Abstract
Background:
It has been shown that glomerulonephritis (GN) recurrence affects graft survival more than acute rejection. Thus, we assessed allograft survival after biopsy-confirmed diagnosis of acute rejection or recurrent GN in current era of immunosuppression.
Methods:
Allograft survival following a biopsy diagnosis of acute rejection or recurrent GN was determined in adult kidney transplant recipients from 1994 to 2013. A total of 306 patients (35%) with IgA, 298 (35%) with FSGS, 177 (21%) with lupus nephritis, and 81 (9%) with membranous nephropathy were followed for a median of 6.3 years.
Results:
Among the 862 transplant recipients with primary GN, allograft loss was similar following a biopsy diagnosis of acute rejection or recurrent glomerular disease (11.5 vs 14.2/100 person-years, P = .15). Differences in allograft survival emerged after 2.5 years following recurrent disease, with significantly higher graft failure in patients with FSGS, MN, or LN compared with IgA after recurrence of disease (16.7 vs 7.5/100 person-years, P = .05). The advantage in allograft survival for IgA patients did not achieve significance after acute rejection (P = .10 for IgA vs FSGS, MN, and LN).
Conclusions:
Allograft survival was similar after disease recurrence or acute rejection after kidney transplant in patients with ESRD due to GN.
Keywords: graft survival, kidney disease, recurrent disease, rejection: acute
1 |. INTRODUCTION
The most recent United States Renal Data System report indicates glomerulonephritis (GN) accounts for approximately 25% of the cases of end-stage renal disease (ESRD) in kidney transplant recipients in the United States.1 In kidney transplant recipients with a history of ESRD due to glomerular disease, the common causes of allograft loss are attributed to recurrent glomerular disease or allograft rejection.2 However, most studies focus on the incidence of recurrent GN and allograft survival.2–9 There are very limited studies comparing the effect of recurrent GN vs acute rejection on allograft survival in patients with GN as a cause of ESRD. Patients with GN often have underlying autoimmune disease or immune dysregulation, and it is unclear whether these alterations in the immune system affect the incidence of acute rejection or impact allograft survival following a diagnosis of rejection or recurrent disease. In 2002, Briganti et al5 showed that 10-year graft survival was worse in patients who had GN recurrence compared with acute rejection.
In this study, we examine the post-transplant course of kidney transplant recipients with GN as the cause of their ESRD in current era of immunosuppression. All patients included in this study had a biopsy-confirmed diagnosis of primary glomerular disease prior to transplant and a biopsy-confirmed diagnosis post-transplant recurrent GN or acute rejection. Whereas previous studies have reported allograft survival in these patients from time of transplant, we assessed allograft survival following the biopsy diagnosis of acute rejection or recurrent GN stratified by primary GN diagnosis that is clinically significant for providers as well as patients.
2 |. MATERIALS AND METHODS
2.1 |. Patient population
All adult (>18 years) renal transplant recipients at the University of Wisconsin Hospital and Clinics between 1994 through 2013 with biopsy-proven primary glomerular disease as the cause of end-stage renal disease were eligible for inclusion in this study. The primary glomerular diseases of transplant recipients included the following four diagnoses: immunoglobulin A nephropathy (IgA), membranous nephropathy (MN), focal segmental glomerulosclerosis (FSGS), or lupus nephritis (LN). Data were obtained from the Wisconsin Allograft Recipient Database (WisARD). This study was approved by the University of Wisconsin Madison Institutional Review Board and the Human Subjects Committee. All clinical and research activities performed were in accordance with the 2000 Declaration of Helsinki and the Declaration of Istanbul 2008 ethical standards for human subjects.
2.2 |. Data collection
The primary outcome was the incidence rate of the first biopsy-confirmed diagnosis of acute rejection or recurrent glomerular disease after renal transplantation. The occurrence of acute rejection was determined by a biopsy diagnosis of either acute cellular rejection or acute antibody-mediated rejection. A diagnosis of recurrent GN was made by pathologic assessment on light microscopy, immunofluorescence, and electron microscopy. Clinical renal pathologists determined the pathologic diagnoses on all allograft biopsies. The post-transplant biopsies were performed for cause (due to a rise in serum creatinine and/or an increase in proteinuria or hematuria). Allograft failure after the event (biopsy-confirmed diagnosis of recurrent disease or acute rejection) was defined as return to dialysis, re-transplantation, or patient death. Patients were followed until graft loss, death, or last available followup.
