Abstract
Objective:
Generalized epileptiform discharges (GEDs) can occur during seizures or without obvious clinical accompaniment. Motor vehicle driving risk during apparently subclinical GEDs is uncertain. Our goals were to develop a feasible, realistic test to evaluate driving safety during GEDs, and to begin evaluating electroencephalographic (EEG) features in relation to driving safety.
Methods:
Subjects were aged ≥15 years with generalized epilepsy, GEDs on EEG, and no clinical seizures. Using a high-fidelity driving simulator (miniSim) with simultaneous EEG, a red oval visual stimulus was presented every 5 minutes for baseline testing, and with each GED. Participants were instructed to pull over as quickly and safely as possible with each stimulus. We analyzed driving and EEG signals during GEDs.
Results:
Nine subjects were tested, and five experienced 88 GEDs total with mean duration 2.31 ± 1.89 (SD) seconds. Of these five subjects, three responded appropriately to all stimuli, one failed to respond to 75% of stimuli, and one stopped driving immediately during GEDs. GEDs with no response to stimuli were significantly longer than those with appropriate responses (8.47 ± 3.10 vs 1.85 ± 0.69 seconds, P < .001). Reaction times to stimuli during GEDs were significantly correlated with GED duration (r = 0.30, P = .04). In addition, EEG amplitude was greater for GEDs with no response to stimuli than GEDs with responses, both for overall root mean square voltage amplitude (66.14 μV vs 52.99 μV, P = .02) and for fractional power changes in the frequency range of waves (P < .05) and spikes (P < .001).
Significance:
High-fidelity driving simulation is feasible for investigating driving behavior during GEDs. GEDs with longer duration and greater EEG amplitude showed more driving impairment. Future work with a large sample size may ultimately enable classification of GED EEG features to predict individual driving risk.
Keywords: absence seizures, consciousness, driving, EEG, epilepsy, spike-wave discharges
1 |. INTRODUCTION
Generalized epileptiform discharges (GEDs) including spike-wave discharges and other abnormal activity patterns are the hallmark of generalized epilepsy syndromes.1 Spike-wave rhythms typically occur in complexes with frequencies > 2.5 Hz and can be elicited by triggers including sleep deprivation, hyperventilation, and intermittent photic stimulation.2,3 These discharges are thought to arise from excitatory and inhibitory circuits in the brain4 and represent thalamocortical oscillations often associated with brief episodes of impaired consciousness like those seen in absence seizures.5,6 Other well-known GED patterns include polyspike discharges and photoparoxysmal responses.
Of the 3.4 million Americans with epilepsy, about 470 000 are children.7 As this demographic approaches adulthood, it will face an extant issue often cited as the top concern among people with epilepsy: driving.8 Previous meta-analyses of drivers with epilepsy showed conflicting results, ranging from no increased risk to a sevenfold increase in risk.9‒12 Evidence of effective seizure control is the main criterion used to allow driving in most parts of the United States.11 Despite a consensus regarding the need for a seizure-free period prior to driving, there is little agreement regarding the necessary duration of such periods.11,13 There is also uncertainty regarding whether a reliable aura or warning should be a favorable modifier to enable driving.14 In addition, many government or professional agency guidelines consider impaired consciousness an important factor in disallowing driving licensure. However, objective means for evaluating loss of consciousness are not available. Thus, further work is needed to improve understanding of how a diagnosis of epilepsy affects risk for motor vehicle collisions.
One important problem is the many patients who are clinically seizure-free yet have epileptiform discharges on electroencephalography (EEG) including GEDs. These epileptiform discharges, with no obvious clinical sequelae, pose a challenge to driver licensing authorities, as well as to clinicians and their patients.13 Despite not being perceived by patients, subclinical epileptiform discharges might be accompanied by transient cognitive impairments, and their effect on driving and potential public safety risk is uncertain.15
Previous work using behavioral tasks has supported the idea that GEDs have varying effects on cognition and behavior.3,6,16,17 Variability in performance on these tasks could be observed from GED to GED between patients as well as within the same patient.3,16 However, GEDs tend to affect simpler tasks such as repetitive tapping less severely than more complex tasks that require decision-making and verbal responses.3,6,16 Understanding the impact of epileptiform discharges on driving is of great importance, especially for those patients who report no clinical seizures and are seeking licensure. Previous studies have also suggested the possibility that EEG features including GED duration and amplitude might help distinguish GEDs with more or less severe behavioral impact.16,18,19
Recent work using a driving game implemented on a laptop recorded reaction times in response to obstacles appearing in car lanes and showed increased crash probability associated with interictal epileptiform activity with particular EEG features.20,21 Using a racecar driving game, another study found impaired driving performance in some epileptiform discharges but not others.22 Although on-road real-world driving testing has been attempted in epilepsy, such an approach presents obvious safety concerns for general implementation.23 These prior studies suggest that the use of a high-fidelity driving simulator designed to closely emulate the experience of driving a real motor vehicle may be a safe and effective means for evaluating driving risk as closely as possible. Therefore, here we provide initial feasibility testing of simultaneous EEG and realistic high-fidelity driving simulation to evaluate driving performance in relation to GED features such as duration and EEG signal amplitude.
2 |. MATERIALS AND METHODS
2.1 |. Subjects
All human research in this project was approved by the Yale University Institutional Review Board, and all subjects underwent written and verbal informed consent. Subjects were recruited through local epilepsy centers and neurologists. Inclusion criteria were as follow: (1) age ≥ 15 years (age for driving learner’s permit in Connecticut), (2) diagnosis of generalized epilepsy, and (3) EEG within year of enrollment showing at least one GED per hour. Exclusion criteria were as follow: (1) any clinical seizures in the month preceding participation and (2) any other neurologic disorder that precludes the ability to drive.
Patients were contacted by phone to conduct an initial screening interview that included a detailed history of their epilepsy including age of onset, types of seizures, description of episodes, medication regimen, and sensitivity to seizure precipitants including photic stimulation and hyperventilation. A summary of participant clinical and demographic characteristics is shown in Table 1.
TABLE 1.
