Abstract
Background
Studies of thyroid function after diet-induced weight loss in patients with obesity have yielded conflicting results. It is not known whether adding exercise to diet affects thyroid function in this patient population. The aim of the study was to prospectively evaluate the effects of a rehabilitation program on weight, body composition and thyroid function in euthyroid patients with obesity.
Methods
Serum levels of thyroid-stimulating hormone (TSH), free thyroxine (FT4), and free triiodothyronine (FT3) in euthyroid patients with severe obesity were analyzed before and at the end of a 3-month rehabilitation program. Relationships between body weight or composition and changes in thyroid function were also investigated. Each study participant acted as his/her own control.
Results
The study population consisted of 34 euthyroid patients with obesity (18 men and 16 women; mean ± SD age: 51 ± 12). The mean BMI was 49.3 ± 12.4 kg/m2 before the program and 46 ± 10.8 (p < 0.005) at the end, with a mean body weight loss of 11 kg (p < 0.05) and a mean fat mass loss of 6.8 kg (p < 0.05). The weight and fat mass losses were not significantly correlated with the serum concentrations of TSH, FT3 and FT4 measured at the end of the program.
Conclusion
A 3-month rehabilitation program combining diet and exercise produced weight and fat mass losses without inducing thyroid dysfunction in patients with obesity.
Keywords: Thyroid function, Obesity, Exercise, Physical activity, Diet, Weight loss
Highlights
-
•
Obesity is closely linked to thyroid dysfunction.
-
•
Weight change interventions on thyroid function yielded mixed results.
-
•
A normal calorie diet plus exercise improve body composition and insulin resistance.
-
•
Maintenance of protein and nutritional status has beneficial effects on thyroid function.
1. Introduction
There is growing scientific and clinical interest in the interactions between obesity and thyroid function [1]. Thyroid hormones are known to be involved in key metabolic pathways in the regulation of basal metabolism and energy expenditure, and also have a role in lipid and glucose metabolism [2]. Thyroid dysfunction has been reported in patients with obesity, although there are conflicting reports on whether the levels of various thyroid hormones are normal, elevated or low in patients with obesity [3]. In most studies, however, a high body mass index (BMI) was found to be associated with elevated serum levels of thyroid stimulating hormone (TSH) and low serum levels of thyroxine (T4) [4,5].
The interactions between thyroid hormone levels, body composition (i.e. fat mass and free fat mass), and insulin resistance have not been extensively characterized. Serum TSH levels were positively associated with visceral adiposity [6] and higher insulin resistance in both men and women, whereas elevated serum FT3 levels were significantly associated with a low lean mass [7]. The mechanisms underlying these findings are not well understood [3]. Given that the thyroid hormones T3 and T4 regulate resting energy expenditure, changes in thyroid hormone levels might suggest that the body is adapting to obesity [8]. Furthermore, caloric restriction may induce alterations in peripheral thyroid hormone metabolism (e.g. low type II deiodinase activity) [9].
The impact of weight change on thyroid function has been explored in several observational studies, although the results were inconsistent [5,10,11]. For example, a caloric restriction diet has been linked to a low serum T3 concentration in lean and weight-stable healthy humans [12]. In patients with obesity, weight loss was associated with a decrease in TSH and T3 levels (relative to baseline levels), regardless of the weight loss strategy used (caloric restriction or bariatric surgery) [[13], [14], [15]]. In contrast, Reinehr et al. evidenced a long-term decrease in thyroid hormones (but not TSH) among children with obesity having lost weight after a year on a normal-calorie diet [16].
It is well established that exercise may promote modest weight loss, and also has an important role in weight regain after initial weight loss. Furthermore, clinical trials of exercise that report no weight loss or modest weight loss (<5 kgs) still report numerous health benefits for overweight and obese adults. These benefits include improving cardiorespiratory fitness, glucose control, endothelial function and quality of life [17].
There are few literature data on the impact of a combination of exercise and diet on thyroid function. Here, we report the results of a study designed to characterize the mid-term thyroid response to a supervised “exercise plus diet” obesity rehabilitation program.
2. Patients and methods
2.1. Design and setting
The study was carried out in the Department of Rehabilitation, Nutrition and Obesity at Berck Maritime Hospital (Berck, France). Most of the Department's patients are referred by nutrition and endocrinology departments throughout northern France. Inpatients with obesity (BMI >30 kg/m2) were invited to participate in the study. All participants provided their informed consent prior to inclusion. The protocol conformed with the principles outlined by the Declaration of Helsinki and was approved by the local ethics committee in Berck. Each participant acted as his/her own control. Participants with a history of thyroid disease, positivity for thyroid autoantibodies, and use of thyroid hormones or iodine-containing drugs were excluded.
