Abstract
Background
Beyond the immediate toll of injuries and deaths, major disasters are often associated with long-term increased risks of chronic disease. We sought to investigate the incidence of metabolic syndrome (MetS) among survivors of the 2011 Great East Japan Earthquake and tsunami.
Methods
Subjects aged ≥18 years from the tsunami-stricken area participated in a prospective cohort study of disaster survivors (the RIAS Study) from 2011 to 2015. After excluding subjects who were previously diagnosed with MetS, we observed the cumulative incidence of MetS across four annual examinations among 7318 subjects (mean age, 59.8 years; 43.5% men). We defined MetS using the International Diabetes Foundation criteria.
Results
The 4-year cumulative incidence of MetS was 18.0% in the overall sample. The incidence was significantly higher among older women survivors relocated to prefabricated temporary housing (40.9%, 95% confidence interval, 36.4–44.6), and other types of housing (36.2%, 95% CI: 32.3–40.6) compared to those who were not relocated (34.1%, 95% CI: 30.9–37.4). An increase in incidence of MetS was not observed for older men, or younger survivors aged ≤64 years.
Conclusion
Relocation to prefabricated temporary housing was a risk factor for increased incidence of MetS in older women.
Keywords: Metabolic syndrome, Great East Japan Earthquake, Natural disaster, Living conditions, Temporary housing, Non-communicable diseases
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDLC, high-density lipoprotein cholesterol; IDF, International Diabetes Federation; MET, metabolic equivalent for task; MetS, metabolic syndrome; SBP, systolic blood pressure; TC, total cholesterol; Temp Housing, prefabricated temporary housing
Highlights
-
•
A 4-year 18.0% cumulative incidence metabolic syndrome (MetS) in a cohort of survivors.
-
•
The increased incidence of MetS was particularly high for older women.
-
•
Older women suffered property loss and were relocated to prefabricated temporary housing.
-
•
No excess incidence was founder for older men or younger survivors.
1. Introduction
On March 11, 2011, a massive earthquake registering a magnitude of 9.0 on the Richter scale struck the northeast coast of Japan (the Great East Japan Earthquake). About 22,000 people lost their lives or were reported missing as a result of the tsunami triggered by the earthquake. The disaster also resulted in widespread property damage and destruction, forcing the relocation of over a quarter of a million residents in coastal areas [[1], [2], [3], [4]]. In the months following the disaster, displaced residents were gradually moved out of emergency shelters and into more stable accommodation, including temporary prefabricated housing that resembled FEMA trailer homes.
The conditions in these temporary homes have been described as cramped, noisy (because of thin walls), and stressful. For example, Sasaki et al. (2018) found that moving into prefabricated housing was uniquely associated with a doubling of the incidence of depression compared to alternative types of accommodation, such as subsidized rental housing on the open market [5]. Some municipalities also built the trailer homes in inconvenient locations such as remote hillside spots, thereby increasing the sense of social isolation for displaced survivors. Even though they were officially referred to as “temporary” housing, people lived in these conditions for almost 6 years before being finally moved to more permanent accommodations. In short, major disasters continue to be associated with lingering health effects long after the immediate toll of injuries and deaths.
Besides the deleterious impacts of living in temporary housing on mental health, previous studies have also indicate that this type of accommodation increases the risk for physical health impairments, including deteriorating physical performance [6], reduced physical activity/sedentarism [7], deteriorating cognitive function [8], and increases in body weight [9].
Metabolic syndrome (MetS) is defined by a constellation of risk factors including abdominal obesity, high cholesterol level, elevated fasting glucose, and elevated blood pressure [10]. Given previous reports of associations between relocation to prefabricated temporary housing and increased risks of sedentarism, worsened diet quality [11], and weight gain, we hypothesized that this type of accommodation would be reflected by adverse shifts in metabolic profiles. The aim of the present study was to therefore document the 5-year cumulative incidence of MetS among survivors of the 2011 disaster according to different types of post-disaster accommodation.
2. Materials and methods
2.1. Study population
The Research Project for Prospective Investigation of Health Problems among Survivors of the Great East Japan Earthquake and Tsunami Disaster (abbreviated to RIAS) is an ongoing prospective follow-up study of survivors enrolled from the coastal municipalities of Yamada, Otsuchi, and Rikuzentakata in the southern part of Iwate Prefecture, which were heavily damaged by the tsunami (Fig. 1) [4]. Post disaster surveys have been conducted annually, beginning 6 months after the disaster (September 2011). The RIAS survey consists of the same items as the annual health check-ups conducted by the National Healthcare Insurance system in Japan, in addition to questions specifically inquiring about disaster-related experiences. The questionnaire surveys are supplemented by annual clinical exams, and collection of anthropometric and biomarker data. Details on the RIAS study have been provided elsewhere [12]. We identified survivors’ addresses from the mandatory residential registration system maintained by the local government in each municipality. We recruited all residents aged 18 years or older by sending out notifications about the RIAS survey on a community bulletin board. Out of the target sample of 42,831, a total of 10,081 participants provided written informed consent to participate (23.5% participation rate).
Fig. 1.
Map of the present study area
The black square shows the study area along the Pacific Ocean coast. The municipalities included in our study are marked in red in Yamada, Otsuchi, and Rikuzentakata. The epicenter of the earthquake is marked as a red bulls-eye.
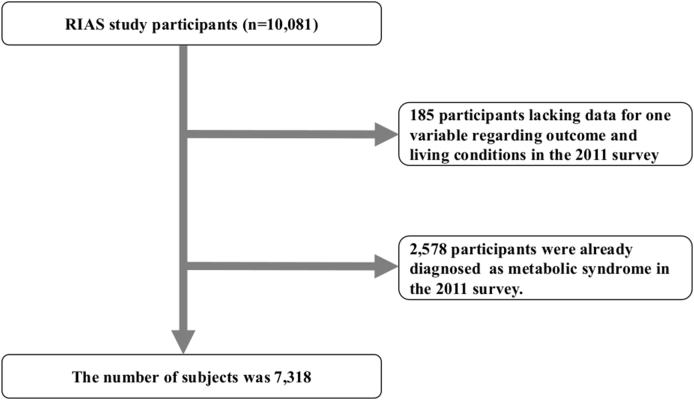
After excluding participants who were missing information on the outcome variable in the 2011 survey (n = 185) and those who had already been diagnosed with metabolic syndrome at baseline (n = 2578), our final analytic sample comprised 7318 participants (mean age, 59.8 years; 43.5% men) (Fig. 2). The research protocol was approved by the Ethics Committee of Iwate Medical University (approval no. H23-69).
