Abstract
Background
The recent identification of the endocannabinoid system in the gastrointestinal tract suggests a role in controlling intestinal inflammation. In addition, the gut chemosensing system has therapeutic applications in the treatment of gastrointestinal diseases and inflammation due to the presence of a large variety of receptors. The purposes of this study were to investigate the presence of markers of the endocannabinoid system and the chemosensing system in the pig gut and, second, to determine if thymol modulates these markers. One hundred sixty 28-day-old piglets were allocated into one of 5 treatment groups (n = 32 per treatment): T1 (control), T2 (25.5 mg thymol/kg feed), T3 (51 mg thymol/kg feed), T4 (153 mg thymol/kg feed), and T5 (510 mg thymol/kg feed). After 14 days of treatment, piglets were sacrificed (n = 8), and then duodenal and ileal mucosal scrapings were collected. Gene expression of cannabinoid receptors (CB1 and CB2), transient receptor potential vanilloid 1 (TRPV1), the olfactory receptor OR1G1, diacylglycerol lipases (DGL-α and DGL-β), fatty acid amine hydrolase (FAAH), and cytokines was measured, and ELISAs of pro-inflammatory cytokines levels were performed.
Results
mRNAs encoding all markers tested were detected. In the duodenum and ileum, the CB1, CB2, TRPV1, and OR1G1 mRNAs were expressed at higher levels in the T4 and T5 groups compared to the control group. The level of the FAAH mRNA was increased in the ileum of the T4 group compared to the control. Regarding the immune response, the level of the tumor necrosis factor (TNF-α) mRNA was significantly increased in the duodenum of the T5 group, but this increase was not consistent with the protein level.
Conclusions
These results indicate the presence of endocannabinoid system and gut chemosensing markers in the piglet gut mucosa. Moreover, thymol modulated the expression of the CB1, CB2, TRPV1, and OR1G1 mRNAs in the duodenum and ileum. It also modulated the mRNA levels of enzymes involved in the biosynthesis and degradation of endocannabinoid molecules. Based on these findings, the effects of thymol on promoting gut health are potentially mediated by the activation of these receptors.
Keywords: Endocannabinoid system, Gut chemosensing, Inflammation, Pig, Thymol, Small intestine
Background
The endocannabinoid system (ECS) comprises three fundamental constituents: receptors, signaling molecules, and enzymes involved in ligand biosynthesis and degradation. The main endocannabinoid receptors are two G protein-coupled receptors (GPCRs) named cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) [1, 2], but additional receptors may also be involved, such as transient receptor potential vanilloid 1 (TRPV1) [3]. The two most widely investigated endocannabinoid signaling molecules are anandamide (AEA) and 2-arachidonoylglycerol (2-AG), which are lipid molecules generated from the breakdown of arachidonic acid [4]. N-Acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) is currently considered the major enzyme responsible for AEA synthesis, while a specific diacylglycerol lipase (DGL) is responsible for 2-AG synthesis [4, 5]. The biological activity of ligands is regulated by intracellular enzymes unique to each endocannabinoid ligand: fatty acid amide hydrolase (FAAH) is the principal enzyme responsible for degrading AEA, whereas monoacylglycerol lipase (MAGL) is responsible for degrading 2-AG [6–8]. In non-neuronal tissues, endocannabinoids act as hormone-like messengers in an autocrine or paracrine mode of action, which is thought to be temporally and spatially restricted [9]. When endocannabinoid molecules are released, they bind receptors with different affinity. After the binding of ligands, receptors mediate the effects, including the stimulation of extracellular-regulated kinases and the inhibition of adenylyl cyclase [10]. The ECS controls a variety of gastrointestinal (GI) functions: it is presumed to regulate GI motility via the enteric nervous system (ENS) [11], to inhibit the secretion of pro-inflammatory cytokines and to attenuate the lipopolysaccharide (LPS)-induced increase in GI transit [12]. For example, CB1 receptors are colocalized with acetylcholine transferase, which is a marker for cholinergic neurons. This observation supports the role of endocannabinoids as inhibitors of intestinal motility and secretion by inhibiting cholinergic neurotransmission [10]. For all these reasons, dysregulation of the ECS clearly plays a role in intestinal disorders, including irritable bowel disease (IBD), irritable bowel syndrome, and obesity [13].
Gut chemosensation represents the ability of the gut to sense chemical and nutrient stimuli at the GI level through the action of enteroendocrine cells (EEC) [14], and it appears to be connected to the presence of chemosensory receptors in the mouth and all along the GI tract [15]. This activity is mediated by a large variety of receptors, most of which are GPCRs, including TRP (Transient Receptor Potential) channels and olfactory receptors (ORs). Transient Receptor Potential channels comprise six related protein subfamilies [16] that include TRPV1, which was previously mentioned as one of the secondary endocannabinoid receptors, whereas ORs have a role in recognizing odorant molecules in the olfactory sensory system [17]. Botanicals such as thymol play a role in regulating the integrity of the intestinal mucosa because of their anti-inflammatory and antioxidant properties [18]. In particular, thymol is a monoterpene and it is a predominant component of several essential oils derived from plant species belonging to the Lamiaceae family. Thymol showed therapeutic potential in reducing oxidative stress, boosting the immune system and fighting against pathogenic bacteria [19], and it is also well known in animal nutrition as a key component of many botanical feed additives [20]. Therefore, the purposes of this study were 1) to investigate the presence of ECS and gut chemosensory markers in the GI tract of piglets and 2) to evaluate the possible modulation of these systems by treatment with a thymol supplement.
Results
Growth performance
Piglets maintained a good health status throughout the experiment and no mortality was recorded. During the experiment, differences in body weight (BW), feed intake (FI), average daily feed intake (ADFI), average daily gain (ADG), and feed to gain ratio (F:G) were not observed among the treatment groups (data not shown).
