Abstract
Objective
Nonalcoholic fatty liver disease (NAFLD) is associated with insulin resistance (IR) and visceral adiposity in adults and boys, but girls with NAFLD are understudied. We sought to evaluate adipose, liver, and skeletal muscle insulin sensitivity in obese adolescent females with or without hepatic steatosis (HS) (intrahepatic triglyceride (IHTG) content >5.5%) along with cardiometabolic components typically associated with IR.
Study design
73 obese adolescent girls at high risk for NAFLD were enrolled. Participants underwent fasting labs, an MRI to measure IHTG and visceral fat, 31phosphorous MR spectroscopy for muscle mitochondrial function, 1H MR spectroscopy for intramyocellular lipid (IMCL), bicycle ergometry to assess VO2peak and a 4-phase hyperinsulinemic euglycemic clamp with isotope tracers to measure hepatic and peripheral IR. 29 participants had HS [age 15 yrs(13,16), BMI%ile 98.7(97.4,99.0), IHTG 10.4%(8.0,13.5)] and 44 did not [age 15 yrs(13,17), BMI%ile 98.5(96.2,99.0), IHTG 2.0%(1.1,3.0)].
Results
During hyperinsulinemia, participants with HS vs. non-HS had failure to suppress free fatty acids (p = 0.008), endogenous glucose release (p = 0.002), and a lower glucose metabolic rate of disappearance (Rd) (p = 0.012). Girls with NALFD also had higher visceral fat (p < 0.001), systolic blood pressure (p = 0.026), triglycerides (p = 0.02), ALT (p < 0.01) and white blood cell count (p < 0.01), and lower adiponectin (p = 0.02). There was no difference between girls with and without HS in systemic glycerol turnover measured with glycerol release, or in IMCL, mitochondrial function or VO2peak.
Conclusions
Obese adolescent girls with HS have evidence of multi-tissue IR, visceral adiposity, inflammation and multiple components of the metabolic syndrome, arguing for close cardiometabolic surveillance over time of girls with HS.
Keywords: Adolescent, NAFLD, Girls, Insulin resistance
Highlights
-
•
We described tissue specific insulin sensitivity in adolescent girls ± NAFLD.
-
•
Girls with NAFLD have higher hepatic and muscular insulin resistance.
-
•
Intramyocellular lipids and muscle mitochondrial function were not different between groups.
-
•
Adipose tissue insulin resistance was not different between groups.
-
•
Girls with NAFLD have worst metabolic profile than those without.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) embodies a spectrum of disease ranging from uncomplicated hepatic steatosis, hepatic steatosis with inflammation (NASH) to cirrhosis. In addition to liver complications, NAFLD is associated with an increased risk of developing type 2 diabetes (T2D), hypertension, and coronary heart disease [1,32]. In adults, NAFLD is associated with increased visceral fat and insulin resistance (IR) in adipose, hepatic and/or peripheral tissues [32,36]. Hepatic steatosis (HS), defined as an intrahepatic triglyceride (IHTG) content >5.5%, is the first manifestation of NAFLD and understanding its pathophysiology may inform future targets for prevention or treatment of NAFLD and associated cardiometabolic disease.
Studies describing the pathophysiology of NAFLD have primarily been done in adults or in boys [9,35,50,51,58]. Youth may demonstrate unique physiologic changes related to the development of NAFLD that differ from adults, due to factors such as growth, puberty and differences in sex steroid concentrations [34,41,55]. Further, intrahepatic lipid deposition patterns are different in youth, and youth may present with higher fibrosis severity and NASH incidence, indicating that the pathologic process is not identical, arguing for separate studies in youth [29,56]. Weight loss both decreases IHTG and improves insulin sensitivity as assessed by hyperinsulinemic euglycemic clamp studies, suggesting IHTG and insulin sensitivity are closely linked [55]. Previous studies found that youth with HS have increased rates of lipolysis, using a palmitate tracer during fasting and hyperinsulinemia, compared to youth without HS [20]. Obese adolescents with NALFD are more IR in the skeletal muscle and liver than obese and lean controls [8,16,17,49]. NAFLD in youth presents more commonly in boys than in girls, with highest rates reported in Hispanic boys [56]. However, NAFLD is a strong risk factor for progression to T2D [16] and T2D risk is uniquely high in teen, suggesting that there may be sex differences in NAFLD pathophysiology, yet most of the studies to-date in youth have included no or small samples of included females [45].
Due to previous under-sampling of females with NAFLD, despite the possibly higher risk for related T2D, it has been unclear if girls with HS have similar metabolic defects and risk factors as their male counterparts. Our group previously demonstrated that adolescent girls with polycystic ovarian syndrome (PCOS) and elevated testosterone concentrations have increased IHTG regardless of obesity status, indicating that sex steroids may play an important role [11,15]. The primary purpose of this study was to evaluate adipose, liver, and skeletal muscle insulin sensitivity and related cardiometabolic processes in obese adolescent females with HS, compared to a cohort of similarly obese adolescent girls without HS. Secondary outcomes included core measures typically thought to be associated with IR, including components of the metabolic syndrome as well as intramyocellular lipid (IMCL), muscle mitochondrial function and exercise capacity. Finally, early NAFLD is hard to detect and understudied in females, and we therefore sought to determine if common clinical assessments could predict the presence of HS.
2. Material and methods
2.1. Study design
Seventy-three obese female adolescent participants were enrolled in either the RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) study which enrolled obese girls with and without T2D or the Androgens and Insulin Resistance Study (AIRS) which enrolled obese girls with and without PCOS using a similar study design [6,11,14]. Obese controls were defined as obese girls without T2D or PCOS. Inclusion criteria for all participants included female sex, age 12–19 years, obesity (BMI >95th percentile) and physically inactive status. Inactive status was defined as <3 h of regular exercise/week (less than half that recommended for youth), as validated with a measure of metabolic equivalents (METS) from a 3-day pediatric activity recall questionnaire and a 7-days of accelerometer wear (Actigraph GT3x, Actigraph Corp, Pensacola, FL) [25]. Exclusion criteria included resting blood pressure >140/90 mmHg, hemoglobin <9 mg/dL, diabetes other than T2D, type 1 diabetes-associated auto antibodies, smoking, antihypertensive medications, medications impacting insulin sensitivity (oral steroids, atypical antipsychotics and metformin unless the participant had T2D), statins, pregnancy, breastfeeding, and any recent illnesses. This was a secondary analysis from the two studies and all girls who had laboratory studies and measures of liver fat were included in the analysis. The University of Colorado Institutional Review Board approved both studies. Parental consent and participant assent was obtained from all participants less than 18 years of age and participant consent was obtained for those over age 18 years.
