Abstract
The Metabolic Syndrome (MS) is a set of alterations that increase the risk of developing type 2 diabetes mellitus (DM2). There is evidence that obesity and the development of metabolic syndrome lead to alterations in cognitive processes. In this work it was proposed to determine if generating the metabolic syndrome produces changes in the electric unitary spontaneous activity in the hippocampus as a possible sustain of the learning alterations. In Wistar rat with a hypercaloric diet, metabolic syndrome was provoked, and this was confirmed with the determination of body and metabolic parameters as a measure of intraperitoneal fat, glucose, triglycerides, and cholesterol. Electrophysiological records were made in the hippocampus and it was determined that rats treated with a hypercaloric diet show a significant decrease in such activity. Thus, it is shown that rats that were caused metabolic syndrome, alter their hippocampal electrophysiological activity.
Keywords: Metabolic syndrome, Hippocampal electrophysiology, Glucose, Triglycerides, Cholesterol, Wistar rat
Highlights
-
•
In Wistar rat a hypercaloric diet produces metabolic syndrome.
-
•
Metabolic syndrome causes decreased hippocampal activity in Wistar rat.
-
•
Metabolic syndrome produces physiological and metabolic alterations in Wistar rat.
1. Introduction
The Metabolic Syndrome (MS) is a set of alterations that increase the risk of developing type 2 diabetes mellitus (DM2), cardiovascular diseases and some types of cancer, which are the main causes of death in patients with this syndrome. Among the important alterations of this syndrome are: obesity, hypertension, dyslipidemia and hyperinsulinemia associated with insulin resistance [[1], [2], [3], [4], [5]].
Insulin is the principal hormone regulating the glucose metabolism in the peripheral tissues and some brain areas as the hypothalamus and the hippocampus, the rest of the brain seems to be an insulin-insensitive tissue. Areas like hippocampus expresses high levels of insulin-sensitive glucose transporter GLUT 4 [6,7].
Evidences have found in patients with different metabolic alterations, varying from obesity to T2DM, that the risk for several cognitive incapacities is increased [8]. In diabetic patients, who have lost the ability to regulate proper glucose management have been found cognitive deficits [9], particularly in the elderly population [10,11]. Obesity in midlife was found to be associated with an increased risk of dementia and Alzheimer Disease in aging [12,13].
The hippocampus has been recognized as a structure that participates, in a determinant way, in some types of learning and memory. Some data suggest that hippocampal cognitive performance is dependent on an adequate supply of glucose, and that providing of additional glucose to the hippocampus, can enhance memory performance [14]. In Alzheimer’s disease patients, seems that insulin may enhance performance on hippocampus-mediated tasks [15]. So, it seems that a good handling of glucose plays a very important role in the cerebral activity [16].
The objective of the present study was to determine if the high consumption of sugars in the diet causes changes or alterations in the spontaneous electrical activity of the hippocampus in the CA1 region in an animal model like the Wistar rat during the establishment of the MS.
2. Method
2.1. Ethical considerations in handling animals
The animal care was carried out according to the protocol published in "NIH guide for the care and use of laboratory animals" and the Official Mexican Standard (NOM-062-ZOO = 1999).
Forty Wistar male rats of 2 months old and an initial weight between 180-200 g were used. Rats were caged in groups of three and maintained under standard laboratory conditions; under light-dark cycles (12:12 h) and temperature controlled 23 ± 2 °C. They had free access to water and food (Purina Rat Chow 5001) during the 24 h a day. The animals were randomly divided, the control group and the experimental group. To the experimental animals the tap water was switched by a solution of 20% sucrose. This treatment was continued for six months.
2.2. Measurement of food and fluids intake and from metabolic parameters
In both groups, the consumption of solid food and liquid (water or solution with 20% sucrose) were quantified to give an average per group per week. The rats from both groups were weighed weekly.
Abdominal circumference of each rat was measured prior to electrophysiological recording. The animals were sacrificed, and the intraperitoneal fat and the brain were extracted for later analysis.
2.3. Determination of metabolic parameters (metabolic syndrome installation)
For the determination of glucose, triglycerides and cholesterol, the animals in both groups, experimental and control, were kept fasted for 12 h, then under general anesthesia with pentobarbital (50 mg/kg IP) was made a puncture in the end of the tail and drops of blood (peripheral) were collected to measure the different metabolic parameters. All measurements were performed in both groups at 3 and 6 months of treatment. The metabolic parameters were measured with Accutrend strips: glucose, triglycerides and cholesterol. All determinations were made following the protocol suggested by the manufacturer.
2.4. Blood pressure measurement
One CODA equipment, connected to a computer for viewing and storing the value of the systolic and diastolic pressure of the animal was used for determining blood pressure. Before the experiment, the animals were placed one by one on a tube of clear acrylic for 2 min, so that they adapt to space and reduce stress.
Experimental test consisted of placing to the rat in the acrylic tube to immobilize it, this tube has a hole where the tail of the rat out, a small ring was placed in the upper third of the tail and this was automatically insufflated, in 60s, the blood pressure was obtained in the computer. The test was performed three times for each animal.
2.5. Extracellular recording
Recordings were made in both control and experimental rats at 3 and 6 months of treatment. For recording, the urethane-anesthetized animal (1.6 mg/kg IP) was placed on a stereotaxic apparatus and following the coordinates of the Pellegrino’s atlas [17], a chloridized silver electrode into a glass pipette filled with NaCl 4M and a dye was introduced to the CA1 hippocampal area. Finding a cell with spontaneous activity in the area of interest, it was recorded for 15 min for its stabilization and then the recording was continued for 40 min more for each cell. Several cells were recorded in each rat. The electrical signal was monitored through an oscilloscope and an audiomonitor. Through a discrimination window the number of spikes generated by the neuron for each 10s was obtained, and frequency histograms were made to determine the firing rate of each cell. The registration area was marked with fast green. The animal was sacrificed under deep anesthesia and the brain was removed for the recording site confirmation.