Endocannabinoid system
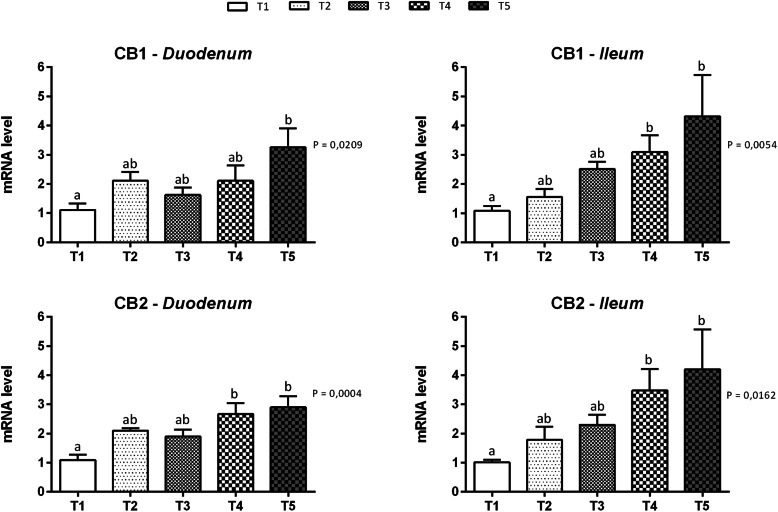
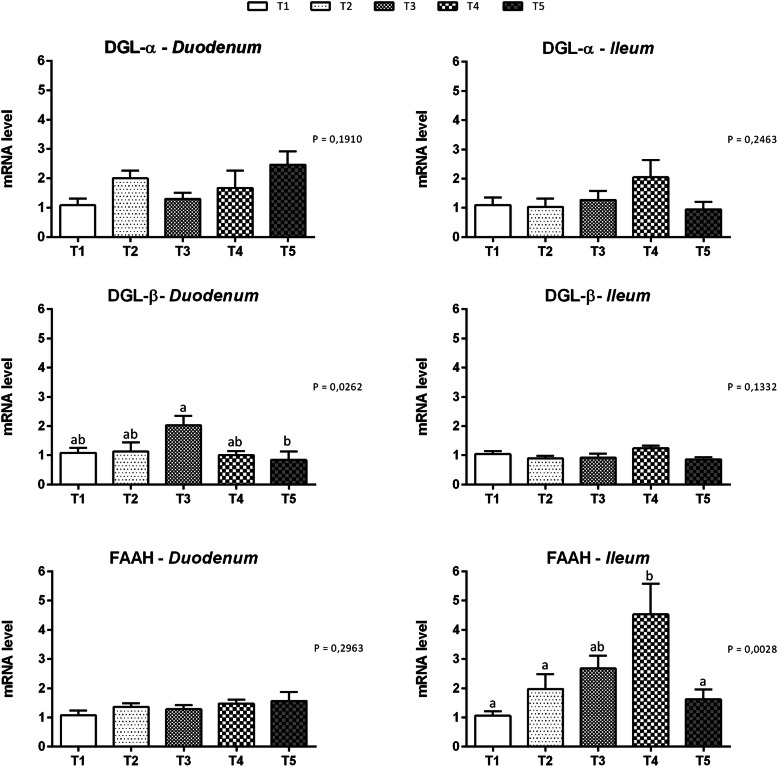
Figure 1 summarizes gene expression data for cannabinoid receptors in the duodenal and ileal mucosa at d14. Cannabinoid receptor 1 and 2 mRNAs were detected in both the duodenal and ileal mucosa. The level of the CB1 mRNA was significantly increased in the duodenum of the T5 group (P = 0.0209) and in the ileum of the T4 and T5 groups (P = 0.0054) compared to the control group. Significantly increased levels of the CB2 mRNA were detected in both the duodenum and ileum of groups T4 and T5 compared to the control group (P = 0.0004 and P = 0.0162 respectively). Data on gene expression for ECS enzymes are reported in Fig. 2. The presence of mRNA for all the enzymes tested was confirmed. The expression of the DGL-α mRNA in both duodenum and ileum was not affected by the treatments. The expression of the DGL-β mRNA was significantly increased in the duodenum of animals fed 51 mg of thymol/kg of feed (T3) compared to animals fed 510 mg of thymol/kg of feed (P = 0.0262). No significant differences were identified in the levels of the DGL-β mRNA in the ileal mucosa. Differences in the levels of the FAAH mRNA were not observed in the duodenum, while mRNA levels were significantly increased in the ileum of the T4 group compared to the control group (P = 0.0028).
Fig. 1.
Gene expression of cannabinoid receptors in duodenal and ileal mucosa of piglets. Data are expressed as means (n = 8) and S.E.M. represented by vertical bars. a, b Values with different superscripts differ significantly at P < 0.05. T1 = basal diet; T2 = basal diet + 25.5 mg of thymol/kg feed; T3 = basal diet + 51 mg of thymol/kg feed; T4 = basal diet + 153 mg of thymol/kg feed; T5 = basal diet + 510 mg of thymol/kg feed (Vetagro SpA, Reggio Emilia, Italy). A modification of the 2–ΔΔCT method was used to analyze the relative expression (fold changes), calculated relative to the control group (control; Livak and Schmittgen, 2001 [21]). CB1 = cannabinoid receptor 1; CB2 = cannabinoid receptor 2
Fig. 2.
Gene expression of endocannabinoid enzymes in duodenal and ileal mucosa of piglets. Data are expressed as means (n = 8) and S.E.M. represented by vertical bars. a, b Values with different superscripts differ significantly at P < 0.05. T1 = basal diet; T2 = basal diet + 25.5 mg of thymol/kg feed; T3 = basal diet + 51 mg of thymol/kg feed; T4 = basal diet + 153 mg of thymol/kg feed; T5 = basal diet + 510 mg of thymol/kg feed (Vetagro SpA, Reggio Emilia, Italy). A modification of the 2–ΔΔCT method was used to analyze the relative expression (fold changes), calculated relative to the control group (control; Livak and Schmittgen, 2001 [21]). DGL-α = diacylglycerol lipase alpha; DGL-β = diacylglycerol lipase beta; FAAH = fatty acid amide hydrolase
Gut chemosensing system
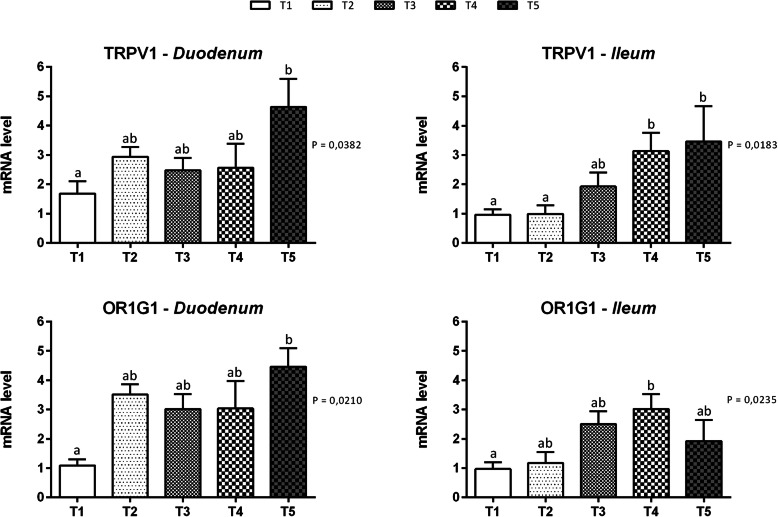
Results for the gut chemosensing are reported in Fig. 3. Concerning the gut chemosensing markers, both the TRPV1 and OR1G1 (Olfactory receptor 1G1) mRNAs were detected in the duodenal and ileal mucosa. Moreover, the supplementation of 510 mg of thymol/kg of feed increased the level of the TRPV1 mRNA in the duodenum (P = 0.0382), while increased mRNA levels were observed in the ileum of the T4 and T5 groups compared to the control group (P = 0.0183). The OR1G1 mRNA was expressed at higher levels in the duodenum of animals provided feed supplemented with 510 mg of thymol/kg of feed (T5) (P = 0.0210) and in the ileum of animals fed 153 mg of thymol/kg of feed (T4) (P = 0.0235) than in the control group.
