Abstract
Background
Taurine upregulated gene 1 (TUG1) has been recognized as a novel oncogenic gene. The current study was established to explore the function and regulatory mechanism of TUG1 in hepatocellular carcinoma (HCC).
Methods
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the expression of TUG1, miR-29c-3p, and COL1A1 in tissues and cell lines. MTT assay, wound-healing and transwell assay were utilized for the detection of cell viability, migration and invasion, respectively. The interactions between miR-29c-3p and TUG1/COL1A1 were predicted by starBase v2.0 (http://starbase.sysu.edu.cn/) and verified by the dual-luciferase reporter or RNA immunoprecipitation assay. Western blot assay was performed to determine the protein levels of COL1A1, cyclin D1, E-cadherin, N-cadherin, Bcl-2, and Bax.
Results
Dramatically increased expression of TUG1 was noticed in HCC tissues and cell lines. TUG1 knockdown restrained the proliferation, migration, and invasion, and promoted the apoptosis of HCC cells. TUG1 targeted miR-29c-3p and inhibited miR-29c-3p expression. Overexpression of miR-29c-3p inhibited the proliferation, migration and invasion of HCC cells. MiR-29c-3p directly targeted COL1A1 and down-regulated COL1A1 expression. In addition, downregulation of miR-29c-3p and upregulation of COL1A1 both reversed the effects of TUG1 knockdown on the proliferation, apoptosis, migration, and invasion of HCC cells.
Conclusion
TUG1 could promote the proliferation, migration and invasion of HCC cells through regulating miR-29c-3p/COL1A1 axis. This novel finding might provide a latent target for the treatment of HCC.
Keywords: TUG1, miR-29c-3p, miR-29c-3p/COL1A1, hepatocellular carcinoma
Introduction
As a common lethal malignant neoplasm, hepatocellular carcinoma (HCC) ranks the third cancer-related mortality all over the world.1 Although great progress has been made in the treatment of HCC using hepatic resection, liver transplantation, and radiofrequency ablation in recent years, the long-term prognosis remains disappointing and the 5-year overall survival is only about 30%.2 Therefore, it is urgent to have a deep understanding of the potential mechanisms involving the occurrence and development of HCC, and develop novel therapeutic targets for HCC.3
Long non-coding RNAs (lncRNAs) are a kind of RNAs with no protein-coding ability at more than 200 nucleotides in length.4 Some lncRNAs have been proved by previous studies to play a critical role in tumorigenesis and metastasis of HCC, such as DBH-AS1,5 MCM3AP-AS16 and CARL.7 LncRNAs may act as competitive endogenous RNA (CeRNA) through sponging miRNAs, thus regulating miRNA expression in cells.8 MiRNAs are an abundant class of highly conserved small non-coding RNAs (ncRNAs) at approximately 20–25 nucleotides in length. MiRNAs can regulate gene expression post-transcriptionally via precisely interacting with the 3ʹ-untranslated regions of mRNAs.9 Intensive researches have proved that miRNAs have inhibitory effects in a variety of malignancies.10–12 Some miRNAs are implicated in the occurrence, growth, and metastasis of HCC, which are considered as prognostic factors for HCC,13 such as miR-449a,14 miR-1296,15 miR-300,16 and miR-874.17 Recently, upregulation of miR-29c-3p has been reported to reverse the epithelial–mesenchymal transition (EMT) and inhibit metastasis of gallbladder carcinoma.18 Additionally, miR-29c-3p restrains the progression of HCC via LATS1-related Hippo and DNMT3B signaling pathways in vivo.19 However, the interaction between TUG1 and miR-29c-3p has not been clarified in HCC.
TUG1 is recognized as a transcript in microarray screening, which is upregulated developing mouse retinal cells during taurine treatment.20 Accumulating evidence has indicated that the dysregulation of lncRNA TUG1 affects cell apoptosis and proliferation in various human cancers. For instance, knockdown of TUG1 restrains cell proliferation and accelerates cell apoptosis in osteosarcoma;21 over-expression of TUG1 shows facilitation effects on cell colony formation, migration, and invasion in colorectal cancer22 and in esophageal squamous cell carcinoma.23 Nonetheless, the biological role of TUG1 in HCC remains unclear.
Collagen type 1 alpha 1 (COL1A1) is a regulator of hepatic fibrosis.24 It has been revealed that COL1A1 is related to the overall survival and disease-free survival of patients with gastric cancer.25,26 In addition, COL1A1 acts as a latent prognostic biomarker in various kinds of malignant tumors, such as gastric cancer,25,26 colorectal cancer,27 and HCC.28 However, the potential role of COL1A1 in HCC remains to be elucidated.
In the current study, TUG1 was identified to be upregulated in HCC tissues and cell lines. TUG1 knockdown showed inhibitory effects on cell proliferation, migration and invasion. Additionally, miR-29c-3p was identified as a target of TUG1. Similarly, COL1A1 was verified to be a target gene of miR-29c-3p. Besides, TUG1 regulated COL1A1 expression by sponging miR-29c-3p. Taken together, our study may bring a latent therapeutic target for HCC.
Materials and Methods
Clinical Tissues
Fifty-one patients with HCC were enrolled in this study between March 2016 and June 2018. HCC was pathologically diagnosed. HCC tissues and paired adjacent non-tumor tissues were obtained by hepatectomy. All samples were preserved in liquid nitrogen prior to further experiments. This study was approved by the Ethics Committee of our hospital and informed consent was obtained from each patient.
Cell Lines
Normal hepatic cell line (L-02) and HCC cell lines (SMMC-7721, HeP3B, HepG2 and sk-Hep-1) were gained from American Type Culture Collection (ATCC, USA). The above cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) at 37°C and 5% CO2.
