Abstract
Albumin has an oxidized form, known as non-mercaptalbumin (HNA), which reflects systemic oxidative stress. The association between serum HNA levels and diabetic complications are yet to be reported. In this cross-sectional study, we investigated 164 diabetic subjects to assess the correlation between HNA% (the proportion in the total albumin) and various clinical parameters. HNA% was significantly associated with the severity of multiple complications including neuropathy (23.3 ± 4.1% vs 26.2 ± 5.1%) and nephropathy (24.1 ± 3.9%, 24.6 ± 4.2%, 28.5 ± 6.1%, 31.3 ± 5.7%, 37.8 ± 2.9%, stage 1/2/3/4/5, respectively). These findings highlight the universal importance of oxidative stress, indicating HNA% potential as a versatile marker of the severity of diabetic complications.
Keywords: Diabetic complication, Neuropathy, Nephropathy, Retinopathy, HNA%, Oxidative stress
Highlights
-
•
Albumin has an oxidized form, known as non-mercaptalbumin (HNA), which reflects systemic oxidative stress.
-
•
This is the first report indicating that HNA% is significantly associated with severity of multiple diabetes complications.
-
•
HNA% may serve as a universal marker representing status of systemic complications in patients with diabetes.
Energy production in living organisms depends on oxidative phosphorylation in mitochondria, where oxygen acts as an electron acceptor. In the course of metabolism, some oxygen molecules become reduced to superoxide or reactive oxygen species (ROS), which can potentially damage DNA. Superoxide dismutase (SOD) eliminates ROS to protect mammalian macromolecules from oxidative damage. An imbalance in this process causes oxidative stress, leading to functional disorders in tissues and organs. Oxidative stress has been shown to play a key role in the pathogenesis of vascular complications of diabetes [1]. Chronic hyperglycemia enhances oxidative stress due to increased ROS generation [2] and impaired antioxidant defense [3]. Previous hyperglycemia has a long-standing impact on the subsequent development of diabetic complications (known as the “legacy effect”). Epigenetic changes have been implicated in this effect [4] and these changes may, theoretically, be mediated by ROS [1]. Various biomarkers of oxidative stress, such as 8-hydroxydeoxyguanosine (8-OHdG) and 8-oxo-7,8-dihydroguanosine (8-oxoGuo) levels in urine and thiobarbituric acid reactive substance (TBARS) levels, catalase activity, and SOD activity in plasma, have been used in clinical trials. However, these biomarkers are rarely applied in clinical practice [5,6].
Albumin is the most abundant protein in the plasma. Several reports have shown that Cys34-cysteinylated albumin (human non-mercaptalbumin: HNA) may regulate redox status in humans [[7], [8], [9]]. Although some studies have reported a correlation between HNA levels and disease severity [[10], [11], [12], [13]], there are no reports assessing their association with specific diseases, such as diabetes mellitus. Until recently, the accurate measurement of HNA levels in blood was technically difficult because of the complex and time-consuming procedures involved. Instability of the oxidation status of the specimen is the major challenge when investigating the relationship between HNA and diabetes complications. HNA gradually increases over time after blood sampling. The recent development of a novel high-performance liquid chromatography (HPLC) method by our group [14] has enabled the rapid and accurate measurement of the degree of oxidized albumin (HNA%). Using this method, HNA% was stable at room temperature for 25 h. Because of stability and simple procedure, large numbers of samples can be analyzed in a clinical setting, with greater ease and precision compared to conventional procedures for general oxidative stress markers.
1. Methods
1.1. Study subjects
In this single-center cross-sectional study, the subjects were inpatients diagnosed with diabetes mellitus, who were admitted to the Department of Diabetes and Metabolic Diseases at The University of Tokyo Hospital between July 17th, 2016 and March 31st, 2017. Patients were excluded if they were pregnant, were lactating, had acute organ failure (e.g., pneumonia, acute myocardial infarction, acute cerebral infarction, diabetic ketoacidosis, and hyperosmolar hyperglycemic state), or had congenital cognitive disorders. This study was performed according to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the University of Tokyo (No. 11171) and informed consent was obtained from all patients. For those patients who scored less than 22 out of 30 on the Mini Mental State Examination (MMSE), consent to participate was obtained from their legal representatives. This analysis was part of an exploratory study that aimed to examine factors related to cognitive function in patients with diabetes mellitus (Study S).
We investigated the correlation between HNA% and the following parameters: sex, age, disease duration, body mass index (BMI), smoking, alcohol consumption, family history of diabetes mellitus, coronary artery disease, stroke, neuropathy, retinopathy, nephropathy, systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure, diabetic treatment, use of antihypertensive drugs, use of lipid-lowering drugs, HbA1c, glycoalbumin (GA), GA/HbA1c ratio, homeostasis model assessment-insulin resistance (HOMA-IR), fasting C-peptide immunoreactivity (CPR), 2-h-after-meal CPR, C-peptide Index (CPI), fasting plasma glucose (FPG), serum albumin (Alb), uric acid (UA), triglycerides (TG), calculated LDL cholesterol (c-LDL), blood urea nitrogen (BUN), creatinine (Cre), estimated glomerular filtration rate (eGFR), and Mini Mental State Examination (MMSE) score. Neuropathy was diagnosed when the patient has at least one of the following findings: (1) coefficient of variation of R-R intervals (CVR-R) under 2%, (2) reduction in Achilles tendon reflex, (3) decreased lower limb vibration sensing, (4) and the presence of obvious sensory impairment. Retinopathy was diagnosed and classified into normal (-), simple diabetic retinopathy (SDR), pre-proliferative diabetic retinopathy (PPDR), or proliferative diabetic retinopathy (PDR), according to the Davis classification [15]. Nephropathy stage was determined by urinary albumin excretion and eGFR, according to the Classification of Diabetic Nephropathy 2014 proposed by the Joint Committee on Diabetic Nephropathy in Japan [16]. Patient background information was collected on the day of hospitalization and fasting blood samples were collected soon after obtaining informed consent.
1.2. Measurement of HNA%
HNA% measurements were performed according to a previously described procedure [14]. Briefly, after sample collection, tubes were kept at -80 °C until assayed. After defrosting, oxidative albumin was measured using a basic HPLC system (LabSolutions System; Shimadzu Co. Ltd, Kyoto, Japan) with an anion-exchange column (50∗7.6 mm I.D.) containing a polyvinyl alcohol cross-linked gel (9 μm in diameter) reacted with diethyl amine. The HPLC conditions were as follows. Eluent A consisted of a solution of 25 mM phosphoric acid buffer, containing 60 mM sulfuric acid, sodium salt (pH 6.0) and eluent B was a 250 mM high-concentration magnesium chloride solution. The flow rate was 1 mL/min after equilibrating the column for 4.5 min with eluent A. The linear gradient time from eluent A (100%) to eluent B (100%) was programmed at 7.5 min. The total measurement time was 12 min per sample, the sample volume was 3 μL, and the temperature was set at 40 °C. The excitation and emission wavelengths were 280 nm and 340 nm, respectively. In addition, we added citrate buffer (pH.6.0) to the blood collection tubes in advance to avoid proceeding oxidization after blood collection. After blood collection, the amount of citrate buffer was adjusted to a final concentration of 70 mM [17].