Fig. 3.
Gene expression of chemosensory receptors in duodenal and ileal mucosa of piglets. Data are expressed as means (n = 8) and S.E.M. represented by vertical bars. a,b Values with different superscripts differ significantly at P < 0.05. T1 = basal diet; T2 = basal diet + 25.5 mg of thymol/kg feed; T3 = basal diet + 51 mg of thymol/kg feed; T4 = basal diet + 153 mg of thymol/kg feed; T5 = basal diet + 510 mg of thymol/kg feed (Vetagro SpA, Reggio Emilia, Italy). A modification of the 2–ΔΔCT method was used to analyze the relative expression (fold changes), calculated relative to the control group (control; Livak and Schmittgen, 2001 [21]). TRPV1 = transient receptor potential vanilloid 1; OR1G1 = Olfactory receptor 1G1
Inflammatory cytokines
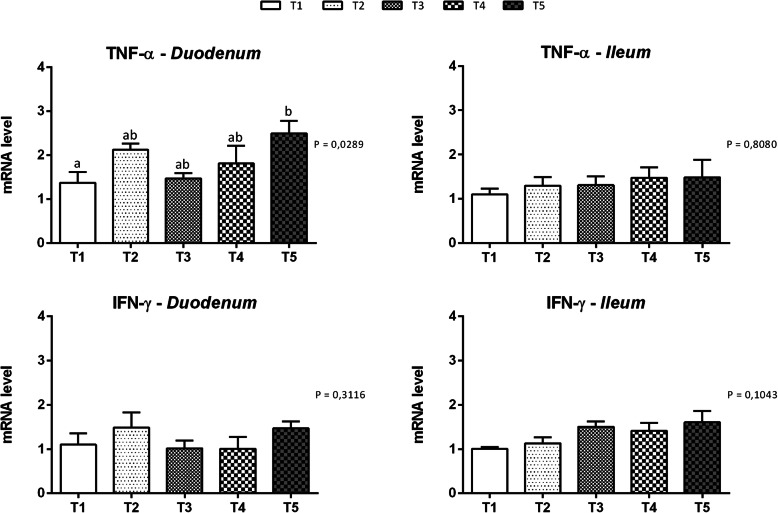
Figures 4 and 5 show the mRNA and protein levels of inflammatory cytokines in the duodenal and ileal mucosa at d14, respectively. Significantly increased levels of the Tumor necrosis factor (TNF)-α mRNA were detected in the duodenum of animals fed thymol; in particular, the T5 group displayed the highest expression of TNF-α (P = 0.0289). On the other hand, the level of the TNF-α mRNA in the ileal mucosa was not affected by the treatments. No difference was reported in interferon (IFN)-γ expression in both the duodenum and ileum. No statistically significant differences in the levels of these proteins were observed among the treatment groups (Fig. 5).
Fig. 4.
Gene expression of inflammatory cytokines in duodenal and ileal mucosa of piglets. Data are expressed as means (n = 8) and S.E.M. represented by vertical bars. a, b Values with different superscripts differ significantly at P < 0.05. T1 = basal diet; T2 = basal diet + 25.5 mg of thymol/kg feed; T3 = basal diet + 51 mg of thymol/kg feed; T4 = basal diet + 153 mg of thymol/kg feed; T5 = basal diet + 510 mg of thymol/kg feed (Vetagro SpA, Reggio Emilia, Italy). A modification of the 2–ΔΔCT method was used to analyze the relative expression (fold changes), calculated relative to the control group (control; Livak and Schmittgen, 2001 [21]). TNF-α = tumor necrosis factor-α; IFN-γ = interferon-γ
Fig. 5.
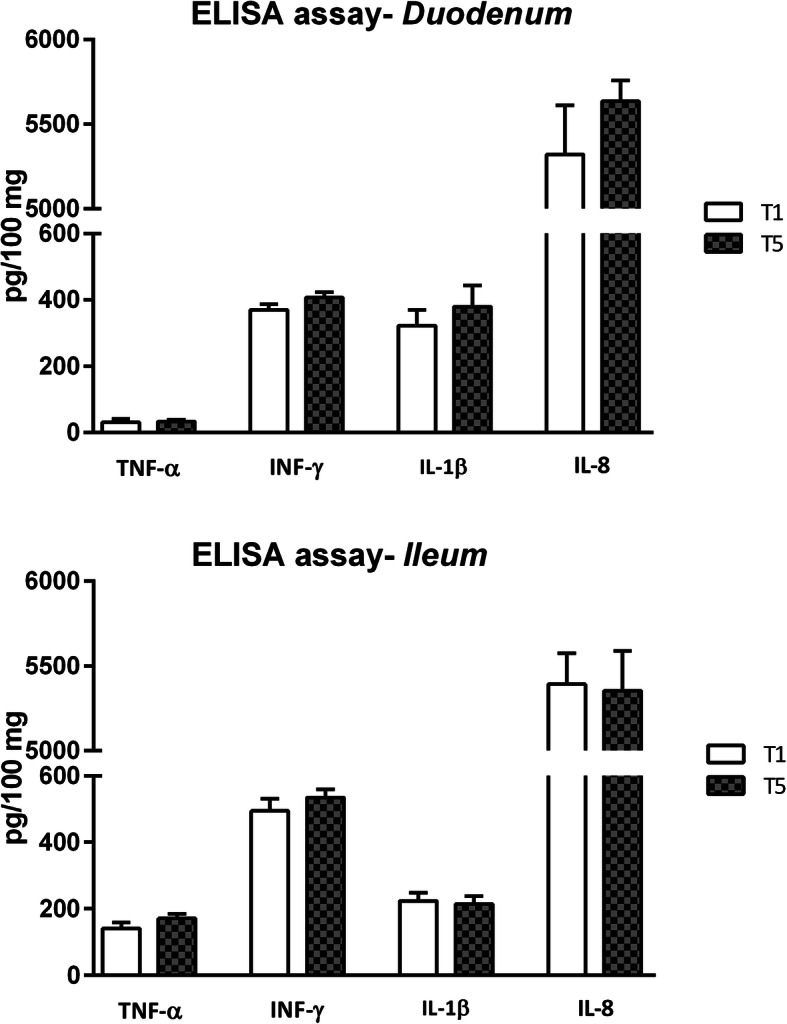
Protein expression of inflammatory cytokines in duodenal and ileal mucosa of piglets. Data are expressed as means (n = 8) and S.E.M. represented by vertical bars. Data refer to picograms of cytokine per 100 mg of tissue (pg/100 mg). T1 = basal diet; T5 = basal diet supplemented with 510 mg of thymol / kg feed (Vetagro SpA, Reggio Emilia, Italy). TNF-α = tumor necrosis factor-α; IFN-γ = interferon-γ; IL-1β = interleukin-1β; IL-8 = interleukin-8
Discussion
Over the last 50 years, the ability of the ECS to modulate the inflammatory status has been investigated in human medicine, due to the pharmacological potential of cannabinoid therapy to treat clinical conditions such as IBD, Crohn’s disease and ulcerative colitis [22]. Similarly, the gut chemosensory system also shows potential as a target for IBD, gluten sensitivity, and obesity [23]. In this paper, we focused our attention on the presence of ECS and gut chemosensing markers in the duodenal and ileal mucosa of piglets, with a particular focus on both the receptors, namely, CB1, CB2, TRPV1 and OR1G1, and the main enzymes involved in the synthesis and degradation of AEA and 2-AG by performing a molecular analysis. To our knowledge, this paper is the first to describe the alleged roles of the ECS and gut chemosensing system on the intestinal functionality of swine and their possible modulation by thymol.
Interesting results were obtained from the analysis of gene expression of ECS markers, as we detected mRNA for all of these markers. Since it was first described in the central nervous system, the ECS is now proposed to regulate different physiological mechanisms. More information is now available about this signaling system, but little evidence is available for its role in the organism. Recently, an increasing number of papers examining the role of the ECS in various pathologies has been published, but the possible roles of ECS in animal medicine, production and nutrition have not been investigated in depth. Notably, the activation of CB1 receptors is involved in inflammation and cell death in different experimental models of disease [22], but evidence for a possible role of