Abstract
Background
Gut microbiota holds a key-role in numerous biological functions and has emerged as a driving force for the development of diabetes. Diet contributes to gut microbiota diversity and functionality providing a tool for the prevention and management of the disease. The study aimed to investigate the effect of a dietary intervention with pistachio nuts, a rich source of monounsaturated fatty acids, dietary fibers and phytochemicals on gut microbiota composition in the rat model of Type 1 Diabetes.
Methods
Male Wistar rats were randomly assigned into four groups: healthy animals which received control diet (CD) or pistachio diet (PD), and diabetic animals which received control diet (DCD) or pistachio diet (DPD) for 4 weeks. Plasma biochemical parameters were determined and histological examination of liver and pancreas was performed at the end of the dietary intervention. Adherent intestinal microbiota populations in jejunum, ileum, caecum and colon were analyzed. Fecal microbiota populations at the beginning and the end of the study were determined by microbiological analysis and 16S rRNA sequencing.
Results
Diabetic animals of both groups exhibited high plasma glucose and low insulin concentrations, as well as characteristic pancreatic lesions. Pistachio supplementation significantly increased lactobacilli and bifidobacteria populations in jejunum, ileum and caecum (p < 0.05) and normalized microbial flora in all examined intestinal regions of diabetic animals. After 4 weeks of supplementation, populations of bifidobacteria and lactobacilli were increased in feces of both healthy and diabetic animals, while enterococci levels were decreased (p < 0.05). Next Generation Sequencing of fecal samples revealed increased and decreased counts of Firmicutes and Bacteroidetes, respectively, in healthy animals that received the pistachio diet. Actinobacteria OTUs were higher in diabetic animals and increased over time in the pistachio treated groups, along with increased abundance of Bifidobacterium. Lactobacillus, Turicibacter and Romboutsia populations were elevated in healthy animals administered the pistachio nuts. Of note, relative abundance of Bacteroides was higher in healthy than in diabetic rats (p < 0.05).
Conclusion
Dietary pistachio restored normal flora and enhanced the presence of beneficial microbes in the rat model of streptozotocin-induced diabetes.
Keywords: Type 1 diabetes, Streptozotocin-induced diabetes, Pistachio nuts, Gut microbiota, Bifidobacteria, Lactobacilli
Highlights
-
•
Pistachio diet balances intestinal adherent microbiota in diabetic rats.
-
•
Fecal lactobacilli and bifidobacteria counts were elevated in rats receiving pistachios.
-
•
Firmicutes population was increased in healthy animals following pistachio diet.
-
•
Bacteroidetes levels were decreased in healthy animals supplemented with pistachio.
-
•
Bifidobacterium genus was more abundant in diabetic rats receiving pistachio nuts.
1. Introduction
Type 1 diabetes (T1D) is characterized by a series of events resulting in autoimmune destruction of insulin-secreting pancreatic β-cells and inability of the body to regulate and use blood glucose. During the last decades, the incidence of T1D has dramatically increased in developed countries, suggesting that beyond the genetic component, environmental factors, such as diet, intestinal microbiota, increased pharmaceutical use and chemical exposure contribute to the onset and the development of the disease [1].
The gastrointestinal (GI) tract constitutes the most important site of interaction between the host immune system and microorganisms. Gut microbes exert both anti- and pro-inflammatory actions, since normal flora community includes members that are capable to induce inflammatory responses [2]. Thus, the composition of microbiota might disturb the normal interaction with the immune system and contribute to altered immune responses having an impact on the development of T1D [1,3]. The intestinal microbiota is a major contributor towards the onset of T1D and/or is also modified as a result of T1D disease progression [4]. The involvement of the intestinal microbiota in the pathophysiology of T1D has been highlighted by several human studies that reported an increased ratio of duodenal Bacteroidetes/Firmicutes [5], a decreased microbial diversity [6] and an overgrowth of opportunistic pathogens [7]. The restoration of normal composition of microbiota populations constitutes a new target for the prevention and treatment of the disease [8,9].
Diet is a major environmental factor contributing to gut microbiota diversity and functionality, as different dietary compositions have diverse effects on bacterial shifts [[10], [11], [12], [13], [14]]. Nuts and seeds are considered important components of healthy dietary patterns. Pistacia vera L., is a dioecious deciduous tree member of the Anacardiaceae family originating in Central and West Asia, which has been distributed throughout the Mediterranean countries. It has been cultivated for centuries due to its fruit, pistachio, and is considered a delicacy. Iran has the highest production worldwide (52%) followed by the United States (24%), Syria (9%), Turkey (7%) and Greece (2%), which is the largest producing country in Europe. Greek pistachios are famous since one of the best varieties in the world is cultivated in the island of Aegina. However, pistachios are also produced in the area of Lamia, as well as in other regions of Central Greece. The Aegina pistachio has been nominated by the European Commission as a Protected Designation of Origin (PDO) product [15], due to its exceptional flavor, shapely form, and full kernel.
Pistachios are rich source of monounsaturated fatty acids (MUFA), dietary fibers and phytochemicals, which are known for their beneficial health effects [16]. Specifically, they contain high amounts of oleic acid, while phenolic compounds like trans-resveratrol, proanthocyanidins, isoflavones daidzein and genistein, as well as catechin and epicatechin have been reported as constituents of pistachio nuts [[17], [18], [19]]. Metabolites of phenolic compounds influence the growth of certain microbial species [20], and a diet high in MUFA has been reported to increase the fecal bifidobacteria in volunteers at Metabolic Syndrome (MetS) risk [21,22]. Fibers form a substrate for microbial fermentation in the gut, facilitating the maintenance and/or selection of a healthy microbiota composition. Pistachio could be suggested as a food with prebiotic properties with significant potential for maintaining health via microbiota regulation [16,23].
The aim of the study was to investigate the effect of dietary supplementation with pistachios on gut microbiota of diabetic animal models that could provide a potential future benefit for T1D patients.
2. Materials and methods
2.1. Animals and induction of diabetes
Eighteen-week old male Wistar rats (bw∼350 g) were intraperitoneally injected with a freshly prepared solution of streptozotocin (STZ) (Sigma-Aldrich, Germany) in citrate buffer (0.1 M, pH 4.5) in a dose of 40mg/kg. The injection was performed early in the morning and animals were in non-fasting state. Seven days after the injection, blood glucose concentration was measured. Animals exhibiting levels above 250 mg/dL [24] accompanied by signs of polyurea and polydipsia were considered diabetic and included in the study. Animal experimentation was reviewed and approved by the Veterinary Directorate of the Athens Prefecture (Ref. Number 2057/05-04-2017) and conducted in compliance with the European Directive 2010/63.
2.2. Dietary treatment
Twenty-four animals were studied. Rats were individually housed in a temperature controlled environment (21 ± 2 °C with 50 ± 5% relative humidity) with a 12-h light/dark cycle (light period between 6:00 and 18:00) in single polypropylene cages in the Laboratory Animal Facility of the Biomedical Research Foundation of the Academy of Athens. Before experimentation, animals were allowed to acclimate for one week and had free access to standard rat chow (Mucedola, Italy, type 4RF22) and tap water.
Rats were randomized into four groups based on dietary treatment: Healthy animals which received the control diet (n = 6, CD) or the pistachio diet (n = 6, PD), and diabetic animals which received the control diet (n = 6, DCD) or the pistachio diet (n = 6, DPD). The number of animals was chosen according to a power analysis (F-test ANOVA) performed by G∗Power 3 software [25], using data regarding bifidobacteria counts derived from a pilot study. Diets contained 10% fat and were isocaloric. Control diet included standard rat chow supplemented with corn oil instead of pistachio, in order to equalize the amount of fat and caloric content. Τhe pistachio diet was prepared using whole fresh pistachio kernels (kindly provided by the Agricultural Pistachios Cooperation of Molos-Thermopyles, Greece), including skin but not the shell. Nutrient composition of the diets is presented on Table 1. The feeding duration lasted 4 weeks. Rats received daily a fixed amount of the appropriate dietary treatment during the entire experimental period. Fresh food was provided to the animals every morning, and if any chow remained from the previous day, it was removed from the cages. Health and general condition of the rats were monitored throughout the study by an expert veterinarian.
Table 1.
Nutrient composition (g in 100 g per diet) and energy content (kcal/100 g) of control and pistachio diet administered to the animals.
| Control | Pistachio | |
|---|---|---|
| Protein | 18.73 | 19.50 |
| Carbohydrate | 39.82 | 38.41 |
| Fat | 10.00 | 10.00 |
| Fiber | 4.05 | 4.98 |
| Starch | 37.18 | 35.59 |
| Sucrose | 2.64 | 2.55 |
| Corn oil | 4.09 | 0.00 |
| Pistachios | 0.00 | 8.05 |
| Energy | ||
| 430.0 | 427.1 |
Blood samples were collected at the beginning and the end of the dietary intervention from the lateral tail vein after 6 h of fasting. Heparinized plasma was stored at −80 °C until analysis. Fresh feces were also collected and stored at −20 °C. Every week, animals were weighted and blood glucose was measured by a glucose meter, in order to ensure that they remained diabetic. At the end of the experimental period, animals were euthanized, in random order, by an overdose of isofluorane. The right liver lobe and pancreatic tissue were excised, rinsed with saline and fixed in 10% neutral buffered formalin. The intestinal segments of the animals in the four groups were dissected, rinsed with saline and samples were taken from jejunum, ileum, caecum, and colon and processed for microbiological examination.
