Abstract
Supplemental Digital Content is available in the text.
Keywords: cardiovascular diseases, COVID-19, heart, myocarditis, severe acute respiratory syndrome coronavirus 2
Coronavirus disease 2019 (COVID-19) is a global pandemic caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 infection occurs predominantly by binding of the viral surface spike protein to the human angiotensin-converting enzyme 2 (ACE2) receptor.1 Hypertension and preexisting cardiovascular disease are risk factors for morbidity from COVID-19, and it remains uncertain whether the use of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers affects infection and disease. This uncertainty has provoked public statements by the American Heart Association, the Heart Failure Society of America, and the American College of Cardiology advising continuation of these agents in the absence of compelling new data.
The predominant portal of entry for SARS-CoV-2 is by ACE2 expression in the upper respiratory epithelium and lungs, yet in a single-center report of 416 patients hospitalized with COVID-19, 19.7% had evidence of cardiac injury, suggesting a possible pathologic role for myocardial ACE2 expression.2 Moreover, the presence of cardiac injury was associated with a 5-fold increase in mechanical ventilation and a 51.2% mortality rate. In this context, the potential for a primary viral myocarditis with SARS-CoV-2 is gaining support.
We aim to shed light on these questions by assessing ACE2 expression by performing bulk and single nucleus RNA-Seq on the left ventricles of 11 individuals with dilated cardiomyopathy, 15 individuals with hypertrophic cardiomyopathy, and 16 controls with nonfailing hearts from the Penn Human Heart Tissue Biobank. All studies were approved by the institutional review boards at each institution. Single-cell sequencing was performed largely as described in our recent study detailing the transcriptional diversity of the cell subtypes and associated gene programs in the normal human heart.3 Data generated by the 10X Genomics 3′ solution v3 were processed with the CellRanger 3.1.0 toolkit followed by strict quality control for transcriptional complexity, mitochondrial read counts, proportion of spliced reads, and entropy. Differential gene expression was performed by summing gene counts across all nuclei in each cell type and applying a limma-voom testing pipeline, adjusting for age and sex.
Consistent with recent reports,4,5 cardiac ACE2 expression is strongest in pericytes, which line the microvasculature, but is also appreciable in vascular smooth muscle cells, fibroblasts, and cardiomyocytes (Figure A). We also evaluated the expression of the proteases encoded by TMPRSS2 and CTSL, which facilitate membrane fusion and viral uptake during SARS-CoV-2 infection. TMPRSS2 was minimally expressed across all cell types, whereas CTSL displayed low levels of expression in all cell types with strongest expression in fibroblasts (18.1% and 56.7% of cells in the 2 populations found in controls with nonfailing hearts), macrophages (39.1%), adipocytes (41.5%), and cardiomyocytes (11.4%; Figure A).
Figure.
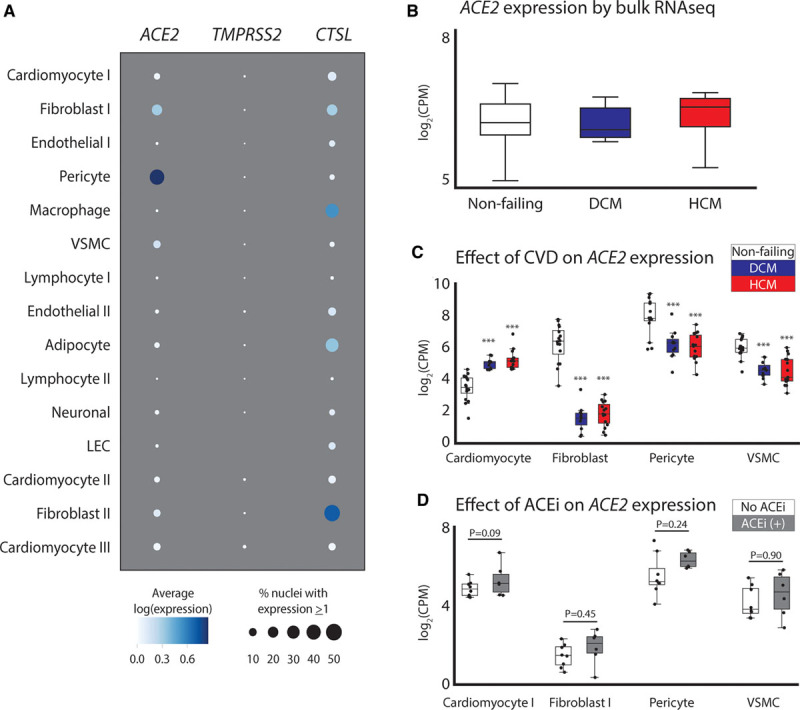
Assessment of ACE2 expression in the human myocardium. A, Dot plot representing the relative expression of ACE2, CTSL, and TMPRSS2 in the left ventricle. Size and hue of the dot indicates the percent of nuclei expressing and the mean of the log-transformed, normalized counts for all nuclei in each cell type. B, Expression of ACE2 by bulk RNA-Seq from nonfailing, dilated cardiomyopathy (DCM), and hypertrophic cardiomyopathy (HCM) ventricles. C, Single nucleus RNA-Seq from the same tissue samples as in B with mean expression of ACE2 in cell subtypes with appreciable expression. D, Effects of angiotensin-converting enzyme inhibitors (ACEis) on ACE2 expression across cell types in individuals with HCM. Boxes represent 25% to 75% and whiskers represent the minimum–maximum range, excluding outliers. ***P<0.001. CVD indicates cardiovascular disease; LEC, lymphatic endothelial cell; and VSMC, vascular smooth muscle cell.
