Abstract
Background
Physical activity and dietary intake of dairy products are associated with improved metabolic health. Dairy products are rich with branched chain amino acids that are essential for energy production. To gain insight into the mechanisms underlying the benefit of the sub-chronic effects of running and intake of milk protein supplements, we studied Low Capacity Runner rats (LCR), a rodent exercise model with risk for metabolic disorders. We especially focused on the role of Sirtuins, energy level dependent proteins that affect many cellular metabolic processes.
Methods
Forty-seven adult LCR female rats sedentary or running voluntarily in wheels were fed normal chow and given supplements of either whey or milk protein drink (PD)-supplemented water, or water only for 21 weeks. Physiological responses were measured in vivo. Blood lipids were determined from serum. Mitochondrial markers and Sirtuins (Sirt1-7) including downstream targets were measured in plantaris muscle by western blotting.
Results
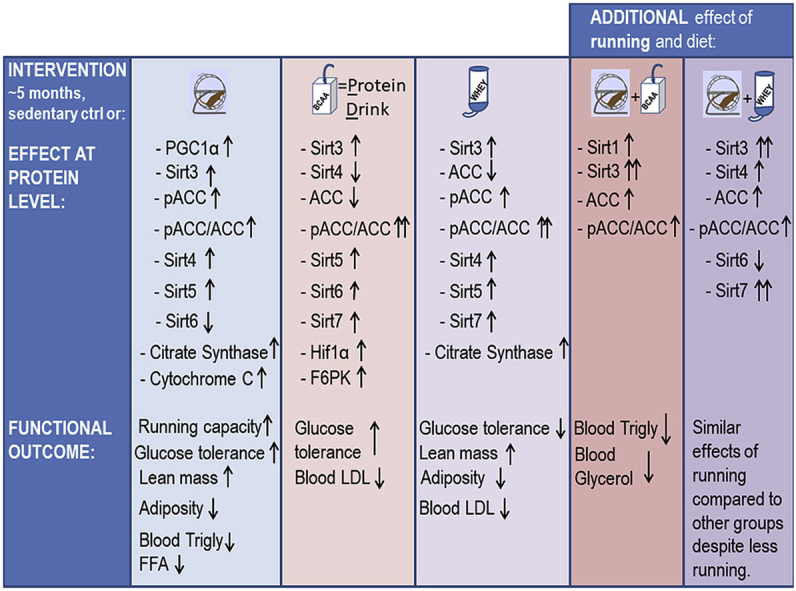
For the first 10 weeks whey-drinking rats ran about 50% less compared to other groups; still, in all runners glucose tolerance improved and triglycerides decreased. Generally, running induced a ∼six-fold increase in running capacity and a ∼8% decrease in % body fat. Together with running, protein supplements increased the relative lean mass of the total body weight by ∼11%. In comparison with sedentary controls, running and whey increased HDL (21%) and whey, with or without running, lowered LDL (−34%). Running increased mitochondrial biogenesis and Sirtuins 3 and 4. When combined with exercise, both whey and milk protein drink induced about a 4-fold increase in Sirt3, compared to runners drinking water only, and about a 2-fold increase compared to the respective sedentary group. Protein supplements, with or without running, enhanced the phosphorylation level of the acetyl-coA-carboxylase, suggesting increased fat oxidation. Both supplemented diets increased Sirt5 and Sirt7 without an additional effect from exercise. Running diminished and PD supplement increased Sirt6.
Conclusion
We demonstrate in rats new sub-chronic effects of milk proteins on metabolism that involve Sirtuins and their downstream targets in skeletal muscle. The results show that running and milk proteins act on reducing the risk factors of metabolic disorders and suggest that the underlying mechanisms may involve Sirtuins. Notably, we found that milk protein supplements have some favorable effects on metabolism even without running.
Keywords: Low capacity running rat (LCR), Metabolism, Muscle, Running, Sirtuins, Whey
Graphical abstract
Highlights
-
•
Interactive effects of running and/or milk protein supplements were studied.
-
•
Milk protein drink enhanced and whey diminished the amount of voluntary running.
-
•
Despite less running whey-supplementation improved metabolic health.
-
•
Almost all Sirtuins in muscle adapted to milk protein and running interventions.
1. Introduction
Epidemiological and experimental evidence suggest that physical activity [1], and consumption of dairy products [2,3] associate with decreased risk for metabolic disorders (MD). Metabolic disorder is a constellation of risk factors that commonly occur together, including high blood glucose, triglycerides and blood pressure, and low high-density lipoproteins (HDL). Low-density lipoproteins (LDL) have been associated with traits of metabolic disorder independent of obesity [4]. The effects of dairy products for improved metabolic health seem to be mediated by the protein components and/or minerals of milk [5]. Specifically, casein and whey are accounted to produce these beneficial effects [5]. Compared to casein, whey is especially rich in branched chain amino acids (BCAAs) and thus, the difference in their metabolic effects may arise from the difference in their essential amino acid compositions [6]. Casein and whey also differ in their postprandial kinetics and satiating effect, exemplified by slower stomach emptying of casein than whey [7,8].
BCAAs are essential amino acids used for energy production especially in the skeletal muscle, but also in other tissues, such as brain and adipose tissue. In contrast to other amino acids, BCAAs do not need the first-pass hepatic catabolism for their degradation. BCAAs have numerous physiological functions, one of those being a regulatory role for protein synthesis in skeletal muscle [9]. Although the beneficial effects of BCAAs on metabolic health [9,10] have been linked with the activity of Sirtuin 1 [11,12], the detailed mechanistic pathways are not thoroughly known. The possible links to all seven Sirtuins with exercise and BCAA-supplementation have not been previously studied.
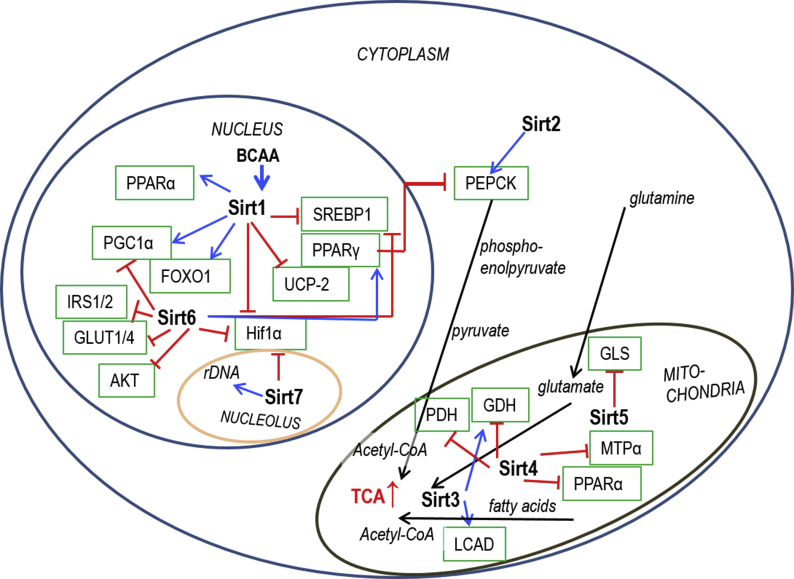
Sirtuins are energy level (NAD+) -regulated enzymes. They can function as e.g. deacetylases, desuccinylases, demalonylases or ADP-ribosylases, to modify post-translationally a large set of proteins from histones to enzymes of energy metabolism [13,14]. Of the sirtuins, Sirt1 has various nuclear and cytosolic targets controlling for instance cell cycle and energy homeostasis [13] and is considered as one of the key regulators of mitochondrial biogenesis [12]. Sirt2 regulates gluconeogenesis [15], and is highly expressed in adipocytes playing a role in metabolic functions [16]. In skeletal muscle, Sirt3 regulates mitochondrial substrate selection and metabolic flexibility [13]. Sirt3 level increases e.g. due to caloric restriction or exercise [17,18] and can be downregulated in MD [19]. The main enzymatic activity of Sirt4 is to control BCAA catabolism: without Sirt4 BCAA metabolism is reduced in hepatic and cardiac mitochondria [20]. Sirt5 desuccinylates, demalonylates and deglutarylates a major portion of proteins related to mitochondrial energy metabolism from Pyruvate Dehydrogenase (PDH) complex, β-oxidation, BCAA catabolism, and Krebs cycle to electron transport chain and ATP synthesis, thus being an important regulator of energy metabolism [21]. Sirt6 is a multifunctional protein [14,22], and acts as one of the key regulators of glucose homeostasis [23], which is disturbed in MD. Sirt7, in addition to its regulatory tasks e.g. in ribosome biogenesis, genome stability, transcription and RNA metabolism, is a regulator of mitochondrial homeostasis and hepatic lipid metabolism [24]. An overview of the roles of Sirtuins in metabolic pathways is shown in Figure S1. Because the protein levels of sirtuins adapt to acute or long-term stimuli like exercise or caloric restriction [14], and skeletal muscles account ∼40% of the body mass and almost 30% of the resting energy consumption in normal human [25], we sought to study the interplay between skeletal muscle metabolism, Sirtuins and BCAAs.
We previously showed that low capacity runner (LCR) rats score high for developing MD [26], have greater adiposity [27] and develop hepatic steatosis even without a high fat challenge [28], have reduced lifespan [29], and express less BCAA degradation and fatty acid metabolism-related genes than the high running capacity (HCR) rats [30]. Thus, it was of special interest to use LCRs to study the separate and combined effects of long-term BCAA supplementation, and exercise on the variables of metabolic health. We hypothesized that either exercise and/or BCAA-rich milk protein supplements would ameliorate the MD risk factors or concurrent regulatory changes would be seen in skeletal muscle Sirtuin levels. HCR/LCR rat model is a polygenic model [26], and thus, the data derived from them are likely more relevant for translational to humans than that of the commonly used inbred laboratory rodents.