Fig. 2.
Flow chart of the procedure used to select participants for the study.
Out of 10,081 persons in the original cohort, we excluded 185 persons who lacked data regarding the outcome and living conditions in the 2011 survey, and 2
578 participants already diagnosed with metabolic syndrome in the 2011 survey. We assessed a total of 7
318 participants.
2.2. Metabolic syndrome
MetS was defined according to the criteria of the International Diabetes Federation (IDF) [10]. Patients with MetS must have central obesity (waist circumference ≥90 cm in men and ≥80 cm in women) plus two or more of the following four components: (1) elevated triglyceride level (≥150 mg/dL or medical history or presence of pharmacological treatment for dyslipidemia); (2) low HDL cholesterol (<40 mg/dL in men and <50 mg/dL in women, or medical history or presence of pharmacological treatment for dyslipidemia); (3) elevated blood pressure (systolic ≥130 mmHg and/or diastolic ≥85 mmHg, or taking antihypertensive agents); (4) impaired glucose metabolism (glycosylated hemoglobin level [HbA1c] ≥5.7% or receiving pharmacological treatment for diabetes mellitus). Because the blood samples we collected were non-fasting, we used HbA1c levels instead of fasting plasma glucose level (HbA1c ≥ 5.7%) [13]. Patients with body mass index (BMI) > 30 kg/m2 were defined as meeting the criteria for central obesity regardless of their waist circumference, according to the criteria of the IDF. Cumulative incidence of MetS was defined by participants who met these criteria at least one time during the 5-year follow-up period.
2.3. Post-disaster housing type
In the RIAS surveys, the housing arrangements of the participants were categorized as living in one’s own home, relocated to prefabricated temporary housing (Temp Housing), or other types of accommodation (Other). The Other group included survivors who moved to subsidized rental housing on the open market, those who rebuilt their own homes, or those who moved in with a family member, friend, or relative. The Home group included those who did not need to relocate after the disaster.
2.4. Measurements
Body weight (kg) was measured with an accuracy of ± 0.1 kg using a standard scale with light clothes without shoes. Height (m) was measured using a stadiometer in digital scales. The BMI (kg/m2) was calculated by dividing the body weight (kg) by the square of the height (m2). Waist circumference (cm) were measured. Systolic blood pressure (SBP, mmHg) and diastolic blood pressure (DBP, mmHg) were measured twice in a sitting position, and average values for SBP and DBP; mmHg were calculated. Fasting or non-fasting blood samples were drawn from the antecubital vein. Serum levels of total cholesterol (TC; mg/dL), high-density lipoprotein cholesterol (HDLC; mg/dL), and triglyceride (TG; mg/dl) and HbA1c levels were measured [14].
2.5. Self-reported questionnaires
Self-reported questionnaires were administered to assess health habits (smoking status, alcohol drinking, daily physical activity, the average number of meals per day and dietary intake), self-assessed economic situation (severely distressed vs. not), employment status after the disaster (unemployment vs. not), and psychosocial factors (psychological distress and insomnia).
Smoking status was classified into two categories (current smokers vs. non-current smokers), and alcohol drinking status was similarly binarized (drinkers vs. non-drinkers). Responses about physical activity were classified into three categories; low physical activity (<19 METs per week), moderate physical activity (≥19 METs per week, <23 METs per week) or high physical activity (≥23 METs per week).
The average number of meals per day during the preceding several days was classified into two categories: <3 times a day versus ≥3 times a day. Dietary intake was classified into two categories: good dietary intake (intake of staple food ≥ three times a day, intake of meat, fish and shellfish eggs, or soybean products ≥ twice a day, vegetable intake ≥ twice a day, and intake of fruit or dairy products ≥ once a day), and poor dietary intake (others) [11].
Psychological distress was dichotomized to significant distress (scores of 5–24) versus low distress (scores of 0–4) based on the Japanese version of the K6 scale [15,16]. Insomnia was defined by scores of 6–24 on the Athens Insomnia Scale (AIS) [[17], [18], [19], [20]]. Social network was assessed by the Lubben’s Social Network Scale [21,22]. Social capital was assessed using 4 questions on social cohesion, including the residents’ perceptions of trust in the community and levels of mutual help [11]. Overweight was defined as a BMI of ≥25 kg/m2. Hypertension was defined as an SBP ≥140 mmHg, DBP ≥90 mmHg, or a diagnosis of hypertension reported on the survey. Diabetes mellitus was defined as plasma glucose level ≥200 mg/dL, plasma HbA1c level (NSGP) ≥6.5%, or a diagnosis of diabetes mellitus reported on the questionnaire. Dyslipidemia was defined as TC ≥ 220 mg/dL, or HDLC <40 mg/dL, or a diagnosis of dyslipidemia reported on the survey [23].
2.6. Statistical analysis
All analyses were stratified by age-group; 64 years or younger, and 65 years or older, considering that insulin resistance increases with age [24]. Baseline characteristics were compared among participants in the living conditions in the 2011 survey. Differences in means/proportions among participants in the living conditions were tested using analysis of variance with Bonferroni correction (continuous variables) or chi-squared test (categorical variables).
We analyzed the cumulative incidence of Mets across four follow-up examinations (2012, 2013, 2014, and 2015) using generalized linear mixed effect models. Because the subjects are non-Mets individuals in 2011, we excluded the data in 2011 to show new onset of Mets. A sequence of two models were run. In Model 1, we included explanatory variables of age, sex, survey year, accommodation type, and an interaction between accommodation type x survey year as fixed effects, while we included the individual as a random effect. In Model 2, we additionally adjusted for smoking status, alcohol drinking, physical activity, daily number of meals, dietary intake, self-assessed economic distress, unemployment in the 2011, psychological distress, insomnia, social network, and social capital. All analyses were stratified by sex. We calculated the cumulative incidence of MetS as well as constituent components of MetS (central obesity, elevated triglyceride level, low HDLC level, elevated blood pressure, and impaired glucose metabolism).