1.3. Statistical analysis
Continuous variables are expressed as the mean ± standard deviation (SD). Categorical and ordinal variables are expressed as percentages. For bivariate analyses, we used a simple linear regression analysis, Fisher’s exact test or analysis of variance (ANOVA), depending on the scale. Continuous variables were analyzed by paired Student’s t-test or Mann-Whitney test. Significant predictors in the univariate analysis were then included in a forward, stepwise multiple logistic regression model to identify the most important risk factors for increased HNA%. Before performing multivariate linear correlation analysis, a stepwise forward selection method was performed, where “In” was defined as a p-value < 0.2 and “Out” was defined as a p-value < 0.1.
To evaluate the impact of HNA% on diabetic complications, we performed bivariate analysis to identify the potential risk factors contributing to diabetic complications. We then eliminated the risk factors that showed multiple collinearity before performing the multivariate linear correlation analysis. For simplicity, we set up dichotomous categorical variables describing each of the following factors and complications: smoking status (current or former versus never), retinopathy (PPDR or PDR versus normal or SDR) and nephropathy (stage 3–5 versus stage 1–2). For nephropathy, we excluded the factors which directly defined renal function due to its redundancy.
A two-sided p-value of less than 0.05 was considered statistically significant. All analyses were performed using JMP Pro 13 software for Windows (SAS Institute Inc, Cary, NC, USA).
2. Results
A total of 235 patients was recruited, of whom 35 did not give consent to participate in this study. Of the remaining 200 participants, 12 were excluded (1 due to a neurological disorder, 1 due to aphasia, 2 due to MMSE refusal, 1 due to impaired fasting glucose/impaired glucose tolerance, 4 due to depression, and 3 due to failure to contact their legal representatives). The remaining 188 patients were eligible for Study S. Blood specimens were not available from 24 patients for HNA% analysis, leaving a total of 164 patients finally enrolled in the study (Study A, Supplemental Fig. S1).163 patients were Japanese and 1 patient was a Chinese. Nine (5.5%) patients were diagnosed with type 1 diabetes and 153 (93.3%) with type 2 diabetes. One (0.6%) patient was suspected to have maturity-onset diabetes of the young (MODY) and one (0.6%) was diagnosed with pancreatic diabetes. One hundred and twenty-three patients scored 28–30 on the MMSE, 40 scored 24–27, and 1 scored below 23. Because of the limited sample size, patients with MMSE scores ≤27 were defined as having mild cognitive impairment (MCI).
Baseline characteristics of the patients are shown in Table 1. Age was 63.5 ± 13.1 years, whereas the duration of diabetes was 13.4 ± 10.7 years. HbA1c levels were generally high (9.03 ± 1.76 %) since the subjects were all inpatients. Due to the relatively long disease duration, complications were frequently observed; 67.7% of the patients had neuropathy and 21.3% had history of coronary artery disease. HNA% was 25.3 ± 5.0 %.
Table 1.
Baseline characteristics of patients.
| Characteristic | Value | Laboratory data | Value |
|---|---|---|---|
| Male | 106 (64.6) | Systolic blood pressure (mmHg) | 122 ± 16 |
| Age | 63.5 ± 13.1 | Diastolic blood pressure (mmHg) | 66 ± 11 |
| Disease duration (years) | 13.4 ± 10.7 | Pulse pressure (mmHg) | 56 ± 15 |
| BMI (kg/m2) | 27.1 ± 5.6 | HbA1c (%) (mmol/mol) | 9.03 ± 1.76 (75 ± 4.3) |
| Smoking (never/former/current) | 70/68/26 | GA (%) | 23.6 ± 6.9a |
| Alcohol consumption (g) | 6.04 ± 14.2 | GA/HbA1c ratio | 2.61 ± 0.56a |
| Diabetes mellitus family history | 101 (61.6) | FPG (mg/dL) | 152 ± 48 |
| Coronary artery disease | 35 (21.3) | HOMA-IR | 3.23 ± 2.57a |
| Stroke | 17 (10.4) | CPR: fasting (ng/mL) | 1.58 ± 1.08 |
| Neuropathy | 111 (67.7) | CPR: 2 h after meal (ng/mL) | 3.69 ± 2.17 |
| Retinopathy (-/SDR/PPDR or PDR) | 95/14/35 | CPI | 1.11 ± 0.80 |
| Nephropathy (1/2/3/4/5)b | 89/44/21/8/2 | Alb (mg/dL) | 4.08 ± 0.37 |
| Diabetes treatment (no drug/oral/GLP-1/insulin) | 19/77/8/60 | UA (mg/dL) | 5.33 ± 1.40 |
| Antihypertensive drug use | 96 (58.5) | TG (mg/dL) | 171 ± 170 |
| Calcium blocker | 65 (39.9) | c-LDL (mg/dL) | 101 ± 33 |
| ACE inhibitor | 13 (7.9) | BUN (mg/dL) | 17.4 ± 7.94 |
| ARB | 64 (39.0) | Cre (mg/dL) | 0.98 ± 0.84 |
| β-blocker | 22 (13.4) | eGFR (mL/min/1.73 m2) | 69.2 ± 26.0a |
| Diuretic | 22 (13.4) | MMSE | 28.4 ± 1.54 |
| Others | 4 (2.4) | HNA% | 25.3 ± 5.0 |
| Lipid-lowering drug use | 97 (59.1) | ||
| Strong statin | 71 (43.3) | ||
| Standard statin | 10 (6.1) | ||
| Fibrate | 9 (5.5) | ||
| EPA/DHA | 11 (6.7) | ||
| Ezetimibe | 10 (6.1) | ||
| Others | 4 (2.4) |
Values are expressed as n (%) or mean ± SD; n = 164.
BMI: body mass index, SDR: simple diabetic retinopathy, PDR: proliferative diabetic retinopathy, PPDR: pre-proliferative diabetic retinopathy, ACE: Angiotensin converting enzyme, ARB: Angiotensin II receptor blocker, EPA: eicosapentaenoic acid, DHA: docosahexaenoic acid, GA: glycoalbumin, FPG: fasting plasma glucose, HOMA-IR: homeostasis model assessment-insulin resistance, CPR: C-peptide immunoreactivity, CPI: C-peptide Index, Alb: serum albumin, UA: uric acid, TG: triglyceride, c-LDL: calculated LDL cholesterol, BUN: blood urea nitrogen, Cre: creatinine, eGFR: estimated glomerular filtration rate, MMSE: Mini Mental State Examination, HNA%: percentage of human non-mercaptalbumin.
Data are missing for some patients. GA (n = 162), GA/HbA1c ratio (n = 162), HOMA-IR (n = 105), eGFR (n = 163).