2. Materials and methods
2.1. Animals and intervention measurements
LCR and HCR rats are produced by two-way artificial selective breeding [26,31]. Shortly, breeding started with a large founder population of genetically heterogeneous, outbred rats (N:NIH stock). After testing the running capacity (i.e. phenotyping) at 11 weeks of age, the individuals with the lowest and highest running distance were mated to produce the two segregating lines for intrinsic aerobic capacity [31]. For this study full-grown, phenotyped, female LCR rats from generation 28 of selection were transported from the University of Michigan, USA to Finland at the age of ∼5 months. Animals were single-housed in standard conditions (temperature 21 ± 2°C, humidity 55 ± 10%, light period from 8.00 to 20.00). The experiment was approved by the Regional State Administrative Agency, Southern Finland, Finland (ESAVI-2010-07989/Ym-23), and the Institutional Animal Care & Use Committee (IACUC) at the University of Michigan. Experiment was conducted in accordance with the Guidelines of the European Community Council directives 86/609/EEC, and European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Council of Europe No 123, Strasbourg 1985). The study is reported according to ARRIVE guidelines.
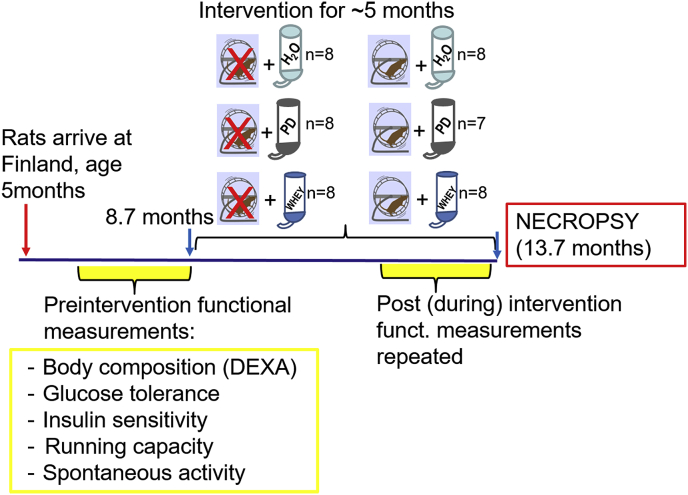
Before the running and diet interventions, we conducted a maximal treadmill running capacity test, and measured body composition, spontaneous activity, and glucose tolerance and insulin sensitivity (see Fig. 1 for study setup). Then, the rats (n = 47, aged 8.7 ± 0.6 months) were divided into six age- and weight-matched groups. Three groups were sedentary and three respective groups were provided running wheels (RW) in their home cages (Fig. 1). During diet intervention, protein supplements were given in drinking water. Sedentary control or running control animals had free access to tap water, whey (W) groups received whey protein (5.28 ± 0.04 g/kg, Valio) and the protein drink (PD) groups received milk protein drink (5.13 ± 0.02 g/kg, Valio) for 21.4 weeks (150 ± 3 days; ∼5months). All rats had aspen-chips (Tapvei, Kaavi, Finland) as a bedding and nesting material. Sedentary animals were housed in Macrolon IV cages (Techniplast 1354G, Buguggiate, Italy). For the exercise animals, running wheels were mounted onto the housing cages (Techniplast 2154F0105, Buguggiate, Italy), and the wheel (Ø 345 mm) revolutions were recorded 24/7 with a self-constructed computerized system [32]. All rats were given pelleted rodent diet (R36, Labfor/Lantmännen, Malmö, Sweden) ad libitum. Both diet drinks were given each day (seven days a week) as fresh and were available ad libitum. Food intake and weight gain were measured at least twice a week. Details of diets are given in the Supplement 1, as are the methods for the functional measurements that were done pre- and post-intervention (Fig. 1).
Fig. 1.
Schematic drawing of the study setup showing the groups and the timeline for the measurements.
2.2. Necropsy
Animals were euthanized in random order at metestrous/diestrous -phase. The estrous stage was determined by vaginal smear, stained fresh with Giemsa on objective glass [33]. Running wheels were blocked 24 h before necropsy to avoid acute effects of running. The rats were fasted overnight, weighed, quickly anesthetized with mixture of air and CO2, and then euthanized by cardiac puncture to collect serum samples. Tissue samples were weighted, immediately snap frozen in liquid nitrogen and stored at −80°C. For the weight of plantaris muscle we used the mean value of the left and right plantaris.
2.3. Western blot
We used previously published protocols [34] for plantaris homogenization, and Western blotting of the samples. Antibodies against the following proteins (with the target function) were: cytochrome C (cytC, mitochondrial hemeprotein, describes number of mitochondria), acetyl-CoA carboxylase (ACC) and its phosphorylated form pACC (enzyme involved in fatty acid biosynthesis), hypoxia inducible factor 1α (HIF1α, transcriptional regulator of cellular response to hypoxia), Pyruvate dehydrogenase lipoamide kinase isozyme 4 (PDK4, regulator of glucose/fatty acid metabolism), Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α, master regulator of mitochondrial biogenesis), phosphofructokinase (F6PK, a key regulatory kinase in the glycolysis), and Sirtuins 1–7. Details of the primary antibodies and methods are given in the Supplement 1. All results were normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, glycolytic enzyme that catalyzes break down of glucose). In addition, the expression of mitochondrial Sirt3-5 were normalized to the markers of mitochondria: number (cytC) and activity (citrate synthase activity).
2.4. Citrate synthase activity, blood lipids, glucose, insulin and HOMA-index
To determine the activity of mitochondria we measured the level of citrate synthase (CS, catalyzes the first reaction of the citric acid cycle) enzyme activity, in plantaris muscle as previously [35]. Blood lipids were assayed from necropsy serum sample by a kinetic photometric method with KoneLab. Fasting blood samples (in vivo) were taken from vena saphena. Blood glucose was measured with glucose analyser (HemoCue Glucose 201+, HemoCue AB, Ängelholm, Sweden). Serum insulin was assayed with ELISA (Mercodia AB, Uppsala, Sweden). Calculation of HOmeostatic Model Assessment for insulin resistance (HOMA-index) is a method to assess function of pancreatic β-cells, and insulin resistance, and was calculated with an equation: (fasting blood glucose/fasting insulin *22.4) [36].
2.5. Statistics
Data were analyzed with MS-Excel 2010 and SPSS (version 22, IBM© SPSS Statictics). Results are shown as mean ± SEM. Data were assessed with Univariate Anova (UniANOVA) or two-way analysis of variance using general linear model (GLM) after verifying homogeneity of variances by the Levene’s test. In case of a statistically significant interactive effect with diet and running, a full-factorial model with contrast coefficient was calculated to assign the statistical differences among the groups. If the interaction was not statistically significant, data were analyzed by the analysis of variance followed by post-hoc testing (Sidak’s test or Dunnett’s 2-sided t-test). The repeated observations from the same rat were accounted using linear mixed model. Type III tests of fixed effects with Sidak’s adjustment for multiple comparisons were used. Pearson’s correlation was calculated to estimate correlation between variables. The level of statistical significance was set at p ≤ 0.05.
3. Results
3.1. Energy intake, body weight and body composition
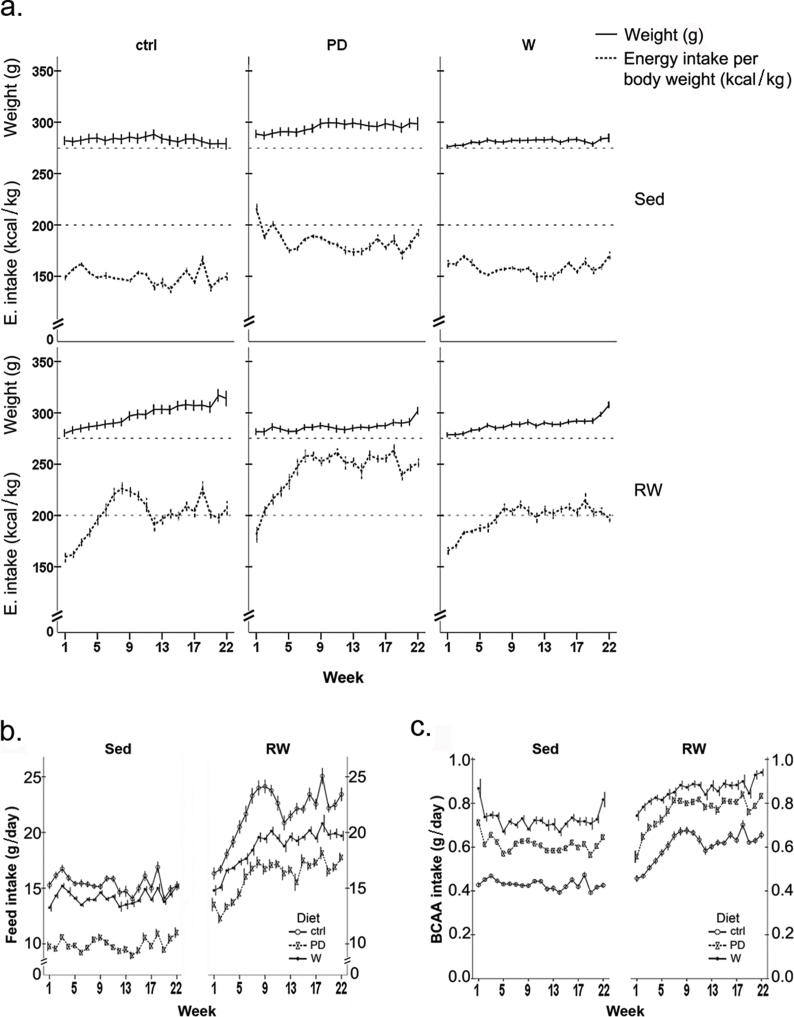
Energy intake [kcal/body weight] was highest in PD groups (Figure S2a), being significantly higher than in water drinking controls (sedentary or running, p < 0.001). Running increased food (p < 0.001, Figure S2b) and BCAA intake (p < 0.001, Figure S2c) in all protein-supplemented groups.