2.3 |. Statistical analysis
Continuous variables were compared between groups using t tests and Kruskal-Wallis tests. Categorical variables were compared between groups with Chi-square or Fisher’s exact tests. Time to event data and survival estimates were obtained using Kaplan-Meier curves and log-rank test. Cox proportional hazard models were used to assess independent associations between demographics and baseline characteristics and recurrence and rejection. All analyses were performed using Stata Statistical Software: Release 13 (StataCorp).
3 |. RESULTS
3.1 |. Baseline characteristics
A total of 862 patients with one of the four major types of GN as the cause for ESRD received a kidney transplant between the years of 1994 and 2013. A total of 306 (35%) patients with IgA, 298 (35%) with FSGS, 177 (21%) with LN, and 81 (9%) with MN were followed for a median of 6.3 years (Table 1). The mean age ranged from 43 years in both IgA and LN, up to 51 years in MN. The proportion of male patients ranged from 22% in LN to 69% in IgA. The peak PRA (panel reactive antibody) was low in IgA patients (5.9%) and FSGS patients (10.2%); peak PRA was moderate in MN (15.3%) and LN (18.3%) patients. The proportion of patients with an HLA (human leukocyte antigen) mismatch greater than two ranged between 72% in LN and 83% in FSGS. As part of their transplant maintenance immunosuppression, all patients were on prednisone and greater than 85% were on calcineurin inhibitors and/or mycophenolate mofetil.
TABLE 1.
Patient characteristics of kidney transplant recipients with primary glomerular disease as the cause of ESRD
| IgA (N = 306) |
FSGS (N = 298) |
MN (N = 81) |
LN (N = 177) |
P-value | |
|---|---|---|---|---|---|
| Age (y), mean | 42.8 | 44.9 | 50.5 | 42.8 | <.005 |
| Sex, N (%) | 212 (69) | 184 (62) | 55 (68) | 38 (22) | <.05 |
| Male | |||||
| Race, N (%) | 259 (84) | 233 (78) | 71 (88) | 140 (79) | .07 |
| Caucasian | |||||
| BMI (kg/m2), mean | 27.4 | 27.6 | 27.0 | 25.1 | .05 |
| Duration of disease prior to transplant (y), mean | 7.1 | 7.6 | 7.5 | 8.3 | .03 |
| Dialysis duration prior to transplant (y), mean | 1.2 | 2.0 | 1.7 | 2.4 | <.005 |
| Prior transplant, % | 17 | 22 | 26 | 25 | .15 |
| Peak PRA, % | 5.9 | 10.2 | 15.3 | 18.3 | .005 |
| HLA mismatch | |||||
| Mismatch >2, % | 79 | 83 | 75 | 72 | |
| HLA A, % | 75 | 78 | 65 | 65 | .005 |
| HLA B, % | 79 | 83 | 74 | 70 | .006 |
| HLA DR, % | 69 | 72 | 72 | 64 | .41 |
| Donor type | |||||
| Deceased, % | 41 | 65 | 61 | 57 | .005 |
| Age (y), mean | 41.5 | 41.7 | 40.2 | 40.1 | .58 |
| Male, % | 48 | 57 | 52 | 50 | .15 |
| Caucasian, % | 94 | 93 | 93 | 91 | .53 |
| Cold ischemia time (h), mean | 16 | 22 | 20 | 29 | .46 |
| Delayed graft function, % | 10 | 21 | 14 | 17 | .01 |
| Maintenance IS | |||||
| Prednisone, % | 100 | 100 | 100 | 100 | NA |
| Calcineurin inhibitor, % | 94 | 87 | 91 | 94 | .01 |
| Mycophenolate, % | 89 | 89 | 85 | 89 | .81 |
Abbreviations: FSGS, focal segmental glomerulosclerosis; IgA, IgA nephropathy; LN, lupus nephritis; MN, membranous nephropathy.
