5.7 | 76.9 ± 5.4 |
| 10 | 41.4 ± 3.4 | 49.8 ± 8.3 | 74.1 ± 7.4 |
| 11 | 35.4 ± 3.2 | 38.9 ± 6.3 | 72.1 ± 7.5 |
| 12 | 55.3 ± 2.9 | 53.7 ± 9.9 | 65.2 ± 7.6 |
| 13 | 32.8 ± 2.3 | 53.7 ± 9.2 | 71.1 ± 8.0 |
| 14 | 50.6 ± 4.7 | 52.7 ± 8.6 | 64.6 ± 8.1 |
| 15 | 30.6 ± 2.7 | 38.7 ± 9.5 | 43.7 ± 3.8 |
| 16 | 47.1 ± 6.1 | 55.9 ± 1.9 | 73.7 ± 8.2 |
| ningnanmycinb | 56.6 ± 6.8 | 54.1 ± 3.4 | 88.3 ± 4.3 |
| ribavirinc | 49.3 ± 7.5 | 48.6 ± 2.5 | 72.7 ± 4.7 |
Average of three replicates at 500 μg/mL.
Ningnanmycin.
Ribavirin was used as the control.
The ± values represent the standard deviation.
Table 2. EC50 of Target Compound Anti-TMV Activity.
| compound | EC50 of inactivation effect activitya | compound | EC50 of inactivation effect activitya |
|---|---|---|---|
| 2 | 84.8 ± 3.2 | 12 | 361.2 ± 5.1 |
| 6 | 419.3 ± 3.8 | 13 | 198.2 ± 8.3 |
| 7 | 205.8 ± 2.3 | 14 | 498.9 ± 4.0 |
| 8 | 542.3 ± 8.9 | 15 | 640.2 ± 5.9 |
| 9 | 238.1 ± 5.5 | 16 | 175.1 ± 11.6 |
| 10 | 265.5 ± 8.7 | ribavirin | 138.3 ± 4.2 |
The ± values represent the standard deviation.
Transmission Electron Microscopy Observation
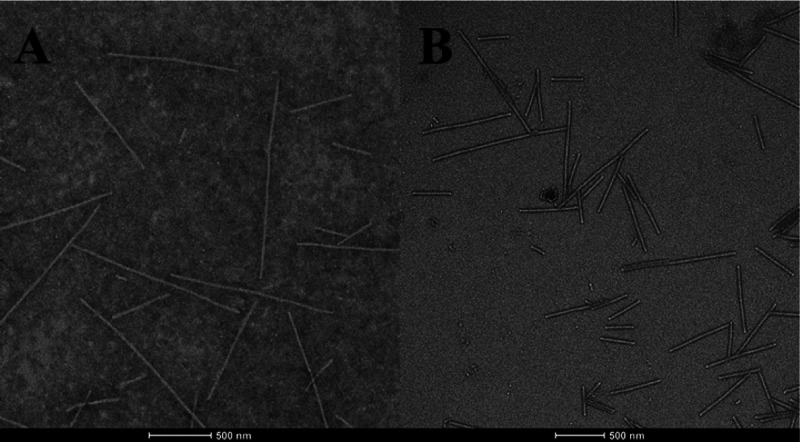
As shown in Figure 4, the morphological observation of TMV virions by transmission electron microscopy (TEM) showed that the complete TMV particles were short, straight, and rod-shaped structures (Figure 4A). Compound 2-treated TMV particles ruptured more severely than blank control TMV particles (Figure 4B). The results indicated that compound 2 can cause certain damage to the morphology and structure of TMV virions and cause the virions to lose their ability to infect tobacco.
Figure 4.
Effects of CK (A) and compound 2 (B) on the morphology of TMV particles.