All statistical analyses were performed using SPSS version 25.0 (IBM Corp. Armonk, NY, USA). All statistical tests described were two-sided, and analysis items with P-values <0.05 were considered statistically significant.
3. Results
We conducted a comparison of characteristics between analytic sample as baseline (2011) and the whole population in 2011 in three municipalities (Supplementary Table 1). Our sample tended to be older and included more women compared with census data.
Baseline characteristics are summarized in Table 1. The number of participants was 4378 in the Home group, 2366 in the Temp Housing group, and 574 in the Other group. Among participants aged ≤64 years, those in the Temp Housing group were significantly younger, higher body weight, and lower SBP than the Home group. The ages of participants aged ≥65 years were similar in the three groups. Although participants aged ≤64 years in the Temp housing and Other groups had higher body weight and BMI compared with those in the Home group, there was no significant difference in body weight among the three groups among participants aged ≥65 years. Among participants aged ≤64 years, those in the Temp Housing group had higher prevalence of current smokers, low physical activity, small number of meals, poor dietary intake, severe economic distress, unemployment, and insomnia compared those in the Home group. Those in the Other group showed the highest prevalence of psychological distress, while those in the Temp Housing group had the second highest prevalence of psychological distress. Among participants aged ≥65 years, those in the Temp Housing group reported more economic distress, and unemployment, while those in the Other group had higher prevalence of low physical activity, small number of meals, psychological distress, and insomnia. There was no significant difference in cardiovascular risk factors among the three groups (e.g., obesity, hypertension, diabetes mellitus, and dyslipidemia) in either age group.
Table 1.
Baseline characteristics of participants in the 2011 survey (n = 7545).
| Age ≤64 years (n = 4094) |
Age ≥65 years (n = 3224) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Missing |
Home (n = 2383) |
Temp Housing (n = 1336) |
Other (n = 375) |
P value | Home (n = 1995) |
Temp Housing (n = 1030) |
Other (n = 199) |
P value | ||
| n (%) | Mean (SD)/n (%) | Mean (SD)/n (%) | Mean (SD)/n (%) | Mean (SD)/n (%) | Mean (SD)/n (%) | Mean (SD)/n (%) | ||||
| Age, yrs | 0 (0.0) | 50.0 (11.7) | 48.3 (11.8) | 48.7 (11.7) | <0.001∗ | 73.2 (5.5) | 73.0 (5.5) | 73.3 (6.1) | 0.556 | |
| Sex | Men | 0 (0.0) | 864 (36.3) | 501 (37.5) | 152 (40.5) | 0.258 ∗ | 1069 (53.6) | 499 (48.4) | 96 (48.2) | 0.017 |
| Anthropometric examination | Body weight (kg) | 0 (0.0) | 57.5 (10.4) | 58.5 (10.7) | 58.7 (10.9) | 0.008 | 55.1 (9.5) | 54.4 (9.6) | 54.5 (9.7) | 0.121 |
| BMI (kg/m2) | 0 (0.0) | 22.6 (3.1) | 22.8 (3.2) | 22.6 (3.3) | 0.241 | 22.9 (2.8) | 22.7 (2.8) | 22.6 (2.9) | 0.062 | |
| Waist circumference (cm) | 0 (0.0) | 79.6 (8.5) | 80.0 (8.4) | 79.7 (8.7) | 0.268 | 80.8 (7.9) | 80.5 (7.8) | 80.5 (7.5) | 0.685 | |
| Blood pressure | SBP (mmHg) | 1 (0.0) | 120.2 (17.9) | 118.5 (16.5) | 118.5 (17.6) | 0.007 ∗ | 129.9 (18.7) | 128.7 (18.5) | 127.9 (19.2) | 0.122 |
| DBP (mmHg) | 1 (0.0) | 72.9 (11.5) | 72.0 (11.3) | 72.1 (11.6) | 0.078 | 74.1 (10.5) | 74.0 (10.3) | 73.5 (11.5) | 0.734 | |
| Blood tests | TC (mg/dl) | 0 (0.0) | 205.2 (36.0) | 205.9 (36.6) | 205.5 (35.9) | 0.837 | 198.9 (34.3) | 200.4 (33.5) | 199.8 (32.4) | 0.507 |
| HDLC (mg/dl) | 0 (0.0) | 67.8 (17.1) | 68.5 (17.3) | 68.7 (16.6) | 0.426 | 64.5 (16.8) | 65.0 (16.7) | 63.4 (15.6) | 0.436 | |
| TG (mg/dl) | 0 (0.0) | 122.8 (81.6) | 125.8 (93.8) | 123.1 (73.2) | 0.589 | 118.5 (65.8) | 118.9 (60.6) | 119.6 (58.2) | 0.965 | |
| HbA1c (%) | 0 (0.0) | 5.52 (0.62) | 5.48 (0.52) | 5.45 (0.50) | 0.031 | 5.71 (0.65) | 5.67 (0.56) | 5.69 (0.66) | 0.201 | |
| Lifestyle | Current smokers | 0 (0.0) | 550 (23.1) | 374 (28.0) | 92 (24.5) | 0.004 | 214 (10.7) | 105 (10.2) | 14 (7.0) | 0.26 |
| Drinkers | 0 (0.0) | 902 (37.9) | 548 (41.0) | 164 (43.7) | 0.033 | 679 (34.0) | 323 (31.4) | 47 (23.6) | 0.007 | |
| Physical activity | Low physical activity | 40 (0.5) | 1103 (46.5) | 636 (47.7) | 174 (46.6) | 0.002 | 901 (45.5) | 566 (55.2) | 123 (62.4) | <0.001 |
