Abstract
Background
Galectin-1, haptoglobin, and nesfatin-1 have recently emerged as promising biomarkers implicated in immunometabolism. However, whether single blood measurements of these analytes could be suitable for large-scale human studies has not yet been evaluated.
Methods
The concentrations of galectin-1, haptoglobin, and nesfatin-1 were measured over a 4-month period in 207 healthy adults with median age of 56.7 years. Biomarker intra-individual reproducibility was assessed based on calculation of intraclass correlation coefficients (ICCs) and examining Bland-Altman plots.
Results
The overall ICCs were excellent for nesfatin-1 (ICC: 0.89 (95% CI: 0.86, 0.92), and good for galectin-1 and haptoglobin (ICCs: 0.70 (95% CI: 0.61, 0.77) and 0.67 (95% CI: 0.57, 0.74), respectively). Bland-Altman plots supported a high level of agreement between repeated biomarker measurements.
Conclusions
Assay measurements of galectin-1, haptoglobin, and nesfatin-1 showed good to excellent within-subject reproducibility over a 4-month period, indicating that they may serve as feasible and reliable biomarkers for assessing metabolic inflammation in population research.
Keywords: galectin-1, haptoglobin, nesfatin-1, Intra-individual reproducibility, Repeated measurements
Abbreviations: BMI, body mass index; EPIC, European Prospective Investigation into Cancer and Nutrition; ELISA– Enzyme-Linked Immunosorbent Assay. Hb, hemoglobin. hsCRP; high sensitivity C-reactive protein. ICC, intraclass correlation coefficient; IQR, interquartile range
1. Introduction
The global syndemic of obesity and metabolic diseases has prompted accelerated research in understanding their etiology and prevention [1]. Immunometabolism has recently emerged as a new field of research aimed to uncover the complex interactions between the metabolic and immune systems [2,3]. While the role of low-grade chronic inflammation has been long recognized, particularly in adipose tissue and liver [4], recent evidence suggested novel links between dysmetabolic conditions and pathologic immune responses [5]. A number of novel pro-inflammatory mediators of metabolism have been described in the literature [6]; however, their potential role as early predictors of metabolic disease-associated inflammation remains unclear. Among a set of candidate molecules, we have identified galectin-1, haptoglobin, and nesfatin-1 as promising biomarkers of immunometabolism.
Galectin-1 is a protein normally secreted in epithelia of many organs and immune cells, including macrophages as well as dendritic cells and Kupfer cells [7]. It is upregulated during inflammation and exhibits a wide range of physiological functions, including regulation of cell differentiation, proliferation, apoptosis and angiogenesis [7,8]. Haptoglobin is a prominent plasma glycoprotein involved in the scavenging of free haemoglobin [9]. Most haptoglobin is produced by the liver, although other organs such as kidney and adipose tissue could be additional sources of its production [9,10]. Haptoglobin also acts as an acute phase protein and is highly induced in the liver by inflammation and injury upon stimulation by IL-6 [9]. Nesfatin-1 is a recently discovered peptide secreted by peripheral tissues but also in the brain, adipose tissue and gastrointestinal tract [11]. Initially identified as a satiety molecule affecting fat metabolism, energy expenditure and glucose homeostasis [12,13], recent research highlights the importance of nesfatin-1 as an immune-mediated biomarker [14,15]. Despite accumulating evidence in support of the inflammatory and metabolic functional associations of these biomarkers, their application in etiological research is limited and whether they could prove useful in clinical practice remains unclear.
This study aimed to evaluate the intra-individual reproducibility of galectin-1, haptoglobin, and nesfatin-1 in a sample of predominantly healthy adults with repeated blood collections over a 4-month period within the European Prospective Investigation into Cancer and Nutrition (EPIC) Potsdam cohort.
2. Methods
2.1. Study population
The study included 407 individuals taking part in a validation study conducted within the EPIC-Potsdam study. Exclusion criteria included history of heart disease and stroke, impaired mobility, used β-blockers, and had systolic or diastolic blood pressure above 180 mmHg or 110 mmHg, respectively. Blood samples were collected on two occasions 4-months apart in the period of October 2007 to March 2008 and between February and July 2008. Of the 407 invited participants, the total number of eligible participants was 207 (11 did not respond, 176 declined participation, 12 used β-blockers, and one provided only one blood sample). From these, repeated measures for each biomarker were available for 166 (galectin-1), 171 (haptoglobin), and 168 (nesfatin-1) participants. Timing of blood collection was conducted from 8 a.m. to 11 a.m., with few exceptions, and 90% of participants were in a fasted state. All participants provided written informed consent and the study procedures were approved by the Ethics Committee of the Medical Association of the State of Brandenburg.