Molecular Docking
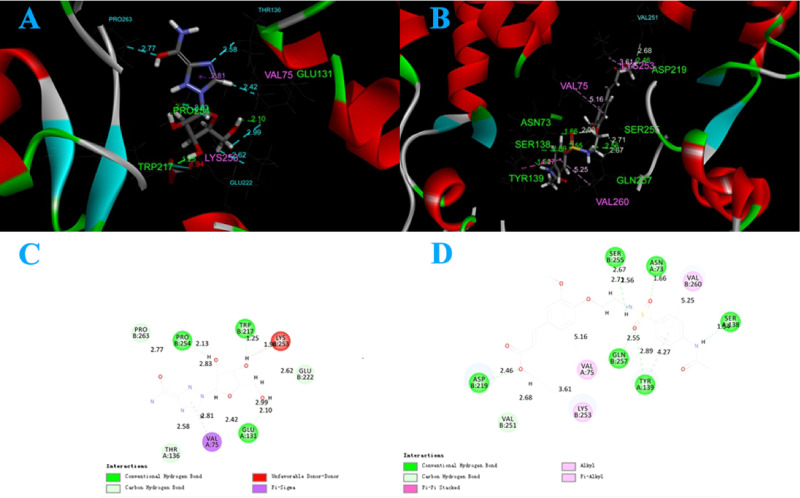
In this study, the molecular docking between compound 2 and TMV-coat protein (TMV-CP) (PDB code: 1EI7) was evaluated. The control agent ribavirin was used as a comparison object in this study. Compound 2 (Figure 5B,D) and ribavirin (Figure 5A,C) are inserted into the active pocket of TMV-CP through amino acid residues. Combined with the analysis of the two-dimensional (2D) and three-dimensional (3D) graphs of molecular docking, we can find that these amino acid residues include tyrosine 139 (TYR139), serine 138 (SER138), serine 255 (SER155), position 75 valine (VAL75), tyrosine at position 72 (TYR139), etc. These amino acid residues play a key role in the self-assembly of TMV-CP (Figure 5). Compound 2 (Figure 5B) showed strong hydrogen bonds with ASP219 (2.46 Å), GLN257 (2.55 Å), SER255 (2.56 Å), ASN 73 (1.54 Å), TYR139 (2.89 Å), and SER 138 (1.66 Å). In addition, some nonbonded interactions (such as π-alkyl and so forth) between certain amino acid residues and compounds are also very important modes of action. For example, π-sigma, alkyl, π-alkyl, and so forth also contribute to disease inactivation. Obviously, compared with the control agent, compound 2 has more hydrogen bonds, and some amino acid residues have more nonbonding than the control agent ribavirin. These binding and nonbinding may enhance the compound for its ability to inhibit the self-assembly process of TMV, thereby achieving the antiviral effect.
Figure 5.
3D graph of molecular docking results of ribavirin (A) and compound 2 (B) with TMV-CP. 2D graph of molecular docking results of ribavirin (C) and compound 2 (D) with TMV-CP.
Binding Affinity Analysis
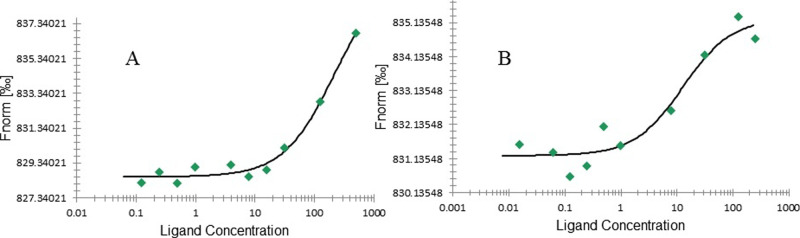
The binding affinity of the compound to TMV-CP was studied using microscale thermophoresis (MST), and the desorption constant (Kd) was obtained (Figure 6). Table 3 lists data comparison and analysis. According to the data in the table, compound 2 has a strong binding affinity with TMV-CP, with a Kd value of 12.6 μM, which is significantly better than that of ribavirin (223.0 μM). This result was consistent with the EC50 value of compound 2. These results indicate that compound 2 has better antiviral activity.
Figure 6.
MST test results of compound 2 (A) and ribavirin (B) with TMV-CP.
Table 3. Interaction Results of Target Compounds with TMV-CPc.
Average of three replicates at 500 μg/mL.
Ribavirin was used as the control.
The ± values represent the standard deviation.
Structure–Activity Relationships
In ferulic acid derivatives containing a sulfonamide moiety, when the substituent on the benzene ring of the sulfonamide is at the 4-position, the anti-TMV therapeutic activity of the electron-donating substituent is superior to that of the electron-withdrawing substituent. For example, compound 7 > compound 14 > compound 1 > compound 5 (4-CH3-Ph > 4-F-Ph > 4-NO2-Ph > 4-OCH3-Ph). At the same time, the anti-TMV protection activity of electron-withdrawing groups is higher than that of electron-donating groups, for example, compound 2 > compound 14 > compound 7 (4-NHCOCH3-Ph > 4-F-Ph > 4-CH3-Ph).