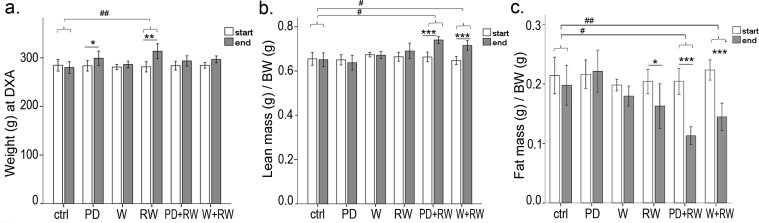
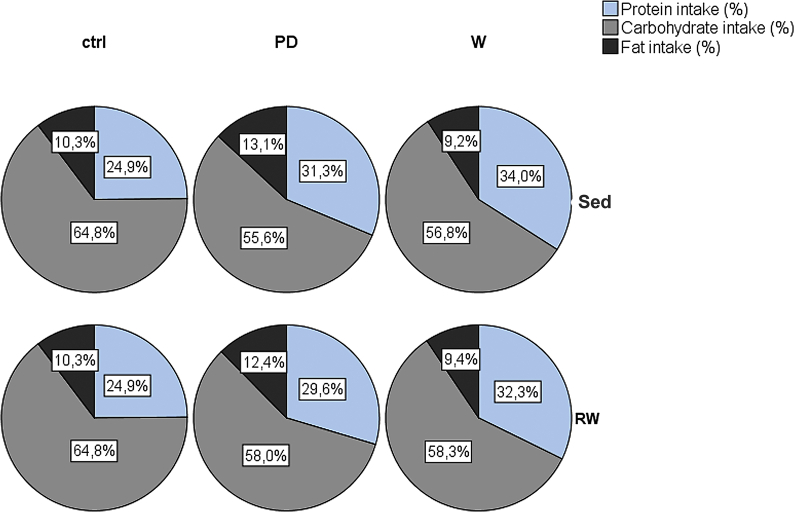
The intake of main nutrients differed between diet-groups (Figure S3), but within groups the levels remained constant throughout the intervention. Intervention did not affect continuous weight gain in any group (Figure S2a), but between the two body composition measurements, both the sedentary PD-group (pre vs. post intervention, p = 0.023) and RW-group (pre vs. post intervention, p = 0.001) gained weight (Fig. 2a). When combined with running, both PD and whey boosted the gain in relative lean mass (of the total body weight, p < 0.05; ∼12 and 10.5%, respectively), while running alone had no effect (Fig. 2b). However, in all runners (Fig. 2c), the amount of fat of the total body mass decreased, the drop being largest in supplemented groups (p < 0.05; PD+RW, −45%; W+RW -35%). Further, absolute lean mass (g) increased in all runners (p < 0.001, data not shown).
Fig. 2.
Changes in body composition during a 5-month intervention. (a) Weight (g) was measured with a scale, while for the (b) lean body mass (g) [relative to body weight, BW (g)], and (c) fat mass (g) [relative to BW (g)] rats were anaesthetized and measured with dual-energy X-ray absorptiometry. In the panels, * indicates significant change within group during intervention, pre vs. post value, linear mixed model,* p < 0.05; ** 0.01 > p ≥ 0.001; *** p < 0.001. # indicates significant difference as compared with ctrl-group, # p < 0.05, ## 0.001 < p < 0.01, ### p < 0.001, GLM.
3.2. Running, aerobic capacity, and spontaneous activity
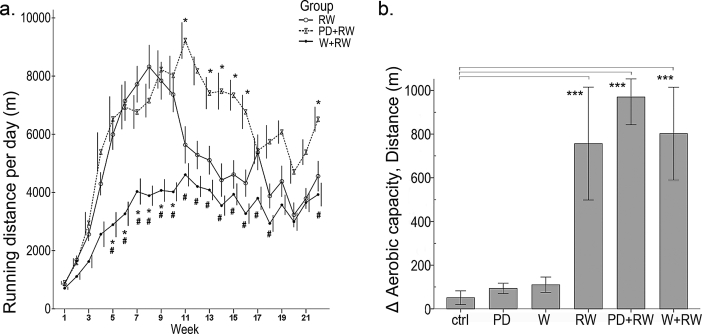
The amount of voluntary running increased in all groups during the first 8–11 weeks (Fig. 3a), but to a lesser extent in the W+RW group. In the RW group, the total running distance was 740 ± 154 km (range 93–1556 km), in PD+RW 932 ± 148 km (range 380–1515 km, PD+RW vs. RW: p = 0.553) and in W+RW 495 ± 97 km (range 70–1023 km, W+RW vs. RW: p = 0.365). Although the total running distance in the end of intervention did not differ between the groups, the average daily distances were different (p < 0.001, Fig. 3a), the PD+RW having the highest and the W+RW the lowest amount of daily running. Voluntary training improved running capacity about 8-fold in all running groups (p < 0.001; Fig. 3b) while diet supplements had no effect.
Fig. 3.
(a) Daily voluntary running distances [meters (m), group average ± SEM] were followed using the computerized recording system, the functioning of which was confirmed using uninterruptible power source. * depicts significant difference of the PD+RW to the RW-group, p < 0.05, # depicts significant difference of the W+RW to the RW-group, p < 0.05, linear mixed model. For the aerobic fitness i.e. running capacity we conducted treadmill exercise tests, and panel (b) shows the difference between the pre and post-values as a change in running distance (m). Effects are shown as *** p < 0.001, linear mixed model.
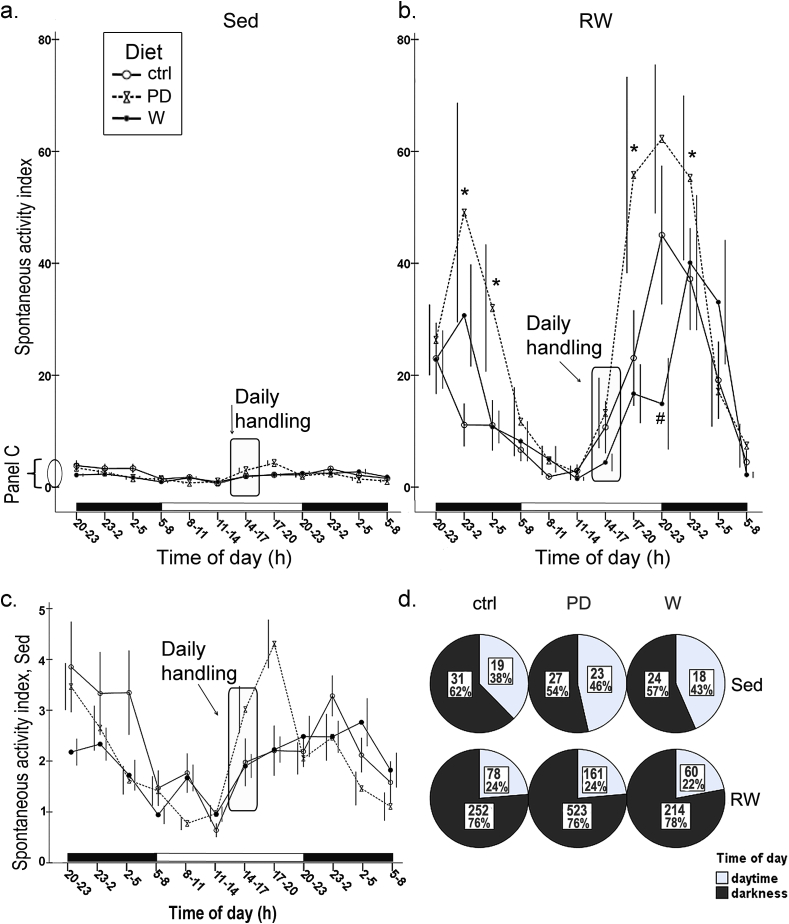
Running in running wheels introduced a 10-fold difference in the spontaneous activity in running rats compared to the sedentary (Fig. 4a, b), the difference being especially clear during the darkness (Sed vs. RW rats, daytime: p = 0.081, and darkness: p < 0.001). Sedentary lifestyle flattened the circadian spontaneous activity pattern (Fig. 4a, b). All rats were handled during the daytime between 14 and 17 h that introduced an insignificant induction in the activity (Fig. 4a–c).
Fig. 4.