2.2. Biomarker measurements
After the blood draw, blood fractions were separated and stored at −80 °C by qualified laboratory technicians. Biomarker concentrations were measured in EDTA-plasma samples (nesfatin-1 and haptoglobin) and serum samples (galectin-1) with commercially available sandwich ELISA kits (BioVendor, Kassel, Germany) at the German Institute of Human Nutrition Potsdam-Rehbrücke, Germany, according to the manufacturer’s instructions. Coefficients of variation reported in the manufacturers’ manuals ranged from 4.3% to 5.9% for intra-assay variation and from 5.9% to 6.9% for inter-assay variation, with no information provided for galectin-1. The repeated samples from each study participant were measured in the same analytical batch. All biomarkers had measurements within the range of quantification.
2.3. Statistical analysis
To avoid skewed results, extreme values of galectin-1, haptoglobin, and nesfatin-1 were excluded from analysis by using the 1st and 99th percentile of the first and second measurement as thresholds for exclusion. Median and interquartile range (IQR) were calculated for both measurements of galectin-1, haptoglobin, and nesfatin-1 for the following strata: overall and stratified by sex, BMI, and hsCRP. Biomarker concentrations between strata were compared using the Wilcoxon-Mann Whitney test. As a measure of reliability, intraclass correlation coefficients (ICCs) were calculated [16]. To evaluate interdependence with the individual characteristics, the ICCs were compared across strata by sex, BMI, and hsCRP. Following established reliability cut points, estimated reproducibility was rated as excellent (ICC ≥ 0.75), good (ICC = 0.74–0.60), fair (ICC = 0.59–0.40), and poor (ICC < 0.40). As a complementing procedure, Bland-Altman plots were generated to visually assess the agreement of measurements for each participant [17]. Associations between baseline biomarker concentrations with body mass index (BMI) and hsCRP were evaluated using partial Spearman correlation analysis adjusted for age, sex, and mutually adjusted for BMI and hsCRP. All analyses were performed in SAS (Version 9.4, Enterprise Guide 6.1, SAS Institute Inc., Cary, NC, USA).
3. Results
The study population had a median age of 56.7 years and consisted of 60% women. At blood draw, 90% of the participants were fasted. Participants had a median BMI of 26.1 kg/m2, waist circumference of 93.0 cm, and serum hsCRP of 1.2 μg/mL.
Table 1 presents the medians and interquartile ranges of repeated measurements of galectin-1, haptoglobin, and nesfatin-1 as well as corresponding ICCs, overall and according to strata by sex, BMI, and hsCRP.
Table 1.
Repeated measurements of biomarker concentrations and estimated ICCs, overall and stratified by sex, BMI and hsCRP.
| Biomarkers | N | Median (IQR) |
ICC (95% CI)∗∗ | ||
|---|---|---|---|---|---|
| First measurement | Second measurement | ||||
| Galectin-1, ng/mL | |||||
| All | 163 | 3.1 (2.7–3.6) | 3.2 (2.8–3.8) | 0.70 (0.61, 0.77) | |
| Sex | Men | 64 | 3.0 (2.6–3.4) | 3.0 (2.8–3.8) | 0.77 (0.64, 0.85) |
| Women | 99 | 3.1 (2.7–3.7) | 3.3 (2.8–3.8) | 0.65 (0.52, 0.75) | |