Conclusions
In summary, we designed and synthesized ferulic acid ester derivatives containing sulfonamide moieties and tested their anti-TMV biological activities in this work. The test results show that most of the target compounds have good anti-TMV activity. Among them, compound 2 has excellent anti-TMV inactivation activity, with an EC50 value of 84.8 μg/mL. Electron microscopy observations showed that compound 2 could cause serious lesion to the morphology of TMV virions, resulting in bending fractures and further inactivation of TMV. The result of the interaction between the MST-titrated compound and TMV-CP found that compound 2 showed significant binding to TMV-CP with Kd = 12.6 μM. The interaction mode between the target compound and TMV-CP was studied through molecular docking, and it was found that compound 2 has excellent binding ability. The above research shows that the compound can better act on virus particles and provide a potential basis for the research of antiviral drugs.
Materials and Methods
Chemicals
The reagents used in the experiments were analytically pure reagents and were used without further purification and drying.
Instruments
The melting point of the target product was measured by using a WRX-4 Micro melting point instrument (Shanghai YiCe Apparatus & Equipment Co., Ltd., China). Using CDCl3 and dimethyl sulfoxide-d6 (DMSO-d6) as a solvent, 1H NMR and 13C NMR spectra of the target compound were acquired using a Brookfield Ascend-400 spectrometer (Brook, Germany) and a JEOL ECX-500 spectrometer (JEOL, Tokyo, Japan). HRMS data have been validated by Thermo Scientific Q Exactive (Thermo, USA). The morphological structure of virus particles was observed using a Talos F200C electron microscope (FEI, USA).
Preparation of Ferulic Acid Ester (A)
Concentrated sulfuric acid was added to the alcohol solution of ferulic acid by the method previously reported,30 and the reaction system was heated to reflux for 9 h and then cooled to room temperature.
Preparation of Sulfonamide (B)
According to the reported method,31 triethylamine was added to a dichloromethane solution of sulfonyl chloride and bromoethylamine hydrobromide, and the reaction system was placed in an ice water bath for 6 h to obtain intermediate B.
General Procedure for the Synthesis of Compounds 1–16
Intermediate A, intermediate B, and potassium carbonate were stirred in 15 mL of acetonitrile at reflux for 6–10 h. The reaction system was quenched with saturated brine and then extracted three times with 50 mL of an organic solvent. The substitution data of compound 2 are shown below.
Methyl (E)-3-(4-(2-((4-acetamidophenyl)sulfonamide)ethoxy)-3-methoxyphenyl)acrylate
1H NMR (500 MHz, DMSO-d6): δ 10.28 (s, 1H), 7.77 (s, 1H), 7.71 (s, 3H), 7.66 (d, J = 6.3 Hz, 1H), 7.55 (d, J = 15.9 Hz, 1H), 7.32 (d, J = 1.7 Hz, 1H), 7.17 (d, J = 8.3 Hz, 1H), 6.88 (d, J = 8.3 Hz, 1H), 6.53 (d, J = 16.0 Hz, 1H), 3.97 (t, J = 5.6 Hz, 2H), 3.77 (s, 3H), 3.68 (s, 3H), 3.06 (t, J = 5.2 Hz, 2H), 2.04 (s, 3H). 13C NMR (125 MHz, DMSO-d6): δ 169.44, 167.44, 150.19, 149.58, 145.12, 143.25, 134.37, 128.15, 127.74, 123.23, 119.03, 115.97, 113.33, 111.27, 67.57, 56.10, 51.79, 42.24, 24.60. HRMS (ESI): calcd for C21H24N2O7KS ([M + K]+), calcd, 487.09358; found, 487.09216.