3. Results
The results presented are 42 animals at three months and 21 animals at 6 months of treatment.
3.1. Solid consumption
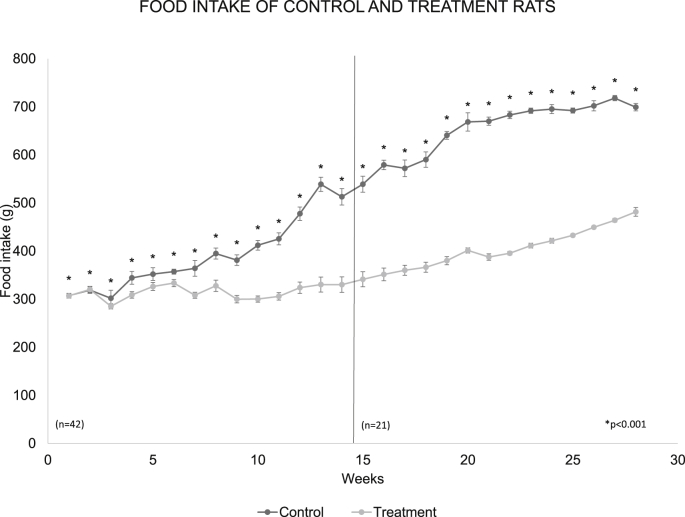
The ingestion of solids in the two groups was very similar during the first three weeks but a separation in such ingestion is observed. From this point, it was gradually increasing in the control group. When the average of the solids consumed per group was made during the 6 months of study, resulted in 74 ± 3.99 g/day/rat in the control group while in rats with treatment the average was 51.27 ± 1.48 g/day/rat, p < 0.001 (Fig. 1).
Fig. 1.
The graph shows the weekly average of the solids consumption in the control group and with treatment during the 6 months of the experiment. The treatment group significantly reduced their solid food intake with respect to the control group p < 0.001 control. The dotted line on the graph indicates week 14, week in which the number of rats was reduced to n = 21.
3.2. Liquids consumption
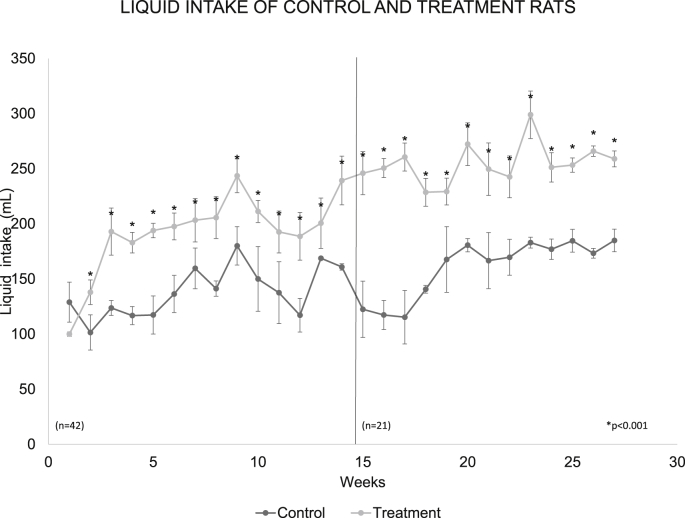
The average fluid consumption in both groups was very similar in the first two weeks, however, a substantial and continuous increase in the experimental group was observed from the third week. When the average of the fluid consumed per group was made during the 6 months of study, in the control rats it was 148.91 ± 5.3 mL/day/rat, while in the rats with treatment it was 212.41 ± 8.2 mL/day/rat, with significant differences between treatment and control at p < 0.001 (Fig. 2).
Fig. 2.
The weekly average of fluid consumption in the control group and with treatment during the 6 months of study. The treatment group increased their consumption of sugar water and there were significant differences with respect to the control p < 0.001. The dotted line in the graph shows week 14, in which the number of rats was reduced to n = 21.
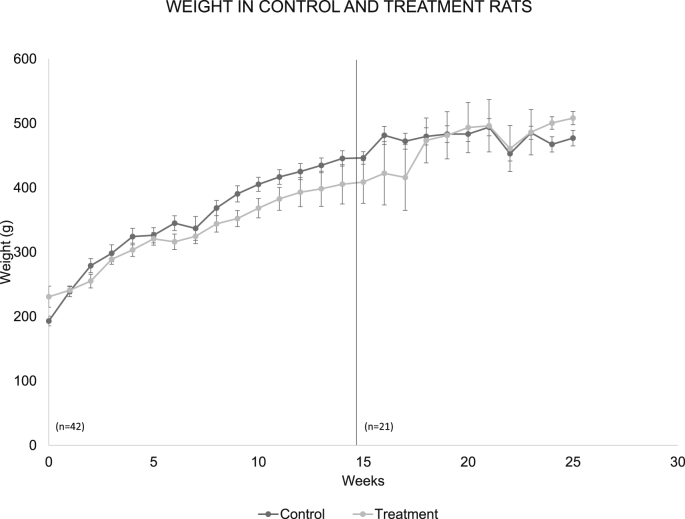
3.3. Body weight
The average body weight in both groups was very similar throughout the experiment. Statistical analysis showed that there are no significant differences between the two groups (Fig. 3).
Fig. 3.
The graph shows the weekly average of the body weight of rats of the control group and of the experimental group, during the six months analyzed. The dotted line points to week 14, week in which the number of rats was reduced to n = 21.