Patient demographic and clinical information
| Pt# | Sex | Age at drive, y | Age at epilepsy onset, y | Treatment at time of study | Clinical seizure type | Driving experience? | EEG description | Total GEDs, n | GEDs tested during drive, n |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 18 | 5 | Lamotrigine, zonisamide |
Absence | Yes | 3.5-Hz generalized SWDs,a often larger amplitude on left side | 9 | 2 |
| 2 | F | 18 | 11 | Lamotrigine, zonisamide |
Absence, GTC, myoclonic | No | No epileptiform discharges | 0 | 0 |
| 3 | F | 20 | 1 | Levetiracetam | Absence, GTC, myoclonic | Yes | 3-Hz generalized SWDs | 7 | 4 |
| 4 | M | 33 | 10 | Lamotrigine | Absence, GTC | Yes | 14- to 18-Hz rhythmic generalized beta activitya | 14 | 0 |
| 5 | F | 43 | 10 | Valproic acid | Absence, GTC | Yes | No discharges | 0 | 0 |
| 6 | M | 18 | 13 | Valproic acid | Absence | Yesb | No discharges | 0 | 0 |
| 7 | F | 76 | 74 | Levetiracetam, lacosamide |
GTC | Yes | 2- to 3-Hz generalized SWDs or polyspike and wave discharges | 30 | 22 |
| 8 | F | 19 | 18 | Lamotrigine, zonisamide |
Absence, GTC | Yes | No discharges | 0 | 0 |
| 9 | F | 20 | 11 | Clobazam | GTC | Yes | 8- to 14-Hz generalized polyspikes evolving into 3- to 4-Hz generalized polyspike and wave discharges | 28 | 27 |
Abbreviations: EEG, electroencephalographic; F, female; GED, generalized epileptiform discharge; GTC, generalized tonic-clonic; M, male; SWD, spike-wave discharge.
Most discharges were evoked by photic stimulation in this patient.
This patient had a learner’s permit but little to no on-road driving experience.
2.2 |. Driving simulation and testing protocol
Test procedures were conducted at the Developmental Neurocognitive Driving Simulation Research Center (DrivSim Lab) in the Department of Emergency Medicine at Yale School of Medicine. Subjects drove in a high-fidelity driving simulator (miniSim, National Advanced Driving Simulator, University of Iowa), which included a midpoint sectioned 2007 Mazda 6 vehicle, accelerator (gas) and brake pedals, steering wheel, and video cameras for capturing driver behavior (Figure 1). Using the simulator’s Interactive Scenario Authoring Tool, we implemented a standard driving scenario consisting of an hour-long drive through urban and rural scenes. The simulator provides a fully immersive environment including responsive driving scenery projected onto a 165° curvilinear screen that wraps around for front and side views, as well as a rear projection screen for naturalistic viewing through side and rear mirrors.
FIGURE 1.
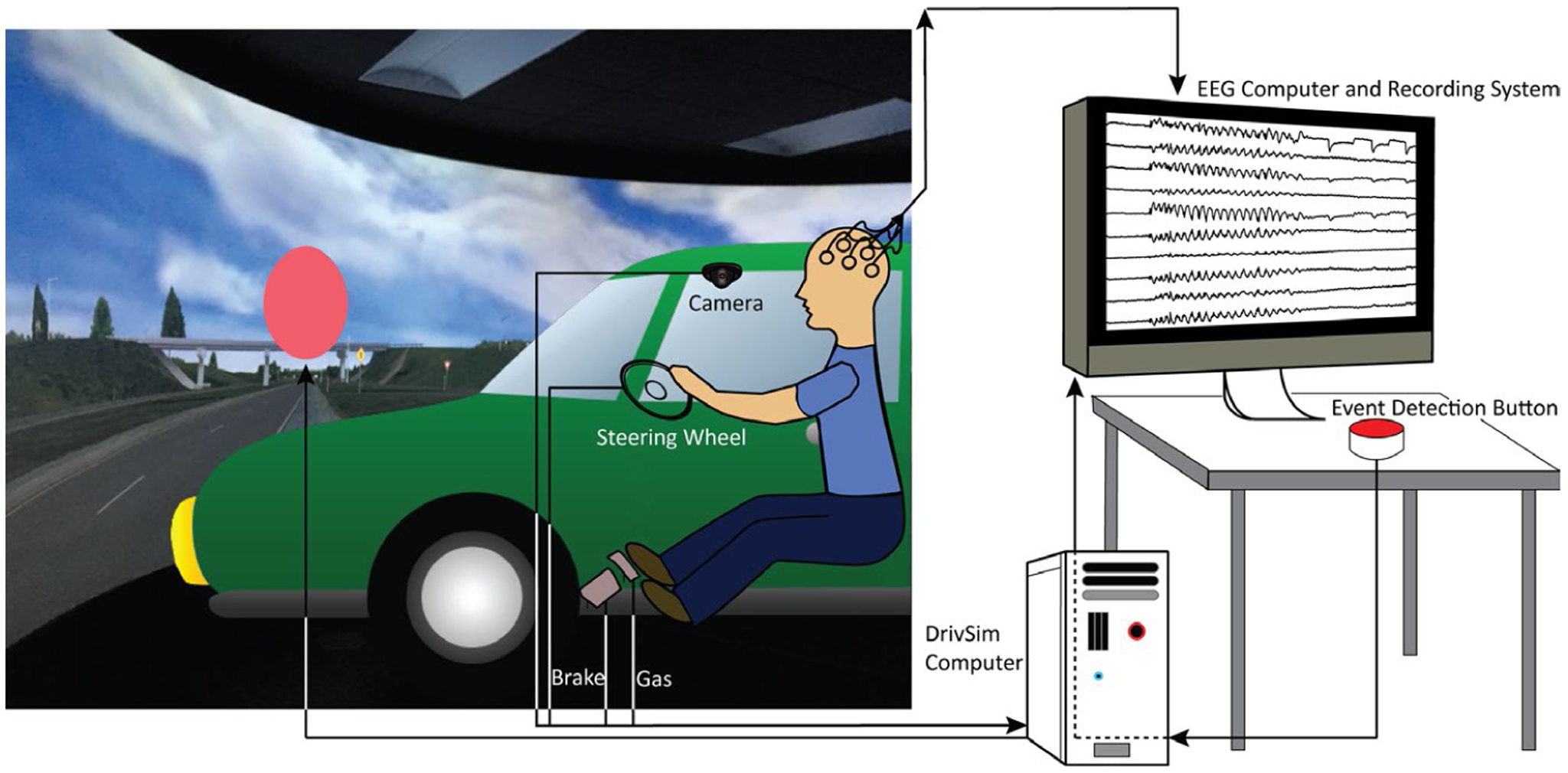
Driving paradigm including electroencephalogram (EEG), video, and behavioral data acquisition. Main components and configuration of the miniSim driving simulator at the DrivSim laboratory are shown. Detection of epileptiform discharge prompts an experienced EEG reviewer to press the Event Detection Button. A red oval stimulus is sent to the screen, and the stimulus time is recorded by the DrivSim computer as well as time-marked as an event on EEG recording. Subject responses including steering wheel, gas, and brake pedal are recorded on the DrivSim computer and analyzed offline. Behavior is also recorded by video cameras in the DrivSim car
Prior to entering the driving simulator, EEG recordings were begun (see next section). Subjects then drove for a 5-minute practice scenario on roadways similar to the full-length drive to become familiar with the testing paradigm. Subjects were shown the visual stimulus consisting of a red oval appearing in the center of the screen for 100 milliseconds (Figure 1). They were instructed to pull over to the shoulder of the road as quickly and safely as possible upon presentation of the stimulus. Before proceeding to the full hour-long drive, the Kennedy Simulator Sickness Questionnaire was administered.24 None of the participants reported any motion sickness–related symptoms during the study.