| pdifference∗ | 0.197 | 0.367 | |||
| BMI | <25 kg/m2 | 67 | 3.0 (2.6–3.2) | 3.1 (2.8–3.5) | 0.45 (0.24, 0.62) |
| ≥25 kg/m2 | 96 | 3.2 (2.7–3.7) | 3.4 (2.9–3.9) | 0.80 (0.71, 0.86) | |
| pdifference∗ | 0.009 | 0.031 | |||
| hsCRP | <1.2 μg/mL | 76 | 3.0 (2.6–3.3) | 3.1 (2.7–3.6) | 0.63 (0.47, 0.75) |
| ≥1.2 μg/mL | 87 | 3.2 (2.8–3.7) | 3.4 (2.9–3.9) | 0.74 (0.63, 0.82) | |
| pdifference∗ | 0.018 | 0.019 | |||
| Haptoglobin, μg/mL | |||||
| All | 168 | 175.0 (139.0–234.0) | 163.5 (137.5–209.5) | 0.67 (0.57, 0.74) | |
| Sex | Men | 69 | 184.0 (144.0–245.0) | 170.0 (140.0–215.0) | 0.55 (0.36, 0.69) |
| Women | 99 | 173.0 (136.0–219.0) | 161.0 (132.0–209.0) | 0.74 (0.63, 0.82) | |
| pdifference∗ | 0.485 | 0.583 | |||
| BMI | <25 kg/m2 | 66 | 158.0 (130.0–195.0) | 156.5 (131.0–190.0) | 0.54 (0.35, 0.69) |
| ≥25 kg/m2 | 102 | 179.5 (147.0–268.0) | 174.0 (140.0–262.0) | 0.72 (0.62, 0.80) | |
| pdifference∗ | 0.013 | 0.083 | |||
| hsCRP | <1.2 μg/mL | 75 | 160.0 (129.0–198.0) | 161.0 (133.0–205.0) | 0.77 (0.65, 0.85) |
| ≥1.2 μg/mL | 93 | 190.0 (153.5–259.0) | 163.5 (141.5–215.0) | 0.57 (0.41, 0.69) | |
| pdifference∗ | 0.008 | 0.543 | |||
| Nesfatin-1, ng/mL | |||||
| All | 163 | 0.3 (0.1–1.5) | 0.3 (0.1–1.6) | 0.89 (0.86, 0.92) | |
| Sex | Men | 63 | 0.3 (0.1–1.3) | 0.3 (0.1–1.5) | 0.84 (0.74, 0.90) |
| Women | 100 | 0.4 (0.1–1.5) | 0.5 (0.1–1.7) | 0.93 (0.90, 0.95) | |
| pdifference∗ | 0.831 | 0.574 | |||
| BMI | <25 kg/m2 | 66 | 0.2 (0.1–1.4) | 0.3 (0.1–1.2) | 0.93 (0.89, 0.96) |
| ≥25 kg/m2 | 97 | 0.4 (0.1–1.5) | 0.3 (0.1–1.7) | 0.86 (0.80, 0.90) | |
| pdifference∗ | 0.207 | 0.206 | |||
| hsCRP | <1.2 μg/mL | 78 | 0.3 (0.1–2.6) | 0.3 (0.1–1.7) | 0.93 (0.89, 0.96) |
| ≥1.2 μg/mL | 85 | 0.4 (0.1–1.3) | 0.3 (0.1–1.3) | 0.85 (0.78, 0.90) | |
| pdifference∗ | 0.917 | 0.834 | |||
∗p value for difference based on Wilcoxon-Mann Whitney test between strata (sex, BMI, hsCRP).
∗∗ICC – intraclass correlation coefficient, based on Box-Cox transformed values of the first and second measurement.
BMI – body mass index; hsCRP – high sensitivity C-reactive protein, IQR – interquartile range.
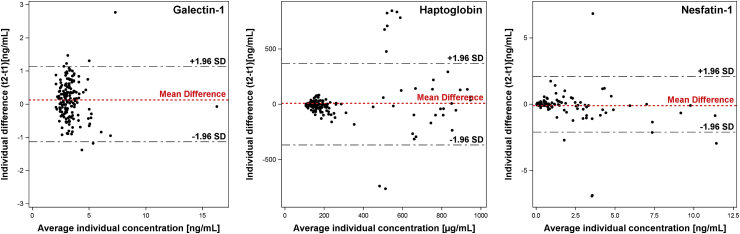
Overall, the ICCs (with 95% confidence intervals) over a 4-month period ranged from good for galectin-1 and haptoglobin (0.70 [0.61–0.77] and 0.67 [0.57–0.74], respectively) to excellent for nesfatin-1 (0.89 [0.86–0.92]). In analyses by strata, no substantial differences in estimated ICCs could be observed. Fig. 1 shows Bland-Altman plots presenting the difference of the repeated biomarker measurements plotted against the mean of the two measurements. Visual inspection of these plots showed evidence of increasing variability of differences between biomarker measurements in participants with medium (480–600 μg/mL) concentrations of haptoglobin. Most individual differences of nesfatin-1 measurements were within the limits of agreement across the full range of concentrations, illustrating an excellent intra-individual reproducibility. Overall, agreements between the two measurements of galectin-1 and haptoglobin were less pronounced, but still within the expected limits.
Fig. 1.