Cell Transfection
SiRNA targeting TUG1 (si-TUG1-1 and si-TUG1-2), siRNA-COL1A1 (si-COL1A1) and their negative control (si-NC); miR-29c-3p mimics/inhibitor and their negative control (miR-NC); overexpression-TUG1 (pcDNA-TUG1), overexpression-COL1A1 (pcDNA-COL1A1) and their negative control (pcDNA-NC) were purchased from GenePharma (Shanghai, China). Lipofectamine 2000 reagent (Invitrogen, Carlsbad, Calif, USA) was used to transfect the above agents into HCC cells.
qRT-PCR
MiRNeasy Mini Kit (Qiagen) was used for the extraction of total RNA from tissue samples and cells. Reverse transcription was carried out using the PrimeScriptTM RT Master Mix (Takara, Dalian, China). The expression levels of target mRNAs and miRNAs were detected in an ABI 7500 PCR system (Applied Biosystems) using the SYBR premix ESx Taq II (Takara Bio Inc.). The PCR amplification condition was 95°C for 5 min, followed by 40 cycles of 95°C for 10 s and 60°C for 20 s. GAPDH and U6 were taken as the internal controls. 2−ΔΔCt method was adopted for the analyses of relative expression of target genes.
MTT Assay
The transfected HeP3B and sk-Hep-1 cells were cultured for 0, 1, 2, 3 or 4 days at 37°C, respectively. Twenty-microliter MTT (5 mg/mL) was then added into cells at different time points. After 4 h of incubation, 150 μL DMSO was added in cells. The absorbance value at 450 nm was measured by a microplate spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
Wound-Healing Assay
Cell migration ability was measured using the scratch assay. HeP3B and sk-Hep-1 cells were planted into 6-well plates at a density of 1×106 cells/well and cultured until 90% confluence. A micropipette tip was then used to make a perpendicular scratch in the middle of each well. After incubation for 24 h, images were taken and wound-healing rate was assessed by ImageJ.
Transwell Assay
The invasion ability was measured using the transwell assay. Briefly, the upper chamber (Corning, Cambridge, MA, USA) was planted with HeP3B and sk-Hep-1 cells suspended in 200 μL serum-free DMEM. The lower chamber was added with DMEM containing 10% FBS. After 24 h of incubation, cells that invaded through the matrigel membrane were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet for 30 min. The stained cells were then observed and counted under a light microscope (Olympus BX53, Tokyo, Japan) at three random fields.
RNA-Binding Protein Immunoprecipitation (RIP) Assay
The binding capacity between TUG1 and miR-29c-3p gene was tested using a Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Bedford, MA, USA). Sk-Hep-1 and HeP3B cells were lysed by RIP buffer and incubated with magnetic beads conjugated with human anti-Ago2 antibody or negative control mouse IgG. Co-precipitated RNAs were purified and further used for qRT-PCR analysis.
Dual-Luciferase Reporter (DLR) Assay
Cells grown in 24-well plates were co-transfected with miR-29c-3p mimics/miR-NC and luciferase reporter vectors TUG1-WT/TUG1-Mut using Lipofectamine 2000 (Invitrogen, Waltham, MA, USA). A Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) was used to test the luciferase activity after the co-transfection for 48 h.
Western Blot
Total protein was extracted from cells using RIPA lysis buffer. The proteins were separated by 10% SDS-PAGE and then transferred to PVDF membranes. The membrane was incubated with primary antibodies COL1A1 (1:1000, Abcam, Cambridge, UK), cyclin D1 (1:1000, Abcam), Bax (1:1000, Abcam), Bcl-2 (1:1000, Abcam), E-cadherin (1:1000, Abcam), N-cadherin (1:1000, Abcam), and β-actin (1:2000, Abcam) at 4°C overnight. The membrane was then incubated with donkey anti-rabbit IgG (1:10,000; Abcam) for another 1 h. The Western blot images were visualized using an ECL reagent (Millipore, Billerica, MA, USA). β-actin was used as an internal control.
Statistical Analysis
Data were expressed as the mean ± SD and analyzed using SPSS 20.0 (SPSS; Chicago, USA). t-test was used for the comparison between two groups. The differences among more than two groups were analyzed by one-way ANOVA, followed by Tukey’s multiple comparisons test. Pearson correlation coefficient (r) was adapted for correlation analysis. P < 0.05 presented statistically significant.
Results
The Expression of TUG1 Was Increased in HCC Tissues and Cell Lines
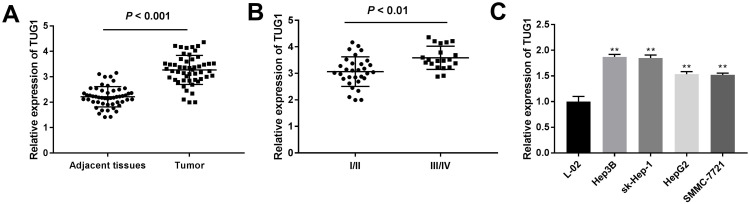
The TUG1 expression in 51 paired HCC tissues and the corresponding adjacent non-cancerous tissues was detected by qRT-PCR. Notably higher expression of TUG1 was found in HCC tissues than that in adjacent non-cancerous tissues (P < 0.001) (Figure 1A). HCC patients were then divided into high-expression (≥median expression) group and low-expression (<median expression) groups. Subsequently, the correlation between TUG1 expression and clinicopathological parameters were further analyzed. The expression of TUG1 was positively associated with the tumor stage (Table 1, P = 0.005), but not associated with the age, gender, tumor diameter, and resection degree. Besides, HCC patients at TNM stage III/IV had a significantly higher TUG1 expression than those at TNM stage I/II (P < 0.01) (Figure 1B). Next, the expression of TUG1 was assessed in HCC cell lines. A remarkable upregulation of TUG1 was discovered in HCC cell lines (sk-Hep-1, HeP3B, HepG2 and SMMC-7721) compared to that in L-02 cells (P < 0.01) (Figure 1C). Sk-Hep-1 and HeP3B cells were selected for subsequent experiments due to relatively high expression of TUG1. The above data suggested that TUG1 was remarkably overexpressed in HCC tissues and cell lines, and the upregulation of TUG1 was correlated with the tumor stage.
Figure 1.
The expression of taurine upregulated gene 1 (TUG1) in primary hepatocellular carcinoma (HCC) tissues and cell lines was detected by quantitative reverse-transcription PCR (qRT-PCR). (A) The expression of TUG1 in 51 paired HCC tissues and matched adjacent non-tumor tissues was examined by qRT-PCR. P < 0.001. (B) Relative expression of TUG1 in HCC patients at tumor-node-metastasis (TNM) stage I/II and TNM stage III/IV. P < 0.01 (C) The expression of TUG1 in four HCC cell lines (sk-Hep-1, HeP3B, HepG2 and SMMC-7721) and normal human liver cell line L-02 was assessed by qRT-PCR. **P < 0.01 vs the L-02 cells group. Data were represented as mean ± standard deviation (SD) of at least two independent experiments.