Throughout the drive, EEG signals were reviewed in real-time by an experienced EEG reader. The stimulus was presented every 5 minutes for baseline response testing (total of approximately 12 stimuli per 1-hour session), as well as whenever the EEG reader observed a GED and pressed an event detection button (Figure 1). The stimulus times were recorded along with the driving behavioral data on the DrivSim computer and were also passed through and recorded with the EEG signals (Figure 1) to allow later synchronization of EEG and driving data. Of note, several “false-positives” occurred throughout the testing periods—events considered by the online review to be GEDs but upon later EEG review were ruled out as such (average = 5.2 stimuli per driving session). These stimuli were included in the baseline testing data for each participant.
If no GEDs were observed in the first 30 minutes of the driving task, provocation was attempted with either hyperventilation or photic stimulation. Hyperventilation was performed by instructing patients to breathe deeply in and out for 3 minutes. If there was a history of photosensitivity then photic stimulation was performed instead of hyperventilation. We used a Siglent (model SDG1025) function generator (Siglent Technologies) connected to a Bio-Logic (model 580-SSPL01) photic stimulator with pulse width = 0.5 seconds, light intensity set to maximum, and light placed 30 cm from subjects’ nasion but positioned above the face so that viewing of the driving scene was not obstructed. Flash frequencies followed an established protocol25 beginning at 2 Hz, going up in even numbers until 20 Hz, then going down by odd numbers until 1 Hz, with alternating periods of 10-second stimulation, then 10 seconds without stimulation.
2.3 |. EEG data acquisition
Recordings were performed with a 128-electrode Ag/Ag-Cl high-density EEG Hydrocel Geodesic Sensor Net (Electrical Geodesics), a Net Amps 400 amplifier, and Netstation EEG acquisition software at a sampling rate of 1000 Hz. Electrode impedance levels were verified before recording to be at 50–100 kΩ using Signagel (Parker Laboratories) applied to each electrode. Throughout the drive, online EEG signals were displayed (Figure 1) using a subset of the 128 electrodes resembling a standard clinical montage (Figure 2A); this subset was also used for subsequent offline visual review.
FIGURE 2.
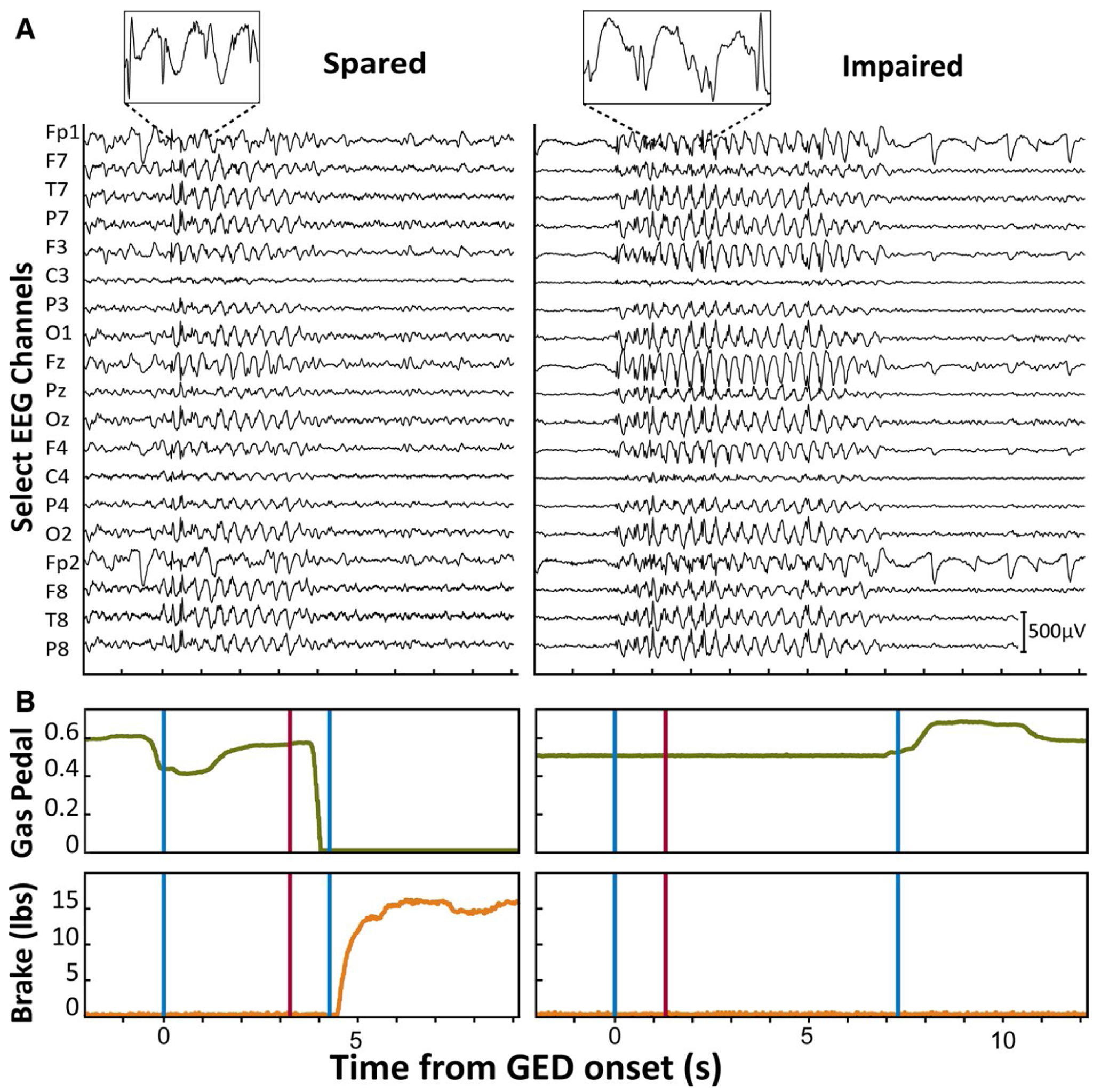
Examples of generalized epileptiform discharge (GEDs) with spared and impaired responses to stimuli while driving. A, Electroencephalographic (EEG) recordings for GEDs associated with spared and impaired response to red oval stimulus. Insets provide an expanded view of the 3-Hz spike-wave morphology of EEG. Selected EEG channels (of 128 recorded) along the vertical axis comprise the viewing montage used during real-time visual detection of GEDs, as well as subsequent offline review. Cz was used as reference. Scale bar is 500 μV. B, Gas pedal position (green traces) and brake pedal force (orange traces) on same time scale as corresponding GED with spared versus impaired responses to stimulus presentation (red vertical lines). GED onset and offset are indicated by vertical blue lines. In the GED with spared response to the stimulus (left traces), there is a prompt decrease in depression of the gas pedal (downward deflection of green trace) followed by increased brake force (upward deflection of orange trace). In contrast, in the GED with impaired response to the stimulus (right traces), there are no appropriate changes in gas pedal or brakes after the stimulus. Gas pedal units are the ratio of downward displacement distance divided by maximal pedal downward displacement. Brake pedal units are in pounds. All example traces shown here (A and B) are from Patient 3 (Table 1)