Continuous diurnal spontaneous activity index, shown as a mean value (±SEM, please note that error bars are slightly dissociated to diminish their overlapping) in 3 h bins, was measured during the intervention in sedentary groups (a) and in running groups (with availability to run during the activity measurement, panel b). Measurement was done for 6 animals in each group, the exception being the water drinking ctrl-group from which 4 animals were measured. In panel (c), activity of sedentary animals is shown in a larger scale. Black-and-white bars near the x-axes indicate the lighting periods in the room (lights were on between 8 a.m. and 8 p.m.). Daily handling of the rats occurred between 2–5 p.m. (rounded rectangle in line graphs a, b, c). In the panel (d), diurnal spontaneous activity (during one representative day, 24 h) is shown as divided into daytime and darkness (12 h). Number within each pie represents the group mean value of the sum of the total activity during daytime (12 h) or darkness, and the percentages show the distribution of the activity across circadian phases in each group. Differences between the groups were statistically assessed by the linear mixed model, * p < 0.05, difference between PD and crtl; # p < 0.05, difference between W and ctrl.
3.3. Glucose tolerance, insulin sensitivity, and fasting blood glucose
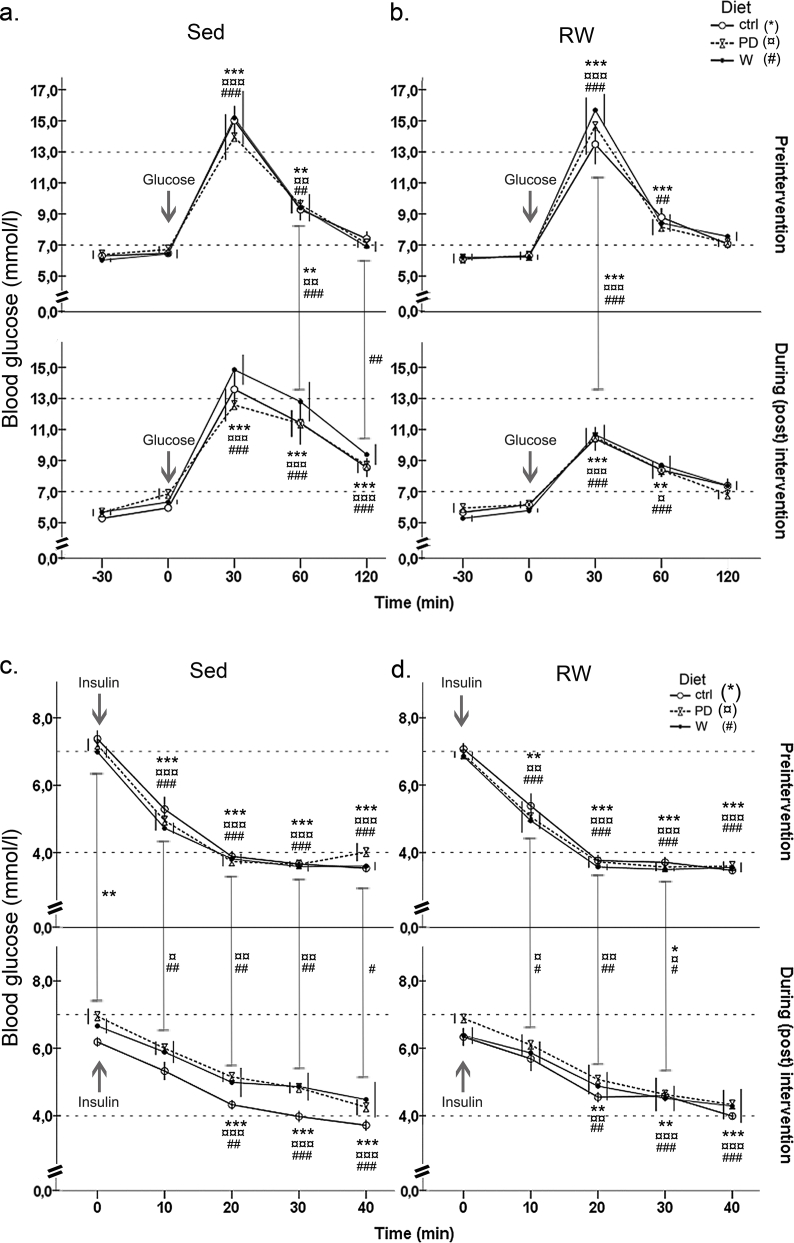
Running improved glucose tolerance in all groups shown by the decreased AUC-values (Table 1; p = 0.001). Whey intervention brought about a ∼18% decrease in the glucose sensitivity (Table 1, glucose tolerance test, pre vs. post value: p = 0.025); whereas running had no effect; there was no difference between pre and post AUC-values (W+RW, pre vs. post: p = 0.115).
Table 1.
AUC-indices: Area under the blood glucose curve was calculated from blood glucose values in glucose tolerance and insulin sensitivity tests (mean ± SEM), before and in the end of the intervention.
| Group | AUC, glucose tolerance test (a |
AUC, insulin sensitivity test |
||
|---|---|---|---|---|
| Before intervention | End of intervention | Before intervention | End of intervention | |
| ctrl | 1187 ± 61 | 1267 ± 107 | 183 ± 6 | 186 ± 5 |
| PD | 1164 ± 64 | 1252 ± 78 | 179 ± 5 | 216 ± 5* |
| W | 1182 ± 90 | 1397 ± 105* | 174 ± 4 | 213 ± 15* |
| RW | 1107 ± 68 | 1003 ± 51 | 181 ± 7 | 200 ± 6 |
| PD+RW | 1115 ± 76 | 989 ± 42 | 176 ± 8 | 214 ± 13* |
| W+RW | 1169 ± 58 | 1020 ± 53 | 172 ± 4 | 206 ± 13* |
Number of animals in each group is 8, except in PD+RW n = 7.
a Significant effect of running, p = 0.001 (GLM).
* Significant effect of intervention, difference between pre and post -value, p < 0.05 (GLM).
All groups became more resistant to exogenous insulin during the intervention (Table 1, Figure S4). At the insulin sensitivity test before intervention, blood glucose plummeted rapidly, within 10 min in all groups, while in the test after intervention, the glucose drop was significant only at 20 min after the insulin injection (Figure S4b). Insulin sensitivity decreased 20–22% in all sedentary and running PD- and W-groups (p < 0.05, Table 1). Fasting blood glucose values did not differ between the groups before or after the intervention (Figure S4).
3.4. Serum lipids, HOMA-index, and glucose levels in the end of intervention
At necropsy, fasting insulin and glucose levels, and thereby HOMA-indices, were equal between the groups (Suppl. Table 2). Diet had a general decreasing effect on serum LDL levels (Table 2; effect of PD, −33%, p = 0.062; W, −34%, p < 0.05) although none of the treatments had a significant effect on serum total cholesterol. Neither the diets nor the running alone affected HDL level, but whey together with running increased HDL by 0.44 mmol/l (p < 0.05, contrast to sedentary controls, Table 2). Running decreased triglycerides (Table 2; −0.18 mmol/l, −25%, p = 0.014), while PD or W did not. In comparison with the sedentary groups, running also decreased free fatty acids (−0.21 mmol/l, p = 0.007, Suppl. Table 2). Total running amount (total distance during intervention) did not associate with blood lipids. Serum LDL was inversely associated with protein intake [r = −0.39, p = 0.008].
Table 2.
Blood lipids (mean ± SEM) in the end of intervention, measured after overnight fasting from serum sample.
| Group | Triglycerides (mmol/l)a | Total cholesterol (mmol/l) | HDL (mmol/l)b | LDL (mmol/l)c,d | |
|---|---|---|---|---|---|
| ctrl | 0.79 ± 0.12 | 3.3 ± 0.2 | 2.06 ± 0.08 | 0.93 ± 0.15 | |
| PD | 0.70 ± 0.08 | 3.4 ± 0.2 (n = 7) | 2.41 ± 0.12 (n = 7) | 0.59 ± 0.13 (n = 7) | |
| W | 0.71 ± 0.10 | 2.8 ± 0.1 | 1.96 ± 0.14 | 0.55 ± 0.10 | |
| RW | 0.64 ± 0.10 | 3.4 ± 0.2 | 2.16 ± 0.09 | 0.96 ± 0.11 | |
| PD+RW | 0.43 ± 0.07* | 3.1 ± 0.3 | 2.19 ± 0.15 | 0.69 ± 0.15 | |
| W+RW | 0.57 ± 0.05 | 3.5 ± 0.2 | 2.50 ± 0.23b | 0.66 ± 0.12 | |
a Significant effect of running, p < 0.05.
b Interactive effect of running and protein supplements, p < 0.05; contrast of W+RW to ctrl p < 0.05).
c Significant effect of whey, p < 0.05.
d Main effect of diet, p < 0.05.
