Abstract
Steatosis, a condition characterized by excessive lipid deposition. Although usually a benign condition, steatosis mays progress to cirrhosis or hepatocellular carcinoma. Recent evidence suggests that SGLT2 inhibitors suppress the development of nonalcoholic steatohepatitis in humans, as well as in rodent models and that SGLT2 inhibitors alleviate hepatic steatosis or steatohepatitis in obese type 2 diabetic rats or mice. 14 Patients with nonalcoholic fatty liver disease used a fixed dose of 10 mg of dapagliflozin for an average of 75 days. ALT, AST, GGT, insulin, HOMA-IR, and weight levels were significantly lower after treatment. There was no significant correlation between the reduction in HOMA and the reduction in ALT values or weight reduction obtained during treatment and ALT values.
Keywords: Nonalcoholic fatty liver disease, Steatohepatitis, Dapagliflozin, SGLT2 inhibitors
Highlights
-
•
Dapagliflozin significantly reduced: alanine transaminase (ALT), aspartate transaminase (AST) and gamma-glutamyltransferase (GGT).
-
•
Dapagliflozin in patients without type 2 diabetes mellitus significantly reduced: insulin, HOMA-IR and weight levels.
-
•
The absence of correlation between reduction of ALT and HOMA-IR or weight indicate possible direct action of dapagliflozin.
-
•
Dapagliflozin was well tolerated in non-diabetic patients without reported adverse effects.
1. Introduction
Steatosis, a condition characterized by excessive lipid deposition, is a widespread condition, with prevalence rates reaching from 9% to 46% in the United States [1,2], depending on diagnostic criteria.
After ruling out alcoholic, deposit, autoimmune or viral diseases or drug hepatotoxicity, we can establish the diagnosis of nonalcoholic fatty liver disease (NAFLD) [3]; the presence of inflammatory activity is, then, referred as nonalcoholic steatohepatitis (NASH) [4,5].
Most patients are asymptomatic, as the initial diagnosis is usually made through incidental imaging and biochemical exams, unraveling increased liver transaminases; the usual pattern is alanine transaminase (ALT) higher than aspartate transaminase (AST), as well as elevated gamma-glutamyltransferase (GGT), if steatohepatitis is already present [5]. The progression from simple steatosis to NASH occurs in about 40% of patients at a 6-year follow-up [6].
Although usually a benign condition, steatosis mays progress to cirrhosis in up to 25% of patients in twenty years [7]; it can also progress to hepatocellular carcinoma (HCC) in up to 3% in a 21-year follow-up [8].
As an initial measure, weight loss of more than 10% is required to achieve an improvement in histologic necroinflammatory activity [3]. Pharmacological treatment aims for patients with necroinflammatory activity; despite best efforts, there is still no label-approved drug for the treatment of NASH. Several drugs have been tested, but the best evidence of necroinflammatory improvement comes from vitamin E and pioglitazone [3].
Recent evidence suggests that SGLT2 inhibitors suppress the development of NASH in humans, as well as in rodent models and that SGLT2 inhibitors alleviate hepatic steatosis or steatohepatitis in obese type 2 diabetic rats or mice [[9], [10], [11], [12]]. Still, the use of this class of anti-diabetic agents is limited in non-diabetic patients.
2. Methods
Data was collected from private practice records, from July 2017 to July 2019, of patients with nonalcoholic fatty liver disease (NAFLD), who were prescribed dapagliflozin. Patients were diagnosed by general Abdominal ultrasound, confirming the presence of steatosis, in the absence of other potential causes, such as excessive alcohol intake (considering a limit of 21 drinks per week for men and 14 drinks per week for women for a period of two years), viral hepatitis (patients had negative hepatitis B and C serology), autoimmune hepatitis (patients had autoantibodies - ANF, anti LKM-1, anti smooth muscle, anti SLA and anti mitochondria dosages), deposit diseases (normal dosages of serum iron, ferritin, copper and ceruloplasmin).
After excluding other causes of hepatic steatosis, we also excluded from our analysis patients with type 2 diabetes mellitus, using the American Diabetes Association [13] diagnostic criteria; patients with altered fasting blood glucose (100 mg/dL to 125 mg/dL or 5.7%–6.4% glycated hemoglobin) would repeated the exams and an oral glucose tolerance test was performed to rule out diabetes.
Patients who had used medications with potential to improve steatohepatitis in the last 6 months, such as vitamin E, metformin, pioglitazone, silymarin or GLP-1 analogs, were also excluded from the analysis.