| Moderate physical activity | 321 (13.5) | 219 (16.4) | 73 (19.6) | 323 (16.3) | 168 (16.4) | 22 (11.2) | ||||
| Food intake | Small number of meals (<3 times) | 47 (0.6) | 228 (9.6) | 161 (12.1) | 44 (11.8) | 0.045 | 28 (1.4) | 32 (3.2) | 7 (3.5) | 0.002 |
| Poor dietary intake | 0 (0.0) | 947 (39.7) | 605 (45.3) | 167 (44.5) | 0.003 | 605 (30.3) | 337 (32.7) | 72 (36.2) | 0.135 | |
| Socioeconomic status | Severe economic distress | 18 (0.2) | 1353 (56.8) | 859 (64.3) | 231 (61.6) | <0.001 | 706 (35.5) | 589 (57.6) | 107 (54.0) | <0.001 |
| Unemployment (2011) | 213 (2.9) | 512 (22.1) | 366 (28.5) | 99 (27.5) | <0.001 | 143 (7.3) | 192 (19.3) | 36 (18.3) | <0.001 | |
| Psychological factors | Psychological distress | 122 (1.7) | 1025 (43.5) | 652 (49.5) | 191 (51.9) | <0.001 | 675 (34.6) | 447 (44.4) | 87 (45.1) | <0.001 |
| Insomnia | 97 (1.3) | 748 (31.7) | 485 (36.7) | 150 (40.4) | <0.001 | 473 (24.2) | 394 (38.7) | 79 (40.1) | <0.001 | |
| Social factors | Low level of social network | 154 (2.1) | 1071 (45.6) | 567 (43.3) | 141 (38.0) | 0.019 | 735 (37.7) | 397 (39.9) | 65 (34.2) | 0.269 |
| Low level of social capital | 20 (0.3) | 239 (10.0) | 138 (10.3) | 43 (11.5) | 0.698 | 160 (8.1) | 84 (8.2) | 25 (12.6) | 0.084 | |
| Cardiovascular risk factors | Obesity | 0 (0.0) | 488 (20.5) | 300 (22.5) | 80 (21.3) | 0.367 | 435 (21.8) | 200 (19.4) | 41 (20.6) | 0.308 |
| Hypertension | 1 (0.0) | 537 (22.5) | 296 (22.2) | 75 (20.0) | 0.547 | 1132 (56.7) | 577 (56.0) | 108 (54.3) | 0.771 | |
| Diabetes mellitus | 0 (0.0) | 110 (4.6) | 60 (4.5) | 18 (4.8) | 0.965 | 223 (11.2) | 120 (11.7) | 25 (12.6) | 0.808 | |
| Dyslipidemia | 0 (0.0) | 914 (38.4) | 516 (38.6) | 147 (39.2) | 0.948 | 727 (36.4) | 393 (38.2) | 74 (37.2) | 0.651 | |
Continuous variables are presented as means (standard deviations). Categorical variables are presented as the number of cases (%).
P values were calculated using analysis of variance for continuous variables using Bonferroni correction, and the chi-squared test for categorical variables. ∗ Statistically significant differences between prefabricated temporary housing group and home group.
Abbreviations: Other, other accommodations; Temp Housing, prefabricated temporary housing; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDLC, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; SD, standard deviation.
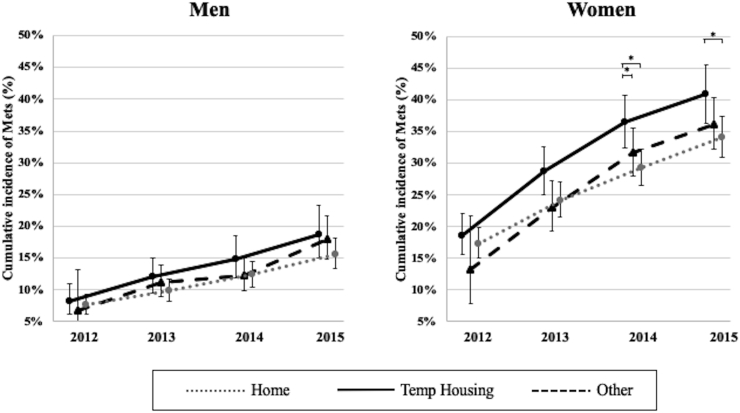
Table 2 shows the cumulative incidence of MetS using generalized linear mixed effects models. There was a significant difference in MetS incidence between accommodation type across time among those aged ≥65 years (Temp Housing in 2014; coefficient [95% confidence interval], 0.20 [0.01 to 0.43]; P = 0.045), but no significant difference among younger participants (aged ≤64 years). Fig. 3 shows the trends in estimated marginal means of the cumulative incidence of MetS among participants aged ≥65 years from 2012 to 2015, stratified by sex. Among men aged ≥65 years, there was no significant difference in the cumulative incidence of MetS from 2012 to 2015. The interaction between accommodation type x time was not statistically significant among men aged ≥65 years (F = 1.04, P = 0.396). Among women, although there was no significant difference in cumulative incidence rate of MetS in 2012 and 2013 by housing type, the cumulative incidence in the Temp Housing group was significantly higher than in the Other group in 2014 and the Home group from 2014 to 2015. The coefficient of the cumulative incidence of MetS was marginally significantly high association with Temp Housing group in 2014 (coefficient [95% confidence interval], 0.24 [-0.01 to 0.48]; P = 0.061). The association did not change after adjustment for covariates (Supplementary Table 2). The cumulative incidence rates of MetS were 40.9% (95% confidence interval: 36.4–44.6) in the Temp Housing group, 36.2% (95% confidence interval: 32.3–40.3) in the Other group, and 34.1% (95% confidence interval: 30.9–37.4) in the Home group in 2015. We observed a dose-response association among three groups. The interaction between housing type x time was not statistically significant among women aged ≥65 years (F = 0.952, P = 0.456).
Table 2.