2.3. Blood analyses
Plasma glucose, total cholesterol (TC), high density lipoproteins’ cholesterol (HDL-C) and triacylglycerols (TAG) were determined on an automated biochemical analyzer (Cobas 8000, Roche) using commercially available kits (Roche). Plasma insulin levels in the beginning and the end of dietary intervention were measured by a sandwich ELISA method using a commercially available rat insulin ELISA kit (EZRMI-13K, Merck Millipore, Germany).
2.4. Analysis of fecal microbiota
Feces (700–1200 mg) were homogenized with sterilized buffered peptone water 0.1% (LaB M, Heywood, UK) and were subjected to serial dilutions using ¼ strength Ringer’s solution (LaB M). The following tests on microbiological analysis were performed: (i) total aerobic counts on plate count agar (LaB M) at 30 °C for 48 h, (ii) staphylococci on Baird Parker (LaB M) enriched with egg yolk tellurite (LaB M) at 37 °C for 24 h, (iii) coliforms on Violet Red Bile agar (LaB M) at 37 °C for 24 h, (iv) Enterobactariaceae on Violet Red Bile Glucose agar (LaB M) at 37 °C for 24 h, (v) streptococci and enterococci on Bile Aesculin agar (LaB M) at 37 °C for 24 h (white or grey colonies were considered streptococci, whereas round black colonies as enterococci), (vi) lactobacilli (Gram positive) on acidified MRS agar (LaB M) at 30 °C for 24 h anaerobically (Merck Millipore Anaerobic Jar 2.5L, Oxoid AnaeroGen 2.5L Sachets), (vii) yeasts/molds on Malt Extract (LaB M) at 30οC for 48 h, (viii) Escherichia coli on MacConkey agar (LaB M) at 37 °C for 24 h (flat, circular, moist, smooth, non-mucoid and of entire margin red and pink colonies, with pink bile precipitation were considered as E. coli), (ix) bifidobacteria on Bifidobacteria agar (22 g/L bacteriological peptone, 5 g/L NaCl, 5 g/L dextrose, 1 g/L starch, 0.3 g l-cysteine HCl, 15 g/L agar) at 37 °C for 24 h anaerobically (Merck Millipore Anaerobic Jar 2.5L, Oxoid AnaeroGen 2.5L Sachets). All incubations were further extended up to 120 h, but no extra colonies were observed. Gram staining and catalase tests were performed for lactobacilli confirmation. Results are presented as log of mean colony-forming units on solid media culture plates containing 30 to 300 colonies per gram of fecal samples.
2.5. Analysis of intestinal tissue adherent microbiota
The small and large intestines were removed aseptically and 3-cm long individual sections were cut longitudinally. After removal of the intestinal fluids, the tissue samples were washed with sterilized buffered peptone water (LaΒ M) mixed with 20% glycerol (Merck Millipore) and then vortex mixed to break down bacterial clumps and to remove loosely attached bacteria. Samples were stored in 20% glycerol in sterilized buffered peptone water (LaB M) at −20 °C until microbiological analysis was performed [26,27].
Intestinal tissues were homogenized with sterilized buffered peptone water (LaB M) and were subjected to serial dilutions using ¼ strength Ringer’s solution (LaB M). The microbiological tests to evaluate the different microbial populations were performed as described in section 2.4.
2.6. DNA extraction, PCR amplification and 16S rRNA sequencing
Duplicate fecal samples from 0 (considered as baseline) and 4th week of every diet group (CD, PD, DCD and DPD) were subjected to DNA isolation and Next-Generation Sequencing (NGS). Total DNA was extracted using the NucleoSpin®Stool Mini Kit (MACHEREY-NAGEL GmbH & Co. KG, Germany), following manufacturer’s instructions.
NGS was performed using MiSeq sequencing by MR DNA (www.mrdnalab.com, Shallowater, TX, USA). The V4 region of the bacterial 16S rRNA gene was amplified from fecal genomic DNA with 27F/519R primers (AGRGTTTGATCMTGGCTCAG/GTNTTACNGCGGCKGCTG). Polymerase Chain Reaction (PCR) amplification was performed using the HotStarTaq Plus Master Mix Kit (Qiagen, Germantown, MD, USA), consisting of 30 cycles with the following steps: 94 °C for 3 min, 30 cycles of 94 °C for 30 s, 53 °C for 40 s, 72 °C for 1 min, and the final elongation step at 72 °C for 5 min. PCR products were then subjected to electrophoresis in 2% agarose gel to confirm the amplification and to determine the relative intensity of bands. The amplicons were then purified using Ampure XP beads (Beckman Coulter, Brea, California, USA). Samples were subsequently prepared for the illumina DNA library using MiSeq sequencing, following the manufacturer’s guidelines. Procession of the sequencing data was held using a proprietary analysis pipeline by MR DNA. Operational taxonomic units (OTUs) were defined by clustering at 3% divergence (97% similarity) and the final OTUs were taxonomically classified using BLASTn against a curated database derived from RDPII and NCBI (www.ncbi.nlm.nih.gov, http://rdp.cme.msu.edu) and compiled into each taxonomic level into both “counts” and “percentage” files. The analysis of raw data in OTUs level and the calculation of a- and b-diversity were performed using Rhea platform [28]. A phylogenetic tree of representative OTUs was constructed with Maximum Likelihood approach and 100 bootstraps, using MEGAX platform [29].
2.7. Histochemistry and microscopic examination
After fixation, liver and pancreatic tissue samples were embedded in paraffin, cut into sections of 3 μm thickness and stained with hematoxylin and eosin for microscopic examination.
Liver sections were examined as previously described [30,31]. Histological features were grouped into five broad categories: steatosis, ballooning, portal inflammation, lobular activity and focal necrosis. For pancreatic lesions, histological features were grouped into six categories [32]: β-cell distortion, nuclei uniformity, vacuolization, necrosis, pycnotic nuclei and fatty infiltration. A score from 0 (absence) to 3 (severe lesion) was assigned to each parameter.
2.8. Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using SPSS 21.0 statistical software. Variables were tested for normal distribution with the Kolmogorov-Smirnov test. One-way analysis of variance (ANOVA) coupled with the Bonferroni post-hoc test was used to compare microbiota populations in segments of intestinal tissue, as well as scores for pancreatic tissue samples. ANOVA for repeated measures coupled with the Bonferroni post-hoc test was employed to compare microbiota populations of fecal samples, blood parameters and body weight in the four groups of animals. The level of statistical significance was set at p < 0.05.
3. Results
3.1. Effect of pistachios on body weight
At the end of dietary intervention, body weight of diabetic animals of both DCD and DPD groups was significantly decreased compared to healthy animals (p < 0.05, Table 2), but no differences between healthy or diabetic groups were noticed (p > 0.05). Dietary pistachios did not affect body weight in PD animals and no differences were observed between PD and CD groups (Table 2).
Table 2.
Body weight and biochemical parameters of the 4 groups of animals in the beginning and the end of the dietary intervention.
| CD |
PD |
DCD |
DPD |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | end | baseline | end | baseline | end | baseline | end | |
| Body weight (g) | 412.3 ± 12.2 | 410.8 ± 11.5 | 412.0 ± 10.3 | 409.5 ± 8.7 | 357.2 ± 15.4 | 333.5 ± 16.5a,b | 377.0 ± 17.2 | 326.0 ± 8.9a,b |
| Glucose (mg/dL) | 124.50 ± 5.35 | 106.67 ± 2.56 | 123.67 ± 8.48 | 112.84 ± 4.17 | 352.17 ± 39.18a,b | 345.67 ± 75.54a,b | 403.17 ± 39.23a,b | 503.00 ± 60.31a,b |
| Insulin (ng/mL) | 4.63 ± 0.23 | 3.60 ± 0.49 | 3.85 ± 0.20 | 2.63 ± 0.48 | 1.81 ± 0.79a,b | 0.82 ± 0.30a,b | 2.07 ± 0.08a,b | 1.72 ± 0.27a |
| TC (mg/dL) | 90.17 ± 4.32 | 102.00 ± 4.35 | 93.5 ± 1.88 | 89.50 ± 2.26 | 99.17 ± 5.99 | 117.34 ± 7.89 | 91.67 ± 1.93 | 115.34 ± 8.52 |
| HDLC (mg/dL) | 71.40 ± 1.89 | 80.84 ± 2.94 | 69.84 ± 4.29 | 74.00 ± 1.57 | 68.17 ± 3.96 | 84.67 ± 4.94 | 73.17 ± 4.36 | 87.20 ± 5.11 |
| TAG (mg/dL) | 103.40 ± 13.08 | 120.00 ± 6.71 | 109.67 ± 14.72 | 118.67 ± 9.34 | 230.4 ± 93.28 | 148.20 ± 40.50 | 210.83 ± 47.74 | 167.67 ± 49.81 |
Values are expressed as mean ± SEM. CD: healthy animals that received the control diet, PD: healthy animals that received the pistachio diet, DCD: diabetic animals that received the control diet, DPD: diabetic animals that received the pistachio diet.
p < 0.05 compared to CD values.
p < 0.05 compared to PD values.