3.4. Intraperitoneal fat and diameter
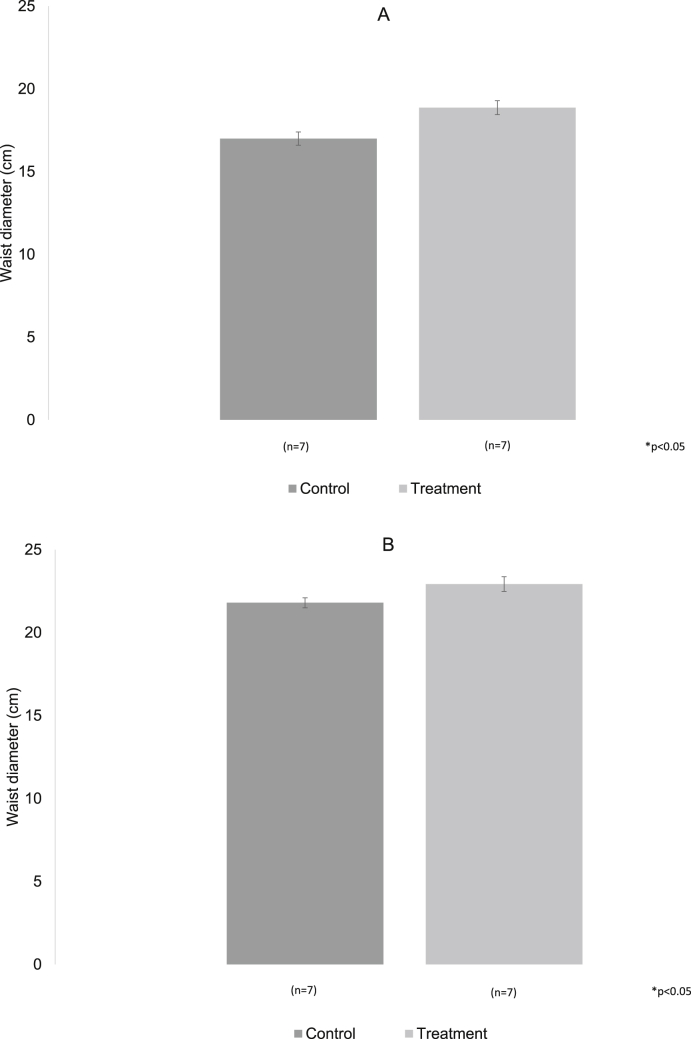
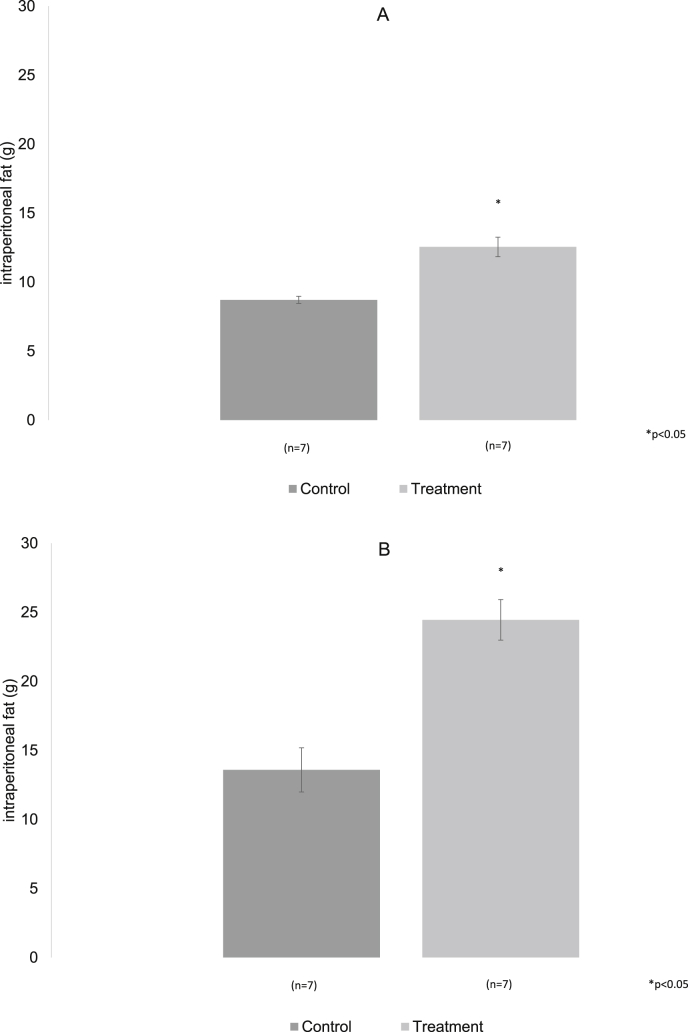
The waist diameter in both groups was similar at three and six months of treatment, even though the experimental group showed a larger diameter, there were no significative differences between the groups (Fig. 4A and B), while the Intraperitoneal fat was significantly increased in the group with hypercaloric diet in the two analyzed moments, p < 0.05 (Fig. 5A and B).
Fig. 4.
A, B. The bars represent the waist diameter without changes in the two periods reported.
Fig. 5.
A, B. Intraperitoneal fat increases significantly in the experimental group both three months and six months after treatment.