2.2. Metabolic studies and imaging
Participants underwent 2 visits: 1) a screening visit which included a complete medical history, physical examination, fasting laboratory testing and a 75-g oral glucose tolerance test (OGTT) as previously described [6,11,14] and 2) a two-day visit which included MRI, MR spectroscopy (MRS), exercise testing, an inpatient overnight fast and a 4-stage hyperinsulinemic euglycemic clamp. Participants with T2D treated with metformin (N = 15) had their metformin withdrawn for 72 h prior to study procedures. For 3 days prior to data collection, participants had restricted physical activity and consumed a Clinical Translational Research Center (CTRC)-prepared fixed-macronutrient, weight-maintenance diet (55% carbohydrates, 30% fat, 15% protein) based upon their weight and activity-determined caloric needs. Studies were performed in the follicular phase of the cycle as possible. Anthropomorphics were determined by standard measures, including waist and hip circumference and a pubertal exam performed by a pediatric endocrinologist (MCG, KJN). A food frequency questionnaire to determine customary macronutrient pattern was completed [37]. A graded cycle ergometer (Lode, Groningen, Netherlands) protocol to exhaustion was used to determine VO2peak, as previously described [42,43]. All participants achieved a peak respiratory exchange ratio of >1.1. Fat mass, fat free mass and body composition were determined by DEXA (Hologic, Marlborough, MA) as performed by standard methods [2]. All MRI and MRS measures were performed on a General Electric (GE) 3T. Hepatic fat was quantified with MRI using a modification of the Dixon method involving a multi-breath-hold double gradient echo T1-weighted sequence, as previously described [12,13,24,43]. Visceral fat was also assessed by MRI as previously described [42,43]. IMCL in the soleus muscle was measured with 1H magnetic MRS as previously described with the lipid peak normalized to creatine in only the ReSISTANCE cohort [13,43]. 31P-MRS muscle mitochondrial data before, during and after isometric calf exercise at 70% of maximal effort were also obtained and analyzed as previously described [[12], [13], [14]].
Participants were then admitted to the inpatient CTRC for an isocaloric evening meal, followed by 12 h of overnight, monitored fasting. Participants with T2D received intravenous insulin during this fast to maintain euglycemia near a target of 100–110 mg/dL. The next morning participants underwent a 4-phase hyperinsulinemic euglycemic clamp paired with a constant infusion of 6,6-2H2 glucose and 2H5 glycerol as previously described [42,43] to determine adipose, hepatic and muscle IR [32,38]. Insulin doses for each phase were 10, 16, and 80mU/m2/min [19,42,43]. Twenty percent dextrose “spiked” with [6,6-2H2] glucose was infused at a variable rate to maintain blood glucose drawn from a heated, arterialized hand vein at 95 mg/dL, as measured every 5 min with a Yellow Springs Instruments (YSI instruments, Yellows Springs, OH). Serum measurements including glucose, FFA, glycerol and insulin, in addition to glucose and glycerol tracer enrichments, were drawn every 10 min during the last 30 min of each of the four clamp phases and the steady-state glucose infusion rate (GIR) was determined from the last 30 min of each phase [3,11]. Indirect calorimetry was performed during the steady-state of each phase using a Vmax Encore metabolic cart system (Carefusion Corp, San Diego, CA) with a hood attachment.
2.3. Laboratory procedures and assays
Serum assays for glucose, insulin, C-peptide, sex steroids, transaminases, HbA1c, high sensitivity C-reactive protein, white blood cell count and lipid panel were performed as previously described [13,24,43]. FFA were measured per enzymatic assay (WaKo Chemicals, USA) and adiponectin and leptin via radioimmunoassay (Millipore Sigma, USA).
Gas chromatography mass spectrometry was performed for the analysis of 2H5glycerol and 6,6-2H2 glucose [3,26]. All isotopic enrichments were corrected for background enrichments as previously described [11,15]. The endogenous glucose production (EGP) during each period were calculated using Steele non-steady-state equations [53,57], modified for stable isotopes. We accounted for the “spiked” glucose in the 20% dextrose infusion as described by Finegood [23]. Glucose and glycerol rate of appearance (Ra) per phase and metabolic clearance rate (MCR) of glucose were calculated as previously described [11]. MCR of glycerol was calculated as glycerol Rd/[glycerol] at each phase (where glycerol Ra = glycerol Rd, because in steady-state).The glycerol Ra at the 10 mU/m2/min phase was used to define adipose IR, EGP at the 16 mU/m2/min phase used to define hepatic IR, and the 80 mU/m2/min phase glucose Rd used to define peripheral IR. IC50 was not calculated as individuals with diabetes received insulin for glucose control overnight and thus do not have a true basal period. Percent suppression was calculated between the maximal Ra value and the Ra during the 80 phase.
2.4. MRS analysis
Phosphorus peak positions and areas of interest were determined by time-domain fitting using jMRUi utilizing AMARES (A Method of Accurate, Robust and Efficient Spectral fitting) [54], a nonlinear least-square-fitting algorithm. All exercise spectra were corrected for saturation using the fully relaxed spectra for that day. The concentration of ATP was calculated as previously described [44], and the rate of oxidative phosphorylation was calculated as ΔPCr/time from the first 10 s following cessation of exercise. Initial PCr synthesis (VPcr) and apparent maximum rate of oxidative ATP synthesis (Qmax) was calculated with the initial rate of PCr resynthesis relative to end-exercise ADP-adenosine diphosphate concentrations using an assumed Km = 30 μM. Time constants for PCr and ADP were calculated via regression analyses with Sigmaplot (Systat Software, Inc, San Jose, CA).
2.5. Statistical analysis
The distributions of all variables were examined and results are presented as mean ± standard error, median (minimum, maximum), or proportions, as appropriate. Group comparisons were made using chi-square or Fisher's exact test for proportions and the t-test or Kruskal-Wallis test for continuous variables. P-values < 0.05 were considered statistically significant.
Instead of IC50 Ra, that cannot be calculated in diabetic population with insulin infusion during basal period, repeated measures mixed-effects models were used to compare the trajectories of EGP and glycerol Ra across the phases of the clamp in the girls with NAFLD compared to the girls without NAFLD, controlling for the insulin concentration at each phase [46]. To describe changes in Ra across the different insulin concentrations of each phase, the intercept and slope of the regression line for each individual's data were also used to calculate the predicted Ra at the average insulin concentration for all participants during the 10 mU/m2/min phase for glycerol Ra (insulin 50 μU/mL) and the 16 mU/m2/min phase for EGP (insulin 68 μU/mL). As that relationship was not linear, data were log transformed. Negative EGP values in the obese group during the 80 mU/m2/min phase could not be log transformed, and thus the most negative EGP value was scaled to 0, and EGP values adjusted accordingly. Data were then reverse transformed for data presentation of the predicted Ra.