2.2. The diet and exercise program
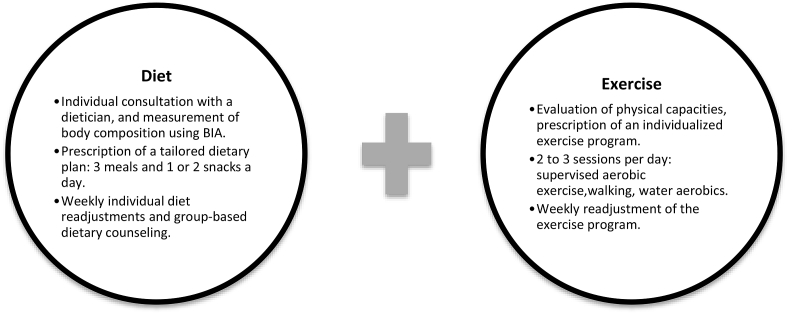
The multidisciplinary inpatient diet and exercise program is summarized in Fig. 1. All participants had an initial one-to-one consultation with a clinical dietician, and then followed a normal-calorie diet. Energy needs were estimated using the Mifflin-St Jeor equation [18]. In energy terms, the diet comprised 55% carbohydrate, 30% fat, and 15% protein. All meals were prepared and provided in our department throughout the three-month program. Clinical dieticians offered individual and group-based dietary counseling on a weekly basis.
Fig. 1.
The 3-month rehabilitation program.
BIA: bio-electrical impedance analysis.
The group-format exercise program involved aerobic exercise training (5 days a week). Patients had every day: two 30-min sessions of land-based exercise in our training room and one 45 min water-based aerobics class (indoor swimming pool). Groups of 4–8 participants performed exercise at 70–80% of their maximum heart rate, with a focus on improving skeletal muscle strength, endurance ability (including walking, cycling and swimming), flexibility, and balance (Pilates-inspired exercise). All exercise sessions were developed and supervised by certified trainers and physiotherapists.
2.3. Body composition and thyroid hormone assays
Clinical, biochemical, and body composition variables were measured before and at the end of the 3-month rehabilitation program. Fasting serum TSH, free T3 and free T4 concentrations were measured using a chemiluminescence immunoassay (ADVIA Centaur XP Immunoassay System, Siemens, USA). Body composition was measured in a bio-electrical impedance analysis (Tanita BC-418 MA, Tanita Corporation).
2.4. Statistical analyses
Statistical analyses were performed with SPSS software (version 10.0; SPSS, Chicago, IL, USA). Data were quoted as the mean ± standard deviation (SD). Changes in clinical and biochemical variables between baseline and at the end of the intervention were assessed in a paired t-test. Spearman's test was used to identify correlations between changes in body weight or composition and thyroid parameters. The threshold for statistical significance was set to p < 0.05.
3. Results
A total of 34 consecutive participants in the rehabilitation program (mean age: 51 ± 12 years) were included in the study. The participants’ demographic and clinical characteristics and pre-/post-intervention changes are summarized in Table 1. All participants tested negative for thyroid antibodies, and had serum TSH levels within the reference range. At baseline, there were no significant sex-related differences in the correlations between thyroid function tests on one hand and clinical variables or body composition on the other (data not shown). At the end of the 3-month rehabilitation program, all the participants presented a substantial body weight loss (mean ± value: 11 kg, p < 0.05); this was mainly due to fat mass loss (mean loss: 6.8 kg, p < 0.05). The mean BMI was 49.3 ± 12.4 kg/m2 before the program and 46 ± 10.8 kg/m2 afterwards (p < 0.05). A small but significant decrease in glycated hemoglobin (HbA1C) was observed (6 ± 0.8 before and 5.8 ± 0.6 after, p < 0.05).
Table 1.