2.4 |. Offline GED marking and behavioral analysis
EEGs were read after the drive by consensus of two reviewers (E.C. and H.B.) in Cz reference (although quantitative data analysis was performed using common average reference), with GED offset and end times marked within 0.1 seconds. EEG and miniSim data were then exported and analyzed in MATLAB 2018b (MathWorks). Synchronization between EEG and driving behavioral data was established by the times of stimulus presentation recorded simultaneously on both systems (Figure 1, Event Detection Button). This enabled the timing of each stimulus relative to GED onset to be determined (Figure 3).
FIGURE 3.
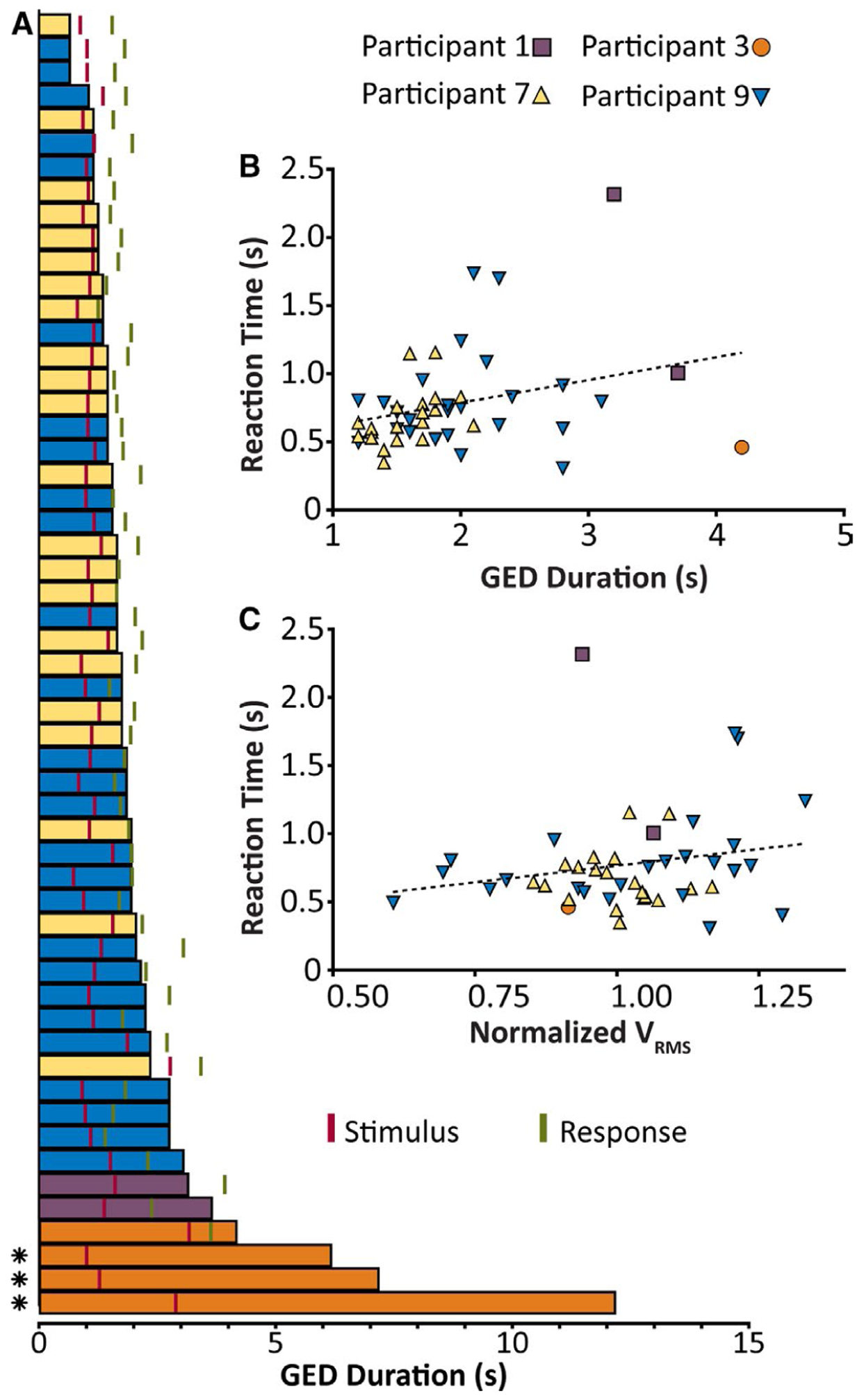
Responses to stimuli in relation to generalized epileptiform discharges (GEDs) while driving. A, GED durations for the four patients with behavioral data during GEDs. Stimuli (marked in red) and time of response (marked in green) are given relative to the onset of GEDs. All GEDs are aligned by onset time and are arranged in ascending order of duration. GED bars are colored by participant (see key). *GED with no response to the stimulus. B, Relationship between GED duration and the reaction time. The dotted line gives best fit by linear least squares regression. Pearson correlation coefficient r = .30, P = .04. C, Relationship between root mean square voltage (VRMS; normalized to mean within participant) for each GED and reaction time. The dotted line gives best fit by least squares regression. Pearson correlation coefficient r = .20, P = .17. For A, all GEDs with behavioral testing from Patients 1, 3, 7, and 9 are shown (Patient 4 lacked behavioral testing; see text). For B and C, only GEDs where the stimulus was presented during the episode (not afterward, see A for examples of this) and where a response occurred either during or after the episode are included
Response or lack of response to stimuli, and reaction times were established using data obtained from the miniSim system including gas pedal position (linear scale with 0 = no displacement and 1 = fully depressed downward), brake pedal force (pounds), and car velocity (miles per hour) following stimuli. We initially analyzed steering wheel movements as well; however, we found that steering movements were often substantially delayed after the appearance of the red oval and had highly variable reaction times in comparison to gas, brake, and velocity changes, which were more immediate and consistent. Therefore, we chose to use only gas, brake, and velocity reactions in our final analysis. The data from the gas pedal were much more variable than the other two parameters, so we first smoothed the 60-Hz recorded gas pedal signal with a four-sample nonoverlapping average followed by spline interpolation. The derivative of the gas pedal signal was then calculated to better discriminate onset of responses. Gas pedal, brake, and velocity signals were then all upsampled to 1000 Hz by linear interpolation to match the EEG sampling rate.