Multiple logistic regression was used to examine the association of covariates with the IHTG. Univariate Spearman analysis was performed first to identify variables to be included in the multivariate models. All models were adjusted for race/ethnicity due to the known ethnic differences in NAFLD (categorized as Caucasian/Asian, Hispanic/American Indian, and Black due to small numbers in some groups) and disease group (T2D, PCOS, control). Participants who were mixed race/ethnicity were categorized by the non-Caucasian race/ethnicity. Several models were constructed: 1) A “clinical” model which contained the ratio of waist:hip circumference, triglyceride:HDL ratio, adiponectin, and ALT; 2) a “research” model which contained visceral fat, EGP, FFA (phase 3 of the hyperinsulinemic euglycemic clamp), GIR (with and without VO2peak per lean kg of body weight), and oxidative phosphorylation; 3) the full model, which contained all covariates above (with and without VO2peak per kg lean mass). Firth's penalized likelihood approach was used for the research model and full model. The likelihood ratio test was used to compare each of the models to a simpler model with only ethnicity and disease as covariates, in order to test whether the more complicated model explained a significantly greater proportion of the variability in the outcome.
All statistical analyses were performed with SAS Software, Version 9.4 (Cary, NC).
3. Results
Twenty nine obese girls with NAFLD and 44 equally obese girls without NAFLD were enrolled. Baseline characteristics and body composition are described in Table 1. By design, the NAFLD group had a significantly higher IHTG content than the non-NAFLD group. The groups also had similar ages, pubertal stage, degree of habitual physical activity, and BMI. Equal percentages of participants with T2D had NAFLD, regardless of metformin treatment status (33% with NAFLD were metformin-naïve vs. 35% with NAFLD were metformin-treated). There was a higher percentage of girls with PCOS in the NAFLD group, consistent with our observation of increased risk for NAFLD in PCOS [11]. However, the free androgen index was not significantly different between the groups when adjusted for PCOS status. Girls with NAFLD had significantly higher systolic blood pressures, although on average they were still within the normal range (p = 0.026). All markers of central obesity were higher in girls with NAFLD with waist/hip ratio (p = 0.005) and MRI-based visceral fat (p < 0.0001) reaching statistical significance. Of note, there was no significant difference in abdominal subcutaneous fat, total body fat or IMCL between groups. Groups also had a similar dietary intake of both micro and macronutrients and physical activity (data not shown) and had no difference in peak exercise capacity (Table 1).
Table 1.
Participant descriptors.
| Non-NAFLD, N = 44 | NAFLD, N = 29 | |
|---|---|---|
| Age (years) | 15 (13,17) | 15 (13,16) |
| Disease state: n (%) | ||
| Type 2 Diabetes | 11 (25) | 6 (21) |
| Polycystic Ovarian Syndrome | 15 (35) | 19 (65) |
| Obese Control (no T2D or PCOS) | 18 (41) | 4 (14) |
| Race/Ethnicity: n (%) | ||
| Non-Hispanic White | 13 (30) | 8 (27) |
| Non-Hispanic Black | 12 (27) | 1 (3) |
| Hispanic | 17 (38) | 20 (70) |
| Other | 2 (5) | 0 (0) |
| Metformin Exposure in T2D n (%) | 9 (20) | 6 (21) |
| Pubertal Tanner Stage | 5 (5,5) | 5 (5,5) |
| Systolic Blood Pressure (mmHg) | 116 ± 1.43 | 121 ± 1.62 * |
| Diastolic Blood Pressure (mmHg) | 68 ± 1 | 73 ± 2 |
| Exercise Capacity (VO2 peak, ml/kg lean mass/min) | 38.5 (34.2, 42.8) | 38.3 (35.9, 39.5) |
| Body Mass Index Percentile | 98.5 (96.4, 99.0) | 98.7 (97.6 99.0) |
| Percent Body Fat Mass from DEXA | 41.9 (39.8, 45.2) | 42.7 (40.2, 46.0) |
| Percent Lean Mass from DEXA | 54.6 (52.1, 57.7) | 53.5 (51.4, 56.7) |
| Waist (cm) | 98.9 (90.5, 110.6) | 105 (99.3, 109.0) |
| Waist/Hip Ratio | 0.89 ± 0.01 | 0.93 ± 0.01 ** |
| IHTG Content (%) from MRI | 2.04 (1.11, 2.94) | 10.4 (8.18, 12.79) ** |
| Subcutaneous Fat (cm3) from MRI | 445 ± 20 | 449 ± 19.0 |
| Visceral Fat (cm3) from MRI | 58 ± 4 | 99 ± 4.82 ** |
| IMCL from 1H-MRS (arbitrary units) | 12.4 (7.7, 21.1) | 13.1 (11.2, 27.1) |
Data presented as mean ± standard error or median (25th,75th percentile). All data are adjusted for PCOS or T2D status and ethnicity. DXA = Dual x-ray absorptiometry, IHTG = intrahepatic triglyceride, IMCL = intramyocellular triglyceride. MRI = Magnetic Resonance Imaging. MRS = magnetic resonance spectroscopy. P-value * = 0.05–0.01, **<0.01–0.001.
Fasting serum laboratory measurements are shown in Table 2. C-peptide (p = 0.04), fasting insulin (p = 0.02) and triglycerides (p = 0.02) were significantly increased in NAFLD girls. ALT (p < 0.01) and WBC (p < 0.01) concentrations were increased in NAFLD, and adiponectin concentrations (p = 0.02) were decreased. There was no difference in the C-reactive protein concentrations.
Table 2.
Participant laboratory measures.
| Non-NAFLD, N = 44 | NAFLD, N = 29 | |
|---|---|---|
| Fasting Glucose (mg/dL) | 83 ± 0.84 | 85 ± 7.3 |
| Fasting Insulin (μU/ml) | 18 (15, 26) | 28 (16, 40) * |
| 2 h OGTT glucose (mg/dL) a | 112 ± 3.3 | 147 ± 5.8** |
| 2 h OGTT insulin (μU/ml) a | 116 (65, 184) | 333 (163, 502)** |
| Hemoglobin A1c (%) | 5.3 (5.2, 5.6) | 5.5 (5.2, 5.9) |
| C-peptide (ng/ml) | 2.7 (1.8, 3.3) | 3.7 (3.1, 4.2)* |
| Aspartate aminotransferase (U/L) | 30 (24, 39) | 36 (31, 45) |
| Alanine aminotransferase (U/L) | 28 (19, 34) | 41 (35, 56)** |
| Hemoglobin (g/dL) | 13.6 ± 0.2 | 14.4 ± 0.1* |
| Triglycerides (mg/dL) | 96 (71, 143) | 162 (111, 209)** |
| High density lipoprotein (mg/dL) | 38 (32, 43) | 34 (32, 38) |
| Low density lipoprotein (mg/dL) | 89 (66, 124) | 97 (81, 129) |
| Sex hormone binding globulin (nmol/L) | 22.0 (13.0, 31.1) | 15.2 (12.1, 22.0) |
| Free Androgen Index | 4.0 (3.4, 9.8) | 8.8 (4.9,15.8) |
| Estradiol (pg/mL) | 43 (28, 55) | 36.5 (30, 44) |
| Progesterone (ng/mL) | 0.9 (0.6, 1.2) | 0.8 (0.6, 1.0) |
| Platelets (k/uL) | 275 (242, 300) | 279 (237, 312) |
| C-reactive protein (mg/L) | 1.6 (0.9, 4.0) | 2.6 (1.5, 4.4) |
| White blood cells (k/μL) | 7.0 (5.6, 8.0) | 8.4 (6.9, 9.6)** |
| Adiponectin (mg/dL) | 6.5 (3.9, 8.5) | 4.5 (3.4, 6.5)* |
Data presented as mean ± standard error or median (25th,75th percentile). All data are adjusted for PCOS or T2D status and ethnicity. P-value* = 0.05–0.01, **<0.01–0.001.