Table 1.
Clinicopathological Characteristics of the HCC Patients
| Characteristics | n | TUG1 Expression | P value | |
|---|---|---|---|---|
| Low (n=25) | High (n=26) | |||
| Age | 0.886 | |||
| <50 years | 25 | 12 | 13 | |
| ≥50 years | 26 | 13 | 13 | |
| Gender | 0.877 | |||
| Male | 28 | 14 | 14 | |
| Females | 23 | 11 | 12 | |
| Diameter | 0.867 | |||
| <5cm | 21 | 10 | 11 | |
| ≥5cm | 30 | 15 | 15 | |
| Resection degree | 0.877 | |||
| Total resection | 28 | 14 | 14 | |
| Subtotal resection | 23 | 11 | 12 | |
| WHO Grade | 0.005* | |||
| I+II | 31 | 20 | 11 | |
| III+IV | 20 | 5 | 15 | |
Note: *P = 0.005.
TUG1 Knockdown Suppressed the Proliferation, Migration, and Invasion of HCC Cells
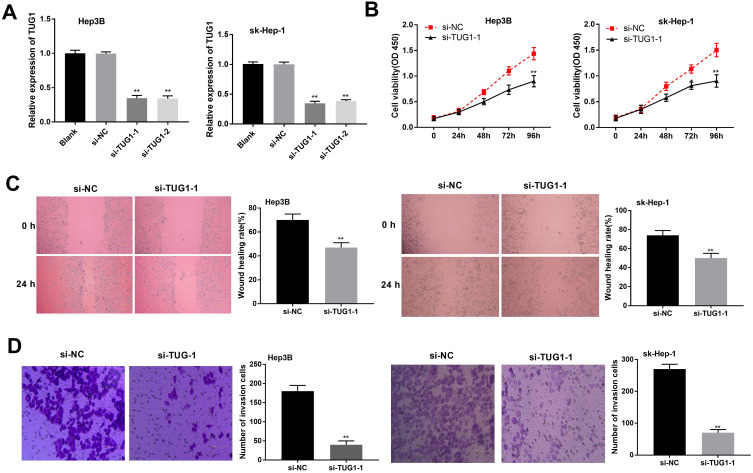
The biological function of TUG1 in HCC cells was further explored. TUG1 knockdown was conducted by using specific siRNAs (si-TUG1-1/si-TUG1-2), and the silence efficiency was determined by qRT-PCR. Both si-TUG1-1 and si-TUG1-2 significantly decreased the expression of TUG1 in HCC cells (P < 0.01) (Figure 2A). MTT assay revealed that si-TUG1-1 caused a significant decrease in cell viability (P < 0.01) (Figure 2B). Furthermore, si-TUG1-1 group showed decreased wound-healing rate and number of invasive cells compared with si-NC group (P < 0.01) (Figure 2C and D). These results indicated that TUG1 could aggravate the proliferation, migration and invasion of HCC cells.
Figure 2.
Taurine upregulated gene 1 (TUG1) knockdown inhibited the proliferation, migration and invasion of hepatocellular carcinoma (HCC) cells. HeP3B and sk-Hep-1 cells were transfected with si-TUG-1, si-TUG-2 or si-NC. (A) qRT-PCR was used to detect the expression of TUG1. (B) 3-(4,5-Dimethyl-2-Thiazolyl)-2, 5-Diphenyl-2-H-Tetrazolium Bromide (MTT) assay was applied to measure cell proliferation at 0, 24, 48, 72 and 96 h in transfected HeP3B and sk-Hep-1 cells. (C) Wound-healing assay was conducted to evaluate the migration of transfected HeP3B and sk-Hep-1 cells. (D) Transwell invasion assay was used to detect the invasion of transfected HeP3B and sk-Hep-1 cells. **P < 0.01 vs the si-NC group. Scale bar = 100 μm, ×400 magnification. Data were represented as mean ± standard deviation (SD) of at least two independent experiments.
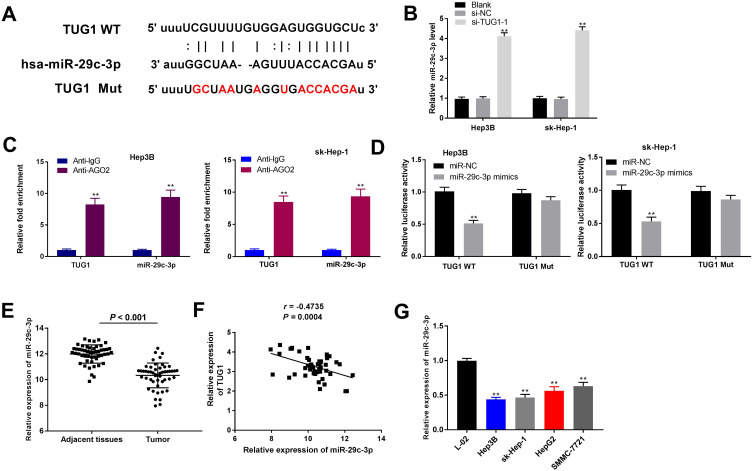
miR-29c-3p Was a TUG1
To elucidate the latent mechanism of TUG1 in HCC cells, the target miRNAs were predicted using the starBase v2.0 (http://starbase.sysu.edu.cn/). A predicted binding site between TUG1 and miR-29c-3p was discovered (Figure 3A). Then, the regulatory role of TUG1 on miR-29c-3p was explored. As a result, TUG1 knockdown significantly upregulate miR-29c-3p expression (P < 0.01) (Figure 3B). Meanwhile, RIP assay implied that both TUG1 and miR-29c-3p were immunoprecipitated by anti-Ago2 (P < 0.01) (Figure 3C). DLR assay illustrated an obviously reduced luciferase activity of TUG1-WT in miR-29c-3p mimics group compared with that in miR-NC group (P < 0.01) (Figure 3D). No notable difference was detected in the luciferase activity of TUG1-Mut between miR-29c-3p mimics and miR-NC groups (Figure 3D). Subsequently, significantly lower expression of miR-29c-3p was observed in HCC tissues than adjacent tissues (P < 0.001) (Figure 3E). Besides, correlation analysis revealed a negative correlation between miR-29c-3p and TUG1 expression (r = −0.4735; P = 0.0004) (Figure 3F). Compared with the L-02 cell line, obviously decreased expression of miR-29c-3p was shown in HCC cell lines (P < 0.01) (Figure 3G). These results suggested that TUG1 directly targeted and negatively regulated miR-29c-3p.
