Abstract
In a variety of solid tumors, the presence of higher densities of tumor-infiltrating lymphocytes or tertiary lymphoid structures (TLS) are correlated with prolonged patient survival. Murine studies are usually required to define mechanisms that govern immunologic infiltrate in tumors. However, few methods have been described that could enable a more comprehensive understanding of the functionality of intratumoral immune infiltrate and TLS in solid murine cancers. In this chapter, we describe multiplex immunohistochemistry and microscopy approaches for identifying, characterizing, and quantifying intra-tumoral immune infiltrate and TLS in murine tumor models.
1. INTRODUCTION
The immune system plays an instrumental role in tumor control and clearance. Newer cancer immune therapies are intended to increase the representation of immune cells in tumors, to promote anti-tumor immune responses and tumor control or eradication. Infiltrating CD8+ T cells are associated with improved prognosis and patient survival in a broad range of tumor types(Barnes and Amir, 2018; Erdag et al., 2012; Fridman et al., 2017), and immune infiltrate has been correlated with response to therapy(Barnes and Amir, 2018; Jochems and Schlom, 2011). In our own studies, we found that three histologic patterns of intra-tumoral immune cell infiltration (termed Immunotypes A, B, C), identified in human melanoma metastases could predict patient survival. Immunotype A tumors harbored little to no immune cell infiltrate; Immunotype B tumors had immune infiltrates found cuffing intra-tumoral blood vessels and located only near blood vessels, and Immunotype C tumors were characterized by diffuse and usually dense immune cell infiltrate throughout the tumor. Immunotypes A, B and C tumors represented 29%, 63%, and 8% of human melanoma metastases with estimated median survival periods of 15, 23, and 130 months, respectively. Higher densities of CD8+ T cells correlated best with survival, and higher densities of CD45+ leukocytes, T cells, and B cells also correlated with increased survival(Erdag et al., 2012). However, the prognostic value of lymphocytes in stromal, peri-tumoral and intra-tumoral locations remains unclear, with conflicting data from different tumor sites(Barnes and Amir, 2018; Galon et al., 2006; Sideras et al., 2018; Tumeh et al., 2014)
Tertiary lymphoid structures (TLS) have been observed in a variety of solid tumors in humans, and their presence is a favorable prognostic indicator for melanoma survival (Dieu-Nosjean et al., 2014). They have also been observed in murine models(Engelhard et al., 2018; Peske et al., 2015). TLS have morphological similarities to conventional secondary lymphoid organs, typically containing organized T- and B-cell compartments, lymphatic vessels, and high endothelial-like vessels that express peripheral node addressin (PNAd)(Engelhard et al., 2018; Germain et al., 2015; Goc et al., 2013). Similar studies evaluating immune infiltrates or TLS in murine tumors could be done to assess the impact of cancer therapies on immune infiltrate in tumors, using multiplex immunohistochemistry, and broadened to incorporate markers informing immune activity such as granzyme-b. Furthermore, understanding the location of immune infiltrate within the tumor microenvironment, or the organization of immune infiltrate relative to other neighboring immune cells, could inform survival, and further elucidate the type of anti-tumor immune response being mounted. Newer, multiplex IHC studies are using spatial proximity analyses, which study the distance or the proximity between two different cell types to predict patient survival(Barua et al., 2018; Mezheyeuski et al., 2018).
Intratumoral immune responses could also be analyzed by flow cytometric analysis. However, since tissue is dissociated for this technique, information about the location of immune infiltrate is lost. Additionally, traditional immunofluorescence histology (IFH) only allows for visualization of 3 antigens in a tissue specimen at best, thus limiting studies of immune response, and is done with frozen tissue sections, which lose some architectural detail compared to fixed tissues. Also, traditional IFH techniques result in signals that are quenched in light and are transient. Traditional bright-field immunohistochemistry is also challenging to perform with multiple antibodies simultaneously, especially when two or more antigens are co-expressed in the same cell or subcellular region, as they can block each other. The availability of techniques that could enable a more comprehensive understanding of the organization of immune infiltrate or TLS in murine cancers has been limited, until now. In this chapter, we describe methods for the characterization of intra-tumoral immune infiltrate and TLS in murine tumor specimens, using multiplex immunohistochemistry which enables the identification, quantification, and stable visualization of six antigens in one tissue specimen.