In contrast to a recent report,4 analyses of bulk RNA-Seq data show no significant alterations in ACE2 expression in the context of dilated or hypertrophic cardiomyopathy (Figure B). However, single nucleus RNA-Seq highlighted a stark downregulation of ACE2 expression in fibroblasts, pericytes, and vascular smooth muscle but a concomitant upregulation in ACE2 expression in cardiomyocytes in dilated cardiomyopathy and hypertrophic cardiomyopathy (Figure C). These results are similar to those seen in a recent report, which examined expression in patients with aortic stenosis and heart failure with reduced ejection fraction.5
Because patients with dilated cardiomyopathy were on an ACEi whereas controls with nonfailing hearts were not, our ability to examine effects of ACEi use was limited to the patients with hypertrophic cardiomyopathy (6 taking an ACEi, 8 not taking an ACEi). As in Nicin et al,5 there was a trend toward increased ACE2 expression with ACEi treatment in all cell types (Figure D), but the present results were not statistically significant. An important distinction between the studies is that our statistical models of differential expression account for the inherent sparsity and intraindividual correlation of single nucleus sequencing data by summing across nuclei within an individual rather than treating the unit of observation as the nucleus. This allows us to test for variability in expression across conditions or treatments more robustly. Future studies with larger patient cohorts to examine cell type–specific ACE2 expression in additional disease states and in donors with nonfailing hearts receiving an ACEi or angiotensin receptor blockers are urgently needed. Transcriptional assessments do not reflect the effects of RNA half-life, translation efficiency, or protein turnover. As such, it will be important that surface localized ACE2 protein is also evaluated in future studies.
These data suggest that previous cardiovascular disease is a predominant driver of cardiomyocyte-specific increased transcription of ACE2. These findings may provide a pathologic link for SARS-CoV–associated viral myocarditis.
Sources of Funding
The Precision Cardiology Laboratory is a joint effort between the Broad Institute and Bayer AG. This work was supported by the Fondation Leducq (14CVD01) and by grants from the National Institutes of Health to Dr Ellinor (1RO1HL092577, R01HL128914, K24HL105780), Dr Tucker (5K01HL140187), and Dr. Margulies (1R01HL105993), as well as the Klarman Cell Observatory and the Manton Foundation (to Dr. Regev). This work was also supported by a grant from the American Heart Association Strategically Focused Research Networks to Dr Ellinor (18SFRN34110082).
Disclosures
Drs Papangeli, Akkad, Hayat, and Stegmann are employees of Bayer US LLC (a subsidiary of Bayer AG) and may own stock in Bayer AG. Dr Ellinor is supported by a grant from Bayer AG to the Broad Institute focused on the genetics and therapeutics of cardiovascular diseases. Dr Ellinor has also served on advisory boards or consulted for Bayer AG, Quest Diagnostics, MyoKardia, and Novartis. Dr Margulies has research grant funding from Sanofi-Aventis and has also served on advisory boards for MyoKardia and Pfizer. The other authors report no conflicts.
Supplementary Material
Footnotes
Drs Tucker and Chaffin contributed equally.
Human Cell Atlas Lung Biological Network study participants are listed in the Appendix in the Data Supplement.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.047911.
All data and materials regarding cell identities and expression of the 3 genes highlighted in this study have been made publicly available at the Single Cell Portal of the Broad Institute and can be accessed at https://singlecell.broadinstitute.org/single_cell/study/SCP846.
References
- 1.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382:1653–1659. doi:10.1056/nejmsr2005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 20205802–810doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tucker NR, Chaffin M, Fleming SJ, Hall AW, Parsons VA, Bedi KC, Jr, Akkad A-D, Herndon CN, Arduini A, Papangeli I, et al. Transcriptional and cellular diversity of the human heart [published online May 14, 2020]. Circulation. doi: 10.1161/CIRCULATIONAHA.119.045401. doi: 10.1161/circulationaha.119.045401. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.119.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 20201161097–1100doi: 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J 2020411804–1806doi: 10.1093/eurheartj/ehaa311 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