3.5. Glucose
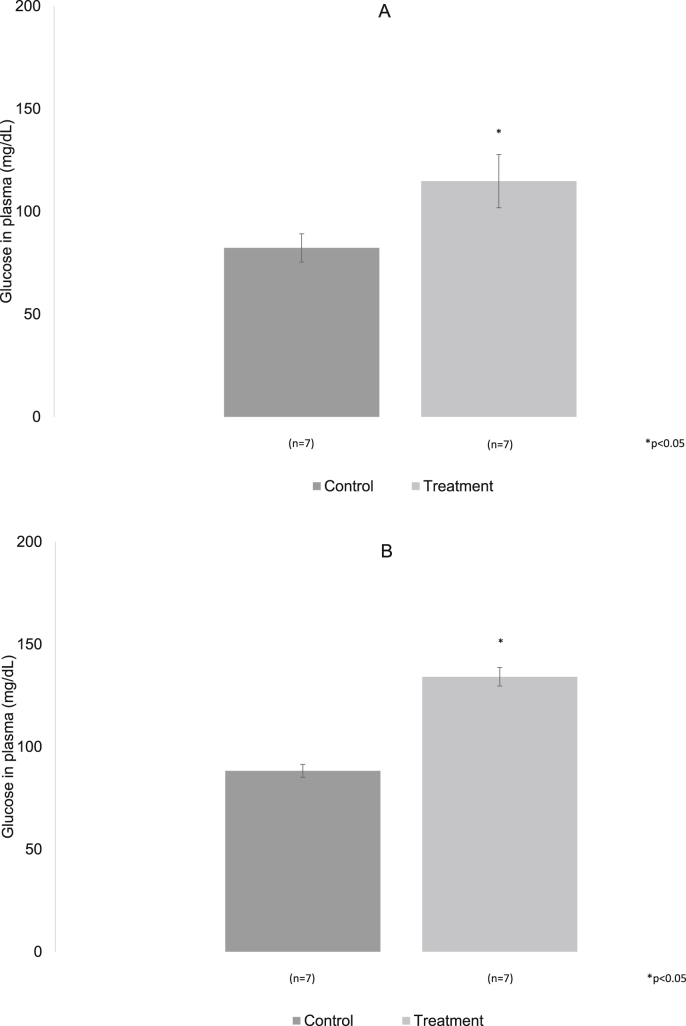
In seven animals of both groups, control and experimental, fasting plasma glucose values were obtained. In control rats to the three months the mean value was 82.25 ± 6.9 mg/dL while in the experimental group the mean value found was 114.75 ± 12.95 mg/dL. At six months of treatment, the plasma glucose mean value in the control animals was 88.28 ± 3.16 mg/dL while in the experimental animals the value found was 134.14 ± 4.51 mg/dL. In the periods analyzed the experimental animals showed a significant increase in glucose p < 0.05 (Fig. 6A and B).
Fig. 6.
A, B. Plasma glucose values with fasting of 12 h, in control and experimental rats with 20% of sugar, are shown at 3 and 6 months of treatment. In both periods a significant increase of the glucose was observed, in the treatment group with respect to the control. p < 0.05.
3.6. Triglycerides
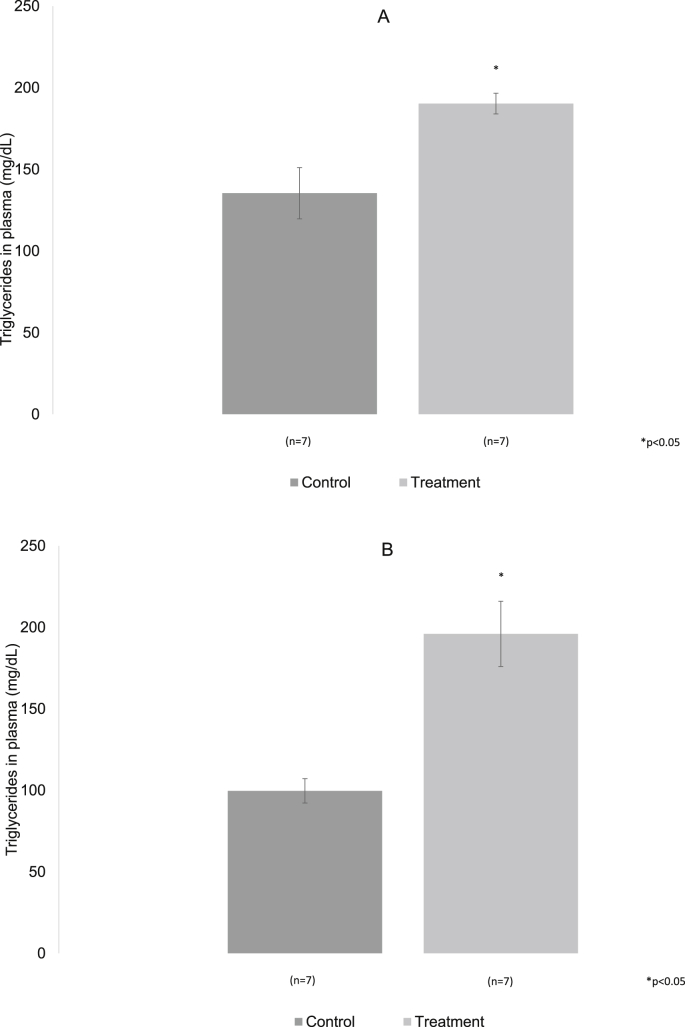
In control and experimental animals, plasma triglyceride values in peripheral blood were obtained. The value for the control animals at three months was 135.42 ± 15.69 mg/dL, and to the six months it was 99.71 ± 7.49 mg/dL. In treated animals the values were 190.28 ± 6.37 mg/dL and 191.8 ± 22.25 mg/dL at three and six months respectively. They were significantly different from the control group, p < 0.05 (Fig. 7A and B).
Fig. 7.
A, B. The averages of triglyceride levels in control and treated with 20% sugar rats at 3 and 6 months are shown. In both periods a significant increase of the triglycerides in the treatment group was observed with respect to the control. p < 0.05.
3.7. Cholesterol
In both groups controls and treatment, plasma cholesterol values were obtained at three months. In the control group it mean value was 177.00 ± 2.92 mg/dL while at the six months these were 180.57 ± 1.73 mg/dL. In the experimental group the mean data were 170.85 ± 1.73 mg/dL and 162.00 ± 10.69 mg/dL at three and six months respectively. No significant differences were found (Fig. 8A and B).
Fig. 8.
A, B. The averages of cholesterol levels in control and with treatment rats at 3 and 6 months. There are no significant differences.