We used several criteria to establish whether a participant had responded to a stimulus and to measure reaction times. First, we required that within 30 seconds of the stimulus the participant slowed to less than a fifth of the average velocity obtained from the 0.5 seconds prior to the stimulus. We allowed a large poststimulus response window (30 seconds), because several participants had little or no driving experience (Table 1), and if they reacted only by taking their foot off the gas without braking, they would slow down very gradually. If this criterion was not met, we classified the stimulus as having no response. If the criterion was met, we required that at least one of the following two criteria was also met, based on brake force and gas pedal derivative. For brake force, we established the reaction time as the first consecutive 10 milliseconds where the brake force was greater than the 95th percentile of break force for the 5 seconds prior to stimulus presentation. For gas pedal, we defined the reaction time as the first consecutive 10 milliseconds where the derivative of gas pedal position was at least 7 SD below the mean gas pedal derivative for the 5 seconds prior to stimulus presentation. We arrived at these criteria after extensive review of the behavioral data following stimulus presentation during baseline periods and during periods where participants had slowed down independently of a stimulus presentation. The criteria were then chosen to avoid assigning responses to small changes in speed and force on the pedals, and only when a concerted attempt was made to slow down quickly. If both the criteria for reaction based on brake and gas pedals were met, we gave the reaction time as the shortest of these two times. In cases where these criteria suggested that no response had been made to the stimulus, we carefully analyzed the video of the participant (which included footage of their feet on the pedals) to assess whether our analysis incorrectly stated they had not responded. This was never the case with the criteria used here.
2.5 |. Analysis of EEG signals during GEDs
Electroencephalography signals were analyzed using MATLAB with EEGLAB (Swartz Center for Computational Neuroscience, University of California, San Diego) toolbox functions. Signals were downsampled to 500 Hz to improve processing time and high-pass filtered at 0.1 Hz to remove baseline drift, and 50/60-Hz line noise was removed by the CleanLine algorithm (notch filter at 60 Hz and its harmonics up to 240 Hz with a bandwidth of 2 Hz) in EEGLAB. A common average reference was applied by subtracting the average potential from each channel. To further reduce artifact, we excluded edge electrodes positioned off the scalp, and excluded any electrodes that exhibited mean fractional power > 5 SD from mean fractional power calculated across all electrodes and across the entire recording for a given participant.
To examine GED physiological severity, we first calculated the root mean square voltage (VRMS) for each electrode and took the average across all included electrodes to obtain a single value for each GED episode. When performing correlation analyses in Figure 3C, to account for intersubject amplitude differences, we calculated “normalized VRMS” by dividing the VRMS of each GED by the mean VRMS for all analyzed GEDs within each patient.
We also analyzed EEG amplitude in the frequency range of spikes and waves of spike-wave discharges as described previously.16 For each GED, the power spectral density for each electrode was acquired using Welch power spectral density estimate with a window length of 250 milliseconds. Power at each frequency was then divided by a segment of baseline data selected for each participant consisting of 30 seconds of quiet artifact-free EEG before the first GED to obtain fractional power. We then calculated mean fractional power across electrodes in the frequency range of waves (2.5–4 Hz) and spikes (10–125 Hz) for each GED. Results were visualized as topo-graphic maps of change in mean fractional power (Figure 4).
FIGURE 4.