After applying the inclusion and exclusion criteria, of the 48 initial medical records, 14 were eligible for analysis. Comparisons were performed by paired Student’s t-test (considering α = 0.05) of the levels of ALT, AST, GGT, glucose, weight and BMI, insulin and HOMA-IR, before and after dapagliflozin. The Pearson test was performed to evaluate the correlation of GPT and HOMA-IR variation with weight, once these factors are known to have a positive impact on the inflammatory profile of steatosis. The t-test (Paired T-Test) calculations were performed by PRISM8 and Pearson by socscistatistics.com.
The patients used a fixed dose of 10 mg of dapagliflozin for an average of 75 days (60–90 days). The sample consisted of 10 men and 4 women with a mean age of 41 years (21–64). 7 patients had hypertension, 2 had dyslipidemia, 1 had asthma and 1 had anxiety disorder.
3. Results
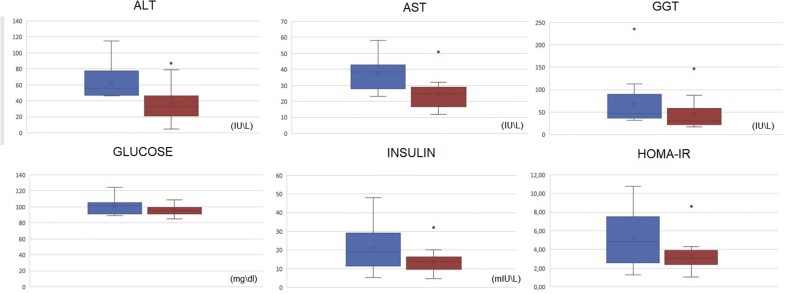
The results of ALT, AST, GGT, glucose, insulin and HOMA-IR levels before and after dapagliflozin (10 mg) for 75 days are shown in Fig. 1.
Fig. 1.
Results of the levels measured before and after dapagliflozin (10 mg) for 75 days presented in boxplots. The upper limits, Q3, median, Q1 and lower limit of the results before (blue) and after (red) use are presented. Results outside these limits were presented as points (outliers). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
ALT (P < 0.0001), AST (P = 0.0006), GGT (P = 0.0009), insulin (P = 0.0004), HOMA-IR (P = 0.0005), and weight (P = 0.0001) levels were significantly lower after treatment. The mean ALT for pre-treatment patients was 62.29 U/L and after, there was a mean reduction to 36.71 U/L. The reduction was from 18.95 to 32.2 U/L (95% CI). The mean AST for pre-treatment patients was 37 U/L and after treatment, there was a mean reduction to 24.64 U/L. The reduction was from 6.4 to 18.31 U/L (95% CI). The average GGT of the pre-treatment patients was 68.5 U/L and after there was a mean reduction to 44.71 U/L. The reduction was from 11.7 to 35.87 U/L (95% CI). The average insulin of the pre-treatment patients was 21.08 mU/L and after there was a mean reduction to 13.79 mU/L. The reduction was from 3.92 to 10.66 mU/L (95% CI). The mean HOMA-IR of the pre-treatment patients was 5.21 and after there was a mean reduction to 3.27 U/L. The reduction was from 1.03 to 2.84 U/L (95% CI). The average weight of the pre-treatment patients was 98.6 kg and after there was a mean reduction to 93.3 kg. The reduction was from 2.53 to 5.89 kg (95% CI). The blood glucose was the only parameter that was unchanged after dapagliflozin use, the average of pre-treatment patients was 100.79 mg/dL and after treatment 95.5 mg/dL being this non-significant reduction (P = 0.075).
Regarding possible confounding factors, there was no significant correlation between the reduction in HOMA and the reduction in ALT values (P = 0.63); similarly, no correlation was found between weight reduction obtained during treatment and ALT values (P = 0.99).
4. Discussion
Different clinical trials performed in patients with T2DM [14] found a reduction in AST and ALT levels with administration of empagliflozin 10 or 25 mg ∖ day. The reduction was more pronounced in patients with elevated liver transaminases levels before use. The authors concluded that such reductions occurred independently of weight and glycemic values, similar to the results obtained in this study. The non-correlation between ALT improvement with weight reduction and HOMA-IR improvement also found in this study indicates a possible direct effect of dapagliflozin on the inflammatory profile of steatosis, refuting an indirect effect of weight reduction.