3.8. Blood pressure
In both groups of animals, the mean values obtained from both systolic and diastolic pressure were very similar to the three months of the experiment. Control group: systolic pressure 115.67 ± 7.6 mm/Hg, diastolic 84.17 ± 7.5 mm/Hg. Experimental group: systolic pressure 114.8 ± 4.5 and diastolic pressure 78.6 ± 3.5 mm/Hg (Fig. 9A and B).
Fig. 9.
A, B. The values of diastolic and systolic pressure at three months of treatment are shown, there were no differences between the groups.
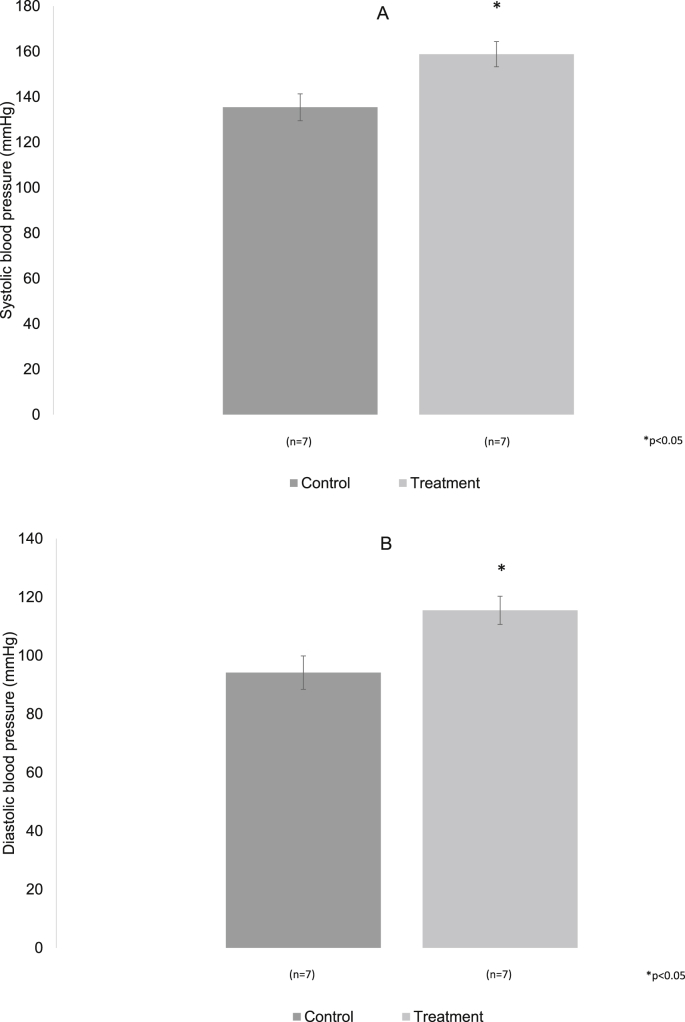
At six months of treatment, in the experimental group there was a significant increase in systolic pressure (158.8 ± 5.5 mm/Hg) and diastolic pressure(115.4 ± 4.8 mm/Hg), while in the control group was systolic pressure (135.4 ± 5.9) and diastolic pressure (94.1 ± 5.7), p < 0.05 (Fig. 10A and B).
Fig. 10.
A, B. At 6 months in the control and treatment groups are shown. There was a significant increase in both pressures in the treatment group versus control p < 0.05.
3.9. Spontaneous unitary activity
The spontaneous unitary activity of the hippocampus in CA1 area was recorded in 18 neurons of each group.
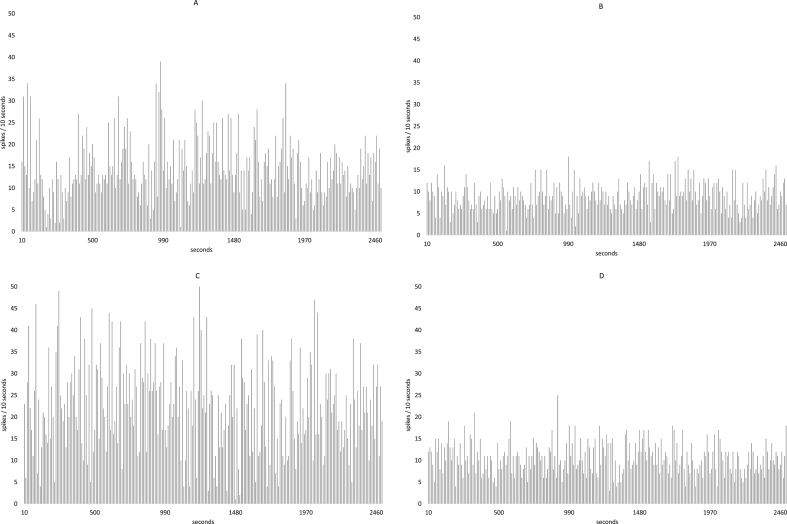
At three months of treatment there was no difference in the frequency of neuronal firing between groups. A frequency of 12.45 ± 1.95/10s was found in the control group, while in the experimental group it was 11.76 ± 1.07/10s. At six months of treatment there were a significant decrease between the trigger frequency that the experimental group showed with respect to the control. So, the control group had 22.7 ± 1.0 and treatment group had 17.2 ± 2.1, p < 0.05.
Fig. 11 shows the frequency histograms of the spontaneous unit activity of a neuron of the hippocampal area CA 1. The histogram A corresponds to the recording of a neuron of the control group, while the histogram B to a neuron of the group with treatment at the 3 months of the study. Histogram C is a neuron of the control group and D is a histogram of a neuron of the group with treatment at 6 months. In both periods a decrease of the firing frequency in the neurons of animals with treatment with respect to the control is shown.
Fig. 11.
Shows the frequency histograms of the spontaneous unit activity from a neuron in the CA 1 area of the hippocampus. Histogram A corresponds to the registration of a neuron in the control group, while histogram B corresponds to a neuron in the group with treatment both at 3 months of the study. Histogram C is a neuron of the control group and D is a histogram of a neuron of the group treated both at 6 months. In both periods a decrease in the firing frequency is shown in the neurons of animals with treatment with respect to the control.