Antiviral Activity Assay
TMV was extracted according to methods in the literature.32 The model plant was selected according to the half-leaf method in the literature33 to evaluate the activity of the synthesized compound for treatment, protection, and inactivation. The curative activity is to first select a five-leaf stage model plant with better growth as the test object, spread a uniform layer of emery on the tobacco leaf surface, and then inoculate the virus by rubbing. After half an hour, rinse off the emery on the surface of the leaves with water, and then let the water on the surface of the leaves dry. The test compound solution was applied to the left half of the lobe, and the right half of the lobe was coated with a blank solution as a control. After 3–4 days, there are obvious disease spots on the leaves. Record the number of disease spots on the left and right halves of the leaves. The protective activity is to first apply pesticide treatment to the model plants. After 24 h, the virus was inoculated by rubbing. For passivation activity, the virus and the test compound were mixed for 0.5 h, and then, the mixed solution was applied to the right half of the model plant, and the left half was inoculated with the same dose of the virus solution. The calculation method of the inhibition rate is as follows
| 1 |
where X (%) is the percentage of inhibition rate, R is the number of spots on the leaf, CK is the number of spots on the blank control, 0 = no activity, and 100 = complete inhibition. All experiments were repeated three times under the same conditions, including the positive control: the commercial drug ribavirin.
TEM Analysis
The impact of compound 2 on TMV morphology was evaluated by TEM.
Molecular Docking
The interaction model of compound 2 with TMV-CP was studied using AutoDock 4.0 software.
Microscale Thermophoresis
The binding force of compound 2 with TMV-CP was analyzed using MST to obtain the dissociation constant (Kd). Ningnanmycin and ribavirin were used as positive controls. All tests were repeated three times.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21867002) and the Key Technologies R&D Program of Guizhou Province in China (no. 2017-5788-1).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02421.
Characterization data and related spectra (PDF)
Author Contributions
X.R., X.L., L.Y., and D.H. conceived and designed the study. X.R. performed the experiments. X.R., X.L., and L.Y. analyzed and interpreted the data. X.R., L.Y., and D.H. wrote the manuscript. X.R., X.L., L.Y., and D.J. provided material support. D.H. supervised and funded the acquisition for this work. All authors have read and reviewed the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Islam W.; Wu Z. Genetic defense approaches against begomoviruses. J. Appl. Virol. 2017, 6, 26–49. 10.21092/jav.v6i3.81. [DOI] [Google Scholar]
- Islam W.; Qasim M.; Noman A.; Tayyab M.; Chen S.; Wang L. Management of tobacco mosaic virus through natural metabolites. Rec. Nat. Prod. 2018, 12, 403–415. 10.25135/rnp.49.17.10.178. [DOI] [Google Scholar]
- Guo W.; Yan H.; Ren X.; Tang R.; Sun Y.; Wang Y.; Feng J. Berberine induces resistance against tobacco mosaic virus in tobacco. Pest Manage. Sci. 2020, 76, 1804–1813. 10.1002/ps.5709. [DOI] [PubMed] [Google Scholar]
- Islam W.; Zaynab M.; Qasim M.; Wu Z. Plant-virus interactions: Disease resistance in focus. Hosts Viruses 2017, 4, 5–20. 10.17582/journal.bjv/2017/4.1.5.20. [DOI] [Google Scholar]
- Li Y.; Zhang Z.; Jia Y.; Shen Y.; He H.; Fang R.; Chen X.; Hao X. 3-Acetonyl-3-hydroxyoxindole: a new inducer of systemic acquired resistance in plants. Plant Biotechnol. J. 2008, 6, 301–308. 10.1111/j.1467-7652.2008.00322.x. [DOI] [PubMed] [Google Scholar]