3.2. Biochemical parameters and insulin
In the beginning of the dietary intervention, plasma glucose concentrations were higher in both DCD and DPD groups compared to CD and PD (p < 0.05), butno differences between healthy or diabetic groups were observed (p > 0.05). Similarly, there were no significant differences in plasma glucose concentrations between DCD and DPD (p > 0.05), as well as between CD and PD groups (p > 0.05) at the end of the dietary intervention.
Dietary pistachio supplementation did not affect plasma lipid profiles of the animals in both PD and DPD groups. TC and HDLC remained within the normal range in the four groups of animals, while TAG levels were increased in diabetic animals, but no significant difference was observed when compared to healthy rats (p > 0.05 compared to baseline values (day 0) and p > 0.05 between groups).
Plasma insulin concentrations were lower in animals of DCD and DPD groups (p < 0.05 compared to CD and PD) in the beginning of the study without difference between them (p > 0.05). At the end of the dietary intervention, insulin levels remained at low levels in both groups of diabetic rats.
3.3. Analysis of the fecal microbiota
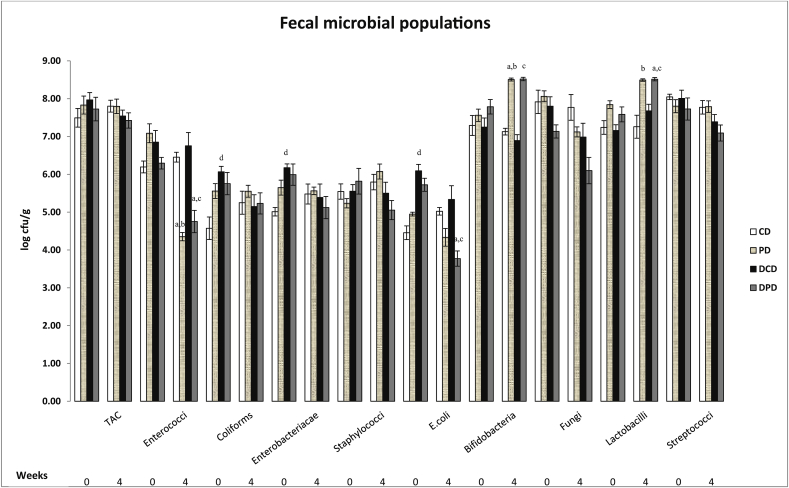
Total aerobic counts (TAC) did not differ between groups (Fig. 1). Staphylococci, streptococci and fungi counts ranged at similar levels in all groups. Coliforms, Enterobacteriacae and E. coli levels were significantly increased in DCD compared to CD group in week 0 (p < 0.05), but the differences were diminished in week 4 (p > 0.05). Pistachio supplementation had a significant impact on population of E. coli in DPD animals at the 4th week, as it was decreased compared to baseline values (p < 0.01), and to DCD group (p < 0.01). A significant reduction in enterococci counts was observed at the 4th week in both PD and DPD compared to the control groups CD (p < 0.01) and DCD (p < 0.01) respectively, and to week 0 (p < 0.01 for PD and p < 0.01 for DPD). Bifidobacteria population in PD group was significantly increased over time (p < 0.01 vs. week 0) and vs. CD group (p < 0.01), whereas it was also higher in DPD group vs. DCD group (p < 0.01). Finally, pistachio diet significantly increased the level of lactobacilli in PD compared to CD group (p < 0.01) at the end of the dietary intervention and in DPD animals compared to baseline and control group (p < 0.01 vs. DPD week 0 and p < 0.05 vs DCD week 4, Fig. 1).
Fig. 1.
Effect of pistachio diet on fecal microbiota population in healthy and diabetic animals at 0 and 4th week of pistachio supplementation. Data are expressed as mean ± SEM. Data with different superscript letters are significantly different p < 0.05, according to the post hoc ANOVA statistical analysis. TAC: total aerobic counts, CD: healthy animals that received the control diet, PD: healthy animals that received the pistachio diet, DCD: diabetic animals that received the control diet, DPD: diabetic animals that received the pistachio diet. ap < 0.05 vs timepoint 0 of the same sample, bp < 0.05 PD vs CD, cp < 0.05 DPD vs DCD, dp < 0.05 CD vs DCD.
3.4. Analysis of the intestinal tissue microbiota
3.4.1. Jejunum microbiota
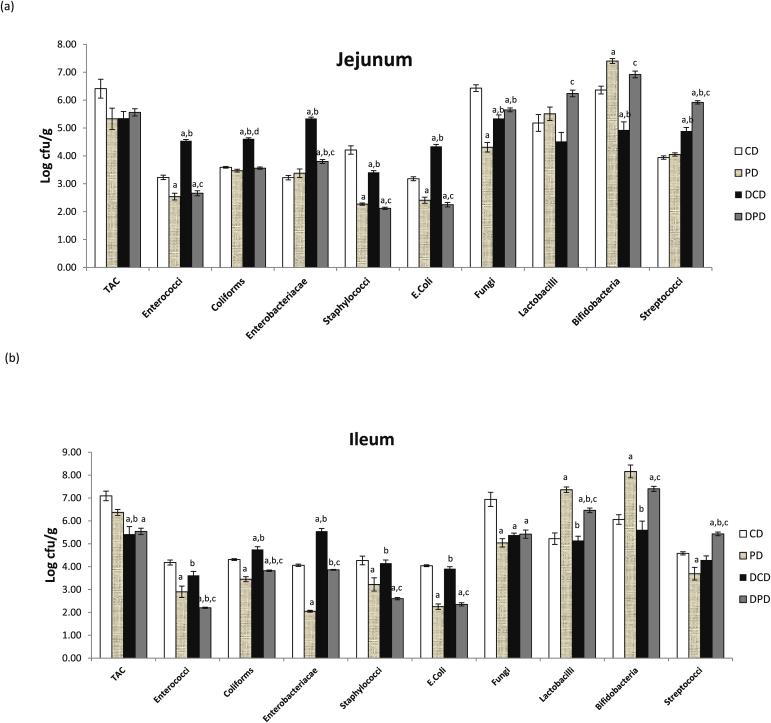
Enterococci, coliforms, Enterobacteriacae, E. coli and streptococci counts were significantly higher in diabetic animals receiving the control diet compared to healthy rats (p < 0.05 between DCD and CD groups, Fig. 2a). Bifidobacteria counts were lower in diabetic animals receiving the control diet, while dietary pistachio significantly increased their levels in both healthy (p < 0.05 vs. CD group) and diabetic rats (p < 0.05 vs. DCD group). Pistachio supplementation caused a significant decrease in enterococci and E. coli populations, as well as in staphylococci counts in both PD and DPD groups (p < 0.05 between DCD and DPD groups). Coliforms and Enterobacteriacae counts were increased in DCD group (p < 0.05 vs. CD and PD) and pistachio treatment decreased the populations to levels similar to those of healthy animals (p < 0.05 vs DCD). Lactobacilli counts were significantly higher in DPD group compared to the other three groups (p < 0.05 vs. CD, PD and DCD groups, Fig. 2a).