Participants’ clinical and biochemical characteristics.
| Variables | Baseline | Post intervention | P-value |
|---|---|---|---|
| n | 34 | 34 | - |
| Gender (M/F) | 18/16 | 18/16 | - |
| Age (years) | 51 ± 12 | 51 ± 12 | - |
| Body weight (kg) | 138.7 ± 38 | 127.7 ± 32 | <0.05 |
| Mean BMI (kg/m2) | 49.3 ± 12.4 | 46 ± 10.8 | <0.05 |
| Body fat (%) | 45.4 ± 9.2 | 43.5 ± 9.8 | <0.05 |
| Fat mass (kg) | 60.1 ± 20.6 | 53.3 ± 18.3 | <0.05 |
| Lean mass (fat-free mass) (kg) | 70.6 ± 17 | 68.3 ± 16.4 | <0.05 |
| Estimated resting energy expenditure (kcal/day) | 2226 | 2135 | <0.05 |
| HbA1C (%) | 6 ± 0.8 | 5.8 ± 0.6 | <0.05 |
| TSH (mUI/L) (N: 0.4–4) | 1.72 ± 0.7 | 1.89 ± 0.86 | 0.2649 |
| Free T4 (ng/dl) (N: 0.80–1.90) | 1.06 ± 0.19 | 1.04 ± 0.17 | 0.9490 |
| Free T3 (pg/ml) (N: 1.80–4.20) | 2.92 ± 0.37 | 2.87 ± 0.39 | 0.1852 |
| Serum Albuminemia (g/l) | 37.8 ± 4.2 | 38.1 ± 3.5 | 0.3304 |
| Serum Creatininemia (mg/l) | 8 ± 1.7 | 8.2 ± 1.4 | 0.0543 |
All values expressed as mean ± SD.
There were no significant pre-/post-differences in thyroid hormone levels. Moreover, the weight loss at the end of the intervention was not significantly correlation with the concentrations of TSH (r = 0.25, P = 0.17), FT3 (r = -0.01; P = 0.94) or FT4 (r = -0.43, P = 0.05). Likewise, there were no significant correlations between the change in fat mass and the concentrations of TSH (r = 0.40; P = 0.05), FT3 (r = −0.16, P = 0.52) or FT4 (r = -0.39, P = 0.13).
4. Discussion
The present study generated two important findings. Firstly, there was no association between baseline thyroid function (i.e. TSH, FT4 and FT3 concentrations) and BMI or body composition. This is in line with some studies [19], whereas others have reported a correlation between the percentage body fat and the TSH level [20,21].
Secondly, thyroid function did not change significantly over the course of the intervention, despite significant weight loss and a fall in BMI. This finding contrasts with Marzullo et al.’s report that weight loss during a 4-week inpatient dieting program was associated with significantly falls in levels of TSH (6.3%), FT3 (3.3%) and FT4 (3.9%; p < 0.001 for all) in 70 patients with obesity [22].
Exercise may affect the hormonal production of many endocrine glands [23]. In line with our study findings, changes in thyroid hormone levels in response to exercise, are in general, small and within the normal range [24]. Increase, decrease, or no change in the levels of thyroid hormones has been reported, regardless of the type of exercise, duration, and intensity [[25], [26], [27]]. These divergent findings might be attributed to several confounding factors, such as variations in body composition, nutritional status and the type of exercise training [24].
It is important to note that the significant body weight loss and fat loss (with mean values of 11 kg and 6.8 kg, respectively) observed in the present study were associated with improved body composition and a reduction in insulin resistance (as indicated by a fall in HbA1C levels, p < 0.05). Furthermore, we also observed a moderate but nevertheless significant decrease in the lean mass (mean value: 2.3 kg). Interestingly, resting energy expenditure and levels of the biomarker serum albumin (reflecting protein and nutritional status) did not change significantly during the program. There are several possible explanations for this stability. Firstly, it has been shown that an exercise plus diet combination reduces muscle mass loss and increases muscle strength during voluntary weight loss in people with obesity [28]. Secondly, insulin is a powerful driver of muscle protein synthesis; hence, the observed decrease in obesity-related insulin resistance after the exercise-diet program might help to preserve muscle mass and resting energy expenditure [29]. Considering that most metabolic processes (energy expenditure, and glucose and lipid homeostasis) occur within the lean mass compartment (i.e. muscle), the relative stability of protein and nutritional status might explain why the thyroid function parameters did not change markedly [30]. We hypothesize that the significant fat mass loss and stable protein and nutritional status observed in our study are related to the provision of an individualized, normal-calorie diet and an appropriate physical activity program.
Our study had a number of strengths – notably its prospective and longitudinal design. Moreover, the study personnel supervised the protocol rigorously, and ensured that patients complied with the dietary and exercise-related recommendations. This close supervision enabled us to reliably evaluate changes over time, with each participant serving as his/her control.