Bland-Altman plot - Agreement of both measurements (y-axis) relative to average concentrations (x-axis) per individual. Agreement was calculated as the difference between both measurements (t2-t1) per individual. The 1.96 SD (standard deviation) thresholds represent the 95% confidence interval of the expected range of differences based on the average difference.
In partial correlation analysis galectin-1, haptoglobin, and nesfatin-1 were weakly positively associated with BMI (ρ = 0.08 [-0.09 – 0.24], ρ = 0.03 [-0.14 – 0.19], and ρ = 0.10 [-0.07 – 0.26]), respectively. The corresponding estimates for associations with hsCRP were the following: ρ = 0.15 [-0.01 – 0.31], ρ = 0.13 [-0.04 – 0.28], and ρ = 0.07 [-0.10 – 0.23], respectively.
4. Discussion
In this study of predominantly healthy participants, circulating concentrations of galectin-1, haptoglobin, and nesfatin-1 showed good to excellent reproducibility over a period of 4 months. Our findings suggest that these biomarkers may serve as reliable biomarkers that could reflect immune-inflammatory pathways associated with metabolic health.
An important prerequisite for investing in biomarker measurements in large-scale human studies is to validate their detectability and reproducibility in healthy individuals over time. The latter aspect enables researchers to use single measurements of these markers as exposure proxies in assessing risk in prospective studies. Several factors can contribute to the intra-individual reproducibility of a biomarker including time of blood collection, laboratory procedures, storage conditions, as well as biological and metabolic individual variance. Accumulating evidence has supported the role of metabolic pathways in immune regulation and metabolic disease development [18]. As a consequence, there has been a growing need to identify immunometabolic biomarkers that serve as risk markers and pharmacological targets [18]. To our knowledge, this is the first study that specifically reports on the intra-individual reproducibility of galectin-1, haptoglobin and nesfatin-1 providing a methodological guidance to researchers potentially interested in measuring these biomarkers in prospective studies. The accelerated interest in these three analytes has been justified by a mounting number of epidemiological and experimental studies suggesting links with immunity, metabolism, and chronic disease development [7,9,11,19]. Galectin-1 was suggested to inhibit metabolic diseases and to suppress T-cell-dependent chronic inflammation in arthritis, hepatitis and colitis [20]. Nesfatin-1 is well known with its potent actions in feeding suppression and inducing pancreatic insulin secretion [21]; however, recent evidence has also uncovered its roles in the regulation of inflammatory responses and cell apoptosis [15]. Haptoglobin reflects hepatic acute phase response and is positively associated with both obesity and chronic low-grade inflammation [22] and to exert important immunomodulatory mechanisms.
Future investigation of relevant biomarker pathways would facilitate a better understanding of their etiological role in immunometabolism and disease development. Circulating levels of the biomarkers could vary by participants’ demographic and lifestyle characteristics including gender, obesity, and ongoing inflammatory status. Accordingly, we examined whether circulating levels of measured biomarkers were associated with sex, BMI, and hsCRP (a surrogate marker of systemic inflammation), and evaluated whether estimated ICCs differed according to strata by these factors. Since all evaluated biomarkers have been previously associated with adipose tissue, we especially anticipated that higher BMI would lead to higher plasma concentrations. However, in our predominantly healthy sample we did not find a strong correlation between BMI, hsCRP, and plasma biomarker concentrations. This observation is likely due to the narrow range of BMI represented in the present study that limits data analysis and interpretation. Our data also did not suggest any major differences in within-subject ICCs when we stratified the analysis according to these variables; therefore, it is unlikely that these factors would be expected to substantially influence future research results. Our study provided assessment of biomarker reliability over several months of time and may not be representative of long-term reliability. Therefore, future studies with a longer time in-between blood collection are warranted to assess long-term reliability of the biomarkers.
In conclusion, this study supports that galectin-1, nesfatin-1 and haptoglobin may serve as feasible and reliable biomarkers for assessing metabolic inflammation in population research.
Funding
This work was funded by Institutional Funds of the German Institute of Human Nutrition Potsdam-Rehbrücke.