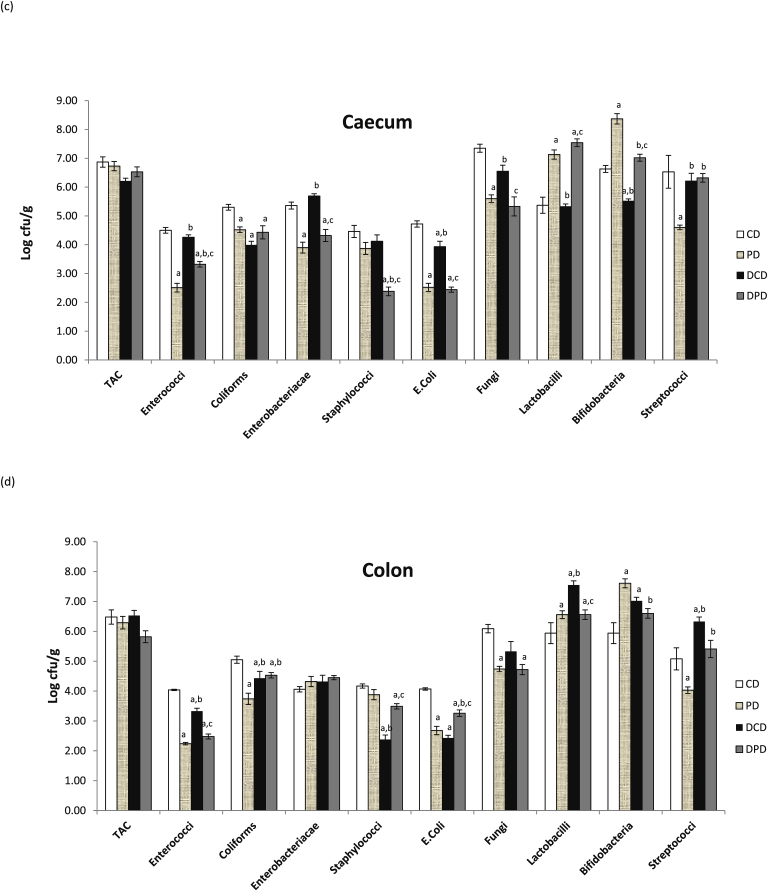
Fig. 2.
Effect of pistachio diet on gut microbiota population of healthy and diabetic animals in: (a) jejunum, (b) ileum, (c) caecum, (d) colon. Data are expressed as mean ± SEM. Data with different superscript letters are significantly different p < 0.05, according to the post hoc ANOVA statistical analysis. TAC: total aerobic counts, CD: healthy animals that received the control diet, PD: healthy animals that received the pistachio diet, DCD: diabetic animals that received the control diet, DPD: diabetic animals that received the pistachio diet. ap < 0.05 vs CD, bp < 0.05 vs PD, cp < 0.05 vs DCD, dp < 0.05 vs DPD.
3.4.2. Ileum microbiota
Coliforms and Enterobacteriacae levels were higher in DCD compared to CD group (p < 0.05) No differences in lactobacilli and bifidobacteria counts were noted in CD and DCD groups. Pistachio supplementation resulted in a significant reduction of coliforms and Enterobacteriacae levels in both diabetic and healthy animals (p < 0.05). A significant increase in lactobacilli and bifidobacteria counts was observed in both healthy and diabetic rats receiving the pistachio nuts (p < 0.05 between CD and PD groups, as well as between DCD and DPD groups, Fig. 2b).
3.4.3. Caecum microbiota
Coliforms, E. coli and bifidobacteria counts were lower in diabetic compared to healthy animals (p < 0.05, Fig. 2c). No differences were observed in lactobacilli population between healthy and diabetic animals, which received the control diet, but pistachio administration increased their levels (p < 0.05 between CD and PD, p < 0.05 between DCD and DPD). Pistachio diet also increased bifidobacteria levels in both healthy and diabetic rats (p < 0.05 between CD and PD, p < 0.05 between DCD and DPD). Counts of enterococci, E. coli and Enterobacteriacae were significantly reduced in PD and DPD groups (p < 0.05 between CD and PD, p < 0.05 between DCD and DPD). Staphylococci population was also significantly lower in DPD compared to DCD group (p < 0.05, Fig. 2c).
3.4.4. Colon microbiota
Enterococci, coliforms, staphylococci and E. coli levels were significantly lower in DCD compared to CD group (p < 0.05, Fig. 2d). Supplementation with pistachio nuts reduced enterococci counts in both healthy and diabetic animals (p < 0.05 between CD and PD, p < 0.05 between DCD and DPD), as well as levels of coliforms and E. coli in healthy animals (p < 0.05 between CD and PD). E. coli counts were increased in DPD compared to DCD group, but yet remained in lower (p < 0.05) levels than in CD animals. Coliforms counts ranged at similar levels in DPD and DCD groups (p > 0.05). Lactobacilli population was lower in DPD vs. DCD group (p < 0.05), but in similar levels to PD rats (which were higher than the CD group, p < 0.05). Pistachio supplementation increased bifidobacteria counts in PD compared to CD animals (p < 0.05). However, they were significantly lower in DPD compared to PD group (p <0.05), but no significant differences between DCD and DPD groups (p > 0.05, Fig. 2d) were recorded.
3.5. Fecal microbiota determined by next generation DNA sequencing
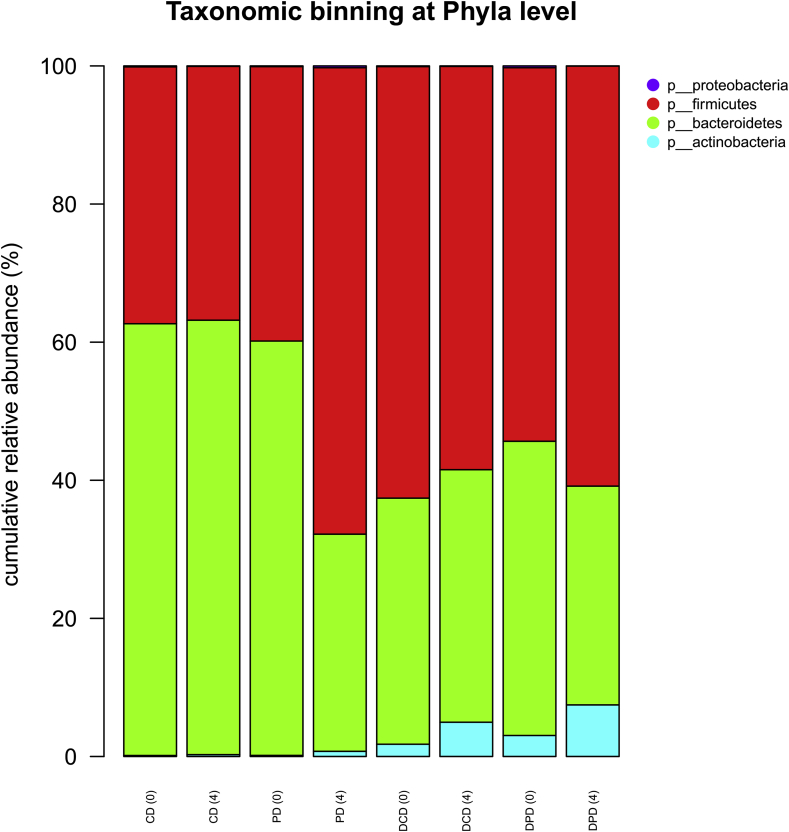
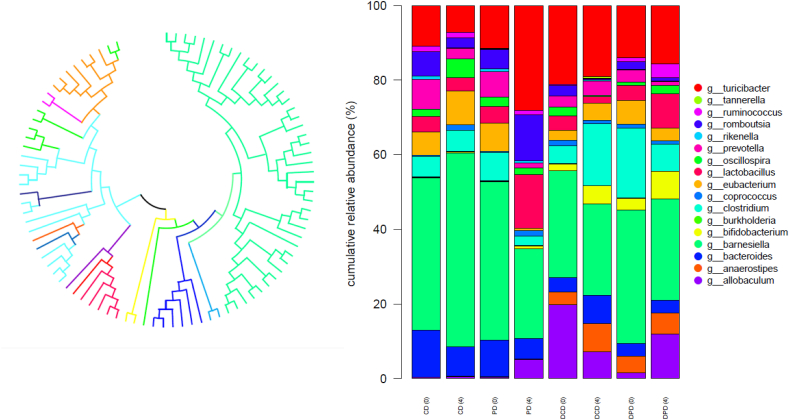
At phyla level, an increased percentage of Actinobacteria in diabetic compared to healthy animals was observed, but yet no significant differences (p > 0.05) in Firmicutes and Bacteroidetes levels between CD and DCD or PD and DPD groups were recorded. At the 4th week of the dietary intervention, a significantly increased population of Firmicutes was observed in healthy animals (PD group) compared to the baseline (p < 0.05, Fig. 3) and to CD group (p < 0.05). Furthermore, Bacteroidetes levels were decreased in PD group at week 4, compared to baseline (p < 0.05) and CD (p < 0.05) group at the same time point. An increased percentage of Actinobacteria was recorded in DPD animals over time (p < 0.01 vs. baseline DPD values). Proteobacteria population, which is the less represented phylum (< 1% abundance), showed no significant differences among the samples (Fig. 3).
Fig. 3.
Taxonomic-binning in phyla level with normalized relative abundances, CD: healthy animals that received the control diet, PD: healthy animals that received the pistachio diet, DCD: diabetic animals that received the control diet, DPD: diabetic animals that received the pistachio diet. Parenthesis indicating the timepoint of the diet, 0: baseline week and 4: after 4 weeks of dietary intervention with pistachio nuts.
The phylogenetic tree showing the evolutionary relationships between the different genera and their distributions among the samples based on the phylogenetic analysis of the representative OTUs is presented in Fig. 4.
Fig. 4.