nutrition in modulating this system has not been available until now. Interestingly, we detected the CB1 and CB2 mRNAs in mucosa scrapings, confirming the presence of these receptors in the mucosa of piglets and suggesting that these receptors are not exclusively localized in the ENS. The classically recognized location of the endocannabinoid receptors is in the ENS, and the only report analyzing their presence on the mucosa was performed in mice. In mouse, Sykaras and colleagues [24] described the presence of the CB1 mRNA in the EEC, where it is suggested to drive the intake of fat-rich foods by inhibiting the release of cholecystokinin (CCK), which normally induces satiation after meals. In addition, CB1 activation inhibits acetylcholine (Ach) and is accompanied by a reduction in GI muscle contraction, resulting in decreased intragastric pressure and the inhibition of gastric emptying, pyloric contraction, and intestinal motility [11].
Cannabinoid receptor 1 also regulates food intake in rats, particularly during starvation periods, by increasing 2-AG synthesis through the activity of DGL [25, 26], suggesting a possible role during weaning. Thymol appears to modulate the expression of these receptors and enzymes. The trend in the use of botanicals in animal feed has increased during the last two decades. Thymol is one of the most well-studied and frequently used molecules in animal nutrition due to its countless beneficial properties [27]. Considering the increase in the levels of the CB1 and CB2 mRNAs in both the duodenum and ileum, we postulated that thymol stimulates the synthesis of the receptors in the GI tract. Cannabinoid receptor 1 and cannabinoid receptor 2 are recognized to play a protective role in IBD, namely, by functioning to control the inflammatory status and are normally present during weaning and starvation [28]. Therefore, the ECS may play a role in the response to stressful situations, such as weaning for piglets, and thymol potentially exerts a positive effect by stimulating the expression of endocannabinoid receptors. Unfortunately, no studies examining this topic have been published to date, but the use of thymol may plausibly support piglets during weaning by activating the ECS.
Additionally, levels of the DGL and FAAH mRNAs were increased; as mentioned above, DGL is involved in the synthesis of 2-AG, while FAAH is responsible for AEA degradation. According to Bashashati and colleagues [29], the inhibition of the degradation of 2-AG decreased whole-gut transit in mice; conversely, Izzo and colleagues [30] concluded that a decrease in the small intestinal content of AEA leads to an increase in transit, suggesting that 2-AG modulates CB1-controlled gut contractility. Thymol may also play a role in modulating gut contractility by regulating enzyme expression and, consequently, the concentrations of the bioactive lipids, resulting in the modulation of gut contractility.
Other interesting data include the detection of mRNAs encoding receptors involved in the gut chemosensory system. Again, we not only detected the TRPV1 and OR1G1 mRNAs in piglet mucosa scrapings but also observed increased levels of the TRPV1 and OR1G1 mRNAs when the diet was supplemented with thymol. The colonic mucosa of both humans and rats express OR mRNAs, and luminal odorants induce serotonin secretion in isolated duodenal enterochromaffin cells and enterochromaffin cell lines [31]. Moreover, the activation of OR1G1 by luminal thymol increases [Ca2+]i. The elevated [Ca2+]i may modulate Ca2+-activated basolateral K+ channels, providing a driving force for the exit of Cl−/HCO3− [32]. As result, thymol activates certain types of the apical odorant receptor OR1G1 and stimulates Cl− secretion in colonic epithelial cells. Recently, human OR1G1 was reported to participate in glucose homeostasis during meal ingestion by inducing gut peptide secretion [33]. A reasonable conclusion is that OR1G1 may play a role in controlling intestinal permeability and nutrient absorption, although only a few studies examining the role of this OR are available, and, moreover, its presence in the swine GI tract is not well documented. Considering that in this study the mRNA of OR1G1 was detected in both duodenum and ileum, it is clear that this receptor, and probably other receptors of this class, are also present in the pig GI tract.