2. MATERIALS
2.1. Tumor Implantation & Harvest Tumor Implantation & Harvest
GL261-luc2 cells (courtesy of Woodworth Lab, University of Maryland)
6-10 week female C57BL/6 mice (The Jackson Laboratory)
U-Frame Stereotaxic Instrument for Mouse (Harvard Apparatus)
Hamilton syringe (Hamilton Company)
Micropump (UltraMicroPump, World Precision Instruments)
2.2. Specimen Preparation
Neutral Buffered Formalin
Ethanol (EtOH)
Superfrost Plus Microscope Slides (Fisher Scientific)
Xylene
2.3. Immunofluorescent staining
Slide humidity chamber
Antigen Retrieval Buffer 9 (Akoya Biosciences).
Opal 7-Color Manual IHC kit (Akoya Biosciences), which includes Opals, blocking/antibody diluent, secondary antibody recognizing mouse or rabbit species, spectral DAPI, and amplification diluent.
Super Picture HRP Polymer Conjugate Broad Spectrum (Life Technologies)
ProLong Diamond Antifade Mountant (ThermoFisher Scientific).
24x50mm cover glass
Opal Staining jar (Akoya Biosciences)
Panasonic Microwave Oven with Inverter Technology and Genius Sensor, 1200W
TBST Wash Buffer containing 0.05% Tween 20, PH 7.5
Table 1:
Antibodies for Immunofluorescence Staining
| Antibody | Clone | Supplier |
|---|---|---|
| CD4 | EPR19514 | Abcam |
| CD8 | CAL38 | Abcam |
| CD19 | D4V4B | Cell Signaling Technology |
| CD34 | EP373Y | Abcam |
| Ki67 | - | Abcam catalog number ab15580 |
| Granzyme-b | - | Abcam catalog number ab4059 |
| PNAd | MECA-79 | BioLegend |
| CD11c | EPR21826 | Abcam |
Table 3:
Tertiary Lymphoid Structures Identification Staining Panel
| Staining Order |
Antigen Retrieval |
Primary Antibody and working dilution |
Secondary Antibody |
OPAL |
|---|---|---|---|---|
| 1. | AR9 | PNAd (1:50) | Super Picture HRP | 620 |
| 2. | AR9 | CD4 (1:100) | Opal Polymer HRP | 520 |
| 3. | AR9 | CD8 (1:200) | Opal Polymer HRP | 540 |
| 4. | AR9 | CD19 (1:50) | Opal Polymer HRP | 650 |
| 5. | AR9 | CD11c (1:100) | Opal Polymer HRP | 570 |
| 6. | AR9 | CD34 (1:100) | Opal Polymer HRP | 690 |
| 7. | AR9 | - | Opal Polymer HRP | Spectral DAPI |
3. METHODS
3.1. Preparation of GL261-luc2 tumors for immunofluorescence microscopy
Part 1: Cell Culture.
Culture GL261 cells stably transfected with luciferase (GL261-luc2) in high glucose 1x Dulbecco modified Eagle medium (DMEM, Gibco) supplemented with 1 mM sodium pyruvate (Gibco), non-essential amino acids (Gibco), 10% fetal bovine serum (Gibco), and 100 μg/mL G418 (GoldBio). Cells are maintained in an incubator at 37°C and 5% CO2. Thawed cells are cultured for up to three passages and maintained in logarithmic growth phase.
Resuspend GL261-luc2 cells (1 x105 cells per 2μL) in sterile PBS for intracranial tumor implantation.
Part 2: Tumor Implantation & Harvest.
Anesthetize 6-10 week female C57BL/6 mice (The Jackson Laboratory) by intraperitoneal injection of Ketamine/Dexdomitor (50/0.25 mg/kg).
Depilate their heads, place mice into stereotaxic frame, and aseptically prepare the skin by disinfecting with 70% alcohol and surgical iodine swabs.
Orthotopically implant 2μL of GL261-luc2 cell resuspension into the right striatum of the brain at a mechanically controlled rate of 0.5μL/min using a Hamilton syringe and micropump. Stereotactic injection coordinates are ~2.0 mm lateral from the sagittal suture, 0.5mm anterior of bregma and 3mm below the dura. Negative control mice undergo sham surgery with intracranial injection of sterile PBS alone.
Excise whole brains at 21 days post-inoculation.
Quarter brains in order to preserve the entirety of the tumor as well as its infiltrative margins. To achieve this, bluntly sever the medial cortex with a scalpel, and resect the anterior right hemisphere of the brain starting at the midline.
This quadrant of the right cerebral cortex containing the GL261-luc2 tumor and some surrounding normal brain tissue is harvested for analysis by multiplex immunohistochemistry.
Place harvested tissue specimens in 10% Neutral Buffered Formalin overnight.
Transfer specimen to 70% EtOH.