Coefficients of cumulative incidence in metabolic syndrome using generalized linear mixed effects models.
| Age ≤64 years |
Age ≥65 years |
|||
|---|---|---|---|---|
| Model 1 (n = 3239) |
Model 2 (n = 3114) |
Model 1 (n = 2837) |
Model 2 (n = 2743) |
|
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | |
| 2012 | Baseline | Baseline | Baseline | Baseline |
| 2013 | 0.39 (0.29–0.50) | 0.41 (0.30–0.52) | 0.38 (0.28–0.47) | 0.37 (0.28–0.47) |
| 2014 | 0.65 (0.53–0.78) | 0.68 (0.55–0.81) | 0.64 (0.53–0.75) | 0.62 (0.51–0.73) |
| 2015 | 0.81 (0.67–0.94) | 0.83 (0.68–0.98) | 0.87 (0.75–1.00) | 0.85 (0.71–0.99) |
| 2013 × Other | 0.34 (−0.04 to 0.72) | 0.34 (−0.04 to 0.72) | 0.28 (−0.17 to 0.72) | 0.25 (−0.18 to 0.68) |
| 2013 × Temp Housing | 0.03 (−0.15 to 0.22) | 0.08 (−0.12 to 0.28) | 0.14 (−0.03 to 0.31) | 0.13 (−0.05 to 0.30) |
| 2014 × Other | 0.22 (−0.18 to 0.61) | 0.21 (−0.19 to 0.60) | 0.32 (−0.15 to 0.79) | 0.31 (−0.15 to 0.77) |
| 2014 × Temp Housing | 0.13 (−0.10 to 0.35) | 0.12 (−0.12 to 0.35) | 0.20 (0.01–0.40) | 0.21 (0.00–0.43) |
| 2015 × Other | 0.21 (−0.19 to 0.62) | 0.21 (−0.19 to 0.61) | 0.39 (−0.10 to 0.88) | 0.35 (−0.13 to 0.83) |
| 2015 × Temp Housing | 0.18 (−0.06 to 0.43) | 0.19 (−0.07 to 0.45) | 0.18 (−0.04 to 0.40) | 0.17 (−0.06 to 0.40) |
| Age | 0.03 (0.02–0.04) | 0.03 (0.02–0.04) | −0.01 (−0.03 to 0.00) | −0.01 (−0.03 to 0.01) |
| Sex (men) | −0.67 (−0.88 to −0.47) | −0.65 (−0.88 to −0.42) | −1.03 (−1.22 to −0.85) | −1.01 (−1.22 to −0.81) |
| Other (ref = Home) | −0.16 (−0.55 to 0.24) | −0.13 (−0.52 to 0.26) | −0.27 (−0.75 to 0.22) | −0.26 (−0.73 to 0.21) |
| Temp Housing | −0.02 (−0.27 to 0.23) | −0.04 (−0.30 to 0.23) | 0.08 (−0.12 to 0.28) | 0.02 (−0.20 to 0.23) |
| Current smokers | −0.10 (−0.33 to 0.14) | −0.26 (−0.59 to 0.06) | ||
| Drinkers | 0.00 (−0.12 to 0.12) | 0.02 (−0.15 to 0.18) | ||
| Low physical activity (ref = High) | 0.14 (0.01–0.27) | 0.01 (−0.09 to 0.11) | ||
| Moderate physical activity | 0.14 (0.02–0.25) | 0.07 (−0.03 to 0.17) | ||
| Small number of meals (<3 times) | −0.07 (−0.23 to 0.10) | −0.21 (−0.44 to 0.03) | ||
| Poor dietary intake | −0.01 (−0.09 to 0.07) | 0.02 (−0.05 to 0.10) | ||
| Severe economic distress | 0.00 (−0.07 to 0.08) | 0.02 (−0.06 to 0.10) | ||
| Unemployment (2011) | 0.14 (−0.06 to 0.35) | 0.26 (−0.01 to 0.54) | ||
| Psychological distress | −0.04 (−0.13 to 0.05) | −0.01 (−0.09 to 0.07) | ||
| Insomnia | −0.02 (−0.12 to 0.07) | 0.07 (−0.02 to 0.15) | ||
| Low level of social network | 0.06 (−0.02 to 0.13) | −0.02 (−0.09 to 0.05) | ||
| Low level of social capital | 0.09 (−0.03 to 0.20) | −0.02 (−0.12 to 0.08) | ||
Abbreviations: CI, confidence interval; Other, other accommodations; Temp Housing, prefabricated temporary housing.
Fig. 3.
Trends in estimated marginal mean of the cumulative incidence rate of metabolic syndrome among participants aged ≥65 years from 2012 to 2015.
∗Statistically significant (P < 0.05). Error bars represent 95% confidence intervals.
We compared the constituent components of MetS among the living conditions (Supplementary Table 3). Among participants aged ≥65 years, the prevalence of obesity tended to be higher in the Temp Housing group than in the Other and the Home groups from 2012 to 2015. Although the percentage of low HDLC was significantly lower in the Temp Housing group than in the Other group in 2011, the pattern reversed after 2012. The percentage of low HDLC was also significantly higher in the Temp Housing group compared to the Home group in 2013 and 2014. On the other hand, the prevalence of elevated triglyceride was significantly higher in the Temp Housing compared to the Home group in 2013 and 2014. There were no significant differences in MetS components such as increased blood pressure or impaired glucose tolerance comparing the two groups.
4. Discussion
In this study, we compared the cumulative incidence of MetS according to the type of housing arrangement among survivors of a major disaster. The cumulative incidence of MetS was significantly higher among women aged ≥65 years who were relocated to prefabricated temporary housing, compared to those who did not move or were housed in other types of accommodation. The excess risk of MetS in this group could not be explained by differences in physical activity, dietary intake, or insomnia. Moreover, although the difference in cumulative incidence of MetS according to housing type was not statistically significant in 2012, differences between accommodation type became marginally significant in 2014.
Previous studies have described the evolution of MetS following natural disasters. DiCastelnuovo et al. [25] found that the 6-month post-disaster prevalence of MetS was higher among people directly affected by the Italian earthquake in L’Aquila compared to the general population. They further reported that the prevalence of MetS was higher among people staying in hotels or camps compared to those who stayed in their homes. Researchers have also investigated MetS in Fukushima following the nuclear disaster caused by the tsunami [[26], [27], [28]]. While the circumstances in Fukushima were quite different from those in our study area, residents who were within the 80 km exclusion zone of the Daiichi Nuclear Power Plant explosion were forcibly relocated. The participants in our study areas were not affected by radiation fallout (about 200 km away, where there was no recorded increase in the background level of radiation).