Thymol has also been linked to the immune system due to its anti-inflammatory and antioxidant properties [19]. Omonijo et al. [34] reported a reduction in LPS-induced ROS (reactive oxygen species) and TNF-α levels in cells pretreated with thymol. In the present study, piglets fed microencapsulated thymol exhibited increased levels of the TNF-α mRNA; this result might appear surprising because this cytokine has a pro-inflammatory function, but no adverse effects on the piglets’ health were observed. Moreover, the level of the TNF-α protein was not altered by the treatments. Interestingly, at the dose where we observed an increase in the level of the TNF-α mRNA, we also registered an increase in the expression of the CB1 and CB2 mRNAs. The cannabinoid receptors act on TNF-α in two opposite directions. Stimulation of CB1 reduces TNF-α release from activated microglia [35]; conversely, CB2 is known to induce the expression of pro-inflammatory cytokines to promote a TH1 immune response [36], which may explain the increase in the gene expression of this cytokine. Finally, thymol increases the levels of the CB1 and CB2 mRNAs, which controls the release and the expression of TNF-α, respectively, providing an explanation for the regulation of the protein and mRNA levels of this pro-inflammatory cytokine. Moreover, the absence of variations in terms of protein levels of inflammatory cytokines obtained with the ELISA assay is not unexpected, considering that all the piglets were healthy and, without a specific challenge or stressors, pro-inflammatory and anti-inflammatory cytokines are normally in equilibrium [37].
Concerning the zootechnical performance, in this study the administration of thymol to weaned piglets at different levels of inclusion did not modify any parameters. Thymol was well tolerated by the animals also at the higher level of inclusion (10x the highest recommended dose in T5 group). This is an interesting result, mainly regarding FI and ADG, which are normally reduced when thymol is supplemented at a high dosage [38–40]. This high tolerability of thymol could be explained thanks to the microencapsulation of the terpene in a lipid matrix. It is well documented that encapsulation technologies are useful not only to deliver the bioactive compound along all the GI tract [41–43], but also to increase the palatability of some pungent molecules, such as thymol [44].
Despite the increasing number of studies analyzing the roles of ECS and chemosensation in the organism, additional studies are required to understand how endocannabinoid receptors and molecules control various pathologies and inflammation. Nevertheless, these results represent a first insight into the potential uses of botanical feed additives to control intestinal inflammation and motility. Further investigations are required to obtain a better understanding of the protein expression and localization of these markers, together with the possible mechanism by which thymol modulates the expression of endocannabinoid markers.
Conclusions
In conclusion, our data not only confirm the presence of the ECS and gut chemosensing markers in the duodenal and ileal mucosa of piglets but also suggest that thymol modulates the gene expression of these markers. Thymol increases the expression of the mRNAs encoding the CB1 and CB2 receptors both in the duodenum and ileum. This compound also modulates the mRNA levels of enzymes involved in the biosynthesis and degradation of endocannabinoid molecules. Moreover, the upregulation of OR1G1 and TRPV1 by thymol throughout the intestine implicates a possible role for these receptors as mediators of the effects of thymol as a feed additive on promoting gut health.
Methods
Ethics statement
The study was conducted at the facilities of the Research Center for Animal Production and Environment (CERZOO), which is Good Laboratory Practices-certified and operates according to the Procedure of Animal Protection and Welfare (Directive No 86/609/EEC). Animals used in the study were raised and treated according to European Union Directive 2010/63/EU. The study was authorized by Italian Health Ministry according to art. Thirty-one of the Italian Legislative Decree No. 26/2014 and to the recommendation of Commission 2007/526/CE, covering the accommodation and care of animals used for experimental and other scientific purposes (authorization n. 341/2017-PR issued on May 3, 2017). Animals were obtained from a breeding farm in Cascina Mandellina, Bergamo, Italy.
Animals and diets
One hundred sixty piglets (Duroc × Large White) weaned at 28 days of age and with a BW of 7.71 ± 1.00 kg were divided into 40 pens (4 piglets per pen, castrated males and females were blocked in separate pens) and randomly assigned to one of the following experimental groups (n = 32): control group fed the basal diet (T1), a group fed the basal diet supplemented with 25.5 mg of thymol/kg feed (T2), a group fed the basal diet supplemented with 51 mg of thymol/kg feed (T3), a group fed the basal diet supplemented with 153 mg of thymol/kg feed (T4) and a group fed the basal diet supplemented with 510 mg of thymol/kg feed (T5). Thymol was provided in a form microencapsulated in a lipid matrix (Vetagro SpA, Reggio Emilia, Italy). Concentrations of thymol were selected to meet or exceed the upper limit of inclusion in food and feed established by the European Agency for the Evaluation of Medicinal Products [45, 46]. The basal feed was formulated to meet or exceed the nutritional requirements of pigs according to National Research Council [47], and feed and water were provided ad libitum (the composition of the basal diet is reported in Table 1). The health status of animals was monitored throughout the study. Piglets were individually weighed at the beginning (day 0) and end of the study (day 14). Growth parameters, such as FI, ADFI, ADG, and F:G, were measured in the animals housed in each pen on d14 of the experiment.
Table 1.
Basal diet composition (%) and nutrient profile
| Ingredient (% of dry matter) | |
| Corn meal | 59.25 |
| Soybean meal, 44% | 21.90 |
| Sweet milk whey | 8.00 |
| Fish meal (Herring 999) | 7.00 |
| Soybean oil | 1.98 |
| Calcium carbonate | 0.35 |
| Vitamin and mineral premix1 | 0.25 |
| L-Lysine HCl | 0.54 |
| NaCl | 0.16 |
| L-Threonine | 0.24 |
| DL-Methionine | 0.26 |
| L-Tryptophan | 0.08 |
| Total | 100 |
| Calculated nutrient composition | |
| Digestible Energy2 (kcal/kg feed) | 3523 |
| Net Energy2–3 (kcal/kg feed) | 2549 |
| Dry matter (%) | 88.53 |
| Crude protein (%) | 21.00 |
| Crude fiber (%) | 2.64 |
| Crude fat (%) | 5.32 |
| Calcium (g/kg) | 7.00 |
| Phosphorus – Total (g/kg) | 5.60 |
| Phosphorus – Available (g/kg) | 1.45 |
| Sodium (g/kg) | 1.80 |
1Content of vitamins and Oligo minerals/kg feed: vit. A: 10,000. UI; vit. D3: 1000UI; vit. E: 100 mg; vit. B1: 3.0 mg; vit. B2: 10.0 mg; vit. B6:5.8 mg; vit. B12: 0.04 mg; Biotin: 0.19 mg; vit. K: 4.8 mg; vit. PP: 35.0 mg; folic acid: 1.4 mg; D-pantothenic acid: 26.1 mg; choline chloride: 120 mg; 49.7 mg Mn from Manganese oxide; 224 mg Fe from Ferrous carbonate; 75.8 mg Cu from Copper sulphate pentahydrate; 139 mg Zn from Zinc oxide; 0.89 mg I from Calcium iodide; 0.64 mg Se from Disodium selenite
2Aritmetic mean
3Net Energy was calculated according to the procedure and equation proposed by Noblet and colleagues [48]
At the end of the study, 8 animals from each treatment group were selected for sacrifice, sample collection, and analysis. Piglets were euthanized by a penetrating captive bolt followed by exsanguination. Duodenal and ileal mucosal scrapings were collected. The duodenum and ileum were longitudinally cut to expose the mucosa, washed with a phosphate-buffered saline solution to remove mucus and digesta, then scraped gently with a glass slide, packed, immediately frozen in liquid N2 and stored at − 80 °C until the analyses of gene and protein expression.