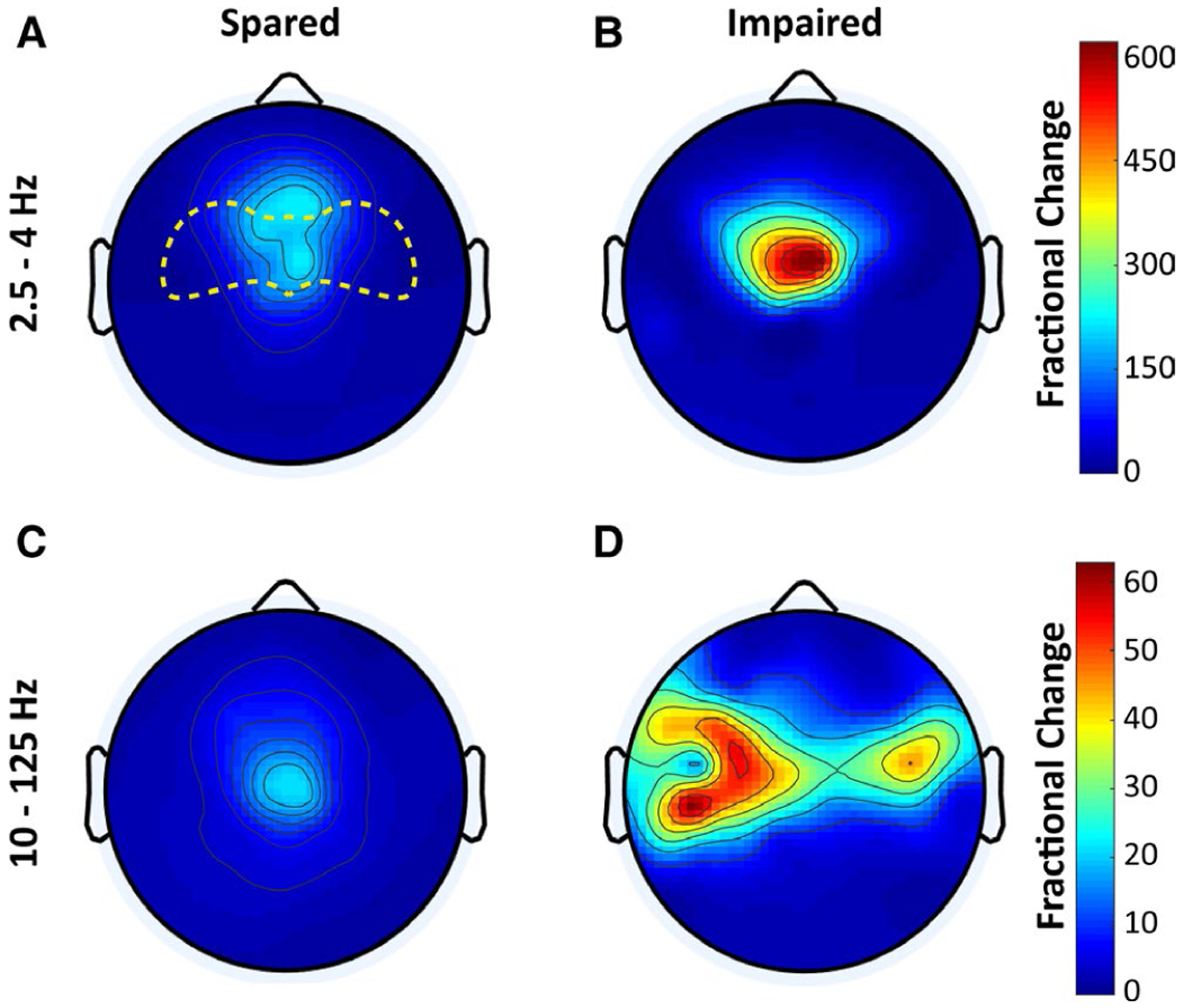
Greater electroencephalographic (EEG) spike amplitude in generalized epileptiform discharge (GED) with impaired responses. A, B, Head maps of 128-channel high-density EEG fractional power change in the 2.5- to 4-Hz frequency range (wave components of spike-wave discharges) for GEDs with spared (A) or impaired (B) response (no response) to stimuli. C, D, Maps of EEG fractional power change in the 10- to 125-Hz frequency range (spike components of spike-wave discharges) for GEDs with spared (C) and impaired (D) responses. Color scale bars represent EEG power during seizures divided by baseline power (fractional power). The top color bar is for A and B, and bottom bar is for C and D. The anterior head region in which fractional power change was compared statistically is outlined in yellow in A. EEG data are from the same patients and GED episodes as in Figure 3A
2.6 |. Statistical analysis
Statistical analysis was done using GraphPad Prism (v7.01) with two-tailed unpaired or paired t tests or analysis of variance (ANOVA) as appropriate for continuous variables, Fisher exact test for proportions, and Pearson correlation for relationships between two variables, all with significance threshold P < .05.
3 |. RESULTS
3.1 |. Study feasibility
Nine subjects (seven female; median age = 20 years) participated in driving simulation for 60 minutes each, and testing was well tolerated by all, without motion sickness or clinical seizures. We obtained a total of 88 GEDs in five of the nine subjects (Table 1). Most GEDs consisted of generalized spike-wave or poly-spike-wave discharges, and one subject had a rhythmic large-amplitude beta-frequency photoparoxysmal response. Of the 88 GEDs, 55 were detected on EEG in real time and tested for response to the red oval stimulus (Figure 1). Mean duration for the detected GED was 2.22 ± 1.78 seconds (mean ± SD), and the stimulus was presented with an average delay of 1.26 ± 0.47 seconds after GED onset, so in most cases the stimulus presentation was within the range of discharge duration (Figure 3A). Mean duration of GEDs that occurred during driving but were only found after post hoc EEG review was 3.11 ± 2.4 seconds, not significantly different in duration from the GEDs detected in real time (P = .09, two-tailed, two-sample t test).
3.2 |. Driving behavior during GEDs
Of the five subjects with GEDs, three responded appropriately to all stimuli during GEDs, one responded to only one of four stimuli (25%), and one stopped driving immediately during GEDs and therefore could not be tested with stimuli. In the three subjects with 100% response rates during GED (Patients 1, 7, and 9 in Table 1), the baseline response rate was very similar without GEDs (mean = 96%). Subject 3’s 25% response rate during GEDs was significantly lower than her 94% baseline response rate without GED (P < .01, Fisher exact test). Examples of EEG and driving behavior during GEDs with spared versus impaired responses to stimuli can be seen in Figure 2.
Durations of GEDs with no response to stimuli were significantly longer than those with spared responses (8.47 ± 3.10 seconds [mean ± SD] vs 1.85 ± 0.69 seconds; P < .001, two-sample, two-tailed t test; Figure 3A). We next examined reaction times to stimuli that elicited responses. Reaction time for stimuli presented at baseline without GEDs was 0.89 ± 1.03 seconds (mean ± SD). Overall mean reaction time for stimuli presented with GEDs was 0.76 ± 0.36 seconds, not significantly different from baseline (P = .09, two-tailed, two-sample t test). Reaction time was 0.67 ± 0.23 seconds when both the stimulus and response occurred during a GED (Figure 3A), 0.84 ± 0.43 seconds when stimulus was during a GED and response after the GED, and 0.64 ± 0.11 seconds when both stimulus and response were after a GED; none was significantly different from another (ANOVA with post hoc pairwise comparison). To evaluate for possible effects of fatigue on reaction times, we analyzed reaction time versus time from session onset and found no significant relationships for stimuli administered either at baseline or during GEDs (data not shown).
To further investigate the relationship between GED duration and severity of impairment, we correlated GED duration with reaction time (Figure 3B). This yielded a slight upward gradient, with significant positive correlation (r = .30; P = .04).