3.2 |. Incidence rate of acute rejection or recurrent GN among transplant recipients with ESRD due to primary GN
We analyzed the first for-cause biopsy post-transplant for all patients with ESRD due to GN (Figure 1). Of the 862 transplant recipients with primary GN, 363 patients (42%) had a biopsy-confirmed diagnosis of acute rejection and 96 patients (11%) had biopsy-confirmed recurrent GN. The incidence rate of acute rejection was 7.2 per 100 person-years compared with 1.4 per 100 person-years for recurrent glomerular disease. Among the 96 patients with recurrent glomerular disease, only 23 patients had both acute rejection and recurrent GN at the first biopsy and the remaining 73 had recurrent glomerular disease (Figure 2). Median time to recurrence was 15 months, and median time to acute rejection was 3 months.
FIGURE 1.
Analyses of first for-cause biopsy post-transplant for kidney transplant recipients with ESRD due to glomerulonephritis
FIGURE 2.
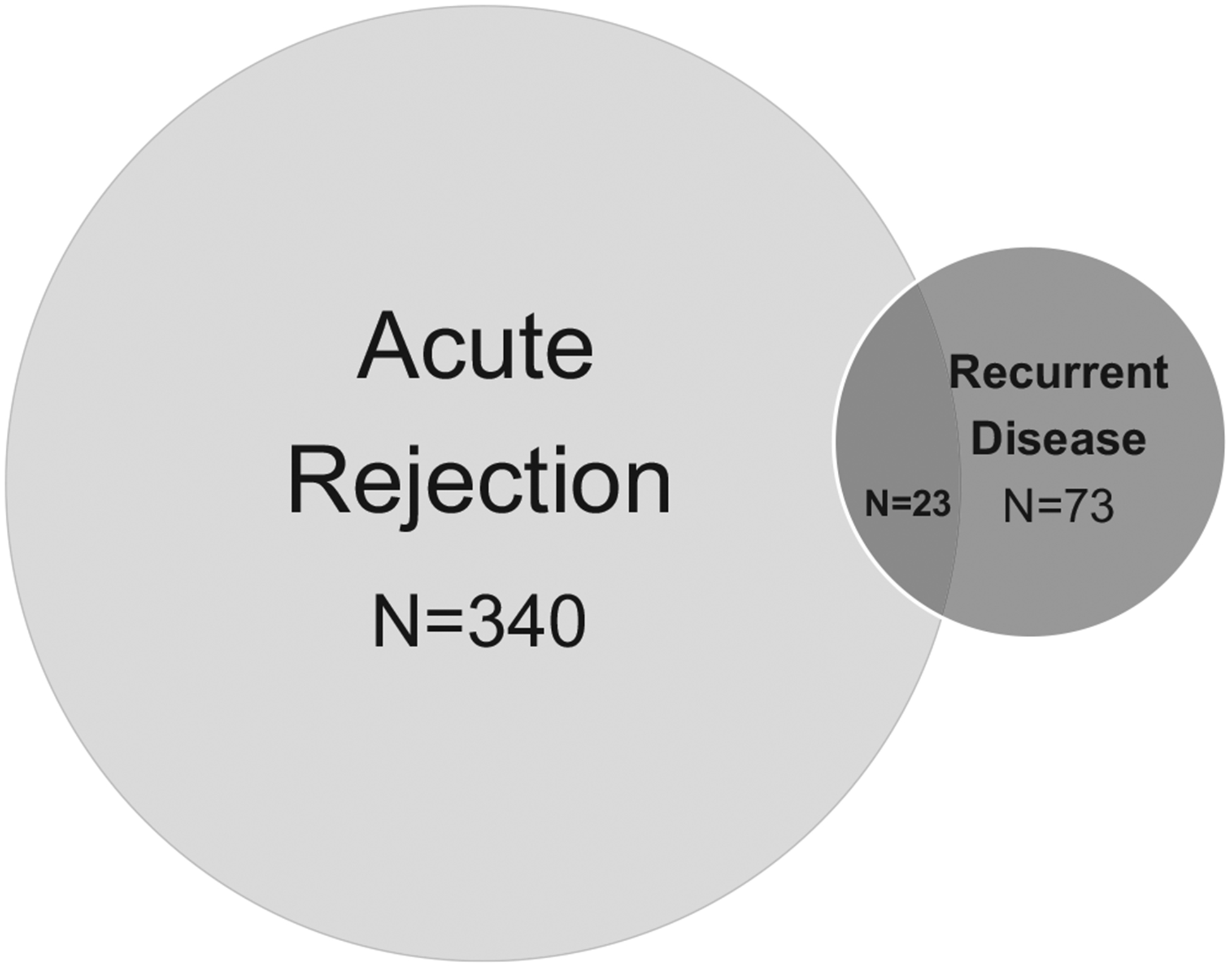
Biopsy-confirmed diagnosis of acute rejection or recurrent glomerular disease in kidney transplant recipients with a history of primary glomerular disease. Acute rejection was the diagnosis in 340 patients. The number of patients with recurrent glomerular disease on biopsy was 96. A minority of patients (N = 23) had both acute rejection and recurrent glomerular disease on biopsy. Median time to acute rejection was 3 mo. Median time to recurrent glomerular disease was 15 mo
3.3 |. Allograft survival was similar after acute rejection or recurrent disease in transplanted patients with ESRD due to primary GN