4. Discussion
Metabolic syndrome (MS) and associated comorbidity are continuously increasing worldwide at an alarming rate. This syndrome is defined as a constellation of interrelated corporal changes and metabolic irregularities, including abdominal obesity, hypertension, hyperglycemia, insulin resistance, leptin resistance and hypercortisolemia [18].
The existing evidence shows that patients with metabolic syndrome often experience a higher prevalence of affective symptoms and cognitive dysfunctions than the general population [19]. Neuropsychiatric disorders resulting from metabolic changes influence the deterioration of the quality of life of patients with MS and the results in additional costs to health systems around the world.
In addition, the metabolic syndrome begins to be related to cognitive alterations that can be associated with cerebrovascular events resulting from alterations in blood pressure, which in turn can cause vascular lesions in the nervous system and in other organs of the economy.
Thus, hypertension is considered as a risk factor to develop brain and cognitive alterations [20]. Hypertension is one of the factors associated with MS. Recently, evidence has emerged that MS is associated with the appearance of silent brain lesions, independently of other risk factors, for ischemic stroke; moreover, there is a directly proportional correlation between the number of components of the MS and the prevalence of silent cerebral lesions [21]. On the other hand, MS has been associated with cerebral and systemic atherosclerosis that can also lead to a cerebrovascular event and cognitive deterioration [22].
In this work the animals studied had a high consumption of sugar water, since the beginning of the experiment. Despite the variability observed in this response, in the first weeks, water with sugar is an appetizing "food" for rats, consumption gradually increased, and it was evident from the 20th week. In rodents there is an addiction to sugar consumption, showing signs of tolerance and abstinence [23]. It has been established that sugar in the rat can produce an addiction that is perhaps more important than addiction to cocaine and heroin [24]. This avid consumption of sugar can result from the release of dopamine in the accumbens nucleus in a similar way to what some drugs of abuse do [23].
Fig. 1 shows that the control animals ingested almost twice as much food as the experimental animals, this result suggests that probably the calories obtained from the sugar water are enough to maintain the body weight of the experimental animals, as shown in Fig. 3. This graphic clearly shows that body weight increased over time but remained very similar in the two groups at the two ages analyzed. The experimental animals exhibited a marked reduction in the consumption of solid food (5001, Rodent diet). It has been suggested, for this strain of rats, that there is a reduction of food consumption when they are exposed to hypercaloric diets [25]. Since the 80’s, it was established [26] that the daily food consumption of the adult rat is usually very stable throughout its life. In rats exposed to high-calorie diets, there is a decrease in the consumption of food as it was showed in rats that had to press a lever to obtain food, the number of times they pressed the lever was reduced when the food was hypercaloric suggesting that the amount of calories can be regulated by the body itself [27], which could be the case in this work.
In several experiments it has been shown that in rodents exposed to high carbohydrate diets, the body weight maybe increases but apparently it does in animals from middle age [28], it is noteworthy that the animals here reported were still growing as indicated in Fig. 3.
Although, the body weight did not change significantly, in the experimental animals, alterations were found in some of the body parameters that were measured as is the case of intraperitoneal fat, this increased significantly from the third month of treatment. There are data [28], which show that rats subjected to high sucrose diet develop visceral adiposity but not weight gain, in those rats, capillary necrosis in striated muscle was found so as hepatic mitochondrial alterations. Even in human has been showed an increase in adiposity and no increase in body weight [29]. It is likely that, in this work, the rats have decreased their muscle mass by necrosis, even when it is not confirmed. It is possible that if some muscles were weighed in these animals, they would weigh less than in the controls. It is proposed to make these measures in an upcoming work.
The abdominal measurements were not different and, in this work, do not seem to be determinants of the fat that is in the organism as it is in the case of the human, in which seems to be a better correlation between abdominal diameter and lipid alterations [30].
The results obtained from the measurement of plasma glucose show that it increased significantly in the experimental animals, in the two periods analyzed (Fig. 6 A, B). The increase indicates that there may already be an insulin resistance since the three month of treatment which prevents glucose from entering to target organs and therefore increases in plasma.
Even though the value of insulin in these animals is unknown, it is suggested that it can be elevated, as in humans, due to the increase in the plasmatic glucose, the adipocytes and the substances released by them (e.g. resistin).
In experimental animals, triglyceride levels were significantly elevated which can be the starting of a prediabetic state as in several studies it has been suggested [31]. The excess of adipose tissue, as observed in the amount of abdominal fat in the animals reported here, releases an increased amount of fatty acids that directly affects the insulin and glucose signals and induces gluconeogenesis in the liver. Additionally, the secretion of resistin is surely increased, which prevents the use of glucose, resulting in high plasmatic levels of glucose.
Cholesterol did not change significantly in animals with a high glucose diet. The afore mentioned metabolic parameters, being elevated in treated animals, can generate vascular alterations [32], that can be reflected in brain changes. Glucose may be causing glucotoxicity in neurons and reduces their death time in the cell. In addition, changes in triglycerides suggest alterations in lipid metabolism that can cause an increase in atheromatous plaque or changes in the vascular endothelium itself [33] leading to a reduction in the diameter of the vessels and therefore, an increase in peripheral resistance that results in an increase in blood pressure both in the systolic and in the diastolic, which is what is observed in the results of this study, particularly in the 6-month-old animals (Fig. 10A and B). In addition, it should be noted that the increase in systolic blood pressure can result from the activation of the autonomic nervous system, increased activity of the renin-angiotensin system (sodium increase), oxidative stress, increase in fatty acids, insulin and leptin.
Animals treated with a diet high in glucose develop metabolic abnormalities identified by hypertriglyceridemia, hyperglycemia and hypertension in addition to showing an increase in abdominal fat. All these parameters were increased in our experimental rats which indicate that they have developed a metabolic syndrome.