Gene expression analysis
Gene expression was analyzed using the method reported by Herfel et al. [49]. Duodenal and ileal scraping samples obtained on d14 of the study were disrupted by grinding them in liquid nitrogen with mortar and pestle, and then homogenized using a TissueLyser (Qiagen, Hilden, Germany). Total RNA was isolated using a NucleoSpin® RNA Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. Genomic DNA contamination was removed by treating the samples with the deoxyribonuclease supplied in the extraction kit (rDNase, RNase-free; Macherey-Nagel). The RNA yield and quality were determined spectrophotometrically by measuring the absorbance at 260 and 280 nm (A260 and A280 nm, respectively) (Microvolume Mode with SmartPath® Technology, Denovix). One microgram of RNA was reverse transcribed with the iScript cDNA Synthesis Kit (Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the manufacturer’s instructions. Real-time PCR was performed using an iCycler Thermal Cycler system and SybrGreen Supermix (Bio-Rad Laboratories Inc.). The thermocycling protocol included an initial denaturation step for 1 min and 30 s at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s and 30 s of annealing and extension at 60 °C. After amplification, a melting curve analysis was performed for all the samples, with slow heating from 55 °C to 95 °C at a rate of 0.5 °C/s to validate the absence of non-specific products. Gene expression was normalized to a housekeeping gene (HK) encoding portions of porcine ribosomal subunit 60 S, in particular ribosomal protein L35 (RPL35). The average threshold cycle (CT) was determined for each gene of interest, and the geometric average was calculated for HK by assuming that CT is the number of cycles needed to reach a fixed arbitrary threshold. Delta CT was calculated, then a modification of the 2–ΔΔCT method [21] was used to analyze the relative expression (fold changes), which was calculated relative to the control group. The sequences, accession numbers in the EMBL database/GenBank, expected product lengths and references for porcine primers are provided in Table 2. Primer oligonucleotides for CB1, DGL-β and OR1G1 were designed using the Primer-BLAST tool (NCBI National Center for Biotechnology Information, www.ncbi.nlm.nih.gov). Primers were obtained from Life Technologies (Life Technologies Italia).
Table 2.
Primer sequence used for gene expression analysis
| Gene | Primer sequence (F and R) 5′ → 3′ | Product length (bp) | Accession N. | Reference |
|---|---|---|---|---|
| CB1 | F: TTCCCCACTTCTTTTCCGCC R: GGGAGTCCCTTCGCATCC | 208 | XM_013992672.2 | Present study |
| CB2 | F: TTTATAGCCTGGCCTCCCCT R: TTTTCCCGTCTGCCTCTGTC | 240 | XM_021095530.1 | [50] |
| FAAH | F: TGCCACCGTGCAAGAAAATG R: CCACTGCCCTAACAACGACT | 234 | XM_013999418.2 | [50] |
| DGL-α | F: GAAACCAAACACGCCTCCAC R: CAACCCAGCAGCAAAGGAAC | 211 | XM_021082924.1 | [50] |
| DGL-β | F: TTTGTAATCCCGGACCACGG R: GACCTGCCGAGGAATACGGA | 255 | XM_021086077.1 | Present study |
| TRPV1 | F: TCACCAACAAGAAGGGGCTC R: GGATAGGTGCCTGCACTCAG | 116 | XM_005669121.1 | [51] |
| OR1G1 | F: CTTGGTTTGTGTGCTCTGCC R: GAAAAGGCTTTCCGCTTCCC | 96 | XM_013990010.1 | Present study |
| INF-γ | F: GGCCATTCAAAGGAGCATGGATGT R: TGAGTTCACTGATGGCTTTGCGCT | 149 | NM_213948.1 | [52] |
| TNF-α | F: GCCCACGTTGTAGCCAATGTCAAA R: GTTGTCTTTCAGCTTCACGCCGTT | 99 | NM_214022.1 | [52] |
| RPL35 | F: AACCAGACCCAGAAAGAGAAC R: TTCCGCTGCTGCTTCTTG | 146 | NM_214326.2 | [53] |
F forward, R reverse, CB1 cannabinoid receptor 1, CB2 cannabinoid receptor 2, FAAH fatty acid amide hydrolase, DGL-α diacylglycerol lipase alpha, DGL-β diacylglycerol lipase beta, TRPV1 transient receptor potential vanilloid 1, OR1G1 olfactory receptor 1G1, IFN-γ interferon-γ, TNF-α tumor necrosis factor-α, RPL35 ribosomal protein L35.
ELISA quantification of inflammatory cytokine concentrations
Duodenal and ileal mucosal scrapings were disrupted by grinding the samples in liquid nitrogen with a mortar and pestle, followed by the addition of lysis buffer (10 mM-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)-HCl, 1 mM-ethylenediaminetetraacetic acid (EDTA), and 0.5%-Triton X100) and homogenization using a TissueLyser (Qiagen). Protein levels of inflammatory cytokines (TNF-α, INF-γ, IL-1β, and IL-8) were analyzed using ELISA kits specific for porcine cytokines (Quantikine ELISA, R&D Systems Inc., Minneapolis, MN, USA). Analyses were performed according to the manufacturer’s instructions. ELISA quantification was performed for samples obtained from the T1 and T5 groups. Results are reported as picograms of cytokine per 100 mg of tissue (pg/100 mg).
Statistical analysis
Animals were blocked in a completely randomized design and data were analyzed using GraphPad Prism® software (GraphPad Software, Inc., La Jolla, CA, USA). Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test to detect differences among treatments. The pen was the experimental unit for growth performance, whereas the pig was the experimental unit for gene expression and ELISA data. Differences were considered significant at P < 0.05, and trends were defined at 0.05 ⩽ P < 0.1.