CRediT authorship contribution statement
Matthew Schenk: Writing - review & editing. Robin Reichmann: Formal analysis, Visualization, Writing - review & editing. Liselot Koelman: Formal analysis, Writing - review & editing. Andreas F.H. Pfeiffer: Writing - review & editing. Natalia N. Rudovich: Writing - review & editing. Krasimira Aleksandrova: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
The authors express thanks to Katrin Ritter (DIfE) for her technical assistance and the Human Study Centre (DIfE) for data collection and logistics. Special thanks to Manuela Bergmann for leading the data generation, to Silke Navia Fruth and Herbert Piechot for their assistance with biosample management, and to Ellen Kohlsdorf for data management. Finally, thanks to all EPIC-Potsdam participants for their valuable contribution in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2020.100034.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Swinburn B.A., Kraak V.I., Allender S., Atkins V.J., Baker P.I., Bogard J.R. The global syndemic of obesity, undernutrition, and climate change: the lancet commission report. Lancet. 2019;393:791–846. doi: 10.1016/S0140-6736(18)32822-8. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D., Shoelson S.E. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011;11:81. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce E.L., Pearce E.J. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil G.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 5.Caligiuri G., Norata G.D. Fuel for thought: immunometabolism is a paradigm shift in understanding immunity in cardiovascular disease. Cardiovasc Res. 2019;115:1383–1384. doi: 10.1093/cvr/cvz155. [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil G.S. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47:406–420. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchard H., Bum-Erdene K., Bohari M.H., Yu X. Galectin-1 inhibitors and their potential therapeutic applications: a patent review. Expert Opin Ther Pat. 2016;26:537–554. doi: 10.1517/13543776.2016.1163338. [DOI] [PubMed] [Google Scholar]
- 8.Rabinovich G.A., Conejo-Garcia J.R. Shaping the immune landscape in cancer by galectin-driven regulatory pathways. J Mol Biol. 2016;428:3266–3281. doi: 10.1016/j.jmb.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 9.MacKellar M., Vigerust D.J. Role of haptoglobin in health and disease: a focus on diabetes. Clin Diabetes. 2016;34:148–157. doi: 10.2337/diaclin.34.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenstein H., Levy N.S., Levy A.P. Haptoglobin genotype and its role in determining heme-iron mediated vascular disease. Pharmacol Res. 2012;66:1–6. doi: 10.1016/j.phrs.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schalla M.A., Stengel A. Current understanding of the role of nesfatin-1. J Endocr Soc. 2018;2:1188–1206. doi: 10.1210/js.2018-00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leivo-Korpela S., Lehtimaki L., Hamalainen M., Vuolteenaho K., Koobi L., Jarvenpaa R. Adipokines NUCB2/nesfatin-1 and visfatin as novel inflammatory factors in chronic obstructive pulmonary disease. Mediat Inflamm. 2014:1–6. doi: 10.1155/2014/232167. 232167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu H., Oh I.S., Okada S., Mori M. Nesfatin-1: an overview and future clinical application. Endocr J. 2009;56:537–543. doi: 10.1507/endocrj.k09e-117. [DOI] [PubMed] [Google Scholar]
- 14.Kvlividze T.Z., Zavodovsky B.V., Akhverdyan Y.R., Polyakova Y.V., Sivordova L.E., Yakovlev A.T. Serum nesfatin-1 as a marker of systemic inflammation in rheumatoid arthritis. Klin Lab Diagn. 2019;64:53–56. doi: 10.18821/0869-2084-2019-64-1-53-56. [DOI] [PubMed] [Google Scholar]
- 15.Leivo-Korpela S., Lehtimaki L., Hamalainen M., Vuolteenaho K., Koobi L., Jarvenpaa R. Adipokines NUCB2/nesfatin-1 and visfatin as novel inflammatory factors in chronic obstructive pulmonary disease. Mediat Inflamm. 2014;2014:232167. doi: 10.1155/2014/232167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartko J.J. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- 17.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 18.Ketelhuth D.F.J., Lutgens E., Back M., Binder C.J., Van den Bossche J., Daniel C. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the working group on atherosclerosis and vascular biology of the European society of cardiology. Cardiovasc Res. 2019;115:1385–1392. doi: 10.1093/cvr/cvz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perillo N.L., Pace K.E., Seilhamer J.J., Baum L.G. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 20.Brinchmann M.F., Patel D.M., Iversen M.H. The role of galectins as modulators of metabolism and inflammation. Mediat Inflamm. 2018;2018:9186940. doi: 10.1155/2018/9186940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayada C., Toru U., Korkut Y. Nesfatin-1 and its effects on different systems. Hippokratia. 2015;19:4–10. [PMC free article] [PubMed] [Google Scholar]
- 22.Maffei M., Barone I., Scabia G., Santini F. The multifaceted haptoglobin in the context of adipose tissue and metabolism. Endocr Rev. 2016;37:403–416. doi: 10.1210/er.2016-1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.