Nephropathy stage was determined according to the Classification of Diabetic Nephropathy 2014 proposed by the Joint Committee on Diabetic Nephropathy in Japan [16]. Stage 1: microalbuminuria <30 mg/g Cre and eGFR ≥30 mL/min/1.73 m2, stage 2: microalbuminuria 30–299 mg/g Cre and eGFR ≥30 mL/min/1.73 m2, stage 3: macroalbuminuria ≥300 mg/g Cre or continuous proteinuria ≥0.5 g/g Cre and eGFR ≥30 mL/min/1.73 m2, stage 4: eGFR <30 mL/min/1.73 m2, and stage 5: requiring dialysis.
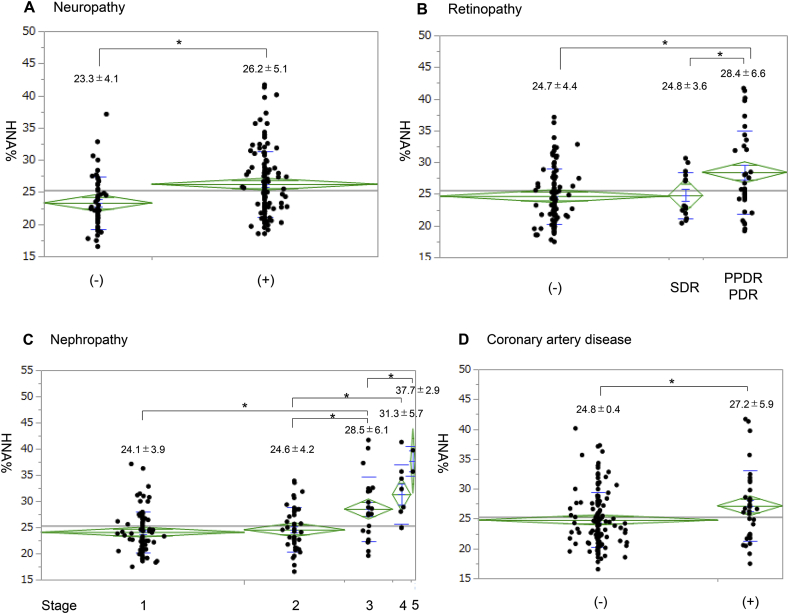
By bivariate analysis, HNA% was positively correlated with age, disease duration, coronary artery disease, progression of neuropathy, PPDR and PDR, nephropathy, use of GLP-1RA or insulin, use of insulin, use of antihypertensive drugs, use of lipid-lowering drugs, pulse pressure, GA/HbA1c ratio, fasting CPR, CPI, BUN, and Cre, while it was negatively correlated with DBP, Alb, and eGFR (Table 2). Of note, HNA% increased with greater severity of diabetic complications (Fig. 1). The average HNA% was 26.2% and it was approximately 3% higher in the neuropathy (+) group than in the neuropathy (-) group. As for retinopathy, HNA% was not significantly different between the normal and SDR groups; however, HNA% was significantly higher in the PPDR plus PDR groups than normal and SDR groups. The average HNA% of PPDR plus PDR groups was 28.5%, which was 3.8% higher than that of the normal and SDR groups. In patients with nephropathy, HNA% increased gradually according to nephropathy progression, averaging 24.1% at stage 1, 24.6% at stage 2, 28.5% at stage 3, 31.3% at stage 4, and 37.7% at stage 5. HNA% was significantly higher in patients with coronary artery disease by 2.3% than those who did not have coronary artery disease. HNA% was significantly associated with the progression of microvascular complications and cardiovascular disease. Using most anti-hypertensive drug classes (Ca blocker, ARB, β-blocker, diuretic) except ACEI indicated significant associations with elevated HNA%. About lipid-lowering drugs, most classes showed no significant differences in HNA% except for ezetimibe; ezetimibe users exhibited higher HNA% than non-users (P = 0.0391).
Table 2.
Bivariate analysis of candidate parameters and HNA%.
| Variable | β coefficient | 95% CI | Standardized regression coefficient | p-value |
|---|---|---|---|---|
| Male | 0.014 | −1.597–1.626 | 0.001 | 0.9862 |
| Age | 0.102 | 0.045–0.159 | 0.268 | 0.0005 ∗ |
| Disease duration | 0.158 | 0.090–0.226 | 0.339 | <0.0001 ∗ |
| BMI | 0.069 | −0.068–0.206 | 0.078 | 0.3217 |
| Smoking | ||||
| Former or current smoker (versus never) | 0.644 | −0.910–2.199 | 0.064 | 0.4144 |
| Current (versus never or former) | −1.364 | −3.463–0.7352 | −0.100 | 0.2013 |
| Alcohol consumption | 0.027 | −0.027–0.081 | 0.077 | 0.3283 |
| Diabetes mellitus family history | 1.322 | −0.249–2.892 | 0.129 | 0.0985 |
| Coronary artery disease | 2.377 | 0.533–4.221 | 0.196 | 0.0119 ∗ |
| Stroke | −0.458 | −2.984–2.069 | −0.0281 | 0.721 |
| Neuropathy | 2.907 | 1.322–4.491 | 0.274 | 0.0004 ∗ |
| Retinopathy | ||||
| PPDR or PDR (versus - or SDR) | 3.739 | 1.857–5.621 | 0.313 | 0.0001 ∗ |
| Nephropathy | ||||
| Stage 3 or 4 or5 (versus stage 1 or 2) | 5.568 | 3.800–7.337 | 0.439 | <0.0001 ∗ |
| Diabetes treatment | ||||
| Oral or GLP-1 or insulin (versus no drug) | 1.161 | −1.240–3.561 | 0.075 | 0.3411 |
| GLP-1 or insulin (versus no drug or oral) | 2.301 | 0.778–3.823 | 0.228 | 0.0033 ∗ |
| Insulin (versus no drug or oral or GLP-1) | 2.256 | 0.696–3.817 | 0.219 | 0.0049 ∗ |
| Antihypertensive drug use | 3.701 | 2.253–5.161 | 0.368 | <0.0001 ∗ |
| Lipid-lowering drug use | 1.68 | 0.134–3.225 | 0.166 | 0.0334 ∗ |
| Blood pressure | ||||
| Systolic | 0.043 | −0.0045–0.091 | 0.138 | 0.078 |
| Diastolic | −0.094 | −0.164∼-0.0234 | −0.202 | 0.0093 ∗ |
| Pulse pressure | 0.103 | 0.052–0.154 | 0.301 | <0.0001 ∗ |
| HbA1c | −0.248 | −0.686–0.191 | −0.087 | 0.266 |
| GA | 0.047 | −0.066–0.160 | 0.065 | 0.413 |
| GA/HbA1c ratio | 1.547 | 0.181–2.913 | 0.174 | 0.027 ∗ |
| FPG | −0.009 | −0.025–0.0070 | −0.087 | 0.269 |
| HOMA-IR | −0.112 | −0.417–0.193 | −0.072 | 0.467 |
| CPR: fasting | 0.888 | 0.185–1.591 | 0.192 | 0.0136 ∗ |
| CPR: 2 h after meal | 0.058 | −0.300–0.414 | 0.025 | 0.719 |
| CPI | 1.076 | 0.120–2.031 | 0.172 | 0.0277 ∗ |
| Alb | −3.102 | −5.096∼-1.109 | −0.235 | 0.0025 ∗ |
| UA | 1.15 | 0.626–1.673 | 0.323 | <0.0001 ∗ |
| TG | −0.00095 | −0.0055–0.00359 | −0.032 | 0.6803 |
| c-LDL | −0.0219 | −0.0459–0.00215 | −0.145 | 0.074 |
| BUN | 0.3418 | 0.260–0.423 | 0.545 | <0.0001 ∗ |
| Cre | 2.859 | 2.050–3.668 | 0.481 | <0.0001 ∗ |
| eGFR | −0.117 | −0.140∼-0.0930 | −0.608 | <0.0001 ∗ |
| MMSE | −0.191 | −0.691–0.310 | −0.059 | 0.4532 |
CI: confidence interval, ∗p < 0.05.