- Chen L.; Xie J.; Song H.; Liu Y.; Gu Y.; Wang L.; Wang Q. Design, synthesis, and biological activities of spirooxindoles containing acylhydrazone fragment derivatives based on the biosynthesis of alkaloids derived from tryptophan. J. Agric. Food Chem. 2016, 64, 6508–6516. 10.1021/acs.jafc.6b02683. [DOI] [PubMed] [Google Scholar]
- Luvisi A.; Panattoni A.; Materazzi A.; Bellis L. D. Chemical outbreak for tobacco mosaic virus control. Int. J. Agric. Biol. 2017, 19, 792. 10.17957/ijab/15.0365. [DOI] [Google Scholar]
- Wang Z.; Wang L.; Ma S.; Liu Y.; Wang L.; Wang Q. Design, synthesis, antiviral activity, and SARs of 1,4-aminophenanthroindolizidines. J. Agric. Food Chem. 2012, 60, 5825–5831. 10.1021/jf3013376. [DOI] [PubMed] [Google Scholar]
- Long C.; Li P.; Chen M.; Dong L.; Hu D.; Song B. Synthesis, anti-tobacco mosaic virus and cucumber mosaic virus activity, and 3D-QSAR study of novel 1,4-pentadien-3-one derivatives containing 4-thioquinazoline moiety. Eur. J. Med. Chem. 2015, 102, 639–647. 10.1016/j.ejmech.2015.08.029. [DOI] [PubMed] [Google Scholar]
- Ji X.; Wang Z.; Dong J.; Liu Y.; Lu A.; Wang Q. Discovery of topsentin alkaloids and their derivatives as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2016, 64, 9143–9151. 10.1021/acs.jafc.6b04020. [DOI] [PubMed] [Google Scholar]
- Qian X.; Lee P. W.; Cao S. China: forward to the green pesticides via a basic research program. J. Agric. Food Chem. 2010, 58, 2613–2623. 10.1021/jf904098w. [DOI] [PubMed] [Google Scholar]
- Seiber J. N. Sustainability and agricultural and food chemistry. J. Agric. Food Chem. 2011, 59, 1–21. 10.1021/jf1046078. [DOI] [PubMed] [Google Scholar]
- Yin L.; Gan X.; Shi J.; Zan N.; Zhang A.; Ren X.; Li M.; Xie D.; Hu D.; Song B. Induced resistance mechanism of novel curcumin analogs bearing a quinazoline moiety to plant virus. Int. J. Mol. Sci. 2018, 19, 4065. 10.3390/ijms19124065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isman M. B. Botanical insecticides: For richer, for poorer. Pest Manage. Sci. 2008, 64, 8–11. 10.1002/ps.1470. [DOI] [PubMed] [Google Scholar]
- Lu A.; Wang Z.; Zhou Z.; Chen J.; Wang Q. Application of “hydrogen bonding interaction” in new drug development: Design, synthesis, antiviral activity, and SARs of thiourea derivatives. J. Agric. Food Chem. 2015, 63, 1378–1384. 10.1021/jf505355r. [DOI] [PubMed] [Google Scholar]
- Ou S.; Kwok K.-C. Ferulic acid: pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. 10.1002/jsfa.1873. [DOI] [Google Scholar]
- Wang Z.; Xie D.; Gan X.; Zeng S.; Zhang A.; Yin L.; Song B.; Jin L.; Hu D. Synthesis, antiviral activity, and molecular docking study of trans-ferulic acid derivatives containing acylhydrazone moiety. Bioorg. Med. Chem. Lett. 2017, 27, 4096–4100. 10.1016/j.bmcl.2017.07.038. [DOI] [PubMed] [Google Scholar]
- Panizzi L.; Caponi C.; Catalano S.; Cioni P. L.; Morelli I. In vitro antimicrobial activity of extracts and isolated constituents of rubusulmifolius. J. Ethnopharmacol. 2002, 79, 165–168. 10.1016/s0378-8741(01)00363-4. [DOI] [PubMed] [Google Scholar]
- Huang G. Y.; Cui C.; Wang Z. P.; Li Y. Q.; Xiong L. X.; Wang L. Z.; Yu S. J.; Li Z. M.; Zhao W. G. Synthesis and characteristics of (Hydrogenated) ferulic acid derivatives as potential antiviral agents with insecticidal activity. Chem. Cent. J. 2013, 7, 33. 10.1186/1752-153x-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.; Wang Z.; Meng C.; Wang K.; Hu Y.; Wang L.; Wang Q. Discovery and SARs of trans-3-Aryl acrylic acids and their analogs as novel anti-tobacco mosaic virus (TMV) agents. PLoS One 2013, 8, e56475 10.1371/journal.pone.0056475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Zhang J.; Chen J.; Pan J.; Zhao L.; Liu D.; Zhang A.; Chen J.; Hu D.; Song B. Design, synthesis, antiviral bioactivity, and 3D-QSAR study of novel ferulic acid ester derivatives containing quinazoline moiety. Pest Manag. Sci. 2017, 73, 2079–2089. 10.1002/ps.4579. [DOI] [PubMed] [Google Scholar]