Figure 3.
MicroRNA (MiR)-29c-3p was a target gene of taurine upregulated gene 1 (TUG1) in hepatocellular carcinoma (HCC). (A) Bioinformatics analysis showed the predicted binding site between TUG1 and miR-29c-3p. (B) Relative expression of miR-29c-3p in HeP3B and sk-Hep-1 cells transfected with si-NC or si-TUG1-1 was measured by quantitative reverse-transcription PCR (qRT-PCR). **P < 0.01 vs the si-NC group. (C, D) RNA-binding protein immunoprecipitation (RIP) and dual-luciferase reporter (DLR) assays were used to verify the targeting relationship between the TUG1 and miR-29c-3p. C, **P < 0.01 vs the anti-IgG group; D, **P < 0.01 vs the miR-NC group. (E) The expression of miR-29c-3p in 51 paired HCC tissues and matched adjacent non-tumor tissues was examined by qRT-PCR. **P < 0.001. (F) Correlation analysis between the expression of miR-29c-3p and TUG1. P = 0.0004, r = −0.4735. (G) The expression of miR-29c-3p in four HCC cell lines (sk-Hep-1, HeP3B, HepG2 and SMMC-7721) and normal human liver cell line L-02 was assessed by qRT-PCR. **P < 0.01 vs the L-02 cells group. Data were represented as mean ± standard deviation (SD) of at least two independent experiments.
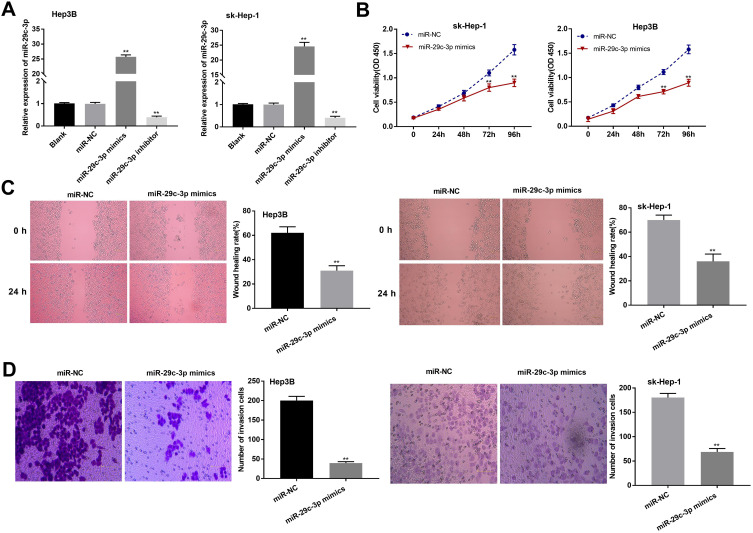
miR-29c-3p Restrained the Proliferation, Migration, and Invasion of HCC Cells
The expression of miR-29c-3p was measured in HCC cells transfected with miR-29c-3p mimics/inhibitors. MiR-29c-3p mimics group showed a strikingly upregulation of miR-29c-3p, while miR-29c-3p inhibitor group exhibited an obvious downregulated expression (P < 0.01) (Figure 4A). MTT assay implied that miR-29c-3p mimics significantly decreased the cell viability (P < 0.01) (Figure 4B). Moreover, wound healing and transwell assays indicated that the transfection of miR-29c-3p mimics resulted in decreased wound-healing rate and number of invasion cells (P < 0.01) (Figure 4C and D). The above results indicated that miR-29c-3p had inhibition effects on cell proliferation, migration, and invasion in HCC.
Figure 4.
MicroRNA (MiR)-29c-3p inhibited the proliferation, migration and invasion of hepatocellular carcinoma (HCC) cells. HeP3B and sk-Hep-1 cells were transfected with miR-negative control (NC), miR-29c-3p mimics or miR-29c-3p inhibitors. (A) Quantitative reverse-transcription PCR (qRT-PCR) was used to detect the expression of miR-29c-3p. (B) 3-(4, 5-Dimethyl-2-Thiazolyl)-2, 5-Diphenyl-2-H-Tetrazolium Bromide (MTT) assay was applied to measure cell proliferation at 0, 24, 48, 72 and 96 h in transfected HeP3B and sk-Hep-1 cells. (C) Wound-healing assay was conducted to evaluate cell migration of transfected HeP3B and sk-Hep-1 cells. (D) Transwell invasion assay was used to detect the invasion of HeP3B and sk-Hep-1 cells. Scale bar = 100 μm, ×400 magnification. **P < 0.01 vs the miR-NC group. Data were represented as mean ± standard deviation (SD) of at least two independent experiments.