The majority of post-disaster studies till now have focused on short-term outcomes, typically up to two years, and the maximum follow up interval was four years (up to 2014). In the present study, we extended follow-up to five years post-disaster, demonstrating that the effects of housing type of MetS persists over the long term. By the end of our last examination wave (in 2015), 19.3% of survivors were still living in prefabricated temporary housing, as more permanent housing became available in 2016. Future monitoring of the population is warranted to determine if the increased incidence of MetS will translate to increased incidence of chronic disease, such as diabetes and cardiovascular disease.
In the present study, a “dose response” pattern in incidence of MetS was observed according to the level of disruption in housing arrangements. The people in the Other group were midway in the incidence of MetS compared to the Temp Housing group (most disruption) and Own Home group (least disruption). People in the Other group were just as likely to have been severely affected by the tsunami as those in the Temp Housing group; but the former group had more choice in selecting their living conditions [29]. The quality of housing was likely to have been higher (e.g., more spacious, more insulated from noise and cold) compared to temporary housing.
MetS is primarily driven by central obesity. In terms of the components of MetS, we found that the prevalence of obesity was significantly higher in the Temp Housing group than in the Other group among older participants aged ≥65 years (Supplementary Table 3). In addition, the prevalence of low HDLC changed to be higher in the Temp Housing group than that in the Other group. Previously we have reported significant weight gain and lower HDLC among survivors who were relocated to prefabricated housing compared to other types of housing arrangements [14]. In the city of Iwanuma (in the neighboring prefecture of Miyagi), Shiba et al. found that tsunami-affected survivors showed increased triglyceride levels after relocation to temporary housing [9]. These are consistent in our results.
Possible reasons for the excess incidence of MetS among people relocated to the Temp Housing include disruptions in health habits including physical activity, alcohol intake, dietary practices, and sleep. The location of some prefabricated homes in remote locations may have discouraged physical activity by increasing the distance to destinations such as grocery stores, post offices, and libraries. Public facilities including park and athletic facility tended to be located in flatland areas before the disaster [30,31]. According to one report, people relocated to temporary housing had lower physical activity levels compared to the national average, particularly at older ages [32]. In our study, older residents of the Temp Housing and Other Housing were significantly more likely to be sedentary compared to Own Home residents. However, adjusting for physical activity could not explain the difference in cumulative incidence of MetS among living conditions.
The cramped conditions inside prefabricated homes may have discouraged the preparation of meals inside the home [33], causing people to eat out more often, or to rely on pre-packaged foods. A higher proportion of older Temp Housing residents reported that they consumed less than 3 meals a day compared to survivors in the home (Table 1). Snacking on foods with poor nutritional quality instead of regular meals, has been linked with higher prevalence of MetS [34]. However, we did not conduct a detailed assessment of dietary quality (e.g. using a food frequency questionnaire), and thus it is possible that the Temp Housing group also consumed a lower quality diet – for example, consisting of store-bought pre-packaged foods that were calorie-dense and high in refined carbohydrates – which may have increased their risk of developing MetS. Residents of Temp Housing may have also engaged in more snacking between meals, which is associated with increased risk of developing MetS [35].
Conditions inside prefabricated homes were also noisy (because of thin walls), which likely accounts for the much higher prevalence of insomnia in the older Temp Housing-residents (Table 1). It has been reported that short sleep duration and psychological distress are associated with a higher prevalence of MetS [36,37]. However, controlling for differences in insomnia could not explain the excess of MetS among Temp Housing-residents in our study.
We found a gender difference in the association between the Temp Housing residence and incidence of MetS. Previous studies have shown that the prevalence of Mets tends to be higher in women than in men [24,38]. Older women may be affected by hormonal changes such as menopause. Menopause is associated with MetS via increase visceral obesity [39]. Also studies have suggested that women report a higher prevalence of psychological distress than men [40]. Some studies suggest that women may be more vulnerable to adverse psychological outcomes following disaster exposure compared to men, e.g. PTSD [41,42]. A meta-analysis indicates that there is a female excess in the risk of insomnia [43]. In the aftermath of the Great East Japan Earthquake, the prevalence of insomnia was higher in women compared to men in disaster affected areas (in Rikuzentakata; men, 27.7% vs. women, 44.4%) [44]. We speculate that the excess risk of MetS in women may be due to gender differences in psychological distress and sleep quality that were not fully captured by our surveys.
The present study had several limitations. First, detailed data on potential mediating variables were not collected, such as precise caloric intake, the amount of daily exercise, and patterns of alcohol consumption. In particular, nutrition status such as excess intake of refined carbohydrate might have affected, for example rice consumption. In addition, we did not control for potential confounding variables such as educational attainment, occupational status, or household income. Thus we cannot rule out the possibility that these unmeasured variables might result in some bias in the present results. Second, due to the use of non-fasting blood sugar level, we could not fully evaluate survivors using the IDF criteria. As a result of using non-fasting blood samples, we might have underestimated the prevalence of abnormal glycosylated hemoglobin level and HDLC levels, and overestimated the prevalence of abnormal triglyceride levels as parameters to classify MetS. Third, the RIAS study was undertaken on a voluntary basis; the findings could potentially be affected by selection bias. The participants were relatively older (average age in the present study, 59.8 years; Iwate prefecture, 49.0 years), and women [45]. Some people moved away from the area affected by the disaster, particularly young family such as those with parents in the child-bearing age [14]. As our subjects tended to be older women, care should be taking when generalizing our findings to other study areas.
5. Conclusion
We found that the cumulative incidence of MetS was higher among participants in the Temp Housing group than among those in the Other and Home groups aged ≥65 years. As having MetS increases the risk of cardiovascular disease over the long term, continued monitoring of disaster survivors may be warranted.
Funding
This work was supported by a Health Labour Sciences Research Grant from the Ministry of Health, Labour, and Welfare of Japan (grant numbers H23-Tokubetsu-Shitei-002, H24-kenki-sitei-001, and H25-Kenki-Shitei-001 [Fukkou]). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The work was also supported by JSPS KAKENHI (grant numbers JP20K18858).
CRediT authorship contribution statement
Shuko Takahashi: Conceptualization, Formal analysis, Investigation, Visualization, Writing - original draft. Yuki Yonekura: Investigation, Methodology, Resources. Kozo Tanno: Investigation, Writing - review & editing. Haruki Shimoda: Methodology, Resources. Kiyomi Sakata: Funding acquisition, Project administration. Akira Ogawa: Project administration. Seiichiro Kobayashi: Funding acquisition, Project administration. Ichiro Kawachi: Investigation, Supervision, Writing - review & editing.