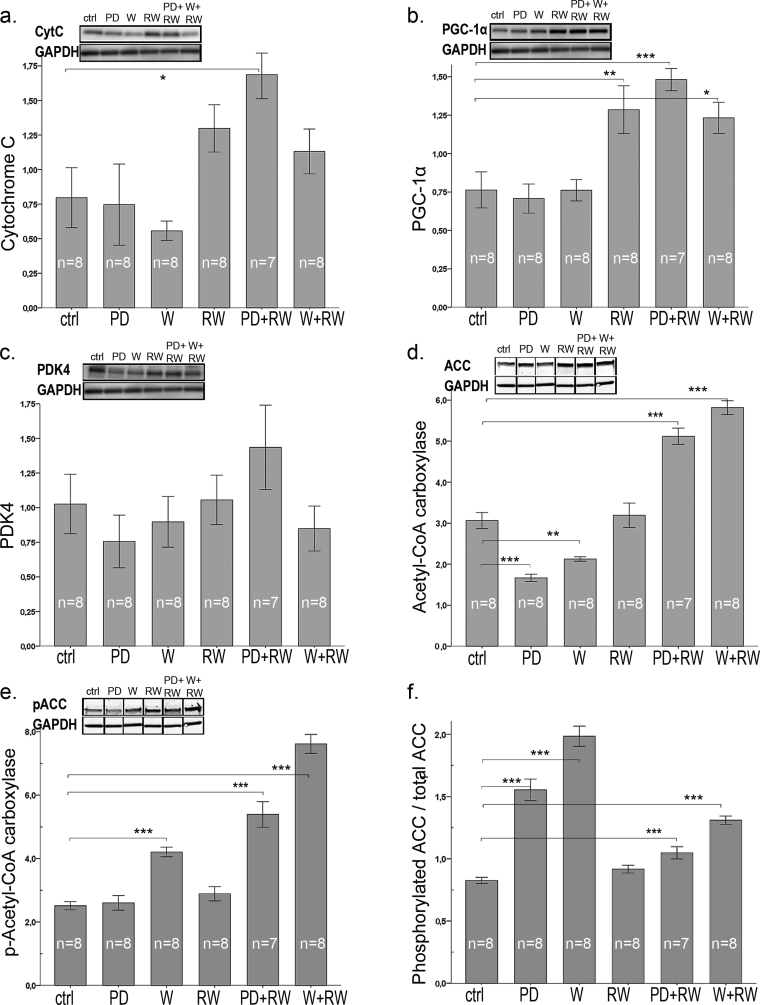
3.5. Mitochondrial markers cytC, CS activity, and PGC-1α
Running increased the expression of cytC by ∼2-fold (p < 0.001) while the diets had no effect on it (Fig. 5a). Generally, both running and diet supplement increased CS activity in plantaris muscle (Suppl. Table 2, running by 1.7-fold, p < 0.001, diets by 1.2-fold, p = 0.043). Especially whey together with running increased CS activity by 1.8 fold (p < 0.001). Expression of PGC-1α increased by 1.8 fold in response to running (Fig. 5b; p < 0.001).
Fig. 5.
Protein content of (a) cytochrome C, (b) PGC-1α, (c) PDK4, (d) Acetyl-CoA-carboxylase (ACC), and (e) phosphorylated Acetyl-CoA-carboxylase (pACC) in plantaris muscle. Panel (f) shows the expression of phosphorylated Acetyl-CoA-carboxylase (pACC) in relation with total ACC. Representative examples of western blots are shown above each graph, gel blots are divided because the order of samples in the gel was not equal for the order of groups in results-section. Nonetheless, all gel figures originate from the samples run in the same gel. Each gel contained protein samples of all groups, and the samples run on different gels were normalized to a control sample, that was repeated in each gel. In the panels, * indicates significant difference to sedentary control (ctrl), * p < 0.05; ** 0.01 > p ≥ 0.001; *** p < 0.001.
Markers of mitochondrial biogenesis and activity were associated with increase in running capacity: PGC-1α (r = 0.56, p < 0.001), cytC (i.e. mitochondrial quantity, r = 0.44, p = 0.002), and CS (i.e. mitochondrial activity, r = 0.74, p < 0.001).
3.6. Intermediary metabolic proteins
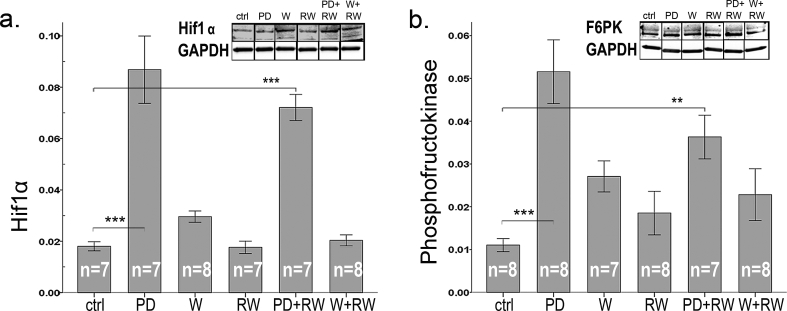
Neither diets nor exercise had an effect on PDK4 (Fig. 5c) that plays a key role in regulation of glucose and fatty acid metabolism. In sedentary animals PD and W diminished ACC while in running groups both PD and W enhanced ACC [Fig. 5d, interactive effect of running and diets (p < 0.001)]. Phosphorylation of ACC, that enhances fat oxidation, increased (Fig. 5e) in response to whey and to running. Thus, both diets (PD +51% and W +89%) and running without supplements (+11%) enhanced the amount of phosphorylated ACC relative to the total amount of ACC (Fig. 5f). PD increased the expression of glucose metabolism-regulating Hif1α by 4.5-fold and 3.2-fold in comparison to controls and whey (respectively) (Fig. 6a). Similarly F6PK, the rate limiting enzyme of glycolysis, was induced by PD by 3-fold and by 1.8-fold (Fig. 6b) in comparison to controls and W, respectively. Running had no effect on either Hif1α or F6PK.
Fig. 6.
Protein content of (a) Hif1α and (b) phosphofructokinase (F6PK) in plantaris muscle. Representative examples of western blots are shown above each graph. Gel blots are divided because the order of samples in the gel was not equal for the order of groups in results-section. Nonetheless, all gel figures originate from the samples run in the same gel. Western blot gels were run equally as in Fig. 5. In the panels, * indicates significant difference to sedentary control (ctrl), * p < 0.05; ** 0.01 > p ≥ 0.001; *** p < 0.001.
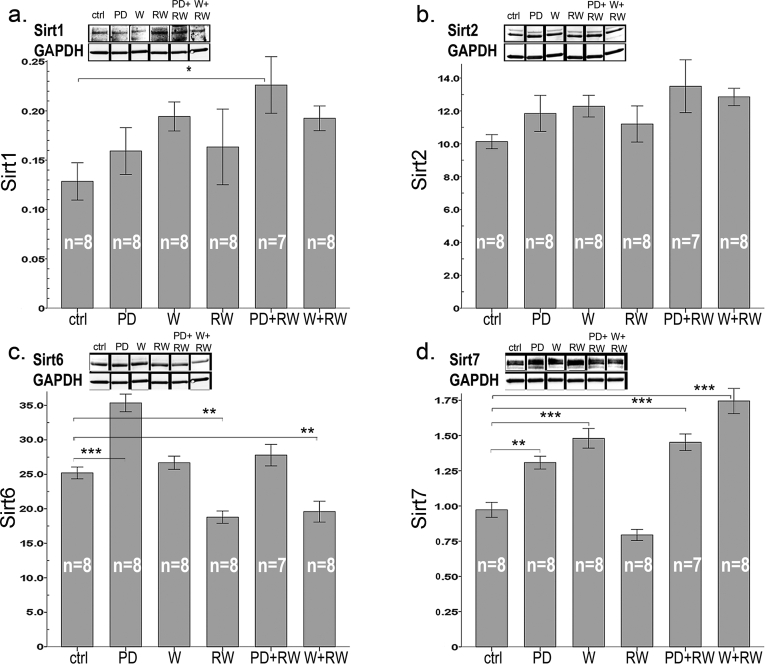
3.7. Sirtuins
Both diets had a similar general tendency to induce Sirt 1 (about +31%, p = 0.092, Fig. 7a), that is one of the key regulators of mitochondrial biogenesis [12]. PD combined with running had most pronounced effect by enhancing Sirt1 level by 1.8-fold in comparison to controls (p = 0.034, Fig. 7a). Neither running nor protein supplements had an effect on Sirt2 (Fig. 7b). Of the effects of intervention on the other non-mitochondrial sirtuins, running decreased Sirt6, which acts as one of the key regulators of glucose homeostasis [23], by 25% (p < 0.001, Fig. 7c), and the difference was significant between all diet-matched, sedentary and RW groups (p < 0.01 for all). However, protein drink increased Sirt6 expression in comparison to both control diet (1.5-fold, p < 0.001) and whey (1.4-fold, p < 0.001). Sirt6 associated with fat intake (r = 0.61, p < 0.001), increased adiposity (r = 0.33, p = 0.023), and with the downstream targets of it: Hif1α (r = 0.60, p < 0.001) and F6PK (r = 0.56, p < 0.001). An inverse association was found with Sirt6 and improved running capacity (r = −0.33, p = 0.023), CS activity (r = −0.44, p = 0.002), and expression of PGC1α (r = −0.33, p = 0.022) and total ACC (r = −0.5, p < 0.001).
Fig. 7.
Protein content of (a) Sirt1, (b) Sirt2, (c) Sirt6, and (d) Sirt7 in plantaris muscle. Representative examples of western blots are shown above each graph. Gel blots are divided because the order of samples in the gel was not equal for the order of groups in results-section. Nonetheless, all gel figures originate from the samples run in the same gel. Western blot gels were run equally as in Fig. 5. In the panels, * indicates significant difference to sedentary control (ctrl), * p < 0.05; ** 0.01 > p ≥ 0.001; *** p < 0.001.
Running and diets had an interactive effect on Sirt7 (Fig. 7d; p = 0.002). Running alone had no effect but both diets increased Sirt7 in comparison with normal diet (PD, +53%, p < 0.001 and W, +82%, p < 0.001, Fig. 7d). Thus, both BCAA (r = 0.68, p < 0.001) and protein (r = 0.77, p < 0.001) intakes were associated with Sirt7, the regulator of mitochondrial homeostasis and hepatic lipid metabolism [24].