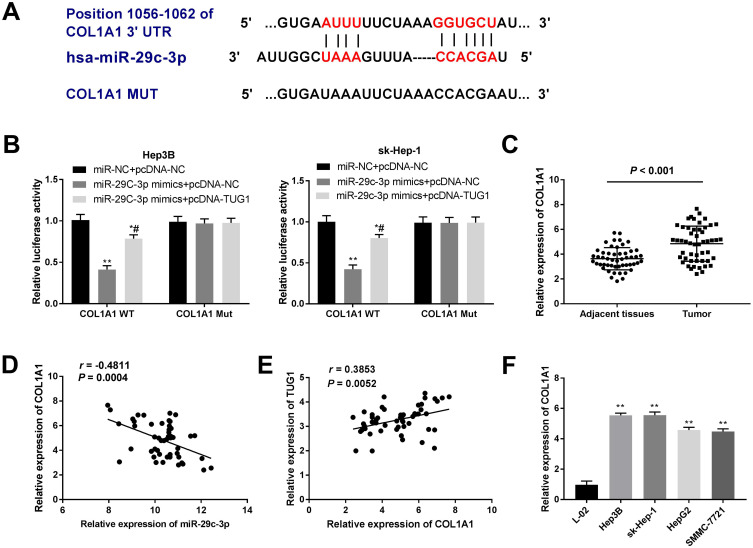
COL1A1 Was a Target Gene of miR-29c-3p
TargetScan software was used to predict the target genes of miR-29c-3p. Figure 5A shows a putative binding site between miR-29c-3p and COL1A1. DLR assay showed that the luciferase activity of COL1A1-WT was obviously decreased in the miR-29c-3p mimics + pcDNA-NC group (P < 0.01). Overexpression of TUG1 restored the suppressed luciferase activity caused by miR-29c-3p mimics (P < 0.05) (Figure 5B). Additionally, HCC tissues had an evidently increased mRNA expression of COL1A1 compared with adjacent tissues (P < 0.001) (Figure 5C). There was a negative correlation between COL1A1 and miR-29c-3p expression (r = −0.4811, P < 0.0004) (Figure 5D), and a positive correlation between TUG1 and COL1A1 expression (r = 0.3853, P < 0.0052) (Figure 5E). Finally, the mRNA expression of COL1A1 was obviously increased in HCC cell lines in comparison to the L-02 cell line (P < 0.01) (Figure 5F). Together, we proved that COL1A1 was a target gene of miR-29c-3p.
Figure 5.
Collagen type 1 alpha 1 (COL1A1) was a target gene of microRNA (miR)-29c-3p. (A) Bioinformatics analysis showed the predicted binding site between miR-29c-3p and COL1A1. (B) dual-luciferase reporter (DLR) assay was used to verify the targeting relationship between miR-29c-3p and COL1A1. *P < 0.05, **P < 0.01 vs the miR-NC + pcDNA-NC group. #P < 0.05 vs the miR-29c-3p mimics + pcDNA-NC group. (C) The expression of COL1A1 in 51 paired hepatocellular carcinoma (HCC) tissues and matched adjacent non-tumor tissues was examined by quantitative reverse-transcription PCR (qRT-PCR). P < 0.001. (D) Correlation analysis between the expression of miR-29c-3p and COL1A1. P = 0.0004, r = −0.4811. (E) Correlation analysis between the expression of COL1A1 and taurine upregulated gene 1 (TUG1). P = 0.0052, r = 0.3853. (F) The expression of COL1A1 in four HCC cell lines and normal human liver cell line L-02 was assessed by qRT-PCR. **P < 0.01 vs the L-02 cells group. Data were represented as mean ± standard deviation (SD) of at least two independent experiments.
TUG1 Acted as a ceRNA to Modulate HCC Progression via miR-29c-3p/COL1A1 AxisAxis
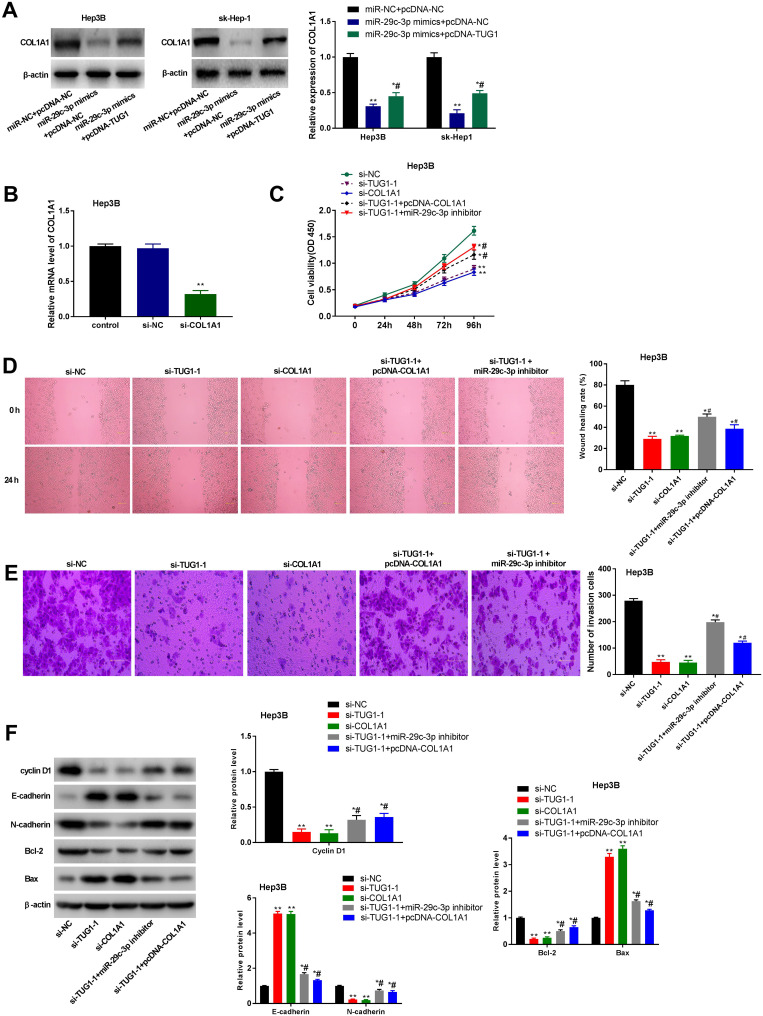
Western blot showed that the protein expression of COL1A1 was significantly decreased by the transfection of miR-29c-3p mimics in Hep3B and sk-Hep-1 cells (P < 0.01). Overexpression of TUG1 significantly reversed the decreased protein expression of COL1A1 induced by miR-29c-3p mimics (P < 0.05) (Figure 6A). In order to reveal the regulatory role of COL1A1 in HCC cells, si-COL1A1 was used to silence COL1A1 in Hep3B cells. qRT-PCR showed that the mRNA expression of COL1A1 was significantly decreased by the transfection of si-COL1A1 (P < 0.01) (Figure 6B). The transfection of si-COL1A1 significantly suppressed the viability, migration and invasion of Hep3B cells (P < 0.01) (Figure 6C–E). The transfection of si-COL1A1 also significantly decreased the expression of Cyclin D1, Bcl-2, and N-cadherin, and increased the expression of E-cadherin and Bax in Hep3B cells (P < 0.01) (Figure 6F). Si-TUG1-1 exhibited consistent results with si-COL1A1 in Hep3B cells (P < 0.01) (Figure 6C–F). Interestingly, downregulation of miR-29c-3p and upregulation of COL1A1 both reversed the suppressive effects of TUG1-1 knockdown on the viability, migration and invasion of Hep3B cells (P < 0.05) (Figure 6C–E). In addition, the suppressive effects of TUG1-1 knockdown on the expression of Cyclin D1, Bcl-2, and N-cadherin, and the promoting effects of TUG1-1 knockdown on the expression of E-cadherin and Bax were significantly reversed by miR-29c-3p downregulation and COL1A1 upregulation (P < 0.05) (Figure 6F). All these results indicated that silencing of TUG1 may inhibit the progression of HCC via regulating miR-29c-3p/COL1A1 axis.