Declarations of a competing interest
None.
Acknowledgments
The authors would like to thank the participants of this study as well as the many health care workers. This project was conducted with the support of the Takemi Program in International Health at the Harvard T.H. Chan School of Public Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2020.100042.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cabinet Office, Government of Japan . 2011. The number of victims in evacuation shelters by the Great East Japan earthquake.www.cao.go.jp/shien/1-hisaisha/pdf/5-hikaku.pdf accessed 1 October 2014. [Google Scholar]
- 2.Geospatial Information Authority of Japan . 2011. The area of the inundation range caused by the tsunami.www.gsi.go.jp/common/000059939.pdf accessed 14 October 2014. [Google Scholar]
- 3.The National Police Agency . The national police agency , Japan; Tokyo: 2014. Damage situation and police measures of the 2011 off the Pacific coast of Tohoku Earthquake. [Google Scholar]
- 4.Japan Science and Technology Agency . 2011. The Great East Japan earthquake information from official websites.http://www.jst.go.jp/saigai.html accessed 12 October 2014. [Google Scholar]
- 5.Sasaki Y., Aida J., Tsuji T., Miyaguni Y., Tani Y., Koyama S. Does type of residential housing matter for depressive symptoms in the aftermath of a disaster? Insights from the Great East Japan Earthquake and Tsunami. Am J Epidemiol. 2017;187:455–464. doi: 10.1093/aje/kwx274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii T., Ochi S., Tsubokura M., Kato S., Tetsuda T., Kato J. Physical performance deterioration of temporary housing residents after the Great East Japan Earthquake. Prev Med Rep. 2015;2:916–919. doi: 10.1016/j.pmedr.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimura E., Ishikawa-Takata K., Murakami H., Tsuboyama-Kasaoka N., Tsubota-Utsugi M., Miyachi M. Relationships between social factors and physical activity among survivors of the Great East Japan earthquake: a cross-sectional study. BMC Geriatr. 2016;16:30. doi: 10.1186/s12877-016-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hikichi H., Aida J., Kondo K., Tsuboya T., Matsuyama Y., Subramanian S.V. Increased risk of dementia in the aftermath of the 2011 Great East Japan earthquake and tsunami. Proc Natl Acad Sci USA. 2016:201607793. doi: 10.1073/pnas.1607793113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiba K., Hikichi H., Aida J., Kondo K., Kawachi I. Long-term associations between disaster experiences and cardiometabolic risk: a natural experiment from the 2011 Great East Japan Earthquake and Tsunami. Am J Epidemiol. 2019;188:1109–1119. doi: 10.1093/aje/kwz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Diabetes Federation . 2006. The IDF consensus worldwide definition of the metabolic syndrome.http://www.idf.org/webdata/docs/MetS_def_update2006.pdf accessed 27 June 2016. [Google Scholar]
- 11.Goryoda S., Nishi N., Shimoda H., Yonekura Y., Sakata K., Kobayashi S. Social capital and dietary intakes following the 2011 Great East Japan earthquake and tsunami. J Epidemiol. 2019;29:92–96. doi: 10.2188/jea.JE20170117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoyama Y., Otsuka K., Kawakami N., Kobayashi S., Ogawa A., Tannno K. Mental health and related factors after the Great East Japan earthquake and tsunami. PloS One. 2014;9 doi: 10.1371/journal.pone.0102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P., Jiang R., Li L., Li X., Liu C., Xu W. Usefulness of hemoglobin A(1c) as a criterion to define metabolic syndrome in nondiabetic Chinese adolescents. J Invest Med. 2013;61:586–592. doi: 10.2310/JIM.0b013e318280ab13. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi S., Nakamura M., Yonekura Y., Tanno K., Sakata K., Ogawa A. Association between relocation and changes in cardiometabolic risk factors: a longitudinal study in tsunami survivors of the 2011 Great East Japan Earthquake. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai K., Nishi A., Kondo K., Yanagida K., Kawakami N. Screening performance of K6/K10 and other screening instruments for mood and anxiety disorders in Japan. Psychiatr Clin Neurosci. 2011;65:434–441. doi: 10.1111/j.1440-1819.2011.02236.x. [DOI] [PubMed] [Google Scholar]
- 16.Kessler R.C., Barker P.R., Colpe L.J., Epstein J.F., Gfroerer J.C., Hiripi E. Screening for serious mental illness in the general population. Arch Gen Psychiatr. 2003;60:184–189. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 17.Soldatos C.R., Dikeos D.G., Paparrigopoulos T.J. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 18.Soldatos C.R., Dikeos D.G., Paparrigopoulos T.J. The diagnostic validity of the Athens insomnia scale. J Psychosom Res. 2003;55:263–267. doi: 10.1016/s0022-3999(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 19.Soldatos C.R., Allaert F.A., Ohta T., Dikeos D.G. How do individuals sleep around the world? Results from a single-day survey in ten countries. Sleep Med. 2005;6:5–13. doi: 10.1016/j.sleep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Okajima I., Nakajima S., Kobayashi M., Inoue Y. Development and validation of the Japanese version of the Athens insomnia scale. Psychiatr Clin Neurosci. 2013;67:420–425. doi: 10.1111/pcn.12073. [DOI] [PubMed] [Google Scholar]
- 21.Kurimoto A., Awata S., Ohkubo T., Tsubota-Utsugi M., Asayama K., Takahashi K. [Reliability and validity of the Japanese version of the abbreviated lubben social network scale] Nihon Ronen Igakkai Zasshi. 2011;48:149–157. doi: 10.3143/geriatrics.48.149. [DOI] [PubMed] [Google Scholar]