Acknowledgments
Not applicable.
Abbreviations
- ECS
Endocannabinoid System
- GPCRs
G protein-coupled receptors (GPCRs)
- CB1
Cannabinoid receptor 1
- CB2
Cannabinoid receptor 2
- TRPV1
Transient receptor potential vanilloid 1
- AEA
Anandamide
- 2-AG
2-arachidonoylglycerol
- NAPE-PLD
N-Acylphosphatidylethanolamine-specific Phospholipase D
- DGL
Diacylglicerol Lipase
- FAAH
Fatty Acid Amide Hydrolase
- MAGL
Monoacylglicerol Lipase
- GI
Gastrointestinal
- ENS
Enteric Nervous System
- LPS
Lipopolysaccharide
- IBD
Irritable Bowel Disease
- EEC
Enteroendocrine Cells
- TRP
Transient Receptor Potential
- ORs
Olfactory Receptors
- BW
Body Weight
- FI
Feed Intake
- ADFI
Average Daily Feed Intake
- ADG
Average Daily Gain
- F: G
Feed to Gain ratio
- OR1G1
Olfactory receptor 1G1
- TNF-α
Tumor Necrosis Factor-α
- IFN-γ
Interferon-γ
- CCK
Cholecystokinin
- Ach
Acetylcholine
- ROS
Reactive Oxygen Species
- HK
Housekeeping
- CT
Threshold cycle
- RPL35
Ribosomal protein L35
- EDTA
Ethylenediaminetetraacetic acid
Authors’ contributions
The authors equally contributed to the project.
A. T., B. T., B. R., A. P. and E. G. equally contributed to the conception of the paper, performed the analysis, and drafted the manuscript. A. P. serves on the Board of Directors of Vetagro. E. G. serves as an advisor of Vetagro. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from Vetagro, Reggio Emilia, Italy. Funder contributed in the study design and in providing the test product.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The sequences used for the primer design are available in the Genbank repository (https://www.ncbi.nlm.nih.gov/genbank/).
All accession numbers are listed in Table 2.
Ethics approval and consent to participate
The study was authorized by Italian Health Ministry according to European Union Directive 2010/63/EU, adopted by Italian legislation with art. Thirty-one of the Italian Legislative Decree No. 26/2014 and to the recommendation of Commission 2007/526/CE, covering the accommodation and care of animals used for experimental and other scientific purposes (authorization n. 341/2017-PR issued on May 3, 2017). Animals were obtained from a breeding farm in Cascina Mandellina, Bergamo, Italy.
Consent for publication
Not applicable.
Competing interests
Andrea Piva serves as professor at the University of Bologna and is a member of the board of directors of Vetagro SpA (Reggio Emilia, Italy) which funded the project. Ester Grilli serves as an advisor of Vetagro.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20:10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 3.Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mol Neurosci. 2019;11. 10.3389/fnmol.2018.00487. [DOI] [PMC free article] [PubMed]
- 4.Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamoto Y, Tsuboi K, Ueda N. Chapter 1 enzymatic formation of Anandamide. In: Vitamins & Hormones: Academic Press; 2009. p. 1–24. 10.1016/S0083-6729(09)81001-7.. [DOI] [PubMed]
- 6.Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/S0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- 8.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 9.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 10.Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med. 2005;83:944–954. doi: 10.1007/s00109-005-0698-5. [DOI] [PubMed] [Google Scholar]
- 11.Vera G, Fichna J, Abalo R. Chapter 98 - cannabinoids and effects on the gastrointestinal tract: a focus on motility. In: Preedy VR, editor. Handbook of Cannabis and related pathologies. San Diego: Academic Press; 2017. pp. 947–957. [Google Scholar]
- 12.Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 13.Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126:21–38. doi: 10.1016/j.pharmthera.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Bertrand PP. The cornucopia of intestinal chemosensory transduction. Front Neurosci. 2009;3. 10.3389/neuro.21.003.2009. [DOI] [PMC free article] [PubMed]
- 15.Höfer D, Asan E, Drenckhahn D. Chemosensory perception in the gut. Physiology. 1999;14:18–23. doi: 10.1152/physiologyonline.1999.14.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi N, Kozai D, Mori Y. TRP channels: sensors and transducers of gasotransmitter signals. Front Physiol. 2012;3. 10.3389/fphys.2012.00324. [DOI] [PMC free article] [PubMed]
- 17.Touhara K. Olfactory receptors. In: Squire LR, editor. Encyclopedia of neuroscience. Oxford: Academic Press; 2009. pp. 163–169. [Google Scholar]
- 18.Du E, Wang W, Gan L, Li Z, Guo S, Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J Anim Sci Biotechnol. 2016;7. 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed]
- 19.Nagoor Meeran MF, Javed H, Al Taee H, Azimullah S, Ojha SK. Pharmacological properties and molecular mechanisms of Thymol: prospects for its therapeutic potential and pharmaceutical development. Front Pharmacol. 2017;8. 10.3389/fphar.2017.00380. [DOI] [PMC free article] [PubMed]
- 20.Rossi B, Toschi A, Piva A, Grilli E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr Res Rev. 2020:1–17. [DOI] [PubMed]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Ambrose T, Simmons A. Cannabis, cannabinoids, and the Endocannabinoid system—is there therapeutic potential for inflammatory bowel disease? J Crohn’s Colitis. 2019;13:525–535. doi: 10.1093/ecco-jcc/jjy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut. 2014;63:179–190. doi: 10.1136/gutjnl-2013-305112. [DOI] [PubMed] [Google Scholar]
- 24.Sykaras AG, Demenis C, Case RM, McLaughlin JT, Smith CP. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS One. 2012;7:e42373. doi: 10.1371/journal.pone.0042373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci U S A. 2011;108:12904–12908. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiPatrizio NV, Igarashi M, Narayanaswami V, Murray C, Gancayco J, Russell A, et al. Fasting stimulates 2-AG biosynthesis in the small intestine: role of cholinergic pathways. American journal of physiology. Regul Integr Comp Physiol. 2015;309:R805–R813. doi: 10.1152/ajpregu.00239.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abd El-Hack ME, Alagawany M, Ragab Farag M, Tiwari R, Karthik K, Dhama K, et al. Beneficial impacts of thymol essential oil on health and production of animals, fish and poultry: a review. J Essent Oil Res. 2016;28:365–382. doi: 10.1080/10412905.2016.1153002. [DOI] [Google Scholar]