Figure 6.
Taurine upregulated gene 1 (TUG1) promotes the proliferation, migration and invasion of hepatocellular carcinoma (HCC) cells via microRNA (miR)-29c-3p/collagen type 1 alpha 1 (COL1A1) axis. (A) Western blot assay was used to detect the expression of COL1A1 in transfected HeP3B and sk-Hep-1 cells. *P < 0.05, **P < 0.01 vs the miR-NC + pcDNA-NC group. #P < 0.05 vs the miR-29c-3p mimics + pcDNA-NC group. (B) The mRNA level of COL1A1 was detected by quantitative reverse-transcription PCR (qRT-PCR). (C) 3-(4,5-Dimethyl-2-Thiazolyl)-2, 5-Diphenyl-2-H-Tetrazolium Bromide (MTT) assay was applied to measure cell proliferation at 0, 24, 48, 72 and 96 h in transfected HeP3B and sk-Hep-1 cells. (D) Wound-healing assay was conducted to evaluate the migration of transfected HeP3B and sk-Hep-1 cells. (E) Transwell invasion assay was used to detect the invasion of HeP3B and sk-Hep-1 cells. (F) Western blot assay was used to detect the protein levels of cyclin D1, E-cadherin, N-cadherin, Bcl-2 and Bax. *P < 0.05, **P < 0.01 vs the si-NC group. #P < 0.05 vs the si-TUG1-1 group. Scale bar = 100 μm, ×400 magnification. Data were represented as mean ± standard deviation (SD) of at least two independent experiments.
Discussion
Since distance migration and drug resistance are two important obstacles to the prognosis of HCC, it is urgent to understand the molecular mechanisms and to find new therapeutic targets for HCC. Previous studies have validated that abnormal expression of lncRNAs is linked to the tumorigenesis and metastasis of cancer.5,29 Overexpression of the lncRNAs has been reported to be closely associated with the advanced TNM stages of patients with malignant tumor.30,31 In the current study, significantly upregulated expression of lncRNA TUG1 was detected in HCC tissues. The increased expression of TUG1 was also identified to be correlated with the TNM stage of HCC.
Dysregulation of lncRNAs has been frequently detected in various cancers and plays vital role in diverse biological processes, including cell proliferation, apoptosis, metastasis, and metabolism.32,33 TUG1 is an important lncRNA that has been identified as an oncogene in various types of cancers. For instance, knockdown of TUG1 suppresses the progress of osteosarcoma.21 Overexpression of TUG1 promotes the metastasis of colorectal cancer in vivo22 and promotes the proliferation and migration of esophageal squamous cell carcinoma cells in vitro.23 A recent study has indicated that TUG1 accelerates cell proliferation, colony formation, and tumorigenicity of HCC through epigenetically inhibiting Kruppel-like factor 2 transcription,34 suggesting a key regulatory role of TUG1 in HCC. In this study, TUG1 was silenced to investigate the effects of TUG1 dysregulation on HCC cells. As a result, TUG1 knockdown showed a suppressive effect on HCC, which was coincided with the oncogenic role of TUG1 reported previously.
A large number of studies have illustrated that miRNAs are vital regulatory factors in cellular processes and involved in the development of HCC.35–37 However, the exact function of miRNAs in the progress of HCC is not entirely understood.38 Here, target prediction was performed based on dramatically increased expression of TUG1 in HCC. MiR-29c-3p was predicated as a target of TUG1. The specific target relation between miR-29c-3p and TUG1 was verified by RIP and DLR assays. Recent studies have illustrated that miR-29c-3p may act as a tumor suppressor in a series of cancers.19,39 Lu et al have found that miR-29c-3p is downregulated in gallbladder carcinoma, and miR-29c-3p overexpression inhibits cell metastasis.18 MiR-29c-3p suppresses cell invasion and migration in colon cancer,40 and inhibits cell proliferation, apoptosis, and tumor growth in HCC.19 Another study has also reported that miR-29c-3p, as a direct target gene of P53, inhibits the invasion and migration of colon cancer cells by targeting PHLDB2.40 Similar inhibitory effect of miR-29c-3p on HCC was obtained in the current study.
As known, miRNAs can downregulate gene expression through base-pairing with the 3ʹ-UTR of the targeted mRNAs.41 In this study, we noticed that miR-29c-3p could directly bind to the 3ʹ-UTR of COL1A1. The involvement of collagen family members has been determined in the carcinogenesis of several tissue types.42 The aberrant expression of COL1A1 and COL1A2 has been discovered in some cancers.28,43,44 Zang et al45 have proved that COL1A1 and COL1A2 are differentially expressed in gastric cancer, which can predict poor clinical outcomes in patients with gastric cancer. Preclinical evidence has shown that COL1A1 is a reliable biomarker and putative therapeutic target for hepatocellular carcinogenesis and metastasis.46 Here, DLR assay showed that overexpression of TUG1 reversed the suppressed luciferase activity caused by miR-29c-3p mimics. It is widely believed that lncRNAs act as sponges to regulate the target miRNAs, thus hindering the inhibitory effect of miRNAs on their target genes. Gao et al have reported that HOXC13-AS enhances HMGA2 expression by sponging miR-383-3p in nasopharyngeal carcinoma.47 PCA3 up-regulates HMGB1 expression through targeting miR-218-5p in prostate cancer.48 HCAL modulates LAPTM4B expression via binding miR-15a, miR-196a and miR-196b in HCC.49 Our results illustrated that overexpression of COL1A1 and inhibition of miR-29c-3p both reversed the suppressive effects of TUG1 knockdown on HCC progression in vitro, suggesting that TUG1 could upregulate COL1A1 expression by sponging miR-29c-3p.