Fig. 1.
Distribution of HNA% in the clinical stages of (A) neuropathy, (B) retinopathy, (C) nephropathy, and (D) coronary artery disease in diabetic patients. The diamond shape shows the 95% CI with measured sample size and the shorter lines marking the lower and upper vertex of the diamond indicate the SD. The horizontal diagonal line indicates the average and the two horizontal lines by the average are overlap marks. The numbers over the plots show the average ± SD. The average values of two groups are significantly different if the two overlap marks do not overlap each other. ∗p < 0.05.
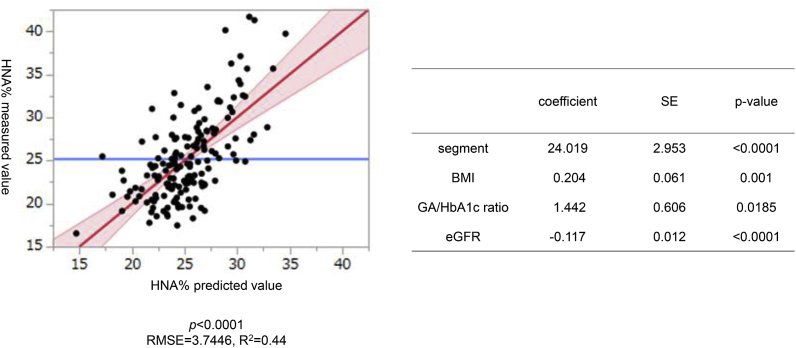
To identify the significant factors contributing to HNA%, we performed multiple regression analysis. For stepwise analysis, applied variables were the followings; gender, age, disease duration, BMI, smoking, amount of alcohol consumed, diabetes family history, coronary artery disease, stroke, neuropathy, retinopathy, nephropathy, diabetic treatment, using depressor drugs, lipid-lowering drugs, SBP, DBP, pulse pressure, HbA1c, GA, GA/HbA1c ratio, FPG, HOMA-IR, 2-h-after-meal CPR, TG, and eGFR. Among the variables tested, BMI, GA/HbA1c ratio, and eGFR remained significantly independent, with the following formula: HNA% = 24.019 + 0.204 × BMI +1.442 × GA/HbA1c - 0.117 × eGFR. Fig. 2 indicates the relationship between predicted values based on the formula and measured values. The coefficient of determination (R2) was 0.44.
Fig. 2.
Clinical parameters affecting HNA% determined by multivariate analysis. Left panel indicates the least-square regression line between the predicted values and the actual measurements of HNA%. Area shaded in red represents the 95% CI confidence interval. The average of the HNA% measurements is displayed as a blue line. The root mean squared error (RMSE) is 3.7446, and the correlation coefficient (R) is 0.663. Right panel shows coefficients and standard errors (SE) of statistically significant parameters. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The bivariate analysis showed neuropathy was significantly correlated with age, disease duration, current smoker (versus never or former), diabetes treatment oral or GLP-1 or insulin (versus no drug), GLP-1 or insulin (versus no drug or oral), insulin (versus no drug or oral or GLP-1), antihypertensive drug use, lipid-lowering drug use, SBP, pulse pressure, c-LDL, BUN, Cre, eGFR<60 mL/min/1.73 m2 and HNA%. Likewise, retinopathy was significantly correlated with disease duration, diabetes treatment GLP-1 or insulin (versus no drug or oral), insulin (versus no drug or oral or GLP-1), antihypertensive drug use, SBP, pulse pressure, GA/HbA1c ratio, BUN, Cre, eGFR<60 mL/min/1.73 m2 and HNA%. Nephropathy was significantly correlated with disease duration, diabetes treatment GLP-1 or insulin (versus no drug or oral), insulin (versus no drug or oral or GLP-1), antihypertensive drug, lipid-lowering drug, SBP, pulse pressure, Alb, UA, BUN, Cre, eGFR<60 mL/min/1.73 m2 and HNA%. Coronary artery disease was significantly correlated with age, disease duration, diabetes treatment insulin (versus no drug or oral or GLP-1), antihypertensive drug use, lipid-lowering drug use, SBP, pulse pressure, c-LDL, BUN, Cre, eGFR<60 mL/min/1.73 m2 and HNA% (Table 3). Multivariate linear correlation analysis showed that HNA%, age, diabetes treatment and lipid-lowering drug use were significantly associated with neuropathy. Likewise, HNA%, antihypertensive drug use and SBP were associated with nephropathy. As for retinopathy and coronary artery disease, HNA% showed relatively weaker association than the other factors (Table 4).
Table 3.
Bivariate analysis of risk factors and diabetic complications.