Allograft survival was similar for transplant patients with a history of ESRD due to glomerular disease when stratified by biopsy-confirmed diagnosis of either recurrent GN or acute rejection (Figure 3). Patients with both rejection and recurrence on the same biopsy had slightly worse allograft survival during the initial 2 years after biopsy. However, this difference in allograft survival did not persist, and at 10 years, survival was similar to those patients with only GN recurrence or only acute rejection even after adjusting for age, sex, race, BMI, dialysis pre-transplant, duration of dialysis pre-transplant PRA, donor age, donor sex, donor race, previous transplant, delayed graft function, CNI use, HLA mismatch (Table 2).
FIGURE 3.
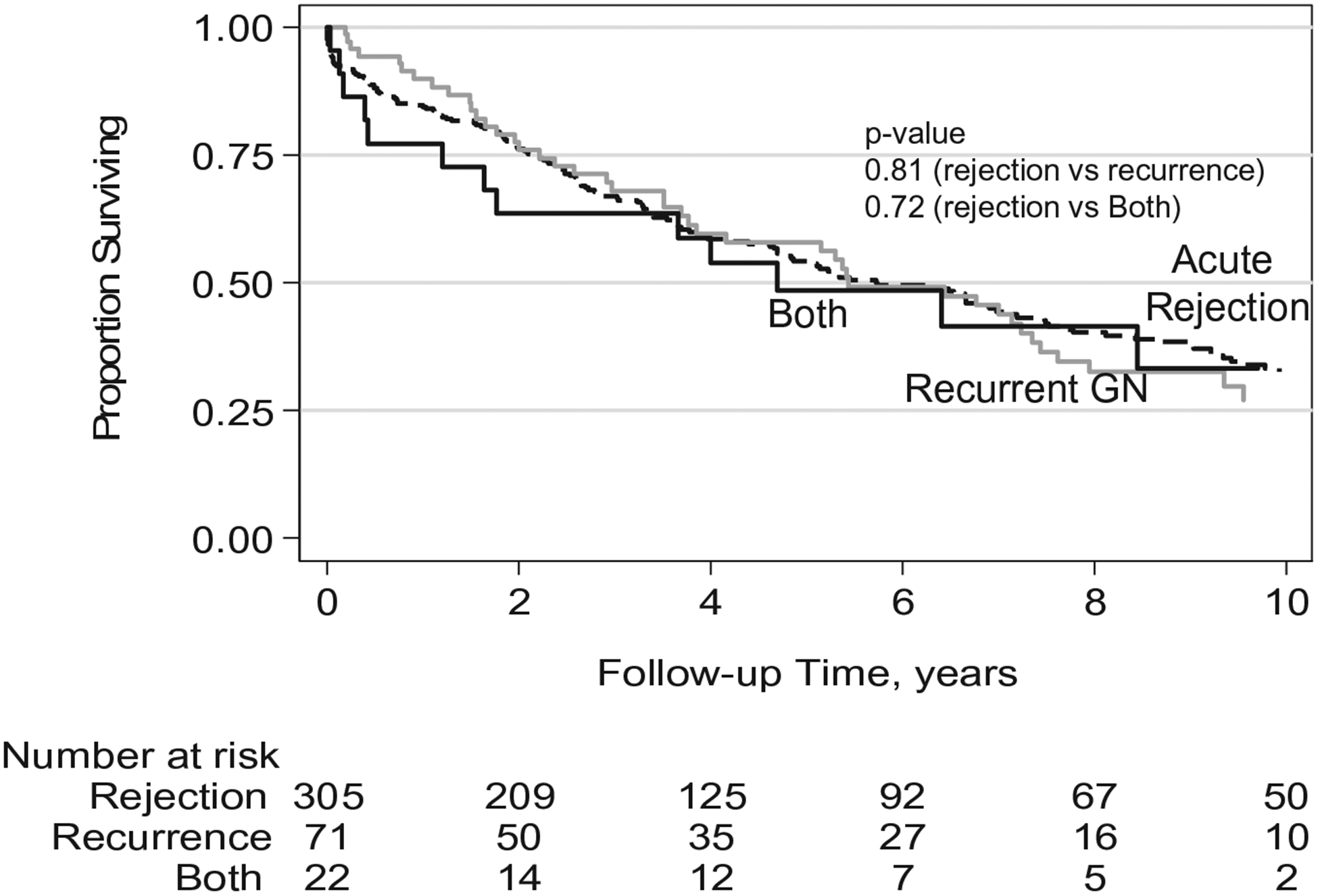
Kaplan-Meier curve of allograft survival for kidney transplant patients with ESRD due to primary glomerular disease. Transplant recipients with a history of ESRD due to primary glomerular disease have similar allograft survival when stratified by diagnosis of acute rejection, recurrent glomerular disease, or both on biopsy following transplantation; acute rejection (dashed line), recurrent glomerular disease (gray line), or both (black line)
TABLE 2.
Hazard ratio for allograft survival in kidney transplant patients with ESRD due to primary glomerular disease
| HR (unadjusted) | aHR | P-interaction | |
|---|---|---|---|
| Recurrence vs rejection | 1.04 (0.75–1.46) | 1.22 (1.71–3.27) | .84 |
| Both vs rejection | 1.10 (0.63–1.95) | 1.21 (1.16–3.65) | .50 |
Note: Adjusted for age, sex, race, BMI, dialysis pre-transplant, duration of dialysis pre-transplant PRA, donor age, donor sex, donor race, previous transplant, delayed graft function, CNI use, HLA mismatch.
3.4 |. Incidence rate of acute rejection or recurrent glomerular disease stratified by primary GN diagnosis