(a) Phylogenetic tree of representative OTUs, which are represented with different colors, from 16S rRNA analysis constructed using Maximum-Likelihood algorithm in MEGAX-program, different colors represent the variant genera, (b) Taxonomic-binning in genus level with normalized relative abundances, CD: healthy animals that received the control diet, PD: healthy animals that received the pistachio diet, DCD: diabetic animals that received the control diet, DPD: diabetic animals that received the pistachio diet, parenthesis indicating the timepoint of the diet, 0:baseline week and 4: after 4 weeks of dietary intervention with pistachio nuts. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
At genera level, Bacteroides OTUs were more abundant in healthy compared to diabetic animals (p < 0.05 between CD and DCD at week 0, Fig. 4). The 4-week pistachio supplementation resulted in a higher percentage of Turicibacter in PD compared to CD animals (p < 0.05) and Romboutsia levels were raised in PD group compared to CD (p < 0.01), DPD (p < 0.01) and PD groups at baseline (p < 0.05). Pistachio diet had also a significant impact on the presence of Lactobacillus genus in healthy animals (PD), which was higher at the end of the study compared to CD (p < 0.01) and samples of week 0 (p < 0.05). In STZ-induced diabetic rats, the population of Bifidobacterium was increased in DPD compared to DCD group (p < 0.01) and baseline values (p < 0.01, Fig. 4).
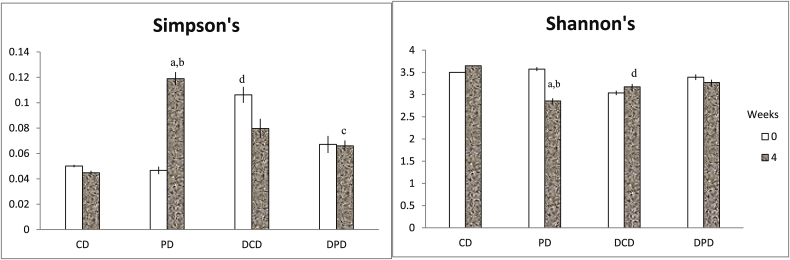
Shannon and Simpson indices were calculated to measure diversity, which reflects the different observed microbiota (Fig. 5), taking into account the number of species present, as well as the relative abundance of each species. Diversity indices provide important information about rarity and commonness of species in a community [22]. The Shannon index assumes all species are represented in a sample and that they are randomly distributed. Increased Shannon indicates increased diversity [33,34]. The Simpson index is based upon the probability that two specimens belong to the same species, and is not affected by rare species [35]. Shannon’s index was decreased in DCD compared to CD group (p < 0.05) at week 4 and Simpson’s index was increased in DCD compared to CD animals (p < 0.01) at week 0. After 4 weeks of dietary supplementation, in PD group, Shannon’s index was significantly decreased compared to baseline (p < 0.01) and to CD group (p < 0.05), and Simpson’s index was increased in both the above mentioned cases (p < 0.01 vs. PD at week 0 and p < 0.01 vs. CD at week 4, Fig. 5).
Fig. 5.
Diversity indices (a) Simpson’s and (b) Shannon’s expressed as mean ± SEM. Data with different superscript letters are significantly different p < 0.05, according to the post hoc ANOVA statistical analysis. ap < 0.05 vs timepoint 0 of the same sample, bp < 0.05 PD vs CD, cp < 0.05 DPD vs PD, dp < 0.05 DCD vs CD.
3.6. Histochemistry
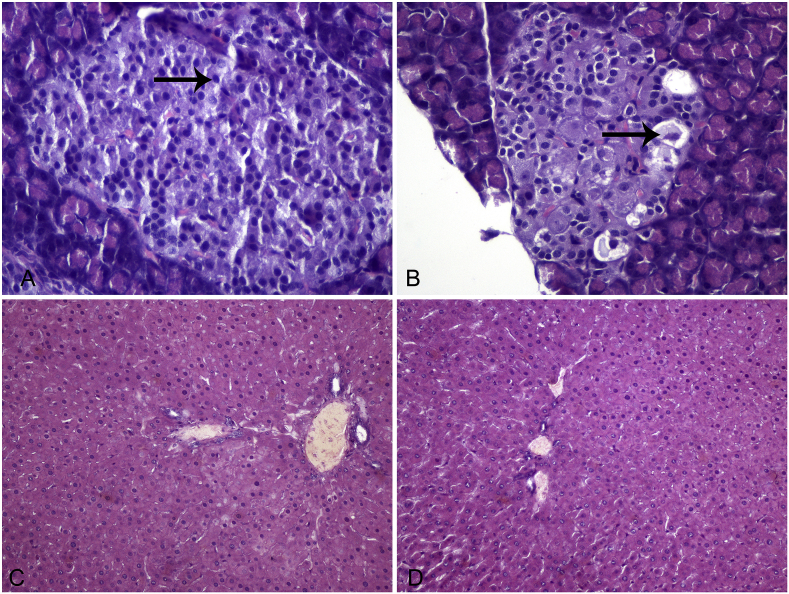
Liver lesions were not observed in any group of animals. Pancreatic lesions were apparent in diabetic animals of both groups, while tissues of CD and PD groups were healthy, as confirmed by the histological examination. Pancreatic lesions were expressed as mean score ± SEM. The scores for β-cell distortion, nuclei uniformity and pycnotic nuclei are presented on Table 3. Vacuolization, necrosis and fatty infiltration were not detected. There were no statistically significant differences between the DCD and DPD groups in any of the measured parameters (p > 0.05 between groups). Photomicrographs of representative liver and pancreatic sections of healthy and diabetic rats are depicted on Fig. 6.
Table 3.
Histological features of pancreas in the animals of the four groups. Values are expressed as mean ± SEM.ap < 0.05 compared to CD, bp < 0.05 compared to PD.
| CD | PD | DCD | DPD | |
|---|---|---|---|---|
| β-cell distortion | 0.34 ± 0.21 | 0.34 ± 0.21 | 2.17 ± 0.31a,b | 1.84 ± 0.17a,b |
| Nuclei uniformity | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.67 ± 0.21a,b | 1.84 ± 0.17a,b |
| Vacuolization | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Necrosis | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Pycnotic nuclei | 0.34 ± 0.21 | 0.34 ± 0.21 | 2.00 ± 0.26a,b | 1.84 ± 0.31a,b |
| Fatty infiltration | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Fig. 6.
Representative sections from pancreas and liver of healthy and STZ-induced diabetic rats. Eosin-hematoxylin stain, 200x. A. Pancreatic section of healthy animal shows a well-preserved cellular architecture. The islets of Langerhans exert a homogenous morphology of β-cells (arrow). B. Pancreatic section of STZ-induced diabetic rat shows degranulation and vacuolation of β-cells (arrow). C, D. Liver sections from healthy (C) and diabetic (D) animals demonstrate normal architecture of the liver parenchyma without evidence of portal inflammation or necrosis.
4. Discussion
Gut microbiota has been recognized as a key environmental factor which contributes to the development of T1D, while the restoration of normal composition of microbial population constitutes a new target for the prevention and treatment of the disease [8,9]. In our study, T1D was induced by intraperitoneal injection of STZ and the effects of a dietary intervention with pistachio nuts on intestinal microbiota composition were determined. The alterations in plasma glucose, serum insulin, body weight, as well as the histopathological examination of pancreas indicated that the T1D model was successful.
STZ is a widely used chemical for the induction of experimental Τ1D in rodents, a disease that does not occur naturally. Its diabetogenic action is primarily caused by the highly specific cytotoxic action on the β-cells of the islets of Langerhans with rapid and irreversible necrosis [36]. The toxicity of STZ to β-cells is observed for a short-time period since it is excreted and further impairment of the surviving β-cells’ function is due to hyperglycemic toxicity [37]. It is currently unclear what the direct effect of STZ-induced T1D development and progression is on the intestinal composition of the host [4]. Although the mechanisms implicated in the onset of T1D in rats may be different than in humans, the events following the β-cell destruction are similar. Therefore, the observed changes in microbial shifts are likely due to the induced diabetes and not to STZ administration.
In STZ-induced diabetic animals, fecal coliforms were significantly higher at baseline, in line with the results of other researchers [38], reporting that fecal coliform counts were increased in the alloxan-induced diabetic rats compared to the control group. E. coli fecal counts were also higher in diabetic rats compared to healthy animals at week 0. Likewise, Ma et al. [39] demonstrated that in STZ-diabetic rats, the E. coli- Shigella genera were increased in fecal samples compared to healthy animals. The results of the present study revealed a functional modulation of gut microbiota by pistachio supplementation. At the end of the 4-week dietary intervention with pistachio nuts, enhancement of beneficial bacteria populations in feces was noticed, such as bifidobacteria and lactobacilli, in both diabetic and healthy animals. In addition, a reduction in enterococci population was observed and E. coli levels were also decreased in diabetic animals. Of note, higher levels of bifidobacteria and lactobacilli have been previously reported in genetically diabetes-resistant rats [40]. Other studies indicated that an increase of lactobacilli levels promotes gut barrier function [41], can reduce body glucose amounts and may be a key-microorganism in the prevention and treatment of T1D. However, a crossover feeding trial [16] with pistachio supplementation in healthy subjects for 18 days showed that although it caused an increase in potentially beneficial butyrate-producing species, it led to decreased levels of lactobacilli. Oleic acid, the monounsaturated fatty acid, which is present in high amounts in pistachio kernels, has been shown to increase fecal bifidobacteria when supplemented in female mice treated with high fat diet [42]. Accordingly, a diet rich in MUFA has been reported to increase the fecal bifidobacteria in volunteers at MetS risk [21,22].