A clinical trial [15] using dapagliflozin in T2DM patients reduced levels of liver injury biomarkers, including ALT, AST, and GGT; combining carboxylic acids (OM-3CA) with dapagliflozin resulted in a significant reduction in fat content in the liver.
Lundkvist et al., 2016 compared the use of dapagliflozin and placebo for 24 weeks in non-diabetic obese patients and found significant differences between groups in weight and systolic pressure, with good tolerability by the patients. Similarly [16], reported a case in a non-diabetic patient who over 13 weeks administering 300 mg canagliflozin once daily achieved reduction: 3.2 kg weight body, visceral fat in 1.5%, waist circumference in 5 cm, uric acid level to 63.01% and a slight increase in bone mineral density.
Moreover, a RCT [17] evaluated 335 patients receiving canagliflozin and phentermine (300 mg and 15 mg respectively), canagliflozin (300 mg) and placebo in overweight and obese non-diabetic subjects for 26 weeks. The medication was well tolerated with a low incidence of discontinuation due to adverse events (mainly caused by fungal infections). All this data reassures the safety of this class of antidiabetic agents, in non-diabetic patients, as well as efficacy in weight loss, another important target in NAFLD.
5. Conclusion
To our extent of knowledge, this is the first report of the potential efficacy of an SGLT-2 inhibitor in the context of NAFLD in patients without diabetes mellitus. Our results were similar to previous results in diabetic patients, showing a significant drop in the ALT levels (a classic inflammation marker in NASH), that appears to be unrelated to weight loss or insulin sensitivity improvement. Our main limitations were the low number of patients and the lack of a control group, but still, this should serve as background for future randomized clinical trials.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution statement
L.R.S performed the extraction of medical records data and performed the final review of the article. R.B.F performed the statistical tests and wrote the article.
CRediT authorship contribution statement
Lucas Ribeiro dos Santos: Conceptualization, Investigation, Methodology, Project administration. Ricardo Baer Filho: Data curation, Formal analysis, Validation, Writing - original draft, Writing - review & editing.
Declaration of competing interest
None.
References
- 1.Goh G.B., McCullough A.J. Natural history of nonalcoholic fatty liver disease. Dig Dis Sci. 2016 May;61(5):1226–1233. doi: 10.1007/s10620-016-4095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams L.A., Lindor K.D. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007 Nov;17(11):863–869. doi: 10.1016/j.annepidem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018 Jan;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 4.Dam-Larsen S., Franzmann M., Andersen I.B., Christoffersen P., Jensen L.B., Sørensen T.I., Becker U., Bendtsen F. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004 May;53(5):750–755. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., Natale S., Vanni E., Villanova N., Melchionda N., Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003 Apr;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 6.McPherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999 Jun;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 8.White D.L., Kanwal F., El-Serag H.B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012 Dec;10(12):1342–1359.e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jojima T., Tomotsune T., Iijima T., Akimoto K., Suzuki K., Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP -4 inhibitor), prevents steatohepatitis in a novel mouse model of non -alcoholic steatohepatitis and diabetes. Diabetol Metab Syndrome. 2016 Jul 26;8:45. doi: 10.1186/s13098-016-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tahara A., Kurosaki E., Yokono M., Yamajuku D., Kihara R., Hayashizaki Y., Takasu T., Imamura M., Li Q., Tomiyama H., Kobayashi Y., Noda A., Sasamata M., Shibasaki M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Komiya C., Tsuchiya K., Shiba K., Miyachi Y., Furuke S., Shimazu N., Yamaguchi S., Kanno K., Ogawa Y. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiang S., Nakatsu Y., Seno Y., Fujishiro M., Sakoda H., Kushiyama A., Mori K., Matsunaga Y., Yamamotoya T., Kamata H., Asano T. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndrome. 2015;7:104. doi: 10.1186/s13098-015-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2019;42(Suppl. 1):S13. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 14.Sattar N., Fitchett D., Hantel S., George J.T., Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia. 2018;61:2155. doi: 10.1007/s00125-018-4702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson J.W., Lundkvist P., Jansson P.A. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923. doi: 10.1007/s00125-018-4675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy S. Administration of once-daily canagliflozin to a non-diabetic patient in addition to standard aerobic exercise: a case report. Cureus. 2019;11(4) doi: 10.7759/cureus.4352. Published 2019 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollander P., Bays H.E., Rosenstock J., Frustaci M.E., Fung A., Vercruysse F., Erondu N. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care. May 2017;40(5):632–639. doi: 10.2337/dc16-2427. [DOI] [PubMed] [Google Scholar]