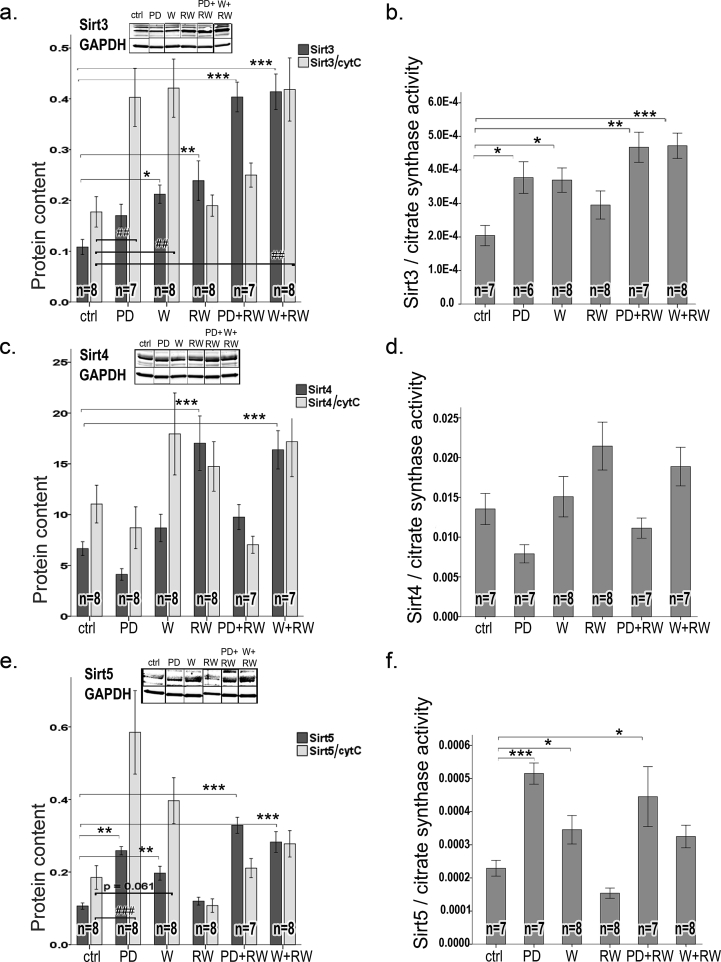
Because running induced expression of mitochondrial markers, the data of all mitochondrial sirtuins (Sirt3, 4 and 5) are shown as expression data, and as normalized to mitochondrial markers CS and cytC (Fig. 8). Sirt3 was associated with an increase in running capacity (r = 0.5, p < 0.001), CS activity (r = 0.75, p < 0.001), PGC1α (r = 0.71, p < 0.001), and cytC expression (r = 0.51, p < 0.001), Both diets (PD, +65% p = 0.001; W, +80% p < 0001) as well as running (+114%, p < 0.001) increased significantly Sirt3 (Fig. 8a). Despite normalization to CS the inducing effect of running and both protein supplements on Sirt3 remained significant (RW, p = 0.011; PD, p = 0.011, and W, p < 0.001), but after normalization to cytC Sirt3 induction by running was not significant. Sirt3 was associated with increased lean mass (r = 0.57, p < 0.001) and an inverse association was found with Sirt3 and ΔAUC in glucose tolerance tests (r = −0.34, p = 0.022). Sirt3 was also inversely associated with increased adiposity (Δfat%, r = −0.51, p < 0.001), and serum triglyceride level (r = −0.35, p = 0.018).
Fig. 8.
Protein content of mitochondrial Sirtuins (a) Sirt3, (c) Sirt4, and (e) Sirt5 in plantaris muscle. Representative examples of western blots are shown above each graph. Gel blots are divided because the order of samples in the gel was not equal for the order of groups in results-section. Nonetheless, all gel figures originate from the samples run in the same gel. Western blot gels were run equally as in Fig. 5. To account the effect of intervention on mitochondria, Sirtuins were normalized to mitochondrial markers: cytochrome C [light grey bars in panels: (a) Sirt3, (c) Sirt4, and (e) Sirt5] and citrate synthase activity [(b) Sirt3, (d) Sirt4, and (f) Sirt5]. In the panels, * indicates significant difference to sedentary control (ctrl), * p < 0.05; ** 0.01 > p ≥ 0.001; *** p < 0.001.
Compared to sedentary controls, Sirt4 (whose the enzymatic activity is controlling BCAA catabolism [20]) was enhanced by 2-fold in response to running (p < 0.001, Fig. 8c). Independent of running, PD diminished Sirt4 expression in comparison to both control diet and whey by 1.7 fold (p < 0.05, Fig. 8c). The diminishing effect of PD on Sirt4 remained the same after normalization to the mitochondrial markers CytC (Fig. 8c) or CS (Fig. 8d) (p = 0.003 and p = 0.002, respectively). An increase in running capacity was associated with the level of Sirt4 (r = 0.36, p = 0.015).
Both PD and W supplements (by 2.6-fold and 2.1-fold, respectively), and running (by 1.3-fold, p < 0.001) increased Sirt5 (Fig. 8e), the regulator of energy metabolism [21]. The effect remained significant even if the Sirt5 expression was normalized to the mitochondrial markers (Fig. 8e, f; p < 0.001 for both).
4. Discussion
Sirtuins are important regulators of energy metabolism, and by finding tools to regulate them in skeletal muscle one could mitigate MD. Because milk proteins are rich in BCAA, and their consumption improves metabolic health [9,10], we hypothesized that milk protein supplements could affect Sirtuins and alleviate MD risk factors in adult, polygenic female LCR rats. LCR rats have poor running capacity based on selective breeding, but also suffer from MD risk factors [[26], [27], [28]]. Because LCR rats have a genetically heterogeneous background they mimic human genetics better than inbred laboratory animals [37]. Previously, it is shown that the protein levels of Sirtuins adapt to an acute or long-term stimuli like exercise or caloric restriction [32,35,[38], [39], [40], [41]]. Therefore we used exercise as a reference group for diet supplements. In addition that exercise induced Sirtuins 3–5 and downregulated Sirtuin 6, we found specific effects of long-term dietary supplementation with milk-based proteins on Sirtuins in skeletal muscle (plantaris), and on blood lipids and body composition. These findings are discussed below.
Voluntary running of the whey-supplemented group was strikingly low, especially during the first 10 weeks of the intervention, amounting to >50% lower daily running distance compared to the other groups. It is of note, that running capacity increased, and skeletal muscle mitochondrial markers (citrate synthase, CS and cytochromeC, cytC) enhanced in a similar manner in all exercising groups despite large difference in the amount of running. Because running differed between the diet groups - especially during the first weeks of the intervention - it warrants further studies to investigate how the diets affect running motivation [42]. Nevertheless, all trained LCR females improved their maximal running capacity, an indicator of aerobic fitness, in response to voluntary training. Neither of the supplemented diets had an effect on aerobic fitness. This agrees well with a human study reporting no effect on treadmill running capacity with whey supplementation [43]. In contrary, a study in mice shows when combined with a different type of exercise, i.e., swimming, whey supplement slightly improved swimming capacity to exhaustion [44].
In our study, energy intake increased as expected [45], in all running groups while body weight increased only in RW and sedentary PD groups. In all running groups lean mass increased and % fat decreased while milk protein supplementation per se had no effects. However, when the body weight was taken into account, only the runners with supplementary diets gained in lean mass during the 5 month intervention. Also randomized clinical trials (RCTs) have shown that – although not always - long-term exercise [45] and diets enriched with milk proteins lead to favorable changes in body composition, albeit the results are depending on experimental settings and dairy protein diet availability (ad libitum or combined with energy restriction) [5,46].
One of the risk factors in metabolic disorders is dyslipidemia, especially heightened triglyceride and lowered HDL level [47,48]. However, elevated LDL cholesterol levels also associate with increased risk of atherosclerotic disease, supported by the finding that exercise reduced the risk via decreasing LDL [48]. Our results show that together with voluntary running, both milk protein drink and whey protein had a similar, lowering effect on fasting blood LDL level (−32%, PD groups and −36%, W groups, in comparison to water drinking groups). Total cholesterol was unaffected by intervention treatments, but whey with running increased HDL. Only running lowered serum triglycerides and free fatty acids compared to sedentary controls. Protein intake was inversely associated with LDL level but without exercise, the diets alone did not affect blood lipids. Previously, only a few studies in obese or MD patients show similar findings of whey supplements on blood lipids, but without exercise and the follow-up times have lasted only 12 weeks or less [47]. In humans, exercise with whey is shown to be effective in lowering triglycerides only [49].
Glucose tolerance and insulin sensitivity were attenuated by whey and PD. However, neither of the diets affected fasting blood glucose, insulin levels, or HOMA-indices. Similar, negative effects on glucose tolerance and insulin sensitivity have been found previously using BCAA-rich diets but also opposite findings exist [42]. Previously some RCTs and epidemiological studies have shown that dairy intake is associated with beneficial effects on lipid metabolism and MD risk factors [50]. To our knowledge, there are no human studies of long-term dietary interventions, our data is the first showing the long-term effects in rat. The 5-month treatment period corresponds to ∼20% of the lifespan of female LCR rat [29]; in human life it would be slightly more than 14 yrs [51].