- 22.Lubben J., Blozik E., Gillmann G., Iliffe S., von Renteln Kruse W., Beck J.C. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontol. 2006;46:503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi S., Yonekura Y., Sasaki R., Yokoyama Y., Tanno K., Sakata K. Weight gain in survivors living in temporary housing in the tsunami-stricken area during the recovery phase following the Great East Japan earthquake and tsunami. PloS One. 2016;11 doi: 10.1371/journal.pone.0166817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arai H., Yamamoto A., Matsuzawa Y., Saito Y., Yamada N., Oikawa S. Prevalence of the metabolic syndrome in elderly and middle-aged Japanese. J Clin Geront Geriatr. 2010;1:42–47. doi: 10.1016/j.jcgg.2010.10.011. [DOI] [Google Scholar]
- 25.Di Castelnuovo A., Di Pietro N., Di Tomo P., Di Silvestre S., Pipino C., Nenna G. Metabolic syndrome in survivors from the 2009 earthquake in Italy. Nutr Metabol Cardiovasc Dis. 2013;23 doi: 10.1016/j.numecd.2012.09.005. e5–8. [DOI] [PubMed] [Google Scholar]
- 26.Ohira T., Nakano H., Nagai M., Yumiya Y., Zhang W., Uemura M. Changes in cardiovascular risk factors after the Great East Japan earthquake. Asia Pac J Publ Health. 2017;29:47s–55s. doi: 10.1177/1010539517695436. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto S., Nagai M., Fukuma S., Ohira T., Hosoya M., Yasumura S. Influence of post-disaster evacuation on incidence of metabolic syndrome. J Atherosclerosis Thromb. 2017;24:327–337. doi: 10.5551/jat.35824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebner D.K., Ohsawa M., Igari K., Harada K.H., Koizumi A. Lifestyle-related diseases following the evacuation after the Fukushima Daiichi nuclear power plant accident: a retrospective study of Kawauchi Village with long-term follow-up. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakazawa A., Konno Y., Meno F., Fujii S., Saito H. Current status and issues of post-disaster public-funded rental accommodation for the designing of the system for the future disasters. Japanese Journal of Real Estate Sciences. 2014;27:121–127. [Google Scholar]
- 30.Hirai H., Kondo N., Sasaki R., Iwamuro S., Masuno K., Ohtsuka R. Distance to retail stores and risk of being homebound among older adults in a city severely affected by the 2011 Great East Japan Earthquake. Age Ageing. 2015;44:478–484. doi: 10.1093/ageing/afu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyama S., Aida J., Kawachi I., Kondo N., Subramanian S.V., Ito K. Social support improves mental health among the victims relocated to temporary housing following the Great East Japan Earthquake and Tsunami. Tohoku J Exp Med. 2014;234:241–247. doi: 10.1620/tjem.234.241. [DOI] [PubMed] [Google Scholar]
- 32.Murakami H., Yoshimura E., Ishikawa-Takata K., Nishi N., Tsuboyama-Kasaoka N., Yokoyama Y. The longitudinal change in physical activity among Great East Japan Earthquake victims living in temporary housing. [Nihon Koshu Eisei Zasshi] Jpn J of Public Health. 2014;61:86–92. doi: 10.11236/jph.61.2_86. [DOI] [PubMed] [Google Scholar]
- 33.Otoki T. 2013. To investigate the change of consciousness for food and to design future dietary life in N village in the natural disaster. Research report of earthquake disaster reconstruction in 2011 and 2012. Iwate; pp. 13–14. [Google Scholar]
- 34.Jung C.-H., Lee J.S., Ahn H.J., Choi J.-S., Noh M.Y., Lee J.J. Association of meal frequency with metabolic syndrome in Korean adults: from the Korea national health and nutrition examination survey (KNHANES) Diabetol Metab Syndrome. 2017;9:77. doi: 10.1186/s13098-017-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pimenta A.M., Bes-Rastrollo M., Gea A., Sayón-Orea C., Zazpe I., Lopez-Iracheta R. Snacking between main meals is associated with a higher risk of metabolic syndrome in a Mediterranean cohort: the SUN Project (Seguimiento Universidad de Navarra) Publ Health Nutr. 2015;19:658–666. doi: 10.1017/s1368980015001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang J.H., Huang P.T., Lin Y.K., Lin C.E., Lin C.M., Shieh Y.H. Association between sleep duration and sleep quality, and metabolic syndrome in Taiwanese police officers. Int J Occup Med Environ Health. 2015;28:1011–1023. doi: 10.13075/ijomeh.1896.00359. [DOI] [PubMed] [Google Scholar]
- 37.Nishina M., Nishina K., Ohira T., Makino K., Iso H. Associations of psychological distress with metabolic syndrome among Japanese urban residents. J Atherosclerosis Thromb. 2011;18:396–402. doi: 10.5551/jat.6692. [DOI] [PubMed] [Google Scholar]
- 38.Ohkubo K., Kiyohara Y. The prevalence of metabolic syndromein Japanese dweller. The Jap J Clin Exp Med. 2004;81:1736–1740. [Google Scholar]
- 39.Janssen I., Powell L.H., Crawford S., Lasley B., Sutton-Tyrrell K. Menopause and the metabolic syndrome: the study of women’s health across the nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drapeau A., Beaulieu-Prevost D., Marchand A., Boyer R., Preville M., Kairouz S. A life-course and time perspective on the construct validity of psychological distress in women and men. Measurement invariance of the K6 across gender. BMC Med Res Methodol. 2010;10:68. doi: 10.1186/1471-2288-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa S., Ishiki M., Nako K., Okamura M., Senda M., Sakamoto T. Effects of the Great East Japan Earthquake and huge tsunami on glycaemic control and blood pressure in patients with diabetes mellitus. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galea S., Nandi A., Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev. 2005;27:78–91. doi: 10.1093/epirev/mxi003. [DOI] [PubMed] [Google Scholar]
- 43.Zhang B., Wing Y.K. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 44.Gender Equality Bureau Co, Government of Japan . Gender Equality Bureau, Cabinet Office, Government of Japan; Tokyo: 2014. Natural disasters and gender statistics: lessons from the Great East Japan earthquake and tsunami. [DOI] [Google Scholar]
- 45.The Portal Site of Official Statistics of Japan . 2015. Population census. 2015 population census.https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00200521&tstat=000001080615&cycle=0&tclass1=000001089055&tclass2=000001089057&tclass3=000001089060; 2015 accessed January 22 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.