As was already noted, one patient’s videos (Patient 4, Table 1) showed that he stopped driving immediately upon GED onset, took his feet off the pedals, and put his head down, making comments such as “Oh, I think I’m supposed to be driving” or “This isn’t good.” Because these behaviors occurred prior to onset of the red oval, it was not possible to reliably evaluate his response to the stimuli. However, based on these abnormal behaviors, his driving during GEDs would be considered severely impaired.
3.3 |. Relating driving performance to GED amplitude
In addition to GED duration, prior work has suggested that GED amplitude may be related to severity of behavioral impairment.16,19 In agreement with this, we found that VRMS voltage was larger on average for GEDs with no response to stimuli than for GEDs with spared responses (66.14 ± 4.23 μV and 52.99 ± 9.01 μV, respectively, mean ± SD, P = .02, two-tailed, two-sample t test). To further examine the relationship between VRMS and behavior, we next correlated VRMS with reaction times for each GED where there was a response. We used normalized VRMS (see Materials and Methods) for this analysis, showing a modest positive relationship with reaction time, which, however, did not reach statistical significance (r = .20, P = .17; Figure 3C).
Prior work has shown differences in signal power in the frequency range of waves (2.5–4 Hz) and spikes (10–125 Hz) for behaviorally spared versus impaired GED spike-wave discharges.16 During driving, fractional power changes in the range of both waves and spikes were larger for GED with no response to stimuli versus spared GED (Figure 4). In the anterior head region in Figure 4 with largest changes for both waves and spikes (region extending approximately from Fz to Cz indicated in Figure 4A), mean fractional EEG power changes during GEDs for waves was 78.5 ± 64.9 for spared and 158.0 ± 151.4 for impaired (P = .04, paired, two-tailed t test across electrodes); mean fractional EEG power change during GED for spikes was 8.9 ± 6.1 for spared and 40.4 ± 18.4 for impaired (P < .001).
4 |. DISCUSSION
The primary aim of this study was to investigate the feasibility of using a realistic driving simulator to evaluate driving safety in patients with apparently asymptomatic generalized epileptiform discharges. A secondary aim was to begin to identify EEG features that may ultimately be used to classify GEDs as “safe” or “unsafe” for driving. We found that GEDs could be obtained relatively easily during simulated driving, and that subjects tolerated the procedures well and could be tested reliably with obstacles presented during GEDs. In the relatively small sample used for this pilot study, we also confirmed previous work16,19 suggesting that GED duration and EEG amplitude are larger on average for episodes that cause behavioral impairment. These findings support further work that should be done to more thoroughly investigate EEG features of GEDs predictive of unsafe driving. Although use of a specialized driving simulator or similar behavioral testing of driving safety may be more direct, ideally if a reliable test based on EEG alone could be developed, it would be more widely available to clinicians.
The approach described here may help solve an important dilemma confronting people with generalized epilepsy who have apparently stopped having clinical seizures either due to outgrowing them or due to effective medications, but where the EEG still shows frequent GEDs.13 The uncertain risk of motor vehicle collisions among people with epilepsy merits restrictions of driving privileges to protect them and the public. However, the inconsistent nature of these restrictions nationally and internationally reflects a need to establish objective criteria for distinguishing between asymptomatic epileptiform activity and potentially driving-impairing discharges in patients, particularly in patients who experience no clinical symptoms of seizures. Previous investigations attempted to characterize the effects of these subclinical discharges. One study done >30 years ago recorded EEG during actual motorway driving, which of course is the most realistic test possible; however, this approach has not been generally practical.23 More recent studies have used driving games to investigate crash rates and reaction times in response to road obstacles.20,21 A reasonable compromise between these approaches may be highly realistic immersive driving simulators as used in the present study and in some other recent investigations.26
Previous work suggests that reaction times during interictal epileptiform activity are significantly prolonged when compared to baseline.20,21 Our testing during GEDs in a driving simulator did not show significant differences in overall reaction times compared to baseline, although we did find that reaction times were positively correlated with GED duration. It is possible that with a larger sample size, including more GEDs with longer durations, we would also replicate prior work showing overall prolonged reaction times during GEDs. It is also possible that there are significant differences in reaction times at baseline versus that of drivers without epilepsy. These relationships have not yet been investigated and represent a potential future direction to evaluate baseline deficits in epilepsy using our novel criteria and paradigm. Future work is also needed to more fully characterize the behavior during GEDs using automatic real-time detection algorithms, which might succeed in attaining faster and more consistent rates of stimulus presentation than we achieved with human visual review. Another factor that could be investigated with a larger sample size is potential effects of antiseizure medications, which are known to influence epileptiform discharges and might also affect behavior.20,27
In conclusion, a serious challenge arises when patients with GEDs on EEG but no clinical seizures request driving privileges. At present, clinicians often decide on driving privileges based on how often GEDs occur or how long they last, but there are no empirically validated guidelines or objective criteria for distinguishing “safe” versus “unsafe” GEDs for driving. More studies are needed, especially given reports that GEDs as brief as 1 second or less can sometimes interfere with behavior, including episodes of complete behavioral arrest.3,18,28 Previous studies of driving during subclinical epileptiform activity including GEDs have provided evidence of impaired driving in some cases, including virtual crashes.20‒23,29 Thus, driving safety remains an open question for patients with GEDs on EEG but no clinically obvious seizures. The present study demonstrates the feasibility of studying this question with a realistic high-fidelity driving simulator, including analysis of EEG features that may ultimately be predictive of driving risk. Further work is needed to determine whether driving simulation is necessary in every patient to determine driving risk, or whether simpler testing or EEG analysis alone may be sufficient. A recent study that analyzed 20 adolescent and 21 adult epilepsy patients with reaction tests of various complexity sheds further light on the relationship between behavioral impairment and EEG features of epileptiform discharges (H.E. Krestel, D.D. Schreier, E. Sakiri, A. von Allmen, C. Bernasconi, M. Steinlin, A. Nirkko, and J. Mathis, in review, 2019). With a large sample of EEG and behavioral data, it may be possible in the future to develop a machine-learning–based classifier that could predict driving safety based on EEG recordings in individual patients. We hope that future work will enable more objective guidelines to be developed that will better inform clinicians and people with GEDs to improve safe decision-making regarding driving.
Key Points.