| Variable | β coefficient | 95% CI | Standardized regression coefficient | p-value |
|---|---|---|---|---|
|
A. Neuropathy | ||||
| Male | 0.6661 | |||
| Age | 9.1195 | 5.0381–13.1998 | 0.3276 | <0.0001 ∗ |
| Disease duration | 4.9794 | 1.5350–8.4239 | 0.2189 | 0.0049 ∗ |
| BMI | 0.4793 | −1.3606–2.3193 | 0.0405 | 0.6076 |
| Smoking | ||||
| Former or current smoker (versus never) | 0.5000 | |||
| Current (versus never or former) | 0.0419 ∗ | |||
| Alcohol consumption | −3.1466 | −7.8111–1.5180 | −0.1041 | 0.1847 |
| Diabetes mellitus family history | 1.0000 | |||
| Stroke | 0.5854 | |||
| Diabetes treatment | ||||
| Oral or GLP-1 or insulin (versus no drug) | 0.0001 ∗ | |||
| GLP-1 or insulin (versus no drug or oral) | 0.0188 ∗ | |||
| Insulin (versus no drug or oral or GLP-1) | 0.0370 ∗ | |||
| Antihypertensive drug | 0.0271 ∗ | |||
| Lipid-lowering drug use | <0.0001 ∗ | |||
| Blood pressure | ||||
| Systolic | 6.4097 | 1.2057–11.6137 | 0.1870 | 0.0161 ∗ |
| Diastolic | −0.8735 | −4.4249–2.6778 | −0.0381 | 0.6278 |
| Pulse pressure | 7.2832 | 2.6001–11.9663 | 0.2346 | 0.0025 ∗ |
| HbA1c | −0.3751 | −0.9529–0.2027 | −0.1002 | 0.2017 |
| GA | −0.0584 | −2.3464–2.2297 | −0.0040 | 0.9599 |
| GA/HbA1c ratio | 0.1058 | −0.0805–0.2920 | 0.0883 | 0.2637 |
| FPG | 5.1795 | −10.6675–21.0264 | 0.0506 | 0.5196 |
| HOMA-IR | −0.0738 | −1.0995–0.9519 | −0.0141 | 0.8868 |
| CPR: fasting | 0.0400 | −0.3168–0.3967 | 0.0174 | 0.8252 |
| CPR: 2 h after meal | −0.3052 | −1.0214–0.4109 | −0.0660 | 0.4012 |
| CPI | −0.0444 | −0.3078–0.2189 | −0.0262 | 0.7395 |
| Alb | −0.1110 | −0.2344–0.0124 | −0.1382 | 0.0776 |
| UA | 0.1306 | −0.3311–0.5924 | 0.0439 | 0.5772 |
| TG | −13.4352 | −69.6593–42.7890 | −0.0371 | 0.6377 |
| c-LDL | −11.4970 | −22.7638∼-0.2301 | −0.1624 | 0.0456 ∗ |
| BUN | 2.9856 | 0.4018–5.5695 | 0.1765 | 0.0238 ∗ |
| Cre | 0.2905 | 0.0171–0.5638 | 0.1627 | 0.0374 ∗ |
| eGFR | −16.1465 | −24.3853∼-7.9076 | −0.2918 | 0.0002 ∗ |
| MMSE | −0.2975 | −0.8053–0.2103 | −0.0905 | 0.2491 |
| HNA% |
2.9069 |
1.3224–4.4914 |
0.2738 |
0.0004 ∗ |
|
B. Retinopathy | ||||
| Male | 0.1612 | |||
| Age | 1.9531 | −2.7006–6.6068 | 0.0695 | 0.4081 |
| Disease duration | 8.3780 | 4.5779–12.1781 | 0.3435 | <0.0001 ∗ |
| BMI | −0.2052 | −2.3405–1.9301 | −0.0160 | 0.8496 |
| Smoking | ||||
| Former or current smoker (versus never) | 0.4311 | |||
| Current (versus never or former) | 0.0657 | |||
| Alcohol consumption | −2.4682 | −8.2174–3.2811 | −0.0710 | 0.3975 |
| Diabetes mellitus family history | 0.1612 | |||
| Stroke | 0.1178 | |||
| Diabetes treatment | ||||
| Oral or GLP-1 or insulin (versus no drug) | 0.0392 | |||
| GLP-1 or insulin (versus no drug or oral) | 0.0008 ∗ | |||
| Insulin (versus no drug or oral or GLP-1) | 0.0029 ∗ | |||
| Antihypertensive drug | 0.0281 ∗ | |||
| Lipid-lowering drug use | 0.1680 | |||
| Blood pressure | ||||
| Systolic | 9.6860 | 3.7748–15.5972 | 0.2623 | 0.0015 ∗ |
| Diastolic | −0.3468 | −4.4033–3.7097 | −0.0142 | 0.8660 |
| Pulse pressure | 10.0328 | 4.5764–15.4891 | 0.2918 | 0.0004 ∗ |
| HbA1c | −0.2245 | −0.8961–0.4471 | −0.0554 | 0.5098 |
| GA | 1.8524 | −0.7956–4.5003 | 0.1157 | 0.1689 |
| GA/HbA1c ratio | 0.2620 | 0.0483–0.4757 | 0.2000 | 0.0166 ∗ |
| FPG | 3.3717 | −15.0290–21.7724 | 0.0304 | 0.7177 |
| HOMA-IR | −0.1977 | −1.6355–1.2400 | −0.0293 | 0.7853 |
| CPR: fasting | −0.0302 | −0.4588–0.3984 | −0.0117 | 0.8894 |
| CPR: 2 h after meal | −0.7483 | −1.5477–0.0511 | −0.1535 | 0.0663 |
| CPI | −0.0326 | −0.3443–0.2791 | −0.0174 | 0.8363 |
| Alb | −0.1376 | −0.2820–0.0067 | −0.1562 | 0.0615 |
| UA | 0.1824 | −0.3453–0.7102 | 0.0572 | 0.4956 |
| TG | −26.2296 | −95.2293–42.7701 | −0.0629 | 0.4536 |
| c-LDL | −3.3586 | −15.9849–9.26776 | −0.0461 | 0.5996 |
| BUN | 7.0286 | 4.0748–9.9823 | 0.3672 | <0.0001 ∗ |
| Cre | 0.7683 | 0.4528–1.0840 | 0.3743 | <0.0001 ∗ |
| eGFR | −21.6629 | −30.4754∼-12.8504 | −0.3788 | <0.0001 ∗ |
| MMSE | 0.0718 | −0.5141–0.6577 | 0.0203 | 0.8089 |
| HNA% |
3.7392 |
1.8572–5.62114 |
0.3130 |
0.0001 ∗ |
|
C.Nephropathy | ||||
| Male | 0.8353 | |||
| Age | 3.4827 | −1.6483–8.6160 | 0.1047 | 0.1820 |
| Disease duration | 5.9971 | 1.8844–10.1098 | 0.2207 | 0.0045 ∗ |
| BMI | 1.5033 | −0.7104–3.7169 | 0.1051 | 0.1818 |
| Smoking | ||||
| Former or current smoker (versus never) | 0.2292 | |||
| Current (versus never or former) | 0.1703 | |||
| Alcohol consumption | −2.5601 | −8.1483–3.0281 | −0.0709 | 0.3670 |
| Diabetes mellitus family history | 0.5397 | |||
| Stroke | 1.0000 | |||
| Diabetes treatment | ||||
| Oral or GLP-1 or insulin (versus no drug) | 0.1290 | |||
| GLP-1 or insulin (versus no drug or oral) | 0.0004 ∗ | |||
| Insulin (versus no drug or oral or GLP-1) | 0.0032 ∗ | |||
| Antihypertensive drug | <0.0001 ∗ | |||
| Lipid-lowering drug use | 0.0256 ∗ | |||
| Blood pressure | ||||
| Systolic | 13.3825 | 7.4040–19.3610 | 0.3287 | <0.0001 ∗ |
| Diastolic | 2.7749 | −1.4484–6.9982 | 0.1014 | 0.1963 |
| Pulse pressure | 10.6076 | 5.0933–16.1218 | 0.2860 | 0.0002 ∗ |
| HbA1c | 0.0867 | −0.6069–0.7803 | 0.0194 | 0.8054 |