Paraffin embed tissue specimen.
Cut 5-μm sections and mount on SuperFrost Plus microscope slides.
3.2. Immunofluorescent staining of cell surface markers on formalin-fixed tumor sections
Part 1: Deparaffinization, Rehydration, Fixation, and Antigen Retrieval.
Immerse slides in Tissue-tek slide staining dishes that are filled with the following solutions: xylene x 3 dishes, 100% EtOH, 95% EtOH, 70% EtOH, DH20 x 3 dishes, TBST x 1 dish, Neutral Buffered formalin x 1 dish. The slide rack containing slides is moved from dish to dish in sequential steps with all steps done at room temperature.
Xylene dish 1 (10 min)
Xylene dish 2 (10 min)
Xylene dish 3 (10 min)
100% EtOH (5 min)
95% EtOH (5 min)
70% EtOH (2 min)
DH20 (2 min)
TBST (2 min)
DH20 (2 min)
Neutral Buffered Formalin (20 min)
DH20 (2 min)
Place the slides in an Opal slide processing jar and add Antigen Retrieval Buffer 9. Rinse the slides for 2 min then discard Buffer.
Completely fill chamber with AR9 Buffer
Microwave slides for approximately 2 min at 100% power until the solution is at a boil. Once the solution is boiling stop the microwave and place at 20% power, allow slides to heat at 20% power for 18 min. During the initial high-power microwave step it is critical to watch the slides so that too much solution does not boil out; if this happens add more buffer. It is critical that the tissue on your slides is completely submerged in buffer during the whole microwaving process and subsequent cooling process.
Allow slides to cool at room temperature for 30 min, then proceed to do further staining steps or store slides in fridge at 4 degrees C overnight.
Part 2: Multiplex Stain.
Specific antibody staining protocols/details are found in Tables 2 and Tables 3 for the Immune Infiltrate and TLS Identification Panels Respectively. All subsequent steps are done at room temperature.
Table 2:
Immune Infiltrate Identification Staining Panel
| Staining Order |
Antigen Retrieval |
Primary Antibody and working dilution |
Secondary Antibody |
OPAL |
|---|---|---|---|---|
| 1. | AR9 | CD4 (1:100) | Opal Polymer HRP | 520 |
| 2. | AR9 | CD8 (1:200) | Opal Polymer HRP | 540 |
| 3. | AR9 | CD19 (1:50) | Opal Polymer HRP | 650 |
| 4. | AR9 | CD34 (1:1k) | Opal Polymer HRP | 620 |
| 5. | AR9 | Ki67 (1:1k) | Opal Polymer HRP | 570 |
| 6. | AR9 | Granzyme-b (1:1k) | Opal Polymer HRP | 690 |
| 7. | AR9 | - | Opal Polymer HRP | Spectral DAPI |
Rinse Slides in TBST in Tissue-Tek staining dish.
Remove slides from TBST and place into humidified chambers.
Pipette 300μl of Antibody Diluent/Block solution on each slide allow to block for 10 min Discard blocking solution by tipping slides over a beaker, place slides back in staining chamber.
Primary Antibody diluted in Antibody Diluent/Block should be pipetted on slides, 300μl per slide, and allowed to incubate for 30 min in a humidified chamber.
Set up 4 Tissue-Tek dishes filled with TBST washing buffer. The first dish should contain a clean slide rack that will be moved from dish to dish.
After Primary antibody incubation discard the primary antibody over a beaker and add slides to staining rack in TBST.
Wash slides in staining rack 4x 2min each in TBST.
Remove slides from TBST and place into humidified staining chambers.
Pipette 300μl of ready to use Secondary Antibody (Opal Polymer HRP or Super Picture HRP) to each slide, and allow to stain for 10 min.
Discard Secondary antibody and wash slides 4x (2min each) in TBST as previously described.
Remove slides from TBST and place into humidified staining chambers.
Pipette 200μl of Opal staining solution that has been diluted in Amplification Diluent and allow to stain for 10 min (Opal staining solution: Opal stock should be made in 150μl DMSO; dilute Opal 1:50 in Amplification Diluent). Use the Opal that you assigned to each primary antibody.
Discard Opal solution and Wash Slides 4x (2min each) in TBST
Rinse Slides in AR9 Buffer in an Opal slide processing jar then discard buffer.
Completely fill jar with AR9 Buffer and Microwave for ~2 min at 100% power until solution is boiling and then, 18 min at 20% power in AR9 Buffer.
Allow Slides to cool at Room temperature for 30 min, then proceed to do further staining steps or store slides in fridge at 4 degrees C overnight.