- Han B. S.; Park C. B.; Takasuka N.; Naito A.; Sekine K.; Nomura E.; Taniguchi H.; Tsuno T.; Tsuda H. A ferulic acid derivative, Ethyl 3-(4’-Geranyloxy-3- methoxyphenyl)-2-propenoate, as a new candidate chemopreventive agent for colon carcinogenesis in the Rat. Jpn. J. Canc. Res. 2001, 92, 404–409. 10.1111/j.1349-7006.2001.tb01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-Y.; Park J.; Lee K.-H.; Lee D.-U.; Kwak J.-H.; Kim Y. S.; Lee S.-M. Ferulic acid protects against carbon tetrachloride-induced liver injury in mice. Toxicology 2011, 282, 104–111. 10.1016/j.tox.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Ou L.; Kong L.-Y.; Zhang X.-M.; Niwa M. Oxidation of ferulic acid by Momordica charantia peroxidase and related anti-inflammation activity changes. Biol. Pharm. Bull. 2003, 26, 1511–1516. 10.1248/bpb.26.1511. [DOI] [PubMed] [Google Scholar]
- Basanagouda M.; Shivashankar K.; Kulkarni M. V.; Rasal V. P.; Patel H.; Mutha S. S.; Mohite A. A. Synthesis and antimicrobial studies on novel sulfonamides containing 4-azidomethyl coumarin. Eur. J. Med. Chem. 2010, 45, 1151–1157. 10.1016/j.ejmech.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Sudhamani H.; Basha S. K. T.; Reddy S. M. C.; Sreedhar B.; Adam S.; Naga R. C. Synthesis and evaluation of biological activities of new sulfonamide and carbamate derivatives of 1H-pyrrolo[2,3-b]pyridine(7-azaindole). Res. Chem. Intermed. 2016, 42, 7471–7486. 10.1007/s11164-016-2547-2. [DOI] [Google Scholar]
- Bungard C. J.; Williams P. D.; Schulz J.; Wiscount C. M.; Holloway M. K.; Loughran H. M.; Manikowski J. J.; Su H.-P.; Bennett D. J.; Chang L.; Chu X.-J.; Crespo A.; Dwyer M. P.; Keertikar K.; Morriello G. J.; Stamford A. W.; Waddell S. T.; Zhong B.; Hu B.; Ji T.; Diamond T. L.; Bahnck-Teets C.; Carroll S. S.; Fay J. F.; Min X.; Morris W.; Ballard J. E.; Miller M. D.; McCauley J. A. Design and synthesis of piperazine sulfonamide cores leading to highly potent HIV-1 protease inhibitors. ACS Med. Chem. Lett. 2017, 8, 1292–1297. 10.1021/acsmedchemlett.7b00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D.; Xie D.; He F.; Song B.; Hu D. Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Bioorg. Med. Chem. Lett. 2018, 28, 2091–2097. 10.1016/j.bmcl.2018.04.042. [DOI] [PubMed] [Google Scholar]
- He F.; Shi J.; Wang Y.; Wang S.; Chen J.; Gan X.; Song B.; Hu D. Synthesis, Antiviral activity, and mechanisms of purine nucleoside derivatives containing a sulfonamide moiety. J. Agric. Food Chem. 2019, 67, 8459–8467. 10.1021/acs.jafc.9b02681. [DOI] [PubMed] [Google Scholar]
- Khatkar A.; Nanda A.; Kumar P.; Narasimhan B. Synthesis and antimi-crobial evaluation of ferulic acid derivatives. Res. Chem. Intermed. 2015, 41, 299–309. 10.1007/s11164-013-1192-2. [DOI] [Google Scholar]
- Bassetto M.; Leyssen P.; Neyts J.; Yerukhimovich M. M.; Frick D. N.; Courtney-Smith M.; Brancale A. In silico identification, design and synthesis of novel piperazine-based anti-HCV agents. Eur. J. Med. Chem. 2017, 125, 1115–1131. 10.1016/j.ejmech.2016.10.043. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Xu F.; Luo D.; Guo S.; He F.; Dai A.; Song B.; Wu J. Synthesis of anthranilic diamide derivatives containing moieties of trifluoromethylpyridine and hydrazone as potential anti-viral agents for plants. J. Agric. Food Chem. 2019, 67, 13344–13352. 10.1021/acs.jafc.9b05441. [DOI] [PubMed] [Google Scholar]
- Gooding G. V.; Hebert T. T. Effect of nitrogen and chloride nutrition on susceptibility and effect of fungicide applications on control of fusicoccum canker of peach. Phytopathology 1967, 57, 1285. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.