Our study also had some limitations. Firstly, only participants with grade III obesity (BMI >40 kg/m2) and a normal serum TSH level were included. It is not therefore possible to generalize the results to other obesity phenotypes or to patients with patent thyroid dysfunction. Secondly, we did not measure levels of hormones or peptides that can be influenced by a weight change (e.g. leptin and ghrelin). Although leptin and TSH levels are reportedly correlated in people with obesity [5], this relationship is subject to debate [2,21].
Unfortunately, the nature of our ward and the patients profile do not allow us to use control group with either diet therapy alone or exercise therapy alone.
In conclusion, our results showed that a 3-month individualized rehabilitation program combining diet and exercise enhanced weight loss without inducing significant thyroid function changes in patients with severe obesity. Further research is warranted to clarify the underlying relationship between thyroid function and body weight change in this population.
Conflicts of interest
The authors declare that there are no conflicts of interest in relation to this study.
Author contribution statement
YK, and RL contributed to the conception and design of the study, and the acquisition of data. MD performed the statistical analysis and interpretation of data; YK wrote the first draft of the manuscript, RD critically reviewed the article; all the authors approved the final version of the manuscript.
Funding information
There was no specific funding for this study.
Acknowledgments
We thank all the study participants for their contribution to the research. We also thank Prof. Jean-Daniel Lalau for his critical review of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2019.100008.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Laurberg P., Knudsen N., Andersen S., Carlé A., Pedersen I.B., Karmisholt J. Thyroid function and obesity. Eur Thyroid J. 2012;1:159–167. doi: 10.1159/000342994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullur R., Liu Y.-Y., Brent G.A. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontenelle L.C., Feitosa M.M., Severo J.S., Freitas T.E.C., Morais J.B.S., Torres-Leal F.L. Thyroid function in human obesity: underlying mechanisms. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2016;48:787–794. doi: 10.1055/s-0042-121421. [DOI] [PubMed] [Google Scholar]
- 4.Michalaki M.A., Vagenakis A.G., Leonardou A.S., Argentou M.N., Habeos I.G., Makri M.G. Thyroid function in humans with morbid obesity. Thyroid Off J Am Thyroid Assoc. 2006;16:73–78. doi: 10.1089/thy.2006.16.73. [DOI] [PubMed] [Google Scholar]
- 5.Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–171. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y., Chen Y., Wang N., Chen C., Nie X., Li Q. Thyroid stimulating hormone within the reference range is associated with visceral adiposity index and lipid accumulation product: a population-based study of SPECT-China. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2018;50:29–36. doi: 10.1055/s-0043-122235. [DOI] [PubMed] [Google Scholar]
- 7.Kwon H., Cho J.-H., Lee D.Y., Park S.E., Park C.-Y., Lee W.-Y. Association between thyroid hormone levels, body composition and insulin resistance in euthyroid subjects with normal thyroid ultrasound: the Kangbuk Samsung Health Study. Clin Endocrinol. 2018;89:649–655. doi: 10.1111/cen.13823. [DOI] [PubMed] [Google Scholar]
- 8.Kiortsis D.N., Durack I., Turpin G. Effects of a low-calorie diet on resting metabolic rate and serum tri-iodothyronine levels in obese children. Eur J Pediatr. 1999;158:446–450. doi: 10.1007/s004310051117. [DOI] [PubMed] [Google Scholar]
- 9.De Andrade P.B.M., Neff L.A., Strosova M.K., Arsenijevic D., Patthey-Vuadens O., Scapozza L. Caloric restriction induces energy-sparing alterations in skeletal muscle contraction, fiber composition and local thyroid hormone metabolism that persist during catch-up fat upon refeeding. Front Physiol. 2015;6:254. doi: 10.3389/fphys.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan B., Chen Y., Yang J., Yang W., Wang C. Effect of bariatric surgery on thyroid function in obese patients: a systematic review and meta-analysis. Obes Surg. 2017;27:3292–3305. doi: 10.1007/s11695-017-2965-2. [DOI] [PubMed] [Google Scholar]