Since Sirtuins are important regulators of energy metabolism responding to metabolic demands, we wanted to determine whether the long-term supplementation of BCAA-rich milk-proteins with or without exercise would induce concurrent changes in muscle Sirtuins and their downstream targets, and improve risk factors for MD. Indeed, several coinciding changes were seen as summarized in the Graphical Abstract. Sirt2 was the only sirtuin that was not affected by exercise or supplements. The actual function of Sirt2 in skeletal muscle remains to be shown.
The combination of voluntary running and protein drink for 21 weeks caused a significant increase in Sirt1 while running alone had no effect. We also found that long-term wheel running increased the content of PGC-1α, the downstream target of Sirt1 deacetylation [12]. The increase in PGC-1α seems to be independent of Sirt1 protein level, but because we did not study Sirt1 activity, the role of Sirt1 in PGC-1α function cannot be concluded. Running induced also an increase in cytC concentration with no effect of diet, suggesting that exercise activates PGC-1α-mediated mitochondrial biogenesis while milk protein does not. Another mitochondrial marker, CS activity increased not only in response to exercise, but also in response to PD and whey diets, indicating that protein supplementation has its own effect on the activity of the mitochondria. While several studies have shown that the expression of Sirt1 increases in skeletal muscle in response to acute and long-term exercise [52,53], some studies found no changes or even a decrease [54]. Moreover, Philp et al. showed that in mice lacking Sirt1 deacetylase activity in skeletal muscle, mitochondrial biogenesis and PGC-1α responses were not impaired during acute exercise [55]. Notwithstanding, exercise is shown to increase Sirt1 activity without changes in its protein level [53], and therefore Sirt1 signaling pathway needs further investigations.
Sirtuins 3, 4 and 5 are located in the mitochondria and function there as the main regulators of mitochondrial metabolic activity and flexibility [13,20,21]. Sirt3 and Sirt4 were upregulated by long-term running, but also whey protein alone increased Sirt3. Combined with exercise both whey and milk protein drink, induced about 4-fold increase in Sirt3 in comparison to water drinking control and about 2-fold increase in comparison to the respective sedentary group. This upregulation remained significant even after accounting for the inductive effect of intervention on mitochondrial biogenesis. Thus, the data favors the hypothesis that exercise and milk protein supplements have additive effects, consequently enhancing mitochondrial energy metabolism. In agreement with previous studies, the running-induced upregulation of Sirt3 was associated with improved glucose tolerance and body composition. Previously, mice with muscle-specific overexpression of Sirt3 were shown to have higher energy expenditure and lower respiratory exchange ratio than wild-type animals indicating increased use of lipids for oxidative energy production [56]. In these mice, Sirt3 increased the activity of AMPK that - among a plethora of other substrates – phosphorylates ACC thus deactivates it [56] turning lipid metabolism on oxidative mode. Accordingly, in our study both whey and milk protein drink alone or combined with running, increased the ratio of p-ACC and total ACC indicating enhanced oxidation of fatty acids. Unfortunately, we could not measure respiratory exchange ratios during the intervention and therefore the data of fuel use is lacking.
Running-induced increase in Sirt4 suggests increased BCAA catabolism in the trained skeletal muscle of LCR rats. However, previously Sirt4 of LCR rats was diminished after 12-week treadmill training [35] and voluntary running for one year in old rats did not have any effect [32] in the gastrocnemius muscle. Since Sirt4 is the regulator of BCAA catabolism [20] further investigation is warranted to show the actual role of Sirt4 in the energy metabolism in different types of muscles, training modes and during the lifespan.
Running alone did not affect Sirt5 expression in skeletal muscle, agreeing with our previous study with old rats [32]. However, milk protein and whey increased Sirt5 expression in plantaris muscle, independent of the normalization of the data, indicating enhanced mitochondrial energy metabolism. Thus, it suggests that specific nutrient supply, and not increased energy demand alone, is required to enhance Sirt5.
The expression patterns of both Sirt6 and Hif1α along with F6PK resembled each other in all diet groups but not in exercised ones. Exercise decreased Sirt6 expression with almost no change in Hif1α. Interestingly, our results show that casein-containing protein supplement (PD), but not whey, induced a large increase in skeletal muscle Hif1α expression, suggesting that different nutritional stimuli may regulate glycolysis via affecting the ratio of Sirt6 and Hif1α. Previously it was shown that casein-containing protein diet had a protective effect on obesity that was not seen with proteins from other sources suggesting increased futile cycling of fatty acids [57]. In skeletal muscle, Sirt6 positively regulates AMPK having influence on glucose uptake, fatty acid uptake and oxidation, and mitochondrial oxidative phosphorylation [58]. It is shown to act as a corepressor of Hif1α thus regulating the expression of glucose transporters and key glycolytic enzymes [23]. In contrast to our findings, previous studies showed no effect on Sirt6 after 6-week treadmill running in the skeletal muscle in young healthy Wistar rats [53] or after one-year running intervention in LCR rats [32]. The effects of different exercise modes, diets, ageing and their combinations on the function of Sirt6 in various muscles remain to be studied.
Protein supplements increased skeletal muscle Sirt7 that was further augmented by exercise - although similarly to our previous finding [32], running alone had no effect. One of the tasks of Sirt7 is to act as regulator of mitochondrial homeostasis and lipid metabolism at least in the liver [24]. However, the effects of Sirt7 on skeletal muscle metabolism are poorly known, and further research is needed.
5. Conclusions
Collectively, our data support the idea that combined, long-term exercise and dietary milk proteins, especially whey, are beneficial for blood lipid profile and body composition. Running increased energy intake and lean mass without affecting body weight. The data adds to existing knowledge by showing the effects of long-term milk protein consumption or whey at cellular level, enabling us to screen the underlying health-beneficial mechanisms in the skeletal muscle. The metabolic effects of long-term running and/or milk protein consumption may be - at least partially - regulated by Sirtuins responding in concert to metabolic challenges. Notably, milk protein supplements had some favorable effects on metabolism, even without running.
Ethics approval
The experimental procedures were approved by the Regional State Administrative Agency, Southern Finland, Finland (permission ESAVI-2010-07989/Ym-23), and the Institutional Animal Care & Use Committee (IACUC) at the University of Michigan. Experiments were done according to the Guidelines of the European Community Council directives 86/609/EEC, and European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Council of Europe No 123, Strasbourg 1985).
Consent for publication
Not applicable.
Availability of data and materials
The datasets of the current study are available from the corresponding author on request.
Funding sources
The study was supported by the SalWe Research Program for Mind and Body (Tekes - the Finnish Funding Agency for Technology and Innovation grant 1104/10) and the Academy of Finland (grant# 298875). The LCR-HCR rat model system is supported by the Office of Research Infrastructure Programs/OD grant P40OD021331 (to LGK and SLB) from the National Institutes of Health, USA. Sponsors had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in decision to submit the article for publication.
Authors’ contributions
HK, SL, ATT, JJH, UMK, LGK, SLB, and SP made contributions to conception and design of the experiment, and drafting and revising the manuscript. SL, SP, HK, AM, NK, JJH, MH, MS, SK were involved in acquisition of data, and/or in analysis and interpretation of data. All authors have read and approved the final version of the manuscript.
Declaration of competing interest
Anu T. Turpeinen is an employee of Valio Ltd, which provided the whey protein and milk protein drinks, but ATT and/or Valio Ltd had no role in the collection and analysis of the data. All other authors have no conflicts of interest.
Acknowledgements
Mervi Matero is acknowledged for the help with the study. Staff of the animal facilities, Jouni Tukiainen, Kaisa-Leena Tulla, Aila Ollikainen and Risto Puurtinen are thanked for the technical assistance.
Satu Pekkala (#308042) and Juha Hulmi (#275922) are Research Fellows of the Academy of Finland. Contact LGK (Lauren.Koch2@UToledo.Edu) or SLB (brittons@umich.edu) for information on the LCR and HCR rats: these rat models are maintained as an international collaborative resource at The University of Toledo, Toledo, Ohio.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2019.100019.
Contributor Information
S. Lensu, Email: sanna.t.k.lensu@jyu.fi.
S.P. Pekkala, Email: satu.p.pekkala@jyu.fi.
A. Mäkinen, Email: annemakinen@outlook.com.
N. Karstunen, Email: niina.karstunen@firstbeat.com.
A.T. Turpeinen, Email: Anu.Turpeinen@valio.fi.
J.J. Hulmi, Email: juha.j.t.hulmi@jyu.fi.
M.M. Silvennoinen, Email: mika.m.silvennoinen@jyu.fi.
H. Ma, Email: ma.hongqiang@helsinki.fi.
U.M. Kujala, Email: urho.m.kujala@jyu.fi.
S. Karvinen, Email: sira.m.karvinen@jyu.fi.
L.G. Koch, Email: Lauren.Koch2@utoledo.edu.
S.L. Britton, Email: brittons@umich.edu.
H. Kainulainen, Email: heikki.s.o.kainulainen@jyu.fi.
List of abbreviations
- ACC
acetyl-Co enzyme A carboxylase
- AUC
Area under the curve
- BCAA
branched chain amino acid
- CS
citrate synthase
- cytC
cytochrome C
- F6PK
Fructose-6-Phosphate Kinase
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HDL
high density lipoprotein
- Hif1α
hypoxia inducible factor
- HOMA
homeostatic model assessment for insulin resistance (fasting blood glucose/fasting insulin *22.4)
- i.p.
intraperitoneal
- LCR
low-capacity runner rat
- LDL
low density lipoprotein
- MD
metabolic disorders
- pACC
phosphorylated acetyl-CoA carboxylase
- PDK4
pyruvate dehydrogenase lipoamide kinase isozyme 4
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1α
- PD
milk protein drink
- RW
running wheel, running group
- Sirt1-Sirt7
Sirtuins 1-7
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Fig. S2.