There is evidence that the metabolic syndrome may be associated with a cognitive decline, particularly in advanced ages, especially in subjects with high levels of inflammation. It has been described that Alzheimer’s disease is associated with inflammation, which is why it has been considered as diabetes mellitus type 3 [34].
It has been observed that there are risks of developing cognitive alterations in patients with type 2 diabetes. In this condition, a "diabetic encephalopathy” [35] can develop which is characterized by electrophysiological, structural, and neurochemical changes that lead to cognitive deterioration, which can include deficiencies in memory. These cognitive variations may result from an alteration in the hippocampal modulation by insulin resistance, which elicits a reduction in the regional metabolism of glucose, which would lead to alterations in spatial memory [36]. In addition, cognitive alterations associated with obesity have also been reported in children [37,38].
The frequency recorded in the neurons of the hippocampus indicates that in the control animals it increases after six months when it is compared to the three months animals. This increase could be due to a higher neuronal excitability generated by changes a greater number of synaptic connections. However, in experimental animals there is a significant decrease in the frequency of neuronal firing at six months of treatment compared to controls, it has been suggested that glucose at concentrations similar to those reached after a food can produce a decrease of the level of firing of dopaminergic neurons, in this work plasma glucose remains high which could result in a similar change. The electrical changes observed here indicate that this part of the hippocampus can be participating in the regulating circuit of hunger and satiety [39].
In pigs [40] it was reported that there is a reduction in brain activity measured by the amount of blood flow in animals that were induced obesity, some regions that decrease their activity are related to limbic areas. The animals studied here showed a reduction of the neuronal frequency, which could result from the decrease in activity. Is there a neurodegeneration? The brain is getting old?
The results shown here indicate that the use of 20% glucose in the daily diet of the Wistar rat significantly modifies metabolic and body parameters leading to a prediabetic state to the animal.
The induction of metabolic syndrome produces alterations in the electrophysiological activity of the hippocampus which in turn can induce cognitive alterations.
CRediT authorship contribution statement
B. Prieto-Gómez: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Supervision, Project administration. M. Díaz-Vázquez: Formal analysis, Investigation, Writing - review & editing, Visualization, Validation. D. Pérez-Torres: Formal analysis, Methodology, Investigation, Validation.
Declaration of competing interest
The authors don’t have any conflict of interest.
Acknowledgements
The authors would like to thank Ana Lilia Ocampo Belmont for helping in the rat care.
References
- 1.Kendall D.M., Harmel A.P. The metabolic syndrome, type 2 diabetes, and cardiovascular disease: understanding the role of insulin resistance. Am J Manag Care. 2002; Dec;8(20 Suppl):S635–S653. [PubMed] [Google Scholar]
- 2.Zimmet K.P., Alberti G.M.M., Serrano-Ríos M. A new international diabetes federation (IDF) worldwide definition of the metabolic syndrome: the rationale and the results. Rev Esp Cardiol. 2005;58(12):1371–1376. [PubMed] [Google Scholar]
- 3.Alberti G., Zimmet P., Shay J. A new IDF worlwide definition of the metabolic syndrome: the rationale and the results. Diabetes Voice. 2005;50(3):31–33. [Google Scholar]
- 4.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr. Circulation. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation. Int Atherosclerosis Soc Int. Assoc Study Obes. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Huang P.L. A comprehensive definition for metabolic síndrome. Dis Model Mech. 2009;2(5- 6):231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen B.S., Reagan L.P. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490(1–3):13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 7.Grillo C.A., Piroli GG G.G., Hendry R.M., Reagan L.P. Insulin-stimulated translocation of glut4 to the plasma membrane in rat hippocampus is pi3-kinase dependent. Brain Res. 2009 Nov 3;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prickett C., Brennan L., Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9(2):93–113. doi: 10.1016/j.orcp.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Wang Ch, Chan J.S.Y., Ren L., Jin H.Y. Review article obesity reduces cognitive and motor functions across the lifespan. Neural Plast. 2016:13. doi: 10.1155/2016/2473081. Article ID 2473081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benito-León J., Mitchell A.J., Hernández-Gallego J., Bermejo-Pareja F. Obesity and impaired cognitive functioning in the elderly: a population-based cross-sectional study (NEDICES) Eur J Neurol. 2013;20(6):899–906. doi: 10.1111/ene.12083. [11] Munshi MN. Cognitive Dysfunction in Older Adults With Diabetes: What a Clinician Needs to Know Diabetes Care 2017; 40:461–467 | DOI: 10.2337/dc16-1229. [DOI] [PubMed] [Google Scholar]
- 11.Munshi M.N. vol. 40. 2017. pp. 461–467. (Cognitive dysfunction in older adults with diabetes: what a clinician needs to know diabetes care). [DOI] [PubMed] [Google Scholar]