These steps are repeated 5 more times until all primary antibodies have been successfully stained with the corresponding assigned Opal.
Part 3: Spectral DAPI Stain, Mount and Coverslip.
Set up 2 Tissue-Tek dishes filled with TBST washing buffer, and 2 Tissue Tek dishes filled with DH20. The first dish should contain a clean slide rack that will be moved from dish to dish.
Wash slides in DH20 (2 min).
Wash slides in TBST (2 min).
Pipette 300μl DAPI staining solution to each slide, allow to incubate for 5 min (DAPI solution: 2 drops of spectral DAPI per ml TBST).
Wash slides in TBST (2 min).
Wash slides in DH20 (2 min).
Move slides to chamber apply ProLong Diamond Antifade Mountant and coverslip.
Allow slides to dry for a few hours to overnight.
Clean slides with EtOH and image with fluorescent microscope.
3.3. Identification and characterization of immune infiltrate and TLS in B16 melanoma or GL261 glioblastoma by immunofluorescence
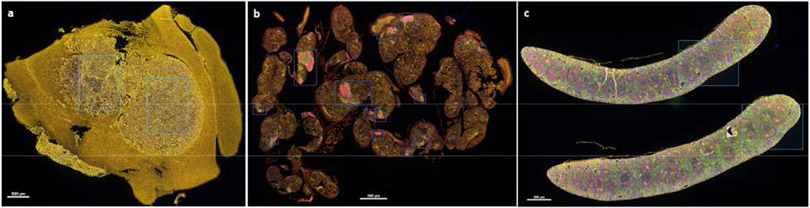
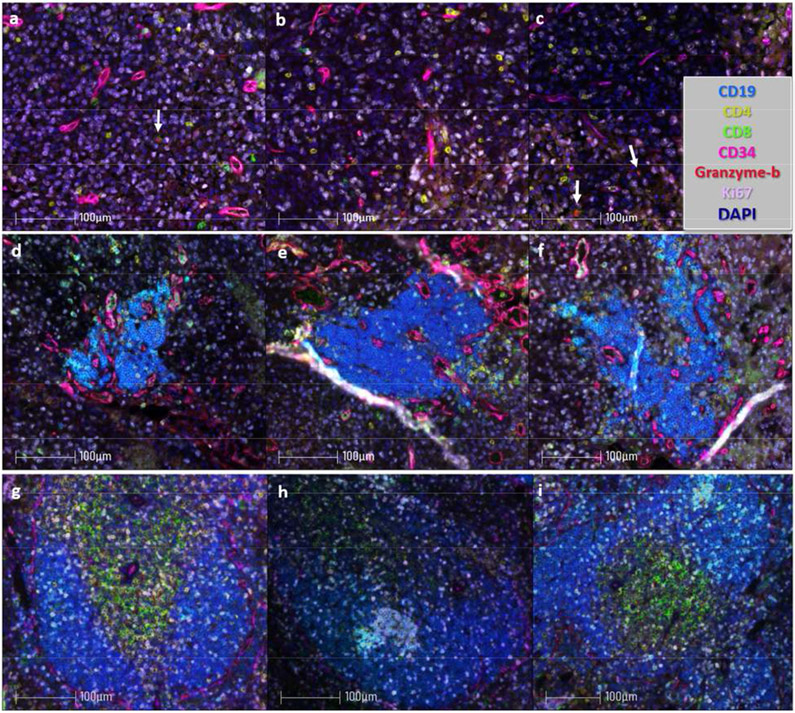
Acquisition and analysis of immunofluorescent images can be performed with a fluorescent microscope and image software package. We use a Vectra 3 (PerkinElmer) with inForm image analysis software (PerkinElmer). Representative staining images are shown in Fig. 1 and Fig. 2 and staining protocols detailed in Tables 2 and 3.
Figure 1:
Tissue specimens were obtained stained for immunofluorescent microscopy as outlined in Sections 3.1-3.3 and Tables 1-3. Images were scanned at 10x and regions of interest (shown in blue) were selected on spectrally uncompensated images. (a-c) Spectrally uncompensated images from a single GL261-luc2 tumor (a), B16-OVA melanoma (b), and Spleen (c). Scale bar = 800 μm. B16-OVA tumor specimens were courtesy of Anthony Rodriguez and Dr. Victor Engelhard; protocols for tumor generation and isolation are published (Rodriguez et al., 2018).
