We read with great interest the invited commentary by Akgul et al [1] on our report on the genomic landscape of urothelial papilloma (UP) and inverted urothelial papilloma (IUP) published in a recent issue of The Journal of Pathology [2]. The authors highlighted the salient findings of our study, namely that IUP and UP have genomic profiles that are distinct from urothelial carcinomas (UC). Unlike UC, IUP and UP have low tumor mutational burden and do not exhibit a predominant APOBEC mutation signature. Furthermore, oncogenic alterations in HRAS and KRAS were present in nearly all cases of IUP and UP, whereas alterations common to UC, including mutations in FGFR3, TP53, chromatin-modifying genes and the TERT promoter, were rarely observed.
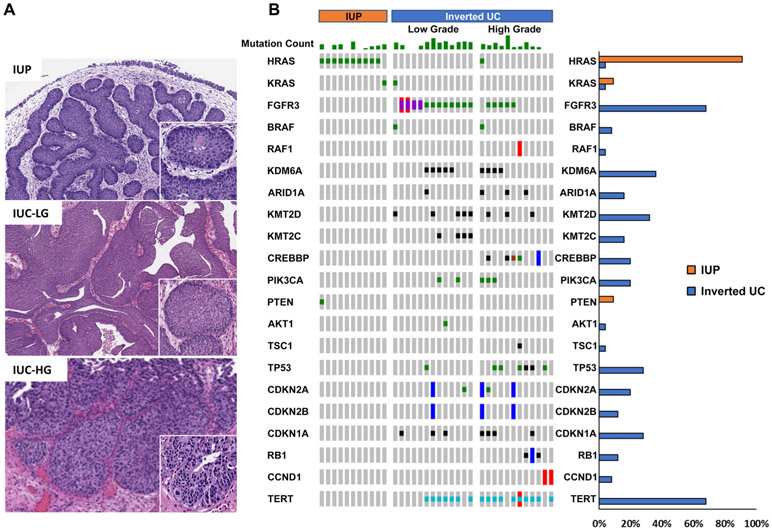
An important point raised by the authors was the lack of comparison between the molecular profiles of IUP and non-invasive UC with inverted growth pattern (IUC), as these entities have overlapping histologic features and may be difficult to distinguish based on histologic review alone. To address this insightful question raised by Akgul and colleagues [1], we reviewed the morphology of patients prospectively sequenced using the MSK-IMPACT platform to identify non-invasive IUC. Twenty-five IUC tumors were identified, of which 13 were low grade and 12 were high grade (Figure 1). Known or likely oncogenic alterations, as defined by OncoKB [3], were identified and compared between our previously reported cohort of 11 IUP and these 25 IUCs [2]. Consistent with other UCs, IUCs frequently harbored mutations in FGFR3 (17/25; 68%), the TERT promoter (17/25; 68%), TP53 (7/25; 28%), CDKN1A (7/25; 28%), PIK3CA (5/25; 20%) and chromatin-modifying genes (19/25; 76%) (Figure 1B). These alterations were rare to non-existent in IUP. As we have previously reported, all 11 IUP tumors harbored an oncogenic HRAS or KRAS missense mutation. In contrast, only two of 25 IUCs (8%) harbored an HRAS or KRAS mutation, both of which additionally harbored oncogenic mutations in chromatin-modifying genes and/or cell cycle regulators. Furthermore, the genetic alterations of low-grade and high-grade IUCs were similar to those reported for papillary UC without an inverted growth pattern [4]. These findings further support that the genomic landscape of IUP is different from that observed in IUC.
Figure 1.
(A) Representative H&E stain of IUP, non-invasive IUC low grade (IUC-LG) and high grade (IUC-HG), original magnification ×100. (B) Oncoprint showing known or likely oncogenic alterations, as grouped by molecular pathway, for 11 IUP and 25 IUC (13 low grade, 12 high grade). Histogram on the right compares the frequency of oncogenic alterations in each gene in IUP and IUC.
The distinct clinical behavior of IUP relative to UC highlights the importance of establishing an accurate diagnosis, which we believe, based on our findings reported here, can be greatly enhanced in morphologically ambiguous or challenging cases through next-generation sequencing-based tumor molecular profiling. UP and IUP are benign neoplasms for which complete transurethral resection is standard treatment, with recurrence observed in only 1–8% of patients and no reported cases of progression to muscle-invasive or metastatic disease across multiple series [5]. Conversely, UC exhibits a high risk of recurrence, ranging from 15 to 61% at 1 year, depending on stage, grade and other prognostic factors, and can carry a substantial risk of progression to muscle-invasive disease [6].
In summary, IUP have a genomic profile (HRAS/KRAS mutant, FGFR3, TERT, TP53 and chromatin-modifying gene wildtype) distinct from that of non-invasive UC with or without an inverted growth pattern. This difference in disease pathogenesis probably underlies the differences in clinical behavior between these neoplasms. Given the differences in clinical behavior, including the risk for recurrence and progression between IUP and low-grade UCs, accurate diagnosis is imperative to ensure optimal clinical management. We therefore believe that tumor molecular profiling is warranted for diagnostic purposes for patients with suspected IUP, particularly for morphologically challenging or ambiguous cases.
Acknowledgements
This work was supported by Cycle for Survival (HA, DBS), R01 CA233899 (HA), a Sloan Kettering Institute for Cancer Research Cancer Center Support Grant (P30CA008748), SPORE in Bladder Cancer P50CA221745 and the Marie-Josée and Henry R Kravis Center for Molecular Oncology.
Footnotes
Conflict of interest statement: There are no conflicts of interest for the work presented in the current study. EJ Pietzak is on the Scientific Advisory Board of Merck. DB Solit is a consultant for Pfizer, Loxo Oncology, Intezyne, Vivideon Therapeutics and Illumina. H Al-Ahmadie is a consultant for Bristol-Myers-Squibb and AstraZeneca.
References
- 1.Akgul M, MacLennan GT, Cheng L. Distinct mutational landscape of inverted urothelial papilloma. J Pathol 2019; 249: 3–5. [DOI] [PubMed] [Google Scholar]
- 2.Isharwal S, Hu W, Sarungbam J, et al. Genomic landscape of inverted urothelial papilloma and urothelial papilloma of the bladder. J Pathol 2019; 248: 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol 2017; 2017. 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietzak EJ, Bagrodia A, Cha EK, et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur Urol 2017; 72: 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picozzi S, Casellato S, Bozzini G, et al. Inverted papilloma of the bladder: a review and an analysis of the recent literature of 365 patients. Urol Oncol 2013; 31: 1584–1590. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006; 49: 466–65; discussion 475–77. [DOI] [PubMed] [Google Scholar]