However, this study has some limitations. First, overexpression experiment is needed to reflect the regulatory role of TUG1 in HCC cells more comprehensively. Second, more assays should be added to verify the results on cell proliferation and apoptosis, such as cell cycle assay, and Western blot on apoptosis-related proteins. Third, this study is limited in cellular level and researches on animal models are urgently needed. Fourth, the pathological significance of COL1A1 in indicating survival and prognosis of patients with HCC is not analyzed. Finally, in-depth mechanisms of TUG1 involving signaling pathways remain need to be studied.
Conclusions
In conclusion, TUG1 was a tumor promoter in HCC. TUG1 positively regulated COL1A1 via sponging miR-29c-3p. Notably, TUG1 could promote the viability, migration and invasion of HCC cells through regulating miR-29c-3p/COL1A1 axis. Our study indicated that TUG1/miR-29c-3p/COL1A1 axis might serve as a therapeutic target for HCC.
Data Sharing Statement
The data in this study can be obtained from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of The Sixth People’s Hospital of Qingdao and informed consent was obtained from each patient. All participants agreed to publish the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–1491. doi: 10.1200/JCO.2008.20.7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morise Z, Kawabe N, Tomishige H, et al. Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20:14381–14392. doi: 10.3748/wjg.v20.i39.14381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47. doi: 10.1038/nrg.2015.10 [DOI] [PubMed] [Google Scholar]
- 5.Bao J, Chen X, Hou Y, et al. LncRNA DBH-AS1 facilitates the tumorigenesis of hepatocellular carcinoma by targeting miR-138 via FAK/Src/ERK pathway. Biomed Pharmacother. 2018;107:824–833. doi: 10.1016/j.biopha.2018.08.079 [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Yang L, Chen T, et al. A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Mol Cancer. 2019;18(1):28. doi: 10.1186/s12943-019-0957-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K, Long B, Zhou L-Y, et al. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun. 2014;5:1–13. [DOI] [PubMed] [Google Scholar]
- 8.Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–369. doi: 10.1016/j.cell.2011.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afum-Adjei Awuah A, Ueberberg B, Owusu-Dabo E, Frempong M, Jacobsen M. Dynamics of T-cell IFN-gamma and miR-29a expression during active pulmonary tuberculosis. Int Immunol. 2014;26:579–582. [DOI] [PubMed] [Google Scholar]
- 10.Huang X, She L, Luo X, Huang S, Wu J. MiR-222 promotes the progression of polycystic ovary syndrome by targeting p27 Kip1. Pathol Res Pract. 2019;215:918–923. doi: 10.1016/j.prp.2019.01.038 [DOI] [PubMed] [Google Scholar]
- 11.Zhang F, Li K, Yao X, et al. A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer. EBioMedicine. 2019;44:311–321. doi: 10.1016/j.ebiom.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu D, Gu L, Li Z, et al. MiR-138-5p suppresses lung adenocarcinoma cell epithelial-mesenchymal transition, proliferation and metastasis by targeting ZEB2. Pathol Res Pract. 2019;215(5):861–872. doi: 10.1016/j.prp.2019.01.029 [DOI] [PubMed] [Google Scholar]
- 13.Zhu Z, Zhang X, Wang G, Zheng H. Role of microRNAs in hepatocellular carcinoma. Hepat Mon. 2014;14(7). doi: 10.5812/hepatmon.18672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han B, Huang J, Yang Z, et al. miR-449a is related to short-term recurrence of hepatocellular carcinoma and inhibits migration and invasion by targeting notch1. Onco Targets Ther. 2019;12:10975. doi: 10.2147/OTT.S216997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Q, Liu X, Liu Z, et al. MicroRNA-1296 inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting SRPK1-mediated PI3K/AKT pathway. Mol Cancer. 2017;16(1):103. doi: 10.1186/s12943-017-0675-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Yu Z, Chen F, et al. miR-300 regulates the epithelial-mesenchymal transition and invasion of hepatocellular carcinoma by targeting the FAK/PI3K/AKT signaling pathway. Biomed Pharmacother. 2018;103:1632–1642. doi: 10.1016/j.biopha.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 17.Jiang T, Guan L-Y, Ye Y-S, Liu H-Y, Li R. MiR-874 inhibits metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma by targeting SOX12. Am J Cancer Res. 2017;7:1310. [PMC free article] [PubMed] [Google Scholar]
- 18.Lu K, Feng F, Yang Y, et al. High-throughput screening identified miR-7-2-3p and miR-29c-3p as metastasis suppressors in gallbladder carcinoma. J Gastroenterol. 2020;55(1):51–66. doi: 10.1007/s00535-019-01627-0 [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Zhang W, Wu Z, et al. miR-29c-3p regulates DNMT3B and LATS1 methylation to inhibit tumor progression in hepatocellular carcinoma. Cell Death Dis. 2019;10(2):48. doi: 10.1038/s41419-018-1281-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15(6):501–512. doi: 10.1016/j.cub.2005.02.027 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Geng P-L, Yin P, et al. Down-regulation of long non-coding RNA TUG1 inhibits osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J Cancer Prev. 2013;14(4):2311–2315. doi: 10.7314/APJCP.2013.14.4.2311 [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Ding C, Yang Z, et al. The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial-mesenchymal transition. J Transl Med. 2016;14(1):42. doi: 10.1186/s12967-016-0786-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y, Wang J, Qiu M, et al. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumor Biol. 2015;36(3):1643–1651. doi: 10.1007/s13277-014-2763-6 [DOI] [PubMed] [Google Scholar]