OGTT not performed in patients with T2D.
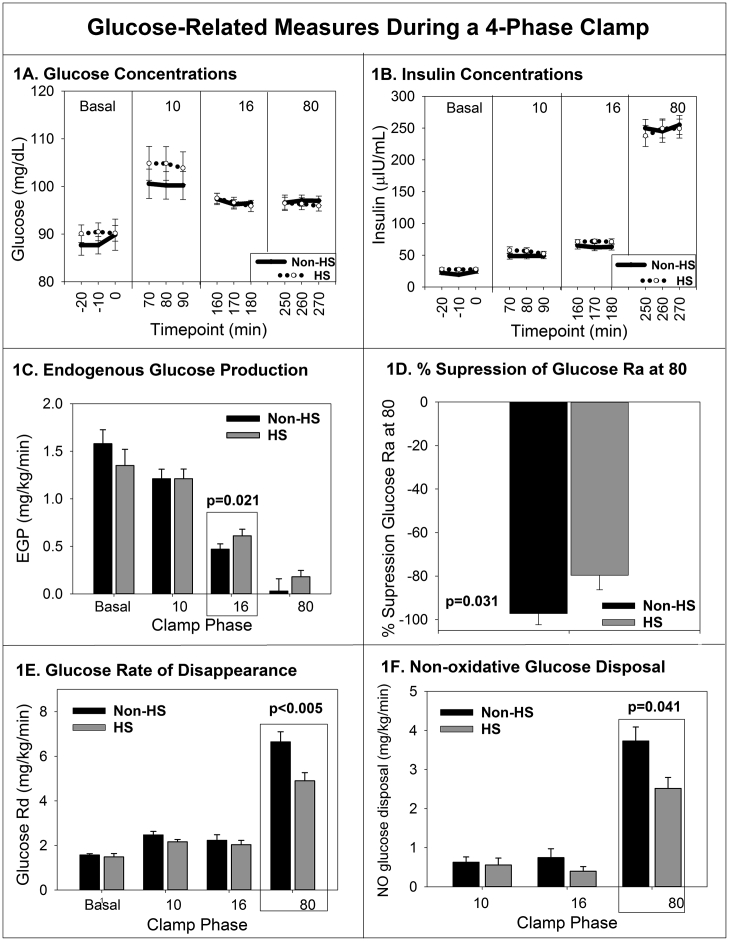
Measurements throughout the hyperinsulinemic euglycemic clamp are shown in Fig. 1. By design, there was no difference in the blood glucose concentrations between the groups in any phase (p = 0.45) (Fig. 1A). The insulin concentration increased similarly in both groups with the administration of increasing doses of insulin, also by design (p = 0.65) (Fig. 1B). The EGP (Fig. 1C) decreased with increases in the insulin dose administered in both groups as expected. In the girls with NAFLD, EGP decreased to the same degree as the girls without NAFLD in the first phase of the clamp (10 mU/m2/min), but EGP decreased less in the hepatic phase (16 mU/m2/min) in girls with NAFLD (p = 0.02), indicating hepatic IR. The percent suppression of EGP was 80 ± 7 in HS, compared to 97 ± 5% in Non-HS, P = 0.031. Girls with NAFLD had a lower glucose Rd during the final phase (80 mU/m2/min) of the clamp, (p < 0.01) (Fig. 1E) than girls without NAFLD indicating peripheral IR. Non-oxidative glucose disposal was significantly lower in HS during the 80 phase (Fig. 1F). Adjustment for race/ethnicity and disease state did not change any of the above results. When the relationship between EGP and insulin concentration were modeled across the curves, the EGP response to insulin was significantly less in those with NAFLD, again indicating hepatic IR (p < 0.01).
Fig. 1.
Glucose (A) and insulin (b) concentrations from each phase of the clamp, per group are shown. Mean endogenous glucose production (c) rate of glucose disappearance (e) and non-oxidative glucose disposal (f) per phase and group are shown. The change in percent suppression (d) from highest EGP to the lowest is shown. Significant P values are shown. Data are mean and standard error of the mean.
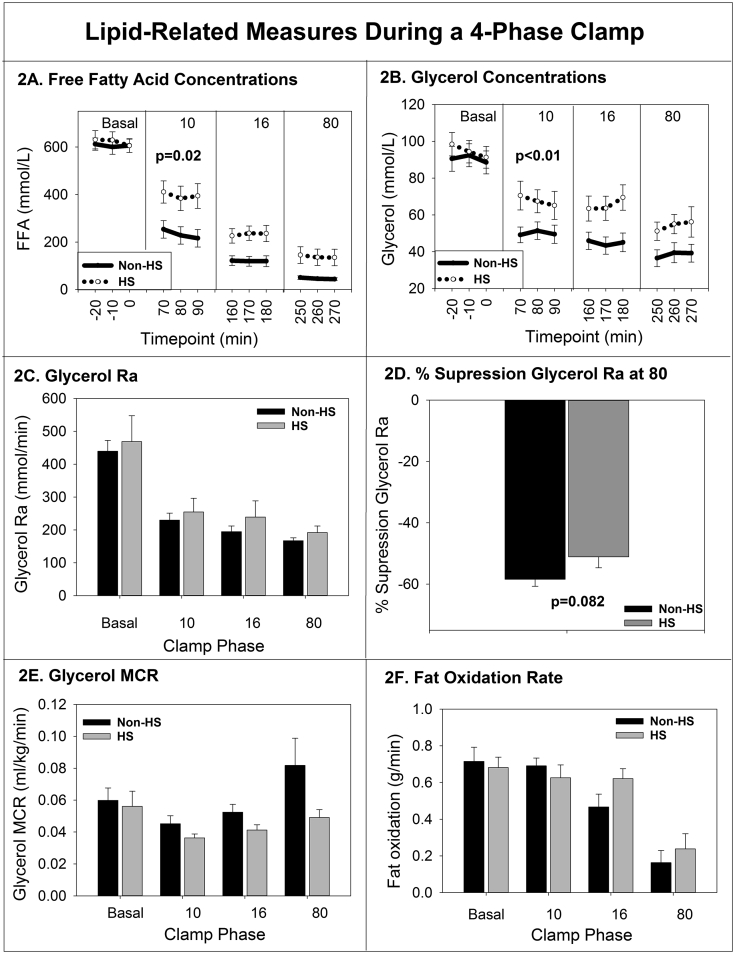
Adipose measurements are shown in Fig. 2. FFA concentrations (Fig. 2A) decreased with increasing insulin doses for both groups as expected, although during the first phase (10 mU/m2/min), those with NALFD had higher FFA (p = 0.02). Glycerol concentrations were also significantly higher in the NAFLD group during the first phase (p < 0.01) (Fig. 2B), but glycerol Ra (Fig. 2C) was not different between groups during the first phase (p = 0.21) with similar results when expressed per kg of body weight (data not shown). The percent suppression of the glycerol Ra at the 80 phase was not different between groups (Fig. 2D). When the glycerol Ra and insulin relationship curves were modeled, there was not a difference in the relationships between the groups (p = 0.88), indicating that systemic adipocyte lipolysis responded equally to insulin. Glycerol MCR during the first phase (10 mU/m2/min) was not lower in girls with HS but was significantly higher during the final phase (80 mU/m2/min) (P = 0.03) (Fig. 2E). The rate of whole body fat oxidation was not different between groups (Fig. 2F).