Figure 2:
Paraffin embedded tissue specimens were sectioned and stained for immunofluorescent microscopy as outlined in Sections 3.1-3.3. Tumor sections were stained with the antibodies and Opals detailed in Table 2. Images were acquired at 20x and spectrally compensated in the Inform Program (PerkinElmer) using single stain positive controls. (a-c) Spectrally compensated images from a single GL261-luc2 (glioblastoma) intracranial tumor; arrows denote CD8+granzyme-b+ tumor infiltrating cells. (e-f) Images from B16-OVA (melanoma) intraperitoneal tumors which harbor TLS. (g-h) Images from murine spleen. Scale bar = 100 μm.
Figure 3:
Tissue specimens were stained with the antibodies and Opals detailed in Table 3. Images were acquired at 20x and spectrally compensated in the Inform Program (PerkinElmer) using single stain positive controls. (a-c) Spectrally compensated images from B16-OVA (melanoma) intraperitoneal tumors which harbor TLS. (g-h) Images from murine spleen.
Acknowledgments
Funding: This work was supported by the Schiff Foundation, Inc., the University of Virginia Cancer Center, NIH R01CA197111, and NIH R01EB020147. NDS was supported by the NCI F99/K00 Predoctoral to Postdoctoral Fellow Transition Award (F99CA234954), NSF Graduate Research Fellowship, and Wagner Fellowship.
Footnotes
We include murine spleen specimens as positive staining controls. Positive staining controls are done side by side with specimens of interest as well as single stain controls that will be used for unmixing; no Opal staining controls (specimen is stained with all reagents/steps except the Opal dye staining steps are omitted) and no primary antibody controls (specimen is stained with all reagents except primary antibodies). Fluorescence Minus One controls (FMO) may also be useful during panel development.
We acquire images of the whole tumor at 10x and select regions of interest to enable infiltrate analyses and identification of all TLS. TLS are usually localized on the tumor border or peritumorally.
Spectral unmixing or compensating is performed using the Inform software and single stain controls. We use CD19, CD4, CD8 and PNAd to identify TLS, CD11c to identify antigen-presenting cells. CD34 is used to identify tumor vasculature and proliferating immune cells and tumor cells are evaluated by Ki67.
We use inForm (PerkinElmer) to enumerate immune infiltrate that can be identified by staining with one antigen such as CD4, CD8, CD20. However, enumeration of immune cells that express multiple markers or granzyme-b or Ki67 can be done manually or by using other software packages such as Halo (Indica Labs).
Conflicts of Interest: The authors have no conflicts of interest to declare.
5. REFERENCES
- Barnes TA, Amir E, 2018. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer 118, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua S, Fang P, Sharma A, Fujimoto J, Wistuba I, Rao AUK, Lin SH, 2018. Spatial interaction of tumor cells and regulatory T cells correlates with survival in non-small cell lung cancer. Lung Cancer 117, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH, 2014. Tertiary lymphoid structures in cancer and beyond. Trends Immunol 35, 571–580. [DOI] [PubMed] [Google Scholar]
- Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD, Slingluff CL Jr., 2018. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J Immunol 200, 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL Jr., 2012. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 72, 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G, 2017. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14, 717–734. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F, 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964. [DOI] [PubMed] [Google Scholar]
- Germain C, Gnjatic S, Dieu-Nosjean MC, 2015. Tertiary Lymphoid Structure-Associated B Cells are Key Players in Anti-Tumor Immunity. Front Immunol 6, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC, 2013. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology 2, e26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochems C, Schlom J, 2011. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med (Maywood) 236, 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezheyeuski A, Bergsland CH, Backman M, Djureinovic D, Sjoblom T, Bruun J, Micke P, 2018. Multispectral imaging for quantitative and compartment-specific immune infiltrates reveals distinct immune profiles that classify lung cancer patients. J Pathol 244, 421–431. [DOI] [PubMed] [Google Scholar]
- Peske JD, Thompson ED, Gemta L, Baylis RA, Fu YX, Engelhard VH, 2015. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun 6, 7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AB, Peske JD, Engelhard VH, 2018. Identification and Characterization of Tertiary Lymphoid Structures in Murine Melanoma. Methods Mol Biol 1845, 241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideras K, Galjart B, Vasaturo A, Pedroza-Gonzalez A, Biermann K, Mancham S, Nigg AL, Hansen BE, Stoop HA, Zhou G, Verhoef C, Sleijfer S, Sprengers D, Kwekkeboom J, Bruno MJ, 2018. Prognostic value of intra-tumoral CD8(+) /FoxP3(+) lymphocyte ratio in patients with resected colorectal cancer liver metastasis. J Surg Oncol 118, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A, 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]