Fig. S3.
Fig. S4.
References
- 1.Leskinen T., Kujala U.M. Health-related findings among twin pairs discordant for leisure-time physical activity for 32 Years: the TWINACTIVE study synopsis. Twin Res Hum Genet. 2015;18:266–272. doi: 10.1017/thg.2015.23. [DOI] [PubMed] [Google Scholar]
- 2.Pal S., Radavelli-Bagatini S. The effects of whey protein on cardiometabolic risk factors. Obes Rev. 2013;14:324–343. doi: 10.1111/obr.12005. [DOI] [PubMed] [Google Scholar]
- 3.Drouin-Chartier J.-P. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7:1026–1040. doi: 10.3945/an.115.011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan J. Small dense LDL cholesterol is associated with metabolic syndrome traits independently of obesity and inflammation. Nutr Metab. 2019;16 doi: 10.1186/s12986-019-0334-y. 7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGregor R.A., Poppitt S.D. Milk protein for improved metabolic health: a review of the evidence. Nutr Metab. 2013;10 doi: 10.1186/1743-7075-10-46. 46-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall W.L., Millward D.J., Long S.J., Morgan L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–248. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- 7.Boirie Y. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson G.H., Tecimer S.N., Shah D., Zafar T.A. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J Nutr. 2004;134:3011–3015. doi: 10.1093/jn/134.11.3011. [DOI] [PubMed] [Google Scholar]
- 9.Kainulainen H., Hulmi J.J., Kujala U.M. Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc Sport Sci Rev. 2013;41:194–200. doi: 10.1097/JES.0b013e3182a4e6b6. [DOI] [PubMed] [Google Scholar]
- 10.Kujala U.M. Branched-chain amino acid levels are related with surrogates of disturbed lipid metabolism among older men. Front Med. 2016;3:1–9. doi: 10.3389/fmed.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Antona G. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metabol. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Li L. Mitochondrial biogenesis and peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) deacetylation by physical activity intact adipocytokine signaling is required. Diabetes. 2011;60:157–167. doi: 10.2337/db10-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nogueiras R. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Phys Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houtkooper R.H., Pirinen E., Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell. 2011;43:33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jing E., Gesta S., Kahn C.R. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metabol. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palacios O.M. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1 alpha in skeletal muscle. Aging-Us. 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tauriainen E. Distinct effects of calorie restriction and resveratrol on diet-induced obesity and Fatty liver formation. J Nutr Metab. 2011;(2011):525094. doi: 10.1155/2011/525094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing E. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108:14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson K.A. SIRT4 is a lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metabol. 2017;25:838–855. doi: 10.1016/j.cmet.2017.03.003. e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkhwanky M.S., Hakkola J. Extranuclear sirtuins and metabolic stress. Antioxidants Redox Signal. 2018;28:662–676. doi: 10.1089/ars.2017.7270. [DOI] [PubMed] [Google Scholar]
- 22.Tasselli L., Zheng W., Chua K.F. SIRT6: novel mechanisms and links to aging and disease. Trends Endocrinol Metab. 2017;28:168–185. doi: 10.1016/j.tem.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong L. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1 alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blank M.F., Grummt I. The seven faces of SIRT7. Transcription. 2017;8:67–74. doi: 10.1080/21541264.2016.1276658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baskin Kedryn K., Winders Benjamin R., Olson Eric N. Muscle as a “mediator” of systemic metabolism. Cell Metabol. 2015;21:237–248. doi: 10.1016/j.cmet.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch L.G., Britton S.L. Theoretical and biological evaluation of the link between low exercise capacity and disease risk. Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wisloff U. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 28.Thyfault J.P. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587:1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karvinen S. Physical activity in adulthood: genes and mortality. Sci Rep. 2015;5:18259. doi: 10.1038/srep18259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kivela R. Gene expression centroids that link with low intrinsic aerobic exercise capacity and complex disease risk. FASEB J. 2010;24:4565–4574. doi: 10.1096/fj.10-157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch L.G., Britton S.L. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genom. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 32.Karvinen S. Effects of intrinsic aerobic capacity, aging and voluntary running on skeletal muscle sirtuins and heat shock proteins. Exp Gerontol. 2016;79:46–54. doi: 10.1016/j.exger.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Goldman J.M., Murr A.S., Cooper R.L. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 34.Hulmi J.J., Silvennoinen M., Lehti M., Kivela R., Kainulainen H. Altered REDD1, myostatin, and Akt/mTOR/FoxO/MAPK signaling in streptozotocin-induced diabetic muscle atrophy. Am J Physiol Endocrinol Metab. 2012;302:E307–E315. doi: 10.1152/ajpendo.00398.2011. [DOI] [PubMed] [Google Scholar]
- 35.Hart N. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food Chem Toxicol. 2013;61:53–59. doi: 10.1016/j.fct.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antunes L.C., Elkfury J.L., Jornada M.N., Foletto K.C., Bertoluci M.C. Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch Endocrinol Metab. 2016;60:138–142. doi: 10.1590/2359-3997000000169. [DOI] [PubMed] [Google Scholar]
- 37.Koch L.G., Britton S.L. Development of animal models to test the fundamental basis of gene-environment interactions. Obesity. 2008;16:S28–S32. doi: 10.1038/oby.2008.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart N. Resveratrol attenuates exercise-induced adaptive responses in rats selectively bred for low running performance. Dose-Response. 2014;12:57–71. doi: 10.2203/dose-response.13-010. Radak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ringholm S. Effect of lifelong resveratrol supplementation and exercise training on skeletal muscle oxidative capacity in aging mice; impact of PGC-1 alpha. Exp Gerontol. 2013;48:1311–1318. doi: 10.1016/j.exger.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Edgett B.A., Bonafiglia J.T., Baechler B.L., Quadrilatero J., Gurd B.J. The effect of acute and chronic sprint-interval training on LRP130, SIRT3, and PGC-1alpha expression in human skeletal muscle. Phys Rep. 2016;4 doi: 10.14814/phy2.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edgett B.A. Dissociation of increases in PGC-1 alpha and its regulators from exercise intensity and muscle activation following acute exercise. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071623. e71623-e71623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karlund A. Protein supplements and their relation with nutrition, microbiota composition and health: is more protein always better for sportspeople? Nutrients. 2019;11:19. doi: 10.3390/nu11040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonio J., Sanders M.S., Van Gammeren D. The effects of bovine colostrum supplementation on body composition and exercise performance in active men and women. Nutrition. 2001;17:243–247. doi: 10.1016/S0899-9007(00)00552-9. [DOI] [PubMed] [Google Scholar]
- 44.Chen W.-C., Huang W.-C., Chiu C.-C., Chang Y.-K., Huang C.-C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med Sci Sport Exerc. 2014;46:1517–1524. doi: 10.1249/MSS.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westerterp K.R. Exercise, energy balance and body composition. Eur J Clin Nutr. 2018;72:1246–1250. doi: 10.1038/s41430-018-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bendtsen L.Q., Lorenzen J.K., Bendsen N.T., Rasmussen C., Astrup A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Adv Nutr. 2013;4:418–438. doi: 10.3945/an.113.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fekete Á.A., Givens D.I., Lovegrove J.A. Can milk proteins be a useful tool in the management of cardiometabolic health? An updated review of human intervention trials. Proc Nutr Soc. 2016;75:328–341. doi: 10.1017/S0029665116000264. [DOI] [PubMed] [Google Scholar]
- 48.Gazi I. LDL cholesterol estimation in patients with the metabolic syndrome. Lipids Health Dis. 2006;5 doi: 10.1186/1476-511X-5-8. 8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J.W. Effect of whey protein on blood lipid profiles: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2016 doi: 10.1038/ejcn.2016.39. [DOI] [PubMed] [Google Scholar]
- 50.Astrup A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: epidemiologic and experimental studies. Am J Clin Nutr. 2014;99:1235S–1242S. doi: 10.3945/ajcn.113.073015. [DOI] [PubMed] [Google Scholar]
- 51.Sengupta, P. The laboratory rat: relating its age with human’s. Int J Prev Med. 4, 624-630, PMID: 23930179 (2013). [PMC free article] [PubMed]
- 52.Huang C.C., Wang T., Tung Y.T., Lin W.T. Effect of exercise training on skeletal muscle SIRT1 and PGC-1alpha expression levels in rats of different age. Int J Med Sci. 2016;13:260–270. doi: 10.7150/ijms.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koltai E. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gurd B.J. Deacetylation of PGC-1 alpha by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metabol. 2011;36:589–597. doi: 10.1139/H11-070. [DOI] [PubMed] [Google Scholar]
- 55.Philp A. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) deacetylation following endurance exercise. J Biol Chem. 2011;286:30561–30570. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin L. Regulation of skeletal muscle oxidative capacity and muscle mass by SIRT3. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085636. e85636-e85636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liisberg U. The protein source determines the potential of high protein diets to attenuate obesity development in C57BL/6J mice. Adipocyte. 2016;5:196–211. doi: 10.1080/21623945.2015.1122855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui X. SIRT6 regulates metabolic homeostasis in skeletal muscle through activation of AMPK. Am J Physiol Endocrinol Metab. 2017;313:E493–E505. doi: 10.1152/ajpendo.00122.2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of the current study are available from the corresponding author on request.