Fig. 2.
Free fatty acid (A) and glycerol (b) concentrations from each phase of the clamp, per group are shown. Mean glycerol rate of appearance (c) glycerol clearance rate (e) and rate of fat oxidation (f) per phase and group are shown. The change in percent suppression (d) from highest glycerol ate of appearance to the lowest is shown. Significant P values are shown. Data are mean and standard error of the mean.
Post-exercise muscle mitochondrial endpoints were not significantly different based on HS status. There was no correlation between oxidative phosphorylation and IHTG (r = −0.16, p = 0.21), nor between ADP time constant and IHTG (r = 0.06, p = 0.66). There also was no relationship between IMCL and IHTG.
In univariate correlations, the following related to IHTG: adiponectin, ALT, visceral fat, GIR, and 80 mIU/m2/min FFA serum concentrations as shown in Table 3. In the clinical model, only ALT was significantly associated with HS (Chi2 7.03, p < 0.01). For a one unit increase in ALT, the odds of HS increased by 1.09 fold (95% CI 1.02–1.16). In the research model without VO2peak, none of the covariates were significantly associated with HS. Similar results were obtained after addition of VO2peak. In the full model of all univariate correlated variables, without VO2peak, again none of the covariates were significantly associated with HS. Similar results were obtained after addition of VO2peak. We also performed likelihood ratio tests for each of the predictive models. Each of the models explained a significantly greater proportion of the variability in the outcome, compared to a simpler model with only ethnicity and disease as covariates, indicating that none of the covariates are independently associated with HS, but that each contributes to it.
Table 3.
Muscle mitochondria measures.
| Non-NAFLD N = 35 | NAFLD N = 24 | p-value | |
|---|---|---|---|
| PCr Time Constant (seconds) | 31.5 ± 6.9 | 29.6 ± 7.2 | 0.40 |
| ADP time Constant (seconds) | 21.5 (18.7, 25.8) | 21.1 (18.2, 24.6) | 0.53 |
| VPCr (mmol/seconds) | 0.23 (0.17, 0.31) | 0.27 (0.17, 0.33) | 0.66 |
| Qmax (mmol/L/sec) | 0.46 (0.36, .055) | 0.57 (0.37, 0.67) | 0.11 |
| OxPhos (mmol/L/sec) | 0.13 (0.10, 0.21) | 0.14 (0.8, 0.19) | 0.67 |
Data presented as mean ± standard error of the mean or median (25th,75th percentile).
4. Discussion
This is the first study to deeply phenotype whole-body metabolic function in obese girls with HS, with and without other metabolic diseases including T2D and PCOS, using the gold standard 4-phase hyperinsulinemic euglycemic clamp. Baseline characteristics including age, BMI, habitual physical activity and dietary intake were similar between the two groups and all results were controlled for both disease-state (PCOS or T2D) and ethnicity. We found that obese adolescent girls with HS have more hepatic and muscle IR compared to girls without HS and had alterations in FFA and glycerol concentrations under hyperinsulinemic conditions. Obese girls with HS also had worse central obesity, as evidenced by higher waist:hip ratio and more visceral fat, and worse cardiometabolic risk markers including higher fasting insulin, c-peptide, triglycerides and systolic blood pressure and lower adiponectin. Girls with HS also had evidence of non-specific inflammation, including higher WBC count and ALT. In multiple logistic regression models, however, we found that only ALT was an independent predictor for HS, likely due to the multi-factorial nature of the disease. Given the multi-tissue IR, inflammation and multiple features of the metabolic syndrome, adolescent girls with HS clearly have increased cardiometabolic risk markers and are in need of more intensive prevention efforts and clinical follow-up over time.
Insulin-stimulated glucose uptake in muscle tissue is altered in girls with HS but is not directly related to IHTG concentrations. These results are in concordance with previous studies that found muscle IR in adults and youth with HS, demonstrating that the glucose abnormalities in girls with HS do not appear to be different from those in adolescent males or adults [16,17,32,33]. This muscle IR translated into clinical findings with higher 2 h OGTT glucose concentrations in girls with HS. Despite demonstrating an increase in muscle IR in adolescent girls with HS in this study, the muscle mitochondrial endpoints of oxidative capacity (muscle oxidative phosphorylation and ADP time constant) did not correlate with IHTG or IMCL. This result implies that the fat accumulation in HS is not directly related to intrinsic oxidation differences in the muscle mitochondria or the IMCL pool size and is a multi-factorial process, as is also suggested by our predictive modeling results.
Girls with HS had multiple abnormalities in fat metabolism and storage, but no detected abnormalities in the rate of lipolysis, with agreement across 4 different models of lipolysis as measured with a glycerol tracer. However, obese girls with HS had higher FFA and glycerol concentrations, although there were no differences in the glycerol Ra nor the response of glycerol Ra to staged insulin doses. This could suggest that there is an alteration in FFA recycling and/or glycerol uptake, and not just a defect in suppression of lipolysis. Previous work in youth and adults has shown that when FFA metabolism was studied with a palmitate tracer, FFA Ra was higher in participants with HS [20,22], which when paired with our data could suggest increased FFA recycling in non-HS. It is know that insulin can drive FFA uptake and resistance to this process may contribute to excess FFA concentrations [47]. Glycerol clearance was lower in HS suggesting decreased hepatic glycerol clearance of glycerol in our participants. Girls with HS also had increased fasting TG concentrations, likely a reflection of increased TG synthesis, which is also upregulated in HS [21,40]. However, this method does assume that glycerol Ra only represents adipose lipolysis and there is some degree of lipolysis from serum TG via lipoprotein lipase. This activity is decreased in the setting of insulin resistance. Thus, it appears that girls with HS have multiple abnormalities in lipid handling, although we were not able to show a difference in adipose tissue lipolysis with our methodology.