Fecal microbiota reflects the microbiology of the colon, particularly the descending colon and rectum [43], but not all microbes thriving in the gut are equally represented in the feces. However, their extensive use relies on the simple sampling method and the non-invasive nature of this approach. Hence, microbiological analysis of the various intestinal segments was performed in order to investigate potential microbial associations of adherent species to the fecal microbiota. Microbial communities’ composition along the GI tract is determined by gut-segment-specific parameters like pH, oxygen, nutrients availability, etc. Rat intestinal segments, i.e. small intestine and large intestine, constitute different microenvironments and harbor variant microbial genera, as diverse genera respond differently in the complex GI environment [44]. Our study revealed distinct alterations in adherent microbiota composition in the different intestinal segments in both diabetic and healthy animals. Importantly, in jejunum and ileum, STZ-induced diabetes caused a significant increase in Enterobacteriacae and coliforms levels. Colon microbiota analysis revealed that enterococci population was significantly lower in diabetic group compared to healthy animals receiving the control diet. Cecal and colonic coliforms and E. coli counts were lower in STZ-induced diabetic rats (DCD group) compared to the control group (CD), a finding that is not consistent with the results of the fecal microbiota and the small intestine. Li et al. [27] reported that despite the large number of overlapping OTUs, the luminal and mucosal microbial communities present notably radial segregation, specifically in the rat lower GI tract. In ileum and jejunum, pistachio supplementation reduced the populations of Enterobacteriacae and coliforms to almost normal levels and resulted in higher counts of bifidobacteria and lactobacilli. In caecum, the dietary intervention with pistachio led to a decrease in the levels of enterococci, coliforms and E. coli, both in diabetic and healthy animals. Reaching the colon of diabetic rats, no significant effects of pistachio treatment were recorded, whereas in healthy animals, increased levels of bifidobacteria and lactobacilli along with reduced counts of enterococci, coliforms, E. coli and staphylococci were observed. In another study [45], similar levels of E. coli and enterococci were reported in ileum, and colon in diabetic prone mice administered with human milk, compared to the control group, while in caecum, E. coli numbers were decreased, proposing the differential behavior of the different intestinal segments to the same treatment. Moreover, fecal bifidobacteria levels were increased in diabetic mice supplemented with human milk, in line with the results of the present study, recording higher numbers of fecal bifidobacteria in DPD rats.
Stool samples are often used to investigate the intestinal microbiota, since they are easily collected. Nevertheless, the degree to which the composition and function of the fecal differ from mucosal microflora remains unclear. In the present study, fecal microbial populations were in agreement with the attached microbial associations at the small intestine (jejunum and ileum), in contrast to the microbiota of the colon. Similar findings were previously published by Kohl et al. [46], suggesting that fecal bacterial communities were significantly different by colonic communities, but were most similar to the small intestine community structure. The colon consists of several distinct parts (proximal, mid and distal) and the term “intestinal microbiota” is used to refer to the different microbial populations, both the luminal and the mucosa-associated microbiota. The mucus varies throughout the colon and consists of variant microenvironments that can host diverse microbial species [47], while fecal microbiota represents a combination of mucosal bacteria and a separate non adherent luminal population [48]. Thus, differences among the fecal microbiota and the microbial populations attached and/or colonized in the intestinal tissues are fully justified [27,46].
Considering the limitations of the culture-based methods, molecular analysis on fecal samples was also carried out, in order to further validate our results. 16S rRNA sequencing of fecal samples revealed that Actinobacteria levels were increased in diabetic animals compared to the control group, in line with other studies [4,39,49], documenting an increased presence of Actinobacteria in STZ-induced T1D rat fecal microbiota. Ma et al. [39], reported that Firmicutes and Bacteroidetes were the main phyla consisting the microbiome of healthy, as long as diabetic rats, but no significant differences between the two groups were noted, in agreement with our results. In contrast, according to Patterson et al. [4], diabetic disease state leads to microbial shifts and STZ-induced T1D is linked with a reduction of Firmicutes and an increase of Bacteroidetes levels accompanied by a diminished diversity. In this vein, pistachio diet had an impact on the main phyla of the microbiome. In healthy animals, after 4 weeks of dietary supplementation, Firmicutes population was increased, while Bacteroidetes levels were decreased. In vitro assays have shown a favorable effect of catechins and epicatechins on the Firmicutes/Bacteroidetes ratio [20,50]. Experiments in the intestinal microbiome of naked-mole rats [51], which are well known for their long life expectancy and cancer resistance, suggested that a diet rich in polyphenols is the principal key factor for their longevity. The intestinal microflora consisted mainly of Firmicutes and Bacteroidetes, but low amounts of Proteobacteria and Actinobacteria were also identified.
Levels of the bacterial genus Bacteroides, a producer of SCFAs which offers a beneficial advantage in the host when present in the gut [52], were increased in healthy compared to STZ-induced diabetic rats. However, in newly diagnosed T1D children, high levels of Bacteroides have been reported [53]. After the 4 weeks of dietary intervention, Turicibacter that is positively related to production of butyric acid and potential probiotic properties [54], and Romboutsia [55], a natural and abundant inhabitant of the rat small intestine, were present in higher levels in healthy animals that received the pistachio nuts compared to the animals of the control group. In a previous dietary intervention study, the above mentioned genera were higher in rats fed with barley malt and were also associated with the production of butyric acid, which is related to improved metabolic parameters [56]. Increase of Bacteroides population has been observed in obese mice after administration of potential prebiotic arabinoxylan from wheat [57]. However, pistachio nuts did not enhance Bacteroides levels in T1D rats. Moreover, the percentage of the beneficial Lactobacillus and Bifidobacterium genera were significantly enhanced in both healthy and STZ-induced diabetic rats receiving the pistachio diet. A positive association between the frequency of consumption of fruits and vegetables with Lactobacillus, Clostridium coccoides and Prevotella populations has been reported previously [20,58], while an increase in the Lactobacillus/Enterococcus group has been observed with polyphenol-rich grape seed extract [20,59]. Phenolic compounds are metabolized by intestinal microbiota, affecting their absorption and determine their functionality, but on the other hand, their metabolites influence the growth of certain bacterial species. In agreement with our study, Liu et al. [60], witnessed a significant increase in the populations of Bifidobacterium and Lactobacillus in fecal samples, as a consequence of almond or almond skin supplementation in healthy humans.
In the present study, the Shannon and Simpson diversity indices highlighted a significant reduction in diversity in diabetic animals, which is in accordance with the results of other researchers [4,8], reporting a reduced diversity in T1D rats compared to the healthy animals [39]. Increased diversity is a characteristic of healthy individuals and low bacterial diversity has been linked to obesity and inflammatory bowel disease [61]. According to our results, the 4-week dietary intervention resulted also in a decrease in diversity. Similarly, inulin-type fructans dietary intervention, considered as prebiotic fibers, revealed a decreased alpha-diversity in both healthy humans and in obese individuals [61,62]. Further research is expected to provide more insights on the effect of dietary intervations on fecal and intestinal microbiome diversity of STZ-induced animal models.
It is well established that Bifidobacterium genus holds a key-role in maintaining GI tract health through supplementation of butyrate-producing species with lactate and acetate, as well as the enhancement of intestinal epithelial barrier function [63,64]. A significant reduction of Bifidobacterium species has been reported for children with β-cell autoimmunity compared to autoantibody-negative children [63]. In addition, in high fat-fed diabetic mice treated with prebiotic oligofructose, Bifidobacterium spp. was positively correlated with improved glucose tolerance, glucose-induced insulin secretion and normalized inflammatory tone [65].
In our study, pistachio did not induce alterations in glucose or insulin of diabetic animals, but it enhanced the presence of microbes that are involved in inflammatory responses. Bacterial metabolites, such as the SCFAs, affect various systems, including gut and immune homeostasis, immunological responses and T-lymphocytes, as well as the control of inflammation [66]. It is likely that pistachio can serve as a potential prebiotic fiber that promotes the balance of microbiota involved in pro- and anti-inflammatory responses implicated in T1D. The duration of the intervention and the high levels of glucose caused by the STZ protocol are factors which could probably be related to the results, indicating no effects on biochemical parameters.