- 12.Xu W.L., Atti A.R., Gatz M., Pedersen N.L., Johansson B., Fratiglioni L L. Midlife overweight and obesity increase late-life dementia risk. A population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzpatrick A.L., Kuller L.H., Lopez O.L. Midlife and late-life obesity and the risk of dementia. Cardiovascular health study. Arch Neurol. 2009;66(3):336–342. doi: 10.1001/archneurol.2008.582. Gold P. Glucose and age-related changes in memory. Neurobiology of Aging. 2005; 26S:S60– 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNay E.C., Gold P.E. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. Biol Sci. 2001;56A(No. 2):B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- 15.Mc Nay E.C., Ong C.T., McCrimmon R.J., Cresswell J., Bogan J.S. Sherwin RS Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010 May;93(4):546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magistretti P.J., Allaman I. Lactate in the brain: from metabolic end-product to signaling molecule. Nat Rev Neurosci. 2018;19:235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- 17.Pellegrino L.J., Pellegrino A.S., Cushman A.J. vol. 4. Plenum Press; 1986. p. 122. (A stereotaxic atlas of the rat brain). [Google Scholar]
- 18.Dik M.G., Jonker C., Comijs H.C. Contribution of metabolic syndrome components to cognition in older individuals. Cardiovasc Metabol Risk. Diabetes Care. 2007;30(10):2655–2660. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- 19.Bora E, Akdede BB, Alptekin K. The relationship between cognitive impairment in schizophrenia and metabolic syndrome: a systematic review and metaanalysis 2018, Psychol Med 48, 1224. [DOI] [PubMed]
- 20.Meissner A. Hypertension and the brain: a risk factor for more than heart disease. Cerebrovasc Dis. 2016;42(3–4):255–262. doi: 10.1159/000446082. Neurol. 2013;20(6):899-906. [DOI] [PubMed] [Google Scholar]
- 21.Bokura H., Yamaguchi S., Iijima K., Nagai A., Oguro H. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke. 2008;39:1607–1609. doi: 10.1161/STROKEAHA.107.508630. [DOI] [PubMed] [Google Scholar]
- 22.De la Torre J.C. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012:367516. doi: 10.1155/2012/367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avena, NM, Rada P, Hoebel, BG.Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 32(1), 20–39. [DOI] [PMC free article] [PubMed]
- 24.Ahmed S.H., Guillem K., Vandaele Y. Sugar addiction: pushing the drug-sugar analogy to the limit. Curr Opin Clin Nutr Metab Care. 2013;16(4):434–439. doi: 10.1097/MCO.0b013e328361c8b8. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento A.F., Sugizaki M.M., Leopoldo A.S., Lima-Leopoldo A.P., Luvizotto R.A.M., Nogueira C.R., Cicogna A.C. A hypercaloric pellet-diet cycle induces obesity and Co-morbidities in Wistar rats. Arq Bras Endocrinol Metabol. 2008;52/6:968–974. doi: 10.1590/s0004-27302008000600007. [DOI] [PubMed] [Google Scholar]
- 26.ANSA. Acuerdo Nacional para la Salud Alimentaria . Secretaria de Salud; 2010. Estrategia contra el sobrepeso y la obesidad. 1a edición. [Google Scholar]
- 27.López-Espinoza A., Galindo A., Martínez A.G., Díaz F., Aguilera V., De la Torre-Ibarra C., Cárdenas A. Regulación de la Conducta Alimentaria ante Cambios en el Contenido Nutricional del Alimento en Ratas. Psicol Iberoam. 2008;16(2):22–28. [Google Scholar]
- 28.Cao L., Liu X., Cao H., Qingguo L., Nanwei T. Modified high-sucrose diet-induced abdominally obese and normal-weight rats developed high plasma free fatty acid and insulin resistance. Oxid Med Cell Longev. 2012:374346. doi: 10.1155/2012/374346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouchi R., Minami I., Ohara N., Nakano Y., Nishitani R., Murakami M., Takeuchi T., Akihisa M., Fukuda T., Fujita M., Yoshimoto T., Ogawa Y. Impact of increased visceral adiposity with normal weight on the progression of arterial stiffness in Japanese patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2015;10(1) doi: 10.1136/bmjdrc-2015-000081. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pouliot M.-C., Desprès J.-P., Lemieux S., Moorjani S., Bouchard C., Tremblay A. Waist circumference and sagittal abdominal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 31.Unger G., Benozzi S.F., Perruzza F. Pennacchiotti GL Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. 2014;61(10):533–540. doi: 10.1016/j.endonu.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Contreras-Leal, Santiago-García Obesidad, síndrome metabólico y su impacto en las enfermedades cardiovasculares. Rev Biomed. 2011;22:103–115. [Google Scholar]
- 33.Maldonado O., Sánchez Ramírez, García J.R., Ceballos Reyes G., Méndez E. Colesterol: función biológica e implicaciones médicas. Rev Mex Ciencias Farm. 2012;43(2) México. [Google Scholar]
- 34.Dolata R., Piwowar A. Neurometabolic evidence supporting the hypothesis of increased incidence of type 3 diabetes mellitus in the 21st century. BioMed Res Int. 2019;21:2019. doi: 10.1155/2019/1435276. 1435276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brands A.M., Henselmans J.M., de Haan E.H., Biessels G.J. Diabetic encephalopathy: an underexposed complication of diabetes mellitus. Ned Tijdschr Geneeskd. 2003;147(1):11–14. 4. [PubMed] [Google Scholar]
- 36.Gladding J.M., Abbott K.N., Antoniadis C.P., Stuart A., Begg D.P. The effect of intrahippocampal insulin infusion on spatial cognitive function and markers of neuroinflammation in diet-induced obesity. Front Endocrinol (Lausanne) 2018;11(9):752. doi: 10.3389/fendo.2018.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornley S., Sundborn G. The case to ban sugary food and drink from schools: these products are addictive, and kids will learn best without them. Pac Health Dialog. 2014;20(1):17–21. [PubMed] [Google Scholar]
- 38.Bozkurt H., Özer S., Yılmaz R., Sönmezgöz E., Kazancı Ö., Erbaş O., Demir O. Assessment of neurocognitive functions in children and adolescents with obesity. Appl Neuropsychol Child. 2017;6(4):262–268. doi: 10.1080/21622965.2016.1150184. [DOI] [PubMed] [Google Scholar]
- 39.Benoit S., Davis J., Davidson T. Learned and cognitive controls of food intake. Brain Res. 2010 September 2;1350:71–76. doi: 10.1016/j.brainres.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Val-Laillet D., Layec S., Guérin S., Meurice P. Charles-henri malbert changes in brain activity after a diet-induced obesity. Obesity. 2011;19:749–756. doi: 10.1038/oby.2010.292. [DOI] [PubMed] [Google Scholar]