Driving safety in generalized epileptiform discharges can feasibly be evaluated in a realistic high-fidelity driving simulator
Generalized epileptiform discharges resulting in impaired driving have longer duration and greater EEG signal amplitude
Future studies with this approach and a large data-set could lead to a classifier to predict driving safety based on EEG features
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health R01 NS055829, the Mark Loughridge and Michele Williams Foundation, and the Betsy and Jonathan Blattmachr family. H.K. has received funding from the European Union’s Framework Program for Research and Innovation Horizon 2020 (2014-2020) under the Marie Sklodowska-Curie Grant Agreement No. 799791. We thank Andrew L. Veit and Shawn F. Allen of the National Advanced Driving Simulator at the University of Iowa, Iowa City, Iowa for their technical support in this study; thank the patients who participated in this study; and the following referring neurologists: Kamil Detyniecki, MD, Pue Farooque, DO, Aline Herlopian, MD, Susan Levy, MD, Richard Mattson, MD, Zubeda Sheikh, MD, and Benjamin Tolchin, MD.
Funding information
National Institutes of Health, Grant/Award Number: R01 NS055829; Mark Loughridge and Michele Williams Foundation
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gil-Nagel A, Abou-Khalil B. Electroencephalography and video-electroencephalography. Handb Clin Neurol. 2012;107:323–45. [DOI] [PubMed] [Google Scholar]
- 3.Berman R, Negishi M, Vestal M, et al. Simultaneous EEG, fMRI, and behavioral testing in typical childhood absence seizures. Epilepsia. 2010;51(10):2011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46:21–33. [DOI] [PubMed] [Google Scholar]
- 5.Avoli M, Biagini G. Thalamocortical synchronization and absence epilepsy In: Schwartzkroin P, editor. Encyclopedia of Basic Epilepsy Research. Cambridge, MA: Academic Press, 2009; p. 28–36. [Google Scholar]
- 6.Bai X, Vestal M, Berman R, et al. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci. 2010;30:5884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilliam F, Kuzniecky R, Faught E, Black L, Carpenter G, Schrodt R. Patient-validated content of epilepsy-specific quality-of-life measurement. Epilepsia. 1997;38:233–6. [DOI] [PubMed] [Google Scholar]
- 9.Sheth SG, Krauss G, Krumholz A, Li G. Mortality in epilepsy: driving fatalities vs other causes of death in patients with epilepsy. Neurology. 2004;63:1002–7. [DOI] [PubMed] [Google Scholar]
- 10.Naik PA, Fleming ME, Bhatia P, Harden CL. Do drivers with epilepsy have higher rates of motor vehicle accidents than those without epilepsy? Epilepsy Behav. 2015;47:111–4. [DOI] [PubMed] [Google Scholar]
- 11.Chen WC, Chen EY, Gebre RZ, et al. Epilepsy and driving: potential impact of transient impaired consciousness. Epilepsy Behav. 2014;30:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansotia P, Broste SK. The effect of epilepsy or diabetes mellitus on the risk of automobile accidents. N Engl J Med. 1991;324:22–6. [DOI] [PubMed] [Google Scholar]
- 13.Antwi P, Atac E, Ryu JH, et al. Driving status of patients with generalized spike-wave on EEG but no clinical seizures. Epilepsy Behav. 2019;92:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Punia V, Farooque P, Chen W, et al. Epileptic auras and their role in driving safety in people with epilepsy. Epilepsia. 2015;56:e182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldenkamp AP, Arends J. Effects of epileptiform EEG discharges on cognitive function: is the concept of “transient cognitive impairment” still valid? Epilepsy Behav. 2004;5:25–34. [DOI] [PubMed] [Google Scholar]
- 16.Guo JN, Kim R, Chen Y, et al. Impaired consciousness in patients with absence seizures investigated by functional MRI, EEG, and behavioural measures: a cross-sectional study. Lancet Neurol. 2016;15:1336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengoku A, Kanazawa O, Kawai I, Yamaguchi T. Visual cognitive disturbance during spike-wave discharges. Epilepsia. 1990;31:47–50. [DOI] [PubMed] [Google Scholar]
- 18.Mirsky AF, Van Buren JM. On the nature of the “absence” in centrencephalic epilepsy: a study of some behavioral, electroencephalographic, and autonomic factors. Electroencephalogr Clin Neurophysiol. 1965;18:334–48. [DOI] [PubMed] [Google Scholar]
- 19.Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res. 2005;150:271–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nirkko AC, Bernasconi C, von Allmen A, Liechti C, Mathis J, Krestel H. Virtual car accidents of epilepsy patients, interictal epileptic activity, and medication. Epilepsia. 2016;57:832–40. [DOI] [PubMed] [Google Scholar]
- 21.Krestel HE, Nirkko A, von Allmen A, et al. Spike-triggered reaction-time EEG as a possible assessment tool for driving ability. Epilepsia. 2011;52:e126–9. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Morland TB, Schmits K, et al. A prospective study of loss of consciousness in epilepsy using virtual reality driving simulation and other video games. Epilepsy Behav. 2010;18:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasteleijn-Nolst Trenité DG, Riemersma JB, Binnie CD, Smit AM, Meinardi H. The influence of subclinical epileptiform EEG discharges on driving behaviour. Electroencephalogr Clin Neurophysiol. 1987;67:167–70. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG. Simulator sickness questionnaire: an enhanced method for quantifying simulator sickness. Int J Aviat Psychol. 1993;3:203–20. [Google Scholar]
- 25.Kasteleijn-Nolst Trenité DG, Rubboli G, Hirsch E, et al. Methodology of photic stimulation revisited: updated European algorithm for visual stimulation in the EEG laboratory. Epilepsia. 2012;53:16–24. [DOI] [PubMed] [Google Scholar]
- 26.Crizzle AM, Classen S, Winter SM, Silver W, LaFranca C, Eisenschenk S. Associations between clinical tests and simulated driving performance in persons with epilepsy. Epilepsy Behav. 2012;23:241–6. [DOI] [PubMed] [Google Scholar]
- 27.Guida M, Iudice A, Bonanni E, Giorgi FS. Effects of antiepileptic drugs on interictal epileptiform discharges in focal epilepsies: an update on current evidence. Expert Rev Neurother. 2015;15:947–59. [DOI] [PubMed] [Google Scholar]
- 28.Tizard B, Margerison JH. Psychological functions during wave-spike discharges. Br J Soc Clin Psychol. 1963;3:6–15. [Google Scholar]
- 29.Kasteleijn-Nolst Trenite DG, Vermeiren R. The impact of subclinical epileptiform discharges on complex tasks and cognition: relevance for aircrew and air traffic controllers. Epilepsy Behav. 2005;6:31–4. [DOI] [PubMed] [Google Scholar]