| GA | 1.2426 | −1.5142–3.9994 | 0.0702 | 0.3747 |
| GA/HbA1c ratio | 0.0895 | −0.1359–0.3149 | 0.0619 | 0.4340 |
| FPG | −3.4463 | −21.9925–15.9033 | −0.0249 | 0.7514 |
| HOMA-IR | 0.3289 | −1.1892–1.8469 | 0.0423 | 0.6684 |
| CPR: fasting | 0.3033 | −0.1204–0.7269 | 0.1104 | 0.1594 |
| CPR: 2 h after meal | −0.1702 | −1.0271–0.6868 | −0.0308 | 0.6954 |
| CPI | 0.1714 | −0.1421–0.4850 | 0.0845 | 0.2820 |
| Alb | −0.2212 | −0.3660∼-0.0763 | −0.2305 | 0.0030 ∗ |
| UA | 0.6138 | 0.0699–1.1577 | 0.1725 | 0.0272 ∗ |
| TG | 40.5494 | −26.3625–107.4612 | 0.0936 | 0.2332 |
| c-LDL | 0.3487 | −13.3428–14.0403 | 0.0041 | 0.9599 |
| BUN | 9.2095 | 6.4183–12.0007 | 0.4557 | <0.0001 ∗ |
| Cre | 0.9543 | 0.6584–1.2502 | 0.4474 | <0.0001 ∗ |
| eGFR | −29.6886 | −38.8731∼-20.5040 | −0.4494 | <0.0001 ∗ |
| MMSE | −0.3371 | −0.9439–0.2697 | −0.0859 | 0.2742 |
| HNA% |
5.5685 |
3.8004–7.3366 |
0.4390 |
<0.0001 ∗ |
|
D. Coronary arterydisease | ||||
| Male | 0.4268 | |||
| Age | 5.5732 | 0.7194–10.4270 | 0.1754 | 0.0247 ∗ |
| Disease duration | 9.2283 | 5.4618–12.9949 | 0.3553 | <0.0001 ∗ |
| BMI | 0.2111 | −1.8894–2.3115 | 0.0156 | 0.8430 |
| Smoking | ||||
| Former or current smoker (versus never) | 0.1771 | |||
| Current (versus never or former) | 1.0000 | |||
| Alcohol consumption | −2.8890 | −8.2238–2.4457 | −0.0837 | 0.2865 |
| Diabetes mellitus family history | 0.6958 | |||
| Stroke | 0.5310 | |||
| Diabetes treatment | ||||
| Oral or GLP-1 or insulin (versus no drug) | 0.0788 | |||
| GLP-1 or insulin (versus no drug or oral) | 0.0017 ∗ | |||
| Insulin (versus no drug or oral or GLP-1) | 0.0006 ∗ | |||
| Antihypertensive drug | <0.0001 ∗ | |||
| Lipid-lowering drug use | <0.0001 ∗ | |||
| Blood pressure | ||||
| Systolic | 8.9890 | 3.1039–14.8735 | 0.2306 | 0.0030 ∗ |
| Diastolic | 0.4707 | −3.5854–4.5274 | 0.0180 | 0.8190 |
| Pulse pressure | 8.5181 | 3.1801–13.8560 | 0.2403 | 0.0019 ∗ |
| HbA1c | −0.1109 | −0.7736–0.5518 | −0.0260 | 0.7415 |
| GA | 1.3445 | −1.3127–4.002 | 0.0788 | 0.3192 |
| GA/HbA1c ratio | 0.1780 | −0.0381–0.3940 | 0.1276 | 0.1057 |
| FPG | −0.7710 | −18.8829–17.3409 | −0.0066 | 0.9331 |
| HOMA-IR | −0.3655 | −1.883–1.1523 | −0.0470 | 0.6340 |
| CPR: fasting | 0.1382 | −0.2685–0.5449 | 0.0526 | 0.5032 |
| CPR: 2 h after meal | −0.5934 | −1.4075–0.2207 | −0.1124 | 0.1520 |
| CPI | 0.1254 | −0.1747–0.4255 | 0.0647 | 0.4104 |
| Alb | −0.0551 | −0.1971–0.0869 | −0.0601 | 0.4449 |
| UA | −0.1337 | −0.6610–0.3935 | −0.0393 | 0.6171 |
| TG | 17.5497 | −46.6157–81.7151 | 0.0424 | 0.5899 |
| c-LDL | −22.6777 | −35.1721∼-10.1833 | −0.2810 | 0.0005 ∗ |
| BUN | 4.0500 | 1.1198–6.9794 | 0.2097 | 0.0070 ∗ |
| Cre | 0.6195 | 0.3183–0.9208 | 0.3040 | <0.0001 ∗ |
| eGFR | −17.0326 | −26.4943∼-7.5709 | −0.2698 | 0.0005 ∗ |
| MMSE | −0.3300 | −0.9098–0.2498 | −0.0880 | 0.2627 |
| HNA% | 2.3766 | 0.5325–4.2206 | 0.1961 | 0.0119 ∗ |
∗p < 0.05.
Table 4.
Logistic regression analysis on diabetic complications.
| Odds ratio | 95% CI | p-value | |
|---|---|---|---|
|
A.Neuropathy | |||
| Age | 1.0545 | 1.0157–1.0948 | 0.0055 |
| Disease duration | 0.9735 | 0.9270–1.0224 | 0.2833 |
| Current smoker (versus never or former) | 1.2483 | 0.4033–3.8641 | 0.7005 |
| Diabetes treatment (oral/GLP-1/Ins use) | 0.1373 | 0.0326–0.5793 | 0.0069 |
| Antihypertensive drug use | 0.9355 | 0.3770–2.3213 | 0.8857 |
| Lipid-lowering drug use | 0.3205 | 0.1170–0.8783 | 0.0269 |
| SBP | 1.0166 | 0.9873–1.0467 | 0.2708 |
| c-LDL | 1.0080 | 0.9933–1.0228 | 0.2881 |
| eGFR< 60 | 1.4002 | 0.4555–4.3040 | 0.5569 |
| HNA% | 1.1561 | 1.0467–0.9873 | 0.0167 |
|
B.Retinopathy | |||
| Disease duration | 1.0385 | 0.9918–1.0874 | 0.1080 |
| Diabetes treatment (GLP-1/Ins use) | 0.5007 | 0.1950–1.2853 | 0.1504 |
| Antihypertensive drug use | 0.9383 | 0.3051–2.8859 | 0.9116 |
| SBP | 1.0320 | 1.0018–1.0632 | 0.0377 |
| GA/HbA1c ratio | 1.5656 | 0.7428–3.2998 | 0.2387 |
| eGFR< 60 | 0.3681 | 0.1279–1.0588 | 0.0638 |
| HNA% | 1.0360 | 0.9366–1.1461 | 0.4919 |
|
C.Nephropathy | |||
| Disease duration | 0.9784 | 0.9298–1.0295 | 0.4407 |
| Diabetes treatment (GLP-1/Ins use) | 0.3410 | 0.1111–1.0463 | 0.0600 |
| Antihypertensive drug use | 0.1734 | 0.0327–0.9187 | 0.0394 |
| Lipid-lowering drug use | 0.9078 | 0.2653–3.1065 | 0.8775 |
| SBP | 1.0497 | 1.0154–1.0851 | 0.0042 |
| Alb | 0.5227 | 2.0144–1.9132 | 0.3459 |
| UA | 1.1461 | 0.7474–1.7574 | 0.5318 |
| HNA% | 1.1585 | 1.0270–1.3069 | 0.0167 |
|
D.Coronaryartery disease | |||
| Age | 0.9882 | 0.9366–1.0427 | 0.6644 |
| Disease duration | 1.0563 | 1.0020–1.1136 | 0.0421 |
| Diabetes treatment (GLP-1/Ins use) | 0.3722 | 0.1294–1.0705 | 0.0668 |
| Antihypertensive drug use | 0.1258 | 0.0267–0.5925 | 0.0088 |
| Lipid-lowering drug use | 0.5483 | 0.1433–2.0984 | 0.3802 |
| SBP | 1.0332 | 1.0008–1.0666 | 0.0443 |
| c-LDL | 0.9805 | 0.9607–1.0007 | 0.0588 |
| eGFR< 60 | 0.9247 | 0.2793–3.0612 | 0.8980 |
| HNA% | 0.9551 | 0.8558–1.0659 | 0.4123 |
“Retinopathy” was defined as presence of PPDR or PDR. “Nephropathy” was defined as stage 3, 4, or 5.