Α limitation of the study was that parameters associated with inflammatory or anti-inflammatory action were not determined. Gut bacteria are an important source of polyamines, which exhibit anti-inflammatory action, such as putrescine and spermine [67]. It has been reported that the administration of Bifidobacteria LKM512 to elderly people increases intestinal polyamine concentrations [68]. It has also been shown that the prevalence of Bacteroides spp. within Finnish and Estonian infants is associated with early-onset autoimmune disease through the production of a type of lipopolysaccharide that inhibits innate immune signaling and endotoxin tolerance [69].
Future studies, including the determination of inflammatory factors, as well as gut microbiota metabolites are needed, in order to clarify the underlying mechanisms. Regular consumption of pistachios by T1D patients could possibly affect gut microbiota composition, leading to beneficial changes for host metabolism with a long-term impact. However, the complexity of gut microbiota, the heterogeneity of bioactive compounds of foods and the fact that the effect of dietary components certainly depends on the immune system of the host, makes the understanding of gut microbiota-food interaction and their relation with the pathogenesis of the disease an intriguing issue.
Ethics approval
Animal experimentation was reviewed and approved by the Veterinary Directorate of the Athens Prefecture (Ref. Number 2057/05-04-2017) and conducted in compliance with the European Directive 2010/63.
Availability of data and materials
The datasets of the current study are available from the corresponding author on request.
Funding sources
Part of the research was co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme “Human Resources Development, Education and Lifelong Learning 2014–2020” in the context of the project “Strengthening Human Resources Research Potential via Doctorate Research – 2nd Cycle” (MIS 5000432).
We acknowledge support of this work by the project “Research Infrastructure on Food Bioprocessing Development and Innovation Exploitation – Food Innovation RI” (MIS 5027222), which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund).
CRediT authorship contribution statement
Amalia E. Yanni: Conceptualization, Writing - original draft, Data curation, Formal analysis. Gregoria Mitropoulou: Data curation, Formal analysis. Ioanna Prapa: Data curation, Formal analysis. Georgios Agrogiannis: Data curation, Formal analysis. Nikolaos Kostomitsopoulos: Data curation, Formal analysis. Eugenia Bezirtzoglou: Writing - original draft. Yiannis Kourkoutas: Conceptualization, Writing - original draft, Data curation. Vaios T. Karathanos: Conceptualization.
Declaration of competing interest
Authors declare that they have no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2020.100040.
List of abbreviations
- GI
gastrointestinal
- HDLC
high density lipoproteins’ cholesterol
- MetS
metabolic syndrome
- MUFA
monounsaturated fatty acids
- NGS
next generation sequencing
- OTU
operational taxonomic unit
- PCR
polymerase chain reaction
- PDO
protected designation of origin
- STZ
streptozotocin
- T1D
type 1 diabetes
- TAC
total aerobic counts
- TAG
triacylglycerols
- TC
total cholesterol
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Round J.L., Mazmanian S.K. The gut microbiome shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galland L. The gut microbiome and the brain. J Med Food. 2014;17(12):1261–1272. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohail M.U., Althani A., Anwar H., Rizzi R., Marei1 H.E. Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. J Diabetes Res. 2017;2017:1–7. doi: 10.1155/2017/9631435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson E., Marques T., O’Sullivan O., Fitzgerald P., Fitzgerald G., Cotter P. Streptozotocin-induced type-1-diabetes disease onset in Sprague–Dawley rats is associated with an altered intestinal microbiota composition and decreased diversity. Microbiology. 2015;161:182–193. doi: 10.1099/mic.0.082610-0. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrini S., Sordi V., Bolla A.M., Saita D., Ferrarese R., Canducci F. Duodenal mucosa of patients with type 1 diabetes shows distinctive inflammatory profile and microbiota. J Clin Endocrinol Metab. 2017;102(5):1468–1477. doi: 10.1210/jc.2016-3222. [DOI] [PubMed] [Google Scholar]
- 6.Leiva-Gea I., Sánchez-Alcoholado L., Martín-Tejedor B., Castellano-Castillo D., Moreno-Indias I., Urda-Cardona A. Gut microbiota differs in composition and functionality between children with type 1 diabetes and MODY2 and healthy control subjects: a case-control study. Diabetes Care. 2018;41(11):2385–2395. doi: 10.2337/dc18-0253. [DOI] [PubMed] [Google Scholar]
- 7.Cinek O., Kramna L., Mazankova K., Odeh R., Alassaf A., Ibekwe M.U. The bacteriome at the onset of type 1 diabetes: a study from four geographically distant African and Asian countries. Diabetes Res Clin Pract. 2018;144:51–62. doi: 10.1016/j.diabres.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Gomes A.C., Bueno A.A., Machado de Souza R.G., Mota J.F. Gut microbiota, probiotics and diabetes. Nutr J. 2014;13:60. doi: 10.1186/1475-2891-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulden E., Wong S.F., Wen L. The gut microbiota and Type 1 Diabetes. Clin Immunol. 2015;159:143–153. doi: 10.1016/j.clim.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh P.J., Ridaura V.K., Faith J.J., Rey F.E., Knight R., Gordon J.I. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6–14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albenberg L.G., Wu G.D. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146(6):1564–1572. doi: 10.1053/j.gastro.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C., Zhang M., Wang S., Han R., Cao Y., Hua W. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 13.Faith J.J., McNulty N.P., Rey F.E., Gordon J.I. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science. 2011;333:101–104. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Legarrea P., Fuller N.R., Zulet M.A., Martinez J.A., Caterson I.D. The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pac J Clin Nutr. 2014;23(3):360–368. doi: 10.6133/apjcn.2014.23.3.16. [DOI] [PubMed] [Google Scholar]
- 15.Commission Regulation (EC) No 1263/96 of 1 July 1996 supplementing the Annex to Regulation (EC) No 1107/96 on the registration of geographical indications and designations of origin under the procedure laid down in Article 17 of Regulation (EEC) No 2081/92.Official Journal of the European Union L 163, 02/07/1996 P. 0019 – 0021..
- 16.Ukhanova M., Wang X., Baer D.J., Novotny J.A., Fredborg M., Mai V. Effects of almond and pistachio consumption on gut microbiota composition in a randomized cross-over human feeding study. Br J Nutr. 2014;111(12):2146–2152. doi: 10.1017/S0007114514000385. [DOI] [PubMed] [Google Scholar]
- 17.Gentile C., Tesoriere L., Butera D., Fazzari M., Monastero M., Allegra M. Antioxidant activity of Sicilian pistachio (Pistacia vera L. var. Bronte) nut extract and its bioactive components. J Agric Food Chem. 2007;55(3):643–648. doi: 10.1021/jf062533i. [DOI] [PubMed] [Google Scholar]
- 18.Tokuşoglu O., Unal M.K., Yemiş F. Determination of the phytoalexin resveratrol (3, 5, 4’-trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatography-mass spectrometry (GC-MS) J Agric Food Chem. 2005;53(12):5003–5009. doi: 10.1021/jf050496+. [DOI] [PubMed] [Google Scholar]
- 19.Kalogeropoulos N., Chiou A., Ioannou M.S., Karathanos V.T. Nutritional evaluation and health promoting activities of nuts and seeds cultivated in Greece. Int J Food Sci Nutr. 2013;64(6):757–767. doi: 10.3109/09637486.2013.793298. [DOI] [PubMed] [Google Scholar]
- 20.Valdés L., Cuervo A., Salazar N., Ruas-Madiedo P., Gueimonde M., González S. The relationship between phenolic compounds from diet and microbiota: impact on human health. Food Funct. 2015;6(8):2424–2439. doi: 10.1039/c5fo00322a. [DOI] [PubMed] [Google Scholar]
- 21.Fava F., Gitau R., Griffin B.A., Gibson G.R., Tuohy K.M., Lovegrove J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes. 2013;37:216–223. doi: 10.1038/ijo.2012.33. [DOI] [PubMed] [Google Scholar]
- 22.Silva Figueiredo P., Carla Inada A., Marcelino G., Maiara Lopes Cardozo C., de CássiaFreitas K., de Cássia Avellaneda Guimarães R. Fatty acids consumption: the role metabolic aspects involved in obesity and its associated disorders. Nutrients. 2017;9(10):1158. doi: 10.3390/nu9101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamuel-Raventos R.M., St Onge M.P. Prebiotic nut compounds and human microbiota. Crit Rev Food Sci Nutr. 2017;57(14):3154–3163. doi: 10.1080/10408398.2015.1096763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu K.K., Huan Y. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2008;40 doi: 10.1002/0471141755.ph0547s40. 5.47.1-5.47.14. [DOI] [PubMed] [Google Scholar]
- 25.Faul F., Erdfelder E., Lang A.G., Buchner A.G. ∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 26.Nagy Z.T. A hands-on overview of tissue preservation methods for molecular genetic analyses. Org Divers Evol. 2010;10:91–105. [Google Scholar]