- 28.DiPatrizio NV. Endocannabinoids in the gut. Cann Cannabinoid Res. 2016;1:67–77. doi: 10.1089/can.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bashashati M, Nasser Y, Keenan CM, Ho W, Piscitelli F, Nalli M, et al. Inhibiting endocannabinoid biosynthesis: a novel approach to the treatment of constipation. Br J Pharmacol. 2015;172:3099–3111. doi: 10.1111/bph.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, et al. Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol. 2009;158:451–461. doi: 10.1111/j.1476-5381.2009.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 32.Kuwahara A. Involvement of the gut chemosensory system in the regulation of colonic anion secretion. Biomed Res Int. 2015. 10.1155/2015/403919. [DOI] [PMC free article] [PubMed]
- 33.Oh SJ. System-wide expression and function of olfactory receptors in mammals. Genom Inform. 2018;16:2–9. doi: 10.5808/GI.2018.16.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omonijo FA, Ni L, Gong J, Wang Q, Lahaye L, Yang C. Essential oils as alternatives to antibiotics in swine production. Anim Nutr. 2018;4:126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi S, Furlan R, Chiara VD, Muzio L, Musella A, Motta C, et al. Cannabinoid CB1 receptors regulate neuronal TNF-α effects in experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;25:1242–1248. doi: 10.1016/j.bbi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Gertsch J. Antiinflammatory cannabinoids in diet – towards a better understanding of CB2 receptor action?: towards a better understanding of CB 2 receptor action? Commun Integr Biol. 2008;1:26–28. doi: 10.4161/cib.1.1.6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cicchese JM, Evans S, Hult C, Joslyn LR, Wessler T, Millar JA, et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev. 2018;285:147–167. doi: 10.1111/imr.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trevisi P, Merialdi G, Mazzoni M, Casini L, Tittarelli C, Filippi SD, et al. Effect of dietary addition of thymol on growth, salivary and gastric function, immune response, and excretion of salmonella enterica serovar Typhimurium, in weaning pigs challenged with this microbe strain. Ital J Anim Sci. 2007;6:374–376. doi: 10.4081/ijas.2007.1s.374. [DOI] [Google Scholar]
- 39.Jugl-Chizzola M, Ungerhofer E, Gabler C, Hagmüller W, Chizzola R, Zitterl-Eglseer K, et al. Testing of the palatability of Thymus vulgaris L. and Origanum vulgare L. as flavouring feed additive for weaner pigs on the basis of a choice experiment. Berlin Munch Tierarztl Wochenschr. 2006;119:238–243. [PubMed] [Google Scholar]
- 40.Michiels J, Missotten J, Hoorick AV, Ovyn A, Fremaut D, Smet SD, et al. Effects of dose and formulation of carvacrol and thymol on bacteria and some functional traits of the gut in piglets after weaning. Arch Anim Nutr. 2010;64:136–154. doi: 10.1080/17450390903499915. [DOI] [PubMed] [Google Scholar]
- 41.Hébert CD, Yuan J, Dieter MP. Comparison of the toxicity of cinnamaldehyde when administered by microencapsulation in feed or by corn oil gavage. Food Chem Toxicol. 1994;32:1107–1115. doi: 10.1016/0278-6915(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 42.Petrujkić BT, Sedej I, Beier RC, Anderson RC, Harvey RB, Epps SVR, et al. Ex vivo absorption of thymol and thymol-β-D-glucopyranoside in piglet everted jejunal segments. J Agric Food Chem. 2013;61:3757–3762. doi: 10.1021/jf401013a. [DOI] [PubMed] [Google Scholar]
- 43.Piva A, Pizzamiglio V, Morlacchini M, Tedeschi M, Piva G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. J Anim Sci. 2007;85:486–493. doi: 10.2527/jas.2006-323. [DOI] [PubMed] [Google Scholar]
- 44.Hultquist KM, Casper DP. Palatability evaluation of free fatty acid encapsulated potassium carbonate as a feed ingredient for lactating dairy cows fed a total mixed ration. Prof Anim Sci. 2016;32:328–332. doi: 10.15232/pas.2015-01485. [DOI] [Google Scholar]
- 45.EMA (European Agency for the Evaluation of Medicinal Products. Committee for Veterinary Medicinal Product. Thymol. 1996. http://www.ema.europa.eu/pdfs/vet/mrls/007596en.pdf.
- 46.EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Scientific Opinion on the safety and efficacy of phenol derivatives containing ring-alkyl, ring-alkoxy and side-chains with an oxygenated functional group (chemical group 25) when used as flavourings for all species: Phenol derivatives (CG 25) EFSA J. 2012;10:2573. doi: 10.2903/j.efsa.2012.2573. [DOI] [Google Scholar]
- 47.National Research Council. Nutrient requirements of swine. Revised edition. National Academies Press; 2012.
- 48.Noblet J, Fortune H, Shi XS, Dubois S. Prediction of net energy value of feeds for growing pigs. J Anim Sci. 1994;72:344–354. doi: 10.2527/1994.722344x. [DOI] [PubMed] [Google Scholar]
- 49.Herfel TM, Jacobi SK, Lin X, Fellner V, Walker DC, Jouni ZE, et al. Polydextrose enrichment of infant formula demonstrates prebiotic characteristics by altering intestinal microbiota, organic acid concentrations, and cytokine expression in suckling piglets. J Nutr. 2011;141:2139–2145. doi: 10.3945/jn.111.143727. [DOI] [PubMed] [Google Scholar]
- 50.Sampaio LS, Silva RTD, Lima D, Sampaio CLC, Iannotti FA, Mazzarella E, et al. The endocannabinoid system in renal cells: regulation of Na+ transport by CB1 receptors through distinct cell signalling pathways. Br J Pharmacol. 2015;172:4615–4625. doi: 10.1111/bph.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown JD, Saeed M, Do L, Braz J, Basbaum AI, Iadarola MJ, et al. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Sci Transl Med. 2015;7:305ra145. doi: 10.1126/scitranslmed.aac6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grilli E, Tugnoli B, Passey JL, Stahl CH, Piva A, Moeser AJ. Impact of dietary organic acids and botanicals on intestinal integrity and inflammation in weaned pigs. BMC Vet Res. 2015;11. 10.1186/s12917-015-0410-0. [DOI] [PMC free article] [PubMed]
- 53.Alexander LS, Seabolt BS, Rhoads RP, Stahl CH. Neonatal phosphate nutrition alters in vivo and in vitro satellite cell activity in pigs. Nutrients. 2012;4:436–448. doi: 10.3390/nu4060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The sequences used for the primer design are available in the Genbank repository (https://www.ncbi.nlm.nih.gov/genbank/).
All accession numbers are listed in Table 2.