- 11.Agnihothri R.V., Courville A.B., Linderman J.D., Smith S., Brychta R., Remaley A. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid. 2014;24:19–26. doi: 10.1089/thy.2013.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L., Klein S., Holloszy J.O., Premachandra B.N. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- 13.Knudsen N., Laurberg P., Rasmussen L.B., Bülow I., Perrild H., Ovesen L. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 14.Lips M.A., Pijl H., van Klinken J.B., de Groot G.H., Janssen I.M., Van Ramshorst B. Roux-en-Y gastric bypass and calorie restriction induce comparable time-dependent effects on thyroid hormone function tests in obese female subjects. Eur J Endocrinol. 2013;169:339–347. doi: 10.1530/EJE-13-0339. [DOI] [PubMed] [Google Scholar]
- 15.Neves J.S., Castro Oliveira S., Souteiro P., Pedro J., Magalhães D., Guerreiro V. Effect of weight loss after bariatric surgery on thyroid-stimulating hormone levels in patients with morbid obesity and normal thyroid function. Obes Surg. 2018;28:97–103. doi: 10.1007/s11695-017-2792-5. [DOI] [PubMed] [Google Scholar]
- 16.Reinehr T., Andler W. Thyroid hormones before and after weight loss in obesity. Arch Dis Child. 2002;87:320–323. doi: 10.1136/adc.87.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swift D.L., McGee J.E., Earnest C.P., Carlisle E., Nygard M., Johannsen N.M. The effects of exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis. 2018;61:206–213. doi: 10.1016/j.pcad.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Madden A.M., Mulrooney H.M., Shah S. Estimation of energy expenditure using prediction equations in overweight and obese adults: a systematic review. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29:458–476. doi: 10.1111/jhn.12355. [DOI] [PubMed] [Google Scholar]
- 19.Manji N., Boelaert K., Sheppard M.C., Holder R.L., Gough S.C., Franklyn J.A. Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin Endocrinol. 2006;64:125–128. doi: 10.1111/j.1365-2265.2006.02433.x. [DOI] [PubMed] [Google Scholar]
- 20.De Pergola G., Ciampolillo A., Paolotti S., Trerotoli P., Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin Endocrinol. 2007;67:265–269. doi: 10.1111/j.1365-2265.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 21.Iacobellis G., Ribaudo M.C., Zappaterreno A., Iannucci C.V., Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol. 2005;62:487–491. doi: 10.1111/j.1365-2265.2005.02247.x. [DOI] [PubMed] [Google Scholar]
- 22.Marzullo P., Minocci A., Mele C., Fessehatsion R., Tagliaferri M., Pagano L. The relationship between resting energy expenditure and thyroid hormones in response to short-term weight loss in severe obesity. PLoS One. 2018;13:e0205293. doi: 10.1371/journal.pone.0205293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinacker J.M., Brkic M., Simsch C., Nething K., Kresz A., Prokopchuk O. Thyroid hormones, cytokines, physical training and metabolic control. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2005;37:538–544. doi: 10.1055/s-2005-870419. [DOI] [PubMed] [Google Scholar]
- 24.Hackney A.C., Davis H.C., Lane A.R. Growth hormone-insulin-like growth factor Axis, thyroid Axis, prolactin, and exercise. Front Horm Res. 2016;47:1–11. doi: 10.1159/000445147. [DOI] [PubMed] [Google Scholar]
- 25.McMurray R.G., Hackney A.C. Interactions of metabolic hormones, adipose tissue and exercise. Sports Med Auckl NZ. 2005;35:393–412. doi: 10.2165/00007256-200535050-00003. [DOI] [PubMed] [Google Scholar]
- 26.Tremblay A., Fontaine E., Poehlman E.T., Mitchell D., Perron L., Bouchard C. The effect of exercise-training on resting metabolic rate in lean and moderately obese individuals. Int J Obes. 1986;10:511–517. [PubMed] [Google Scholar]
- 27.Baylor L.S., Hackney A.C. Resting thyroid and leptin hormone changes in women following intense, prolonged exercise training. Eur J Appl Physiol. 2003;88:480–484. doi: 10.1007/s00421-002-0737-7. [DOI] [PubMed] [Google Scholar]
- 28.FRIMEL T.N., SINACORE D.R., VILLAREAL D.T. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujita S., Rasmussen B.B., Cadenas J.G., Grady J.J., Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745. doi: 10.1152/ajpendo.00271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloise F.F., Cordeiro A., Ortiga-Carvalho T.M. Role of thyroid hormone in skeletal muscle physiology. J Endocrinol. 2018;236:R57–R68. doi: 10.1530/JOE-16-0611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.