- 24.Koilan S, Hamilton D, Baburyan N, et al. Prevention of liver fibrosis by triple helix-forming oligodeoxyribonucleotides targeted to the promoter region of type I collagen gene. Oligonucleotides. 2010;20(5):231–237. doi: 10.1089/oli.2010.0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang K, Liu H, Xie D, Xiao Q. Differentially expressed genes ASPN, COL1A1, FN1, VCAN and MUC5AC are potential prognostic biomarkers for gastric cancer. Oncol Lett. 2019;17:3191–3202. doi: 10.3892/ol.2019.9952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Ding Y, Li A. Identification of COL1A1 and COL1A2 as candidate prognostic factors in gastric cancer. World J Surg Oncol. 2016;14:297. doi: 10.1186/s12957-016-1056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Wang Y, Zhang J, Zhong J, Yang R. COL1A1 promotes metastasis in colorectal cancer by regulating the WNT/PCP pathway. Mol Med Rep. 2018;17:5037–5042. doi: 10.3892/mmr.2018.8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi M, Nomoto S, Hishida M, et al. Identification of the collagen type 1 alpha 1 gene (COL1A1) as a candidate survival-related factor associated with hepatocellular carcinoma. BMC Cancer. 2014;14:108. doi: 10.1186/1471-2407-14-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong H, Hu J, Zou K, et al. Activation of LncRNA TINCR by H3K27 acetylation promotes trastuzumab resistance and epithelial-mesenchymal transition by targeting MicroRNA-125b in breast cancer. Mol Cancer. 2019;18:1–18. doi: 10.1186/s12943-018-0931-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Wu D-M, Wang S, Wen X, et al. LncRNA SNHG15 acts as a ceRNA to regulate YAP1-hippo signaling pathway by sponging miR-200a-3p in papillary thyroid carcinoma. Cell Death Dis. 2018;9(10):1–15. doi: 10.1038/s41419-018-0975-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong P, Qiao F, Wu H, et al. LncRNA UCA1 promotes tumor metastasis by inducing miR-203/ZEB2 axis in gastric cancer. Cell Death Dis. 2018;20(12):1–14. doi: 10.1038/s41419-018-1170-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Li X, Wu S, Xue M, Chen W. Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci. 2014;105:951–955. doi: 10.1111/cas.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang MD, Chen WM, Qi FZ, et al. Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 2015;14:165. doi: 10.1186/s12943-015-0431-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohamed AA, Ali-Eldin ZA, Elbedewy TA, et al. MicroRNAs and clinical implications in hepatocellular carcinoma. World J Hepatol. 2017;9(23):1001–1007. doi: 10.4254/wjh.v9.i23.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X, Yang Z, Li G, et al. The role and clinical implications of microRNAs in hepatocellular carcinoma. Sci China Life Sci. 2012;55(10):906–919. doi: 10.1007/s11427-012-4384-x [DOI] [PubMed] [Google Scholar]
- 37.Peng S, Geng J, Sun R, Tian Z, Wei H. Polyinosinic-polycytidylic acid liposome induces human hepatoma cells apoptosis which correlates to the up-regulation of RIG-I like receptors. Cancer Sci. 2009;100:529–536. doi: 10.1111/j.1349-7006.2008.01062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurnherr T, Mah W-C, Lei Z, et al. Differentially expressed miRNAs in hepatocellular carcinoma target genes in the genetic information processing and metabolism pathways. Sci Rep. 2016;6(1):20065. doi: 10.1038/srep20065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S, Jin J, Tian X, Wu L. hsa-miR-29c-3p regulates biological function of colorectal cancer by targeting SPARC. Oncotarget. 2017;8(61):104508. doi: 10.18632/oncotarget.22356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G, Zhou T, Li Y, Yu Z, Sun L. p53 target miR-29c-3p suppresses colon cancer cell invasion and migration through inhibition of PHLDB2. Biochem Biophys Res Commun. 2017;487(1):90–95. doi: 10.1016/j.bbrc.2017.04.023 [DOI] [PubMed] [Google Scholar]
- 41.Bartel DP. MicroRNAs: target recognition and regulatory functions. cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33(1):49–54. doi: 10.1038/ng1060 [DOI] [PubMed] [Google Scholar]
- 43.Ibanez de Caceres I, Dulaimi E, Hoffman AM, et al. Identification of novel target genes by an epigenetic reactivation screen of renal cancer. Cancer Res. 2006;66(10):5021–5028. doi: 10.1158/0008-5472.CAN-05-3365 [DOI] [PubMed] [Google Scholar]
- 44.Bonazzi VF, Nancarrow DJ, Stark MS, et al. Cross-platform array screening identifies COL1A2, THBS1, TNFRSF10D and UCHL1 as genes frequently silenced by methylation in melanoma. PLoS One. 2011;6(10):e26121. doi: 10.1371/journal.pone.0026121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zang S, Guo R, Xing R, et al. Identification of differentially-expressed genes in intestinal gastric cancer by microarray analysis. Genomics Proteomics Bioinformatics. 2014;487(6):276–283. doi: 10.1016/j.gpb.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma HP, Chang HL, Bamodu OA, et al. Collagen 1A1 (COL1A1) is a reliable biomarker and putative therapeutic target for hepatocellular carcinogenesis and metastasis. Cancers. 2019;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao C, Lu W, Lou W, Wang L, Xu Q. Long noncoding RNA HOXC13‐AS positively affects cell proliferation and invasion in nasopharyngeal carcinoma via modulating miR‐383‐3p/HMGA2 axis. J Cell Physiol. 2019;234:12809–12820. doi: 10.1002/jcp.27915 [DOI] [PubMed] [Google Scholar]
- 48.Zhang G, He X, Ren C, Lin J, Wang Q. Long noncoding RNA PCA3 regulates prostate cancer through sponging miR-218-5p and modulating high mobility group box 1. J Cell Physiol. 2019;234(8):13097–13109. doi: 10.1002/jcp.27980 [DOI] [PubMed] [Google Scholar]
- 49.Xie C-R, Wang F, Zhang S, et al. Long noncoding RNA HCAL facilitates the growth and metastasis of hepatocellular carcinoma by acting as a ceRNA of LAPTM4B. Mol Ther Nucleic Acids. 2017;9:440–451. doi: 10.1016/j.omtn.2017.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]