3. Discussion
In this study, we showed that HNA% was significantly associated with the presence and/or the severity of multiple diabetic complications, including neuropathy, retinopathy, nephropathy, and coronary artery disease. In a multivariate analysis, eGFR had a strong negative correlation with HNA%, whereas BMI and the surrogate index of glycemic variability, GA/HbA1c ratio, were independently, significantly and positively correlated with HNA%. HNA% had a stronger correlation with neuropathy and nephropathy than other factors.
The pathogenesis of diabetes and its complications are influenced by oxidative stress [1]; however, there is little clinical data supporting this. This study showed that HNA% had a strong negative correlation with eGFR and a strong positive correlation with diabetic complications. Previous reports have shown that HNA% increases in hepatic and/or renal failure [[10], [11], [12], [13]], suggesting that oxidized albumin may reflect systemic oxidation status. It has also been reported that HNA% may be elevated in patients with cardiovascular disease [12].
Most studies of 8-OHdG have simply compared normal subjects with diabetes patients, regardless of the severity or stage of the complications. Suzuki et al. reported that 8-OHdG content in muscle tissue correlates with retinopathy and nephropathy [18]. Nishikawa et al. reported that 8-OHdG levels in urine are associated with HbA1c, microalbuminuria, the occurrence of simple retinopathy, and coronary heart disease risk score [19]. Fukuhara et al. reported HNA% by our method is a strong determinant for activities of daily living disability in elderly patients with diabetes [20]. To our knowledge, our study is the first to report that three major microvascular complications (neuropathy, retinopathy, and nephropathy) and coronary artery disease are all correlated with one oxidative stress biomarker in the blood. Our new method, which enabled the rapid and accurate measurement of HNA in multiple specimens, revealed the relationship between HNA% and diabetic complications.
Using a stepwise method, we narrowed possible predictors of HNA% down to three factors, i.e. BMI, GA/HbA1c ratio, and eGFR. Moderate correlation was observed between the actual values and the predicted values based on the formula.
No correlations were found between HNA% and glycemic markers, such as HbA1c and GA, although some studies have reported positive correlations between HbA1c and oxidative stress markers [19]. However, we found a significant correlation between HNA% and GA/HbA1c ratio. GA is significantly associated with increased ROS production in experimental systems [21]. HbA1c reflects the average plasma glucose level but not the acute elevation of plasma glucose levels [[22], [23], [24]], and thus, GA is often used as an alternative measure of acute hyperglycemia [25]. GA has attracted attention recently as a useful marker of postprandial glucose excursion [26,27]. Because acute hyperglycemia or postprandial hyperglycemia may trigger oxidative stress rather than chronic sustained hyperglycemia [28,29], we employed the GA/HbA1c ratio as a surrogate marker of plasma glucose variability. In fact, several recent studies have reported that this ratio is a better marker of glucose excursion than GA or HbA1c alone in continuous glucose monitoring systems [22,27]. The independent association between GA/HbA1c ratio and HNA% identified by a stepwise method suggests that repeated high postprandial glucose levels may affect oxidative stress more than chronic sustained hyperglycemia. This result is consistent with a previous report that HNA% is strongly correlated with daily glucose profile in diabetes patients [30]. As for the diabetic complications, the factors extracted in the bivariate analysis were consistent with those reported in the previous studies (Table 3) [[31], [32], [33], [34]]. As shown in Fig. 1 and Table 3, HNA% was associated with all the diabetic complications; especially neuropathy and nephropathy, suggesting the potentially significant involvement of oxidative stress in the etiology. These findings are consistent with the previous reports indicating that pentosidine (a major advanced glycation end product) levels are relevant to complications in diabetes patients [35,36]. Serum pentosidine levels were increased in diabetic patients with retinopathy and nephropathy [35].
We found that both HNA% and eGFR were useful to predict the status of systemic complications in diabetes. Although they were strongly correlated with each other, HNA% and eGFR represent different statuses. HNA% can be directly altered by redox metabolites, while eGFR is not fundamentally affected by these metabolites. Because of its reversibility, HNA% is potentially a unique biomarker for evaluating the risk of complications in diabetic patients.
There are several limitations in this study. Firstly, there may be a selection bias, as all the subjects were selected from the inpatient pool at a single university hospital, where there tend to be more patients with more complicated medical backgrounds, such as relatively long medical histories. Further multicenter studies are required to enable more general conclusions. Secondly, more information should be collected from not only diabetes patients but also from patients with other diseases to understand the general role of HNA% in predicting oxidative stress in humans. Thirdly, this is a cross-sectional study. We didn’t obtain histories about intake of supplements or antioxidative agents. Further investigation is necessary to identify specific agents that influence HNA% and to determine whether decreasing HNA% can be an effective therapeutic strategy to prevent the complications of diabetes.
In conclusion, our newly developed method enabled the rapid and accurate measurement of HNA% in a large number of specimens. HNA% measured with this method reflected the status of multiple diabetic complications. HNA% may have clinical application as a universal marker of the status of systemic complications in patients with diabetes.
CRediT authorship contribution statement
Yuka Kobayashi: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Visualization. Ryo Suzuki: Conceptualization, Methodology, Formal analysis, Supervision. Keiko Yasukawa: Investigation, Resources. Koji Oba: Formal analysis. Toshimasa Yamauchi: Supervision. Yutaka Yatomi: Resources, Supervision. Takashi Kadowaki: Conceptualization, Methodology, Supervision.