Patients with primary MN had a significantly higher incidence rate of acute rejection compared with IgA (12.4 vs 7.2 per 100 person-years, respectively, P < .05, Table 3). The timing of acute rejection was similar among all four subgroups. FSGS and MN patients had significantly higher rates of glomerular disease recurrence compared with IgA (1.7 and 1.9, respectively, vs 1.2 per 100 person-years, P < .05). The shortest time to disease recurrence was seen in FSGS (3.7 months in FSGS vs 33.7 months in IgA, P < .0005).
TABLE 3.
Incidence rate of acute rejection or recurrent glomerular disease following transplant stratified by primary glomerular disease diagnosis
| Primary glomerular disease | Incidence rate of acute rejection (per 100 person-y) | Time to acute rejection (median, in mo) | Incidence rate of recurrent glomerular disease (per 100 person-y) | Time to recurrent glomerular disease (median, in mo) |
|---|---|---|---|---|
| IgA | 7.2 | 3.9 | 1.2 | 33.7 |
| FSGS | 7.4 | 2.1 | 1.7* | 3.7*** |
| MN | 12.4* | 3.1 | 1.9* | 11.7 |
| LN | 7.9 | 2.5 | 1.0 | 32.7 |
P < .05 compared to IgA.
P < .0005 compared to IgA.
3.5 |. Allograft survival after acute rejection or recurrence by primary GN diagnosis
Among all the primary GN diagnoses, IgA patients had better allograft survival after glomerular disease recurrence than FSGS, MN, or LN (Figure 4A). The differences in allograft survival emerged after 2.5 years following a diagnosis of recurrent disease; with significantly more graft failures in patients with biopsy-confirmed recurrent FSGS, MN, or LN compared with IgA (16.7 vs 7.5 per 100 person-years, P = .04). The advantage in allograft survival for IgA patients did not achieve significance following rejection (P = .10 for IgA vs FSGS, MN, and LN; Table 4 and Figure 4B).
FIGURE 4.