- 27.Li D., Chen H., Mao B., Yang Q., Zhao J., Gu Z. Microbial biogeography and core microbiota of the rat digestive tract. Sci Rep. 2017;7:e45840. doi: 10.1038/srep45840. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagkouvardos I., Fischer S., Kumar N., Clavel T. Rhea: a transparent and modular R pipeline for microbial profiling based on 16S rRNA gene amplicons. Peer J. 2017;5 doi: 10.7717/peerj.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGAX: molecular evolutionary genetics analysis accros computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanni A.E., Efthymiou V., Lelovas P., Agrogiannis G., Kostomitsopoulos N., Karathanos V.T. Effects of dietary Corinthian currants (Vitis vinifera L., var. Apyrena) on atherosclerosis and plasma phenolic compounds during prolonged hypercholesterolemia in New Zealand White rabbits. Food Funct. 2015;6:963–971. doi: 10.1039/c4fo01106f. [DOI] [PubMed] [Google Scholar]
- 31.Brunt E.M., Kleiner D.N., Wilson L.A., Belt P., Neuschwander-Tetri B.E. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandhi G.R., Ignasimuthu S., Paulraj M.G. Hypoglycemic and β-cells regenerative effects of Aeglemarmellos (L.) Corr. bark extract in streptozotocin induced diabetic rats. Food ChemToxicol. 2012;50:1667–1674. doi: 10.1016/j.fct.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 33.Spellerberg I.F., Fedor J.P. A tribute to Claude Shannon (1916-2001) and a plea for more rigorous use of species richness, species diversity and the’Shannon-Wiener’ Index. Global Ecol Biogeogr. 2003;12:177–179. [Google Scholar]
- 34.Shannon C.E. A mathematical theory of communication. ACM SIGMOB - Mob Comput Commun Rev. 2001;5:3–55. [Google Scholar]
- 35.Simpson E.H. Measurement of diversity. Nature. 1949;163:688. [Google Scholar]
- 36.Junod A., Lambert A.E., Orci L., Pictet R., Gonet E.A., Renold A.E. Studies of the diabetogenic action of streptozotocin. Proc. Soc. Exp. Biol. Med. 1967;126:201. doi: 10.3181/00379727-126-32401. [DOI] [PubMed] [Google Scholar]
- 37.Wu J., Yan L.J. vol. 8. Dovepress; 2015. pp. 181–188. (STZ-induced T1D in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangwan V., Tomar S.K., Alo B., Singh R.R.B., Ashish S. Hypoglycaemic effect of galactooligosaccharides in alloxan-induced diabetic rats. J Dairy Res. 2015;82:70–77. doi: 10.1017/S0022029914000582. [DOI] [PubMed] [Google Scholar]
- 39.Ma Q., Li Y., Wang J., Li P., Duan Y., Dai H. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomed Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109873. [DOI] [PubMed] [Google Scholar]
- 40.Roesch L.F.W., Lorca G.L., Casella G., Giongo A., Natanjo A., Pionzio A.M. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. ISME J. 2009;3:536–548. doi: 10.1038/ismej.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen K., Chen H., Faas M.M., de Haan B.J., Li J., Xiao P. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function and microbiota homeostasis. Mol Nutr Food Res. 2017;61(8):1–35. doi: 10.1002/mnfr.201601006. [DOI] [PubMed] [Google Scholar]
- 42.Mujico J.R., Baccan G.C., Gheorghe A., Diaz L.E., Marcos A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110:711–720. doi: 10.1017/S0007114512005612. [DOI] [PubMed] [Google Scholar]
- 43.Ignyś I., Szachta P., Gałęcka M., Schmidt M., Pazgrat-Patan M. Methods of analysis of gut microorganism--actual state of knowledge. Ann Agric Environ Med. 2014;21(4):799–803. doi: 10.5604/12321966.1129936. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki T.A., Nachman M.W. Spatial heterogeneity of gut microbial composition along the gastrointestinal tract in natural populations of house mice. PloS One. 2016;11(9) doi: 10.1371/journal.pone.0163720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sane F., Scuotto A., Pierrat V., Kacet N., Hober D., Romond M.B. Diabetes progression and alterations in gut bacterial translocation: prevention by diet supplementation with human milk in NOD mice. J Nutr Biochem. 2018;62:108–122. doi: 10.1016/j.jnutbio.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Kohl K.D., Miller A.W., Marvin J.E., Mackie R., Dearing M.D. Herbivorous rodents (Neotoma spp.) harbour abundant and active foregut microbiota. Environ Microbiol. 2014;16(9):2869–2878. doi: 10.1111/1462-2920.12376. [DOI] [PubMed] [Google Scholar]
- 47.Tropini C., Earle K.A., Huang K.C., Sonnenburg J.L. The gut microbiome: connecting spatial organization to function. Cell Host Microbe. 2017;21:433–442. doi: 10.1016/j.chom.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheng Y., Zheng S., Ma T., Zhang C., Ou X., He X. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microfora. Sci Rep. 2017;7 doi: 10.1038/s41598-017-12245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuohy K.M., Conterno L., Gasperotti M., Viola R. Upregulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J Agric Food Chem. 2012;60:8776–8782. doi: 10.1021/jf2053959. [DOI] [PubMed] [Google Scholar]
- 51.Debebe T., Holtze S., Morhart M., Hildebrandt B., Rodewald S., Huse K. Analysis of cultivable microbiota and diet intake pattern of the long-lived naked mole-rat. Gut Pathog. 2016;8(25):1–9. doi: 10.1186/s13099-016-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wexler M.H. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20(4):529–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mejía-León M.E., Petrosino J.F., Ajami N.J., Dominguez M.G., de la Barca A.M.C. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci Rep. 2014;4:1–5. doi: 10.1038/srep03814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo X., Li J., Tang R., Zhang G., Zeng H., Wood R.J. High fat diet alters gut microbiota and the expression of Paneth cell-antimicrobial peptides preceding changes of circulating inflammatory cytokines. Mediat Inflamm. 2017;2017 doi: 10.1155/2017/9474896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerritsen J., Hornung B., Renckens B., van Hijum S., Martins dos Santos V., Rijkers G. Genomic and functional analysis of Romboutsia ilealis CRIBT reveals adaptation to the small intestine. Peer J. 2017;5 doi: 10.7717/peerj.3698. e3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong Y., Nyman M., Fak F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res. 2015;59:2066–2076. doi: 10.1002/mnfr.201500187. [DOI] [PubMed] [Google Scholar]
- 57.Neyrinck A.M., Possemiers S., Druart C., Van de Wiele T., De Backer F., Cani P.D. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, roseburia and Bacteroides/Prevotella in diet-induced obese mice. PloS One. 2011;6(6) doi: 10.1371/journal.pone.0020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.La-Ongkham O., Nakphaichit M., Leelavatcharamas V., Keawsompong S., Nitisinprasert S. Distinct gut microbiota of healthy children from two different geographic regions of Thailand. Arch Microbiol. 2015;197:561–573. doi: 10.1007/s00203-015-1089-0. [DOI] [PubMed] [Google Scholar]
- 59.Cueva C., Sanchez-Patan F., Monagas M., Walton G.E., Gibson G.R., Martin-Alvarez P.J. In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: changes in microbial groups and phenolic metabolites. FEMS Microbiol Ecol. 2013;83:792–805. doi: 10.1111/1574-6941.12037. [DOI] [PubMed] [Google Scholar]
- 60.Liu Z., Lin X., Huang G., Zhang W., Rao P., Ni L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe. 2014;26:1–6. doi: 10.1016/j.anaerobe.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Byerley L.O., Samuelson D., Blanchard I.V.E., Luo M., Lorenzen B.N., Banks S. Changes in the gut microbial communities following addition of walnuts to the diet. J Nutr Biochem. 2017;48:94–102. doi: 10.1016/j.jnutbio.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicolucci A.C., Hume M.P., Martínez I., Mayengbam S., Walter J., Reimer R.A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153:711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 63.de Goffau M.C., Luopajärvi K., Knip M., Ilonen J., Ruohtula T., Härkönen T. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. 2013;62(4):1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitsuoka T. Bifidobacteria and their role in human health. J Ind Microbiol Biotechnol. 1990;6 263–267.34. [Google Scholar]
- 65.Cani P.D., Neyrinck A.M., Fava F., Knauf C., Burcelin R.G., Tuohy K.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 66.Mackay C.R., Rose I.R. sixth ed. Academic Press; 2020. The autoimmune diseases; pp. 331–342. [Google Scholar]
- 67.Blander J.M., Longman R.S., Iliev I.D., Sonnenberg G.F., Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. 2017;18(8):851–860. doi: 10.1038/ni.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kibe R., Kurihara S., Sakai Y., Suzuki H., Ooga T., Sawaki E. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep. 2014;4 doi: 10.1038/srep04548. e4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vatanen T., Kostic A.D., d’Hennezel E., Siljander H., Franzosa E.A., Yassour M. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of the current study are available from the corresponding author on request.