Acknowledgments
The authors have no conflicts of interest to disclose. We would like to thank Mr. Tomoaki Miyamoto for helping with HNA% measurements in specimen transportation. This work was supported by the Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering.
Y. K., R. S. and T. K. designed the study. Y. K. performed the experiments. K. Y. measured HNA in blood samples. Y. K., R. S., K. Y., T. Y., Y. Y., and T. K. interpreted the results. Y. K. and R. S wrote the initial manuscript. R.S. and T.K. take responsibility for the content of the paper. K.O. provided advice on statistical analysis. All authors reviewed the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2020.100032.
Contributor Information
Ryo Suzuki, Email: ryosuzuki-tky@umin.ac.jp.
Takashi Kadowaki, Email: kadowaki-3im@h.u-tokyo.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cooper M.E. Metabolic karma-the atherogenic legacy of diabetes: the 2017 Edwin Bierman award Lecture. Diabetes. 2018;67(5):785–790. doi: 10.2337/dbi18-0010. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa T. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 3.Di Marco E. Are reactive oxygen species still the basis for diabetic complications? Clin Sci (Lond) 2015;129(2):199–216. doi: 10.1042/CS20150093. [DOI] [PubMed] [Google Scholar]
- 4.El-Osta A. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho E. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjær L.K. Cardiovascular and all-cause mortality risk associated with urinary excretion of 8-oxoGuo, a biomarker for RNA oxidation, in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2017;40(12):1771–1778. doi: 10.2337/dc17-1150. [DOI] [PubMed] [Google Scholar]
- 7.Nagumo K. Cys34-cysteinylated human serum albumin is a sensitive plasma marker in oxidative stress-related chronic diseases. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruschi M. Oxidized albumin. The long way of a protein of uncertain function. Biochim Biophys Acta. 2013;1830(12):5473–5479. doi: 10.1016/j.bbagen.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Anraku M. Redox properties of serum albumin. Biochim Biophys Acta. 2013;1830(12):5465–5472. doi: 10.1016/j.bbagen.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe A. Problems in serum albumin measurement and clinical significance of albumin microheterogeneity in cirrhotics. Nutrition. 2004;20(4):351–357. doi: 10.1016/j.nut.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Oettl K. Oxidative albumin damage in chronic liver failure: relation to albumin binding capacity, liver dysfunction and survival. J Hepatol. 2013;59(5):978–983. doi: 10.1016/j.jhep.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Terawaki H. Decrease in reduced-form albumin among chronic kidney disease patients: new insights in cardiovascular complications. Ther Apher Dial. 2011;15(2):156–160. doi: 10.1111/j.1744-9987.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 13.Mera K. The structure and function of oxidized albumin in hemodialysis patients: its role in elevated oxidative stress via neutrophil burst. Biochem Biophys Res Commun. 2005;334(4):1322–1328. doi: 10.1016/j.bbrc.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Yasukawa K. A simple, rapid and validated high-performance liquid chromatography method suitable for clinical measurements of human mercaptalbumin and non-mercaptalbumin. Ann Clin Biochem. 2018;55(1):121–127. doi: 10.1177/0004563217693257. [DOI] [PubMed] [Google Scholar]
- 15.Davis M.D. Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol. 1965;74(6):741–751. doi: 10.1001/archopht.1965.00970040743003. [DOI] [PubMed] [Google Scholar]
- 16.Haneda M. A new classification of diabetic nephropathy 2014: a report from Joint committee on diabetic nephropathy. J Diabetes Investig. 2015;6(2):242–246. doi: 10.1111/jdi.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasukawa K. Establishment of a stable sampling method to assay mercaptoalbumin/non-mercaptoalbumin and reference ranges. Pract Lab Med. 2019;17 doi: 10.1016/j.plabm.2019.e00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki S. Oxidative damage to mitochondrial DNA and its relationship to diabetic complications. Diabetes Res Clin Pract. 1999;45(2–3):161–168. doi: 10.1016/s0168-8227(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa T. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care. 2003;26(5):1507–1512. doi: 10.2337/diacare.26.5.1507. [DOI] [PubMed] [Google Scholar]
- 20.Fukuhara S. Clinical usefulness of human serum nonmercaptalbumin to mercaptalbumin ratio as a biomarker for diabetic complications and disability in activities of daily living in elderly patients with diabetes. Metabolism. 2019:153995. doi: 10.1016/j.metabol.2019.153995. [DOI] [PubMed] [Google Scholar]
- 21.Rodiño-Janeiro B.K. Glycated albumin, a precursor of advanced glycation end-products, up-regulates NADPH oxidase and enhances oxidative stress in human endothelial cells: molecular correlate of diabetic vasculopathy. Diabetes Metab Res Rev. 2010;26(7):550–558. doi: 10.1002/dmrr.1117. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa A. New indices for predicting glycaemic variability. PloS One. 2012;7(9) doi: 10.1371/journal.pone.0046517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Association A.D. Postprandial blood glucose. American Diabetes Association. Diabetes Care. 2001;24(4):775–778. doi: 10.2337/diacare.24.4.775. [DOI] [PubMed] [Google Scholar]
- 24.Kohnert K.D. Chronic hyperglycemia but not glucose variability determines HbA1c levels in well-controlled patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;77(3):420–426. doi: 10.1016/j.diabres.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Koga M. Glycated albumin; clinical usefulness. Clin Chim Acta. 2014;433:96–104. doi: 10.1016/j.cca.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Suwa T. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM) Endocr J. 2010;57(2):135–140. doi: 10.1507/endocrj.k09e-234. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto H. Glycated albumin to glycated hemoglobin ratio is a sensitive indicator of blood glucose variability in patients with fulminant type 1 diabetes. Intern Med. 2012;51(11):1315–1321. doi: 10.2169/internalmedicine.51.7236. [DOI] [PubMed] [Google Scholar]
- 28.Monnier L. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. J Am Med Assoc. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 29.Ceriello A. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki E. Increased oxidized form of human serum albumin in patients with diabetes mellitus. Diabetes Res Clin Pract. 1992;18(3):153–158. doi: 10.1016/0168-8227(92)90140-m. [DOI] [PubMed] [Google Scholar]
- 31.Retnakaran R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 32.Tesfaye S. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39(11):1377–1384. doi: 10.1007/s001250050586. [DOI] [PubMed] [Google Scholar]
- 33.Gaede P. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 34.Solomon S.D. Diabetic retinopathy: a position statement by the American diabetes association. Diabetes Care. 2017;40(3):412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerkeni M. Pentosidine as a biomarker for microvascular complications in type 2 diabetic patients. Diabetes Vasc Dis Res. 2013;10(3):239–245. doi: 10.1177/1479164112460253. [DOI] [PubMed] [Google Scholar]
- 36.Sternberg M. Skin collagen pentosidine and fluorescence in diabetes were predictors of retinopathy progression and creatininemia increase already 6 years after punch-biopsy. Clin Biochem. 2016;49(3):225–231. doi: 10.1016/j.clinbiochem.2015.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.