Consistent with previous observations in both adults and adolescents [18,32,52,58], girls with HS had more visceral fat but not more abdominal subcutaneous fat or total body fat as compared to controls. Hypertrophied visceral adipocytes are characterized by lower adiponectin concentrations and induce a hyperlipolytic state with decreased insulin responsiveness [4,18,39,52]. This results in excess FFA delivered directly to the liver via the portal circulation [30]. Without placing hepatic or portal catheters, it is not currently possible to distinguish the source of FFA or glycerol in the peripheral circulation. As has been suggested, it is possible that the increase FFA in the HS group actually arise from the viscera rather than the peripheral adipose, and that this is the reason for higher FFA concentrations [20,22]. Thus, in girls with HS, mechanisms of adipose IR in the peripheral and visceral tissues are likely to be similar to other populations with HS.
Obese girls with HS also failed to suppress EGP during the last two phases of the clamp indicating hepatic IR. The general association of persistent EGP during hyperinsulinemia has been demonstrated in youth with HS [17]. However, in girls there was not a strong correlational relationship with the rate of EGP per se and amount of IHTG, indicating that hepatic IR may not relate strongly to the accumulation of IHTG in girls. Previous results have also demonstrated a dichotomy between hepatic IR and IHTG, which have been proposed to relate to individual variances in the cellular signaling [5].
Obese girls with HS had higher white blood cell count and C-reactive protein concentrations as compared to controls indicating a non-specific elevated inflammatory state. Previous research has noted that the serum C-reactive protein concentrations, as a surrogate for overall systemic inflammation, is a strong predictor of atherosclerotic events though the exact mechanism of this relationship is still under investigation [48]. Obese participants with HS in the study also had higher ALT and lower adiponectin concentrations. Adiponectin has been associated with improved insulin signaling and potential protection against atherosclerosis [18,52]. However, differences in markers of inflammation did not predict HS, which may be due to more mild disease from a protocol weight limit of 250 pounds and recruitment from endocrinology clinics as opposed to hepatology clinics. Therefore, the study may not have included the most ill youth with the highest degree of inflammation. In addition, hemoglobin was higher in the HS group, which has also been reported to be associated with the presence of hepatic inflammation (NASH) and fibrosis in youth [27]. Future work with more specific markers, or tissue measured samples of inflammatory markers is needed.
Obese girls with HS have clinical markers of IR in addition to tissue-specific IR as measured by the hyperinsulinemic euglycemic clamp and we attempted to determine which, if any, clinical measures best related to IHTG or presence of HS in our female cohort. Obese girls with HS had higher fasting insulin and c-peptide concentrations compared to those without HS, indicating that underlying IR may be similar between populations at high risk for diabetes and those that have converted to type 2 diabetes. Unfortunately, other than ALT, we did not find any independent predictors of disease; however, when multiple measures were combined, there was a significant association with HS. This is similar to other clinical models developed, such as the HS fibrosis score which includes age, BMI, glucose status, platelets, AST/ALT ratio and albumin, and has been shown to correlate with prognosis for hepatic complications and mortality in HS [10]. Thus, while it does not appear that there are any unique predictors for HS detection in obese female adolescents, the entire clinical picture must be considered to fully understand disease state and progression.
The mechanisms behind the development of HS in obese girls with obesity may be similar to those in other populations. Adiponectin has been shown to have a direct role in hepatic lipid regulation in animals as adiponectin knockouts have increased HS whereas those that overproduce adiponectin are protected from a high fat diet [28,31]. Further the adiponectin knockout animal had dysglycemia and evidence of insulin resistance [28]. Further work is needed to understand the role of adiponectin in the coordinated pathology of dysglycemia and development of hepatic steatosis.
There are several strengths and weaknesses to the study. We enrolled a fairly large cohort of girls for a mechanistic study of this intensity. We also performed the gold standard measurement for insulin sensitivity with a hyperinsulinemic euglycemic clamp, and used multiple concentrations of insulin and stable isotopes to measure tissue-specific IR. We used a strict definition of HS, in only including girls with IHTG content greater than 5.5% as measured with MRI proton density fat fraction. We did not use liver biopsy; however, it may be that our results with MRI, which evaluated the fat content of the entire liver, are more accurate than a biopsy of only a small section of the liver [7]. Another weakness of the study was the trend toward uneven distribution of comorbid disease statuses within the groups and the inclusion of some T2D youth on metformin, which has been reported in some studies to improve HS [41]. However, ethnicity and disease-state adjustments did not change the outcomes, and equal numbers of participants with and without metformin-exposure had HS. Unfortunately, we do not have genetic information in these youth, as there are several genetic variants that are more common in Hispanic patients and are associated with HS [56]. Ideally, we would have been able to include a palmitate tracer as well as the glycerol tracer to better understand the differences in lipolysis vs re-esterification of FFA, but there are increased safety concerns with FFA tracers and co-administered albumin in youth. While we were not powered for all of our outcomes, by deeply phenotyping this population we have provided hypothesis-generating information to inform future studies.
5. Conclusion
In conclusion, we found that obese adolescent females with HS have multi-tissue IR, and multiple components of the metabolic syndrome as seen in adults and boys with HS, indicating that girls with HS also likely have high CVD risk. Alterations in glucose and fat metabolism in girls are similar to that reported for boys and adults, suggesting that adolescent girls likely share the same NAFLD pathophysiology as their male and adult counterparts. Obese female adolescents with HS must be treated as a higher risk category for future potential cardiometabolic disease as compared to obese females without HS. These findings should encourage increased diligence in screening for NAFLD in girls. Further research is needed to better understand the clinical implications in this at-risk population especially as pharmacologic treatment for NAFLD becomes available.
Funding
K.J.N.: NCRR K23 RR020038-01, NIH/NCRR Colorado CTSI Co-Pilot Grant TL1 RR025778, NIH/NIDDK 1R56DK088971-01, JDRF5—2008-291, ADA 7-11-CD-08.
M.C.G.: AHA 13CRP 14120015, Thrasher Pediatric Research Foundation Mentored Pilot Grant, NIH/NCRR Colorado CTSI Co-Pilot Grant TL1 RR025778, Pediatric Endocrinology Society Fellowship, Endocrine Society Fellowship in Women’s health, NIDDK T32 DK063687, BIRCWH K12HD057022; NIDDK K23DK107871.
Institution
Colorado CTSA Grant UL1TR002535.
Disclosure statement
The authors have nothing to disclose.
Author contributions
M.C.G designed the study, researched data and wrote the manuscript, S.R. researched data and wrote the manuscript, AMC researched data and edited the manuscript, R.S. researched data and wrote the manuscript, A.B. researched data and edited the manuscript, G.C. researched data and edited the manuscript, B.B. researched data and edited the manuscript, A.S. researched data and edited the manuscript, T.J researched data and edited the manuscript, L.P. performed statistical analyses and edited the manuscript, K.J.N designed the study, researched data, contributed to discussion and edited the manuscript.
Acknowledgements
The authors would like to thank the participants, their families and the CTRC nurses and staff.
References
- 1.Adams L.A., Lymp J.F., St Sauver J., Sanderson S.O., Lindor K.D., Feldstein A., Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Arslanian S.A., Lewy V.D., Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86:66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 3.Bergman B.C., Howard D., Schauer I.E., Maahs D.M., Snell-Bergeon J.K., Eckel R.H., Perreault L., Rewers M. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab. 2012;97:1663–1672. doi: 10.1210/jc.2011-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman R.N., Kim S.P., Catalano K.J., Hsu I.R., Chiu J.D., Kabir M., Hucking K., Ader M. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity. 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 5.Birkenfeld A.L., Shulman G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjornstad P., Truong U., Pyle L., Dorosz J.L., Cree-Green M., Baumgartner A., Coe G., Regensteiner J.G., Reusch J.E., Nadeau K.J. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: a RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) Study. J Diabetes Complicat. 2016;30:1103–1110. doi: 10.1016/j.jdiacomp.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonekamp S., Tang A., Mashhood A., Wolfson T., Changchien C., Middleton M.S., Clark L., Gamst A., Loomba R., Sirlin C.B. Spatial distribution of MRI-Determined hepatic proton density fat fraction in adults with nonalcoholic fatty liver disease. J Magn Reson Imaging. 2014;39:1525–1532. doi: 10.1002/jmri.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprio S., Perry R., Kursawe R. Adolescent obesity and insulin resistance: roles of ectopic fat accumulation and adipose inflammation. Gastroenterology. 2017;152:1638–1646. doi: 10.1053/j.gastro.2016.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan D.F., Li A.M., Chu W.C., Chan M.H., Wong E.M., Liu E.K., Chan I.H., Yin J., Lam C.W., Fok T.F., Nelson E.A. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004;28:1257–1263. doi: 10.1038/sj.ijo.0802734. [DOI] [PubMed] [Google Scholar]
- 10.Cheah M.C., McCullough A.J., Goh G.B. Current modalities of fibrosis assessment in non-alcoholic fatty liver disease. J Clin Transl Hepatol. 2017;5:261–271. doi: 10.14218/JCTH.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cree-Green M., Bergman B.C., Coe G.V., Newnes L., Baumgartner A.D., Bacon S., Sherzinger A., Pyle L., Nadeau K.J. Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity. 2016;24:2399–2406. doi: 10.1002/oby.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cree-Green M., Newcomer B.R., Brown M., Hull A., West A.D., Singel D., Reusch J.E., McFann K., Regensteiner J.G., Nadeau K.J. Method for controlled mitochondrial perturbation during phosphorus MRS in children. Med Sci Sport Exerc. 2014;46:2030–2036. doi: 10.1249/MSS.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cree-Green M., Newcomer B.R., Brown M.S., Baumgartner A.D., Bergman B., Drew B., Regensteiner J.G., Pyle L., Reusch J.E., Nadeau K.J. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2015;64:383–392. doi: 10.2337/db14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cree-Green M., Newcomer B.R., Coe G., Newnes L., Baumgartner A., Brown M.S., Pyle L., Reusch J.E., Nadeau K.J. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. Am J Physiol Endocrinol Metab. 2015;308:E726–E733. doi: 10.1152/ajpendo.00619.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cree-Green M., Rahat H., Newcomer B.R., Bergman B.C., Brown M.S., Coe G.V., Newnes L., Garcia-Reyes Y., Bacon S., Thurston J.E., Pyle L., Scherzinger A., Nadeau K.J. Insulin resistance, hyperinsulinemia, and mitochondria dysfunction in nonobese girls with polycystic ovarian syndrome. J Endocr Soc. 2017;1:931–944. doi: 10.1210/js.2017-00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Adamo E., Cali A.M., Weiss R., Santoro N., Pierpont B., Northrup V., Caprio S. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33:1817–1822. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deivanayagam S., Mohammed B.S., Vitola B.E., Naguib G.H., Keshen T.H., Kirk E.P., Klein S. Nonalcoholic fatty liver disease is associated with hepatic and skeletal muscle insulin resistance in overweight adolescents. Am J Clin Nutr. 2008;88:257–262. doi: 10.1093/ajcn/88.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 19.Druet C., Tubiana-Rufi N., Chevenne D., Rigal O., Polak M., Levy-Marchal C. Characterization of insulin secretion and resistance in type 2 diabetes of adolescents. J Clin Endocrinol Metab. 2006;91:401–404. doi: 10.1210/jc.2005-1672. [DOI] [PubMed] [Google Scholar]
- 20.Fabbrini E., deHaseth D., Deivanayagam S., Mohammed B.S., Vitola B.E., Klein S. Alterations in fatty acid kinetics in obese adolescents with increased intrahepatic triglyceride content. Obesity. 2009;17:25–29. doi: 10.1038/oby.2008.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabbrini E., Magkos F., Mohammed B.S., Pietka T., Abumrad N.A., Patterson B.W., Okunade A., Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finegood D.T., Bergman R.N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36:914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- 24.Fishbein M.H., Miner M., Mogren C., Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36:54–61. doi: 10.1097/00005176-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Freedson P.S., Melanson E., Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sport Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Gilker C.D., Pesola G.R., Matthews D.E. A mass spectrometric method for measuring glycerol levels and enrichments in plasma using 13C and 2H stable isotopic tracers. Anal Biochem. 1992;205:172–178. doi: 10.1016/0003-2697(92)90595-x. [DOI] [PubMed] [Google Scholar]
- 27.Giorgio V., Mosca A., Alterio A., Alisi A., Grieco A., Nobili V., Miele L. Elevated hemoglobin level is associated with advanced fibrosis in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2017;65:150–155. doi: 10.1097/MPG.0000000000001614. [DOI] [PubMed] [Google Scholar]
- 28.Holland W.L., Adams A.C., Brozinick J.T., Bui H.H., Miyauchi Y., Kusminski C.M., Bauer S.M., Wade M., Singhal E., Cheng C.C., Volk K., Kuo M.S., Gordillo R., Kharitonenkov A., Scherer P.E. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metabol. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holterman A.X., Guzman G., Fantuzzi G., Wang H., Aigner K., Browne A., Holterman M. Nonalcoholic fatty liver disease in severely obese adolescent and adult patients. Obesity. 2013;21:591–597. doi: 10.1002/oby.20174. [DOI] [PubMed] [Google Scholar]
- 30.Jacome-Sosa M.M., Parks E.J. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr Opin Lipidol. 2014;25:213–220. doi: 10.1097/MOL.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 31.Kim J.Y., van de Wall E., Laplante M., Azzara A., Trujillo M.E., Hofmann S.M., Schraw T., Durand J.L., Li H., Li G., Jelicks L.A., Mehler M.F., Hui D.Y., Deshaies Y., Shulman G.I., Schwartz G.J., Scherer P.E. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Investig. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korenblat K.M., Fabbrini E., Mohammed B.S., Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotronen A., Vehkavaara S., Seppala-Lindroos A., Bergholm R., Yki-Jarvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709–E1715. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 34.Lavine J.E., Schwimmer J.B., Van Natta M.L., Molleston J.P., Murray K.F., Rosenthal P., Abrams S.H., Scheimann A.O., Sanyal A.J., Chalasani N., Tonascia J., Unalp A., Clark J.M., Brunt E.M., Kleiner D.E., Hoofnagle J.H., Robuck P.R., Nonalcoholic Steatohepatitis Clinical Research N. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. Jama. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manco M., Bedogni G., Monti L., Morino G., Natali G., Nobili V. Intima-media thickness and liver histology in obese children and adolescents with non-alcoholic fatty liver disease. Atherosclerosis. 2010;209:463–468. doi: 10.1016/j.atherosclerosis.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M., McCullough A.J., Natale S., Forlani G., Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 37.Mayer-Davis E.J., Nichols M., Liese A.D., Bell R.A., Dabelea D.M., Johansen J.M., Pihoker C., Rodriguez B.L., Thomas J., Williams D., Group SfDiYS Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Am Diet Assoc. 2006;106:689–697. doi: 10.1016/j.jada.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Miranda P.J., DeFronzo R.A., Califf R.M., Guyton J.R. Metabolic syndrome: evaluation of pathological and therapeutic outcomes. Am Heart J. 2005;149:20–32. doi: 10.1016/j.ahj.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Mittelman S.D., Van Citters G.W., Kirkman E.L., Bergman R.N. Extreme insulin resistance of the central adipose depot in vivo. Diabetes. 2002;51:755–761. doi: 10.2337/diabetes.51.3.755. [DOI] [PubMed] [Google Scholar]
- 40.Mittendorfer B., Yoshino M., Patterson B.W., Klein S. VLDL triglyceride kinetics in lean, overweight, and obese men and women. J Clin Endocrinol Metab. 2016;101:4151–4160. doi: 10.1210/jc.2016-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nadeau K.J., Ehlers L.B., Zeitler P.S., Love-Osborne K. Treatment of non-alcoholic fatty liver disease with metformin versus lifestyle intervention in insulin-resistant adolescents. Pediatr Diabetes. 2009;10:5–13. doi: 10.1111/j.1399-5448.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 42.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, and Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab 95: 513-521. [DOI] [PMC free article] [PubMed]
- 43.Nadeau K.J., Zeitler P.S., Bauer T.A., Brown M.S., Dorosz J.L., Draznin B., Reusch J.E., Regensteiner J.G. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94:3687–3695. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newcomer B.R., Boska M.D. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve. 1997;20:336–346. doi: 10.1002/(SICI)1097-4598(199703)20:3<336::AID-MUS11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Newton K.P., Hou J., Crimmins N.A., Lavine J.E., Barlow S.E., Xanthakos S.A., Africa J., Behling C., Donithan M., Clark J.M., Schwimmer J.B., Nonalcoholic Steatohepatitis Clinical Research N. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA pediatrics. 2016;170 doi: 10.1001/jamapediatrics.2016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pyle L., Bergman B.C., Nadeau K.J., Cree-Green M. Modeling changes in glucose and glycerol rates of appearance when true basal rates of appearance cannot be readily determined. Am J Physiol Endocrinol Metab. 2016;310:E323–E331. doi: 10.1152/ajpendo.00368.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos-Roman M.A., Lapidot S.A., Phair R.D., Parks E.J. Insulin activation of plasma nonesterified fatty acid uptake in metabolic syndrome. Arterioscler Thromb Vasc Biol. 2012;32:1799–1808. doi: 10.1161/ATVBAHA.112.250019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridker P.M., Rifai N., Rose L., Buring J.E., Cook N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 49.Santoro N., Feldstein A.E., Enoksson E., Pierpont B., Kursawe R., Kim G., Caprio S. The association between hepatic fat content and liver injury in obese children and adolescents: effects of ethnicity, insulin resistance, and common gene variants. Diabetes Care. 2013;36:1353–1360. doi: 10.2337/dc12-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwimmer J.B., Deutsch R., Kahen T., Lavine J.E., Stanley C., Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 51.Schwimmer J.B., Deutsch R., Rauch J.B., Behling C., Newbury R., Lavine J.E. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 52.Silveira L.S., Monteiro P.A., Antunes Bde M., Seraphim P.M., Fernandes R.A., Christofaro D.G., Freitas Junior I.F. Intra-abdominal fat is related to metabolic syndrome and non-alcoholic fat liver disease in obese youth. BMC Pediatr. 2013;13:115. doi: 10.1186/1471-2431-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 54.van den Boogaart A., MRUI MANUAL V. Delft Technical University Press; Delft: 1997. 96.3. A user's guide to the magnetic resonance user interface software package. [Google Scholar]
- 55.Vitola B.E., Deivanayagam S., Stein R.I., Mohammed B.S., Magkos F., Kirk E.P., Klein S. Weight loss reduces liver fat and improves hepatic and skeletal muscle insulin sensitivity in obese adolescents. Obesity. 2009;17:1744–1748. doi: 10.1038/oby.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vos M.B., Abrams S.H., Barlow S.E., Caprio S., Daniels S.R., Kohli R., Mouzaki M., Sathya P., Schwimmer J.B., Sundaram S.S., Xanthakos S.A. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the expert committee on NAFLD (ECON) and the north American society of pediatric gastroenterology, hepatology and nutrition (NASPGHAN) J Pediatr Gastroenterol Nutr. 2017;64:319–334. doi: 10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winkler B., Steele R., Altszuler N. Relationship of glycerol uptake to plasma glycerol concentration in the normal dog. Am J Physiol. 1969;216:191–196. doi: 10.1152/ajplegacy.1969.216.1.191. [DOI] [PubMed] [Google Scholar]
- 58.Yang H.R., Chang E.J. Insulin resistance, body composition, and fat distribution in obese children with nonalcoholic fatty liver disease. Asia Pac J Clin Nutr. 2016;25:126–133. doi: 10.6133/apjcn.2016.25.1.15. [DOI] [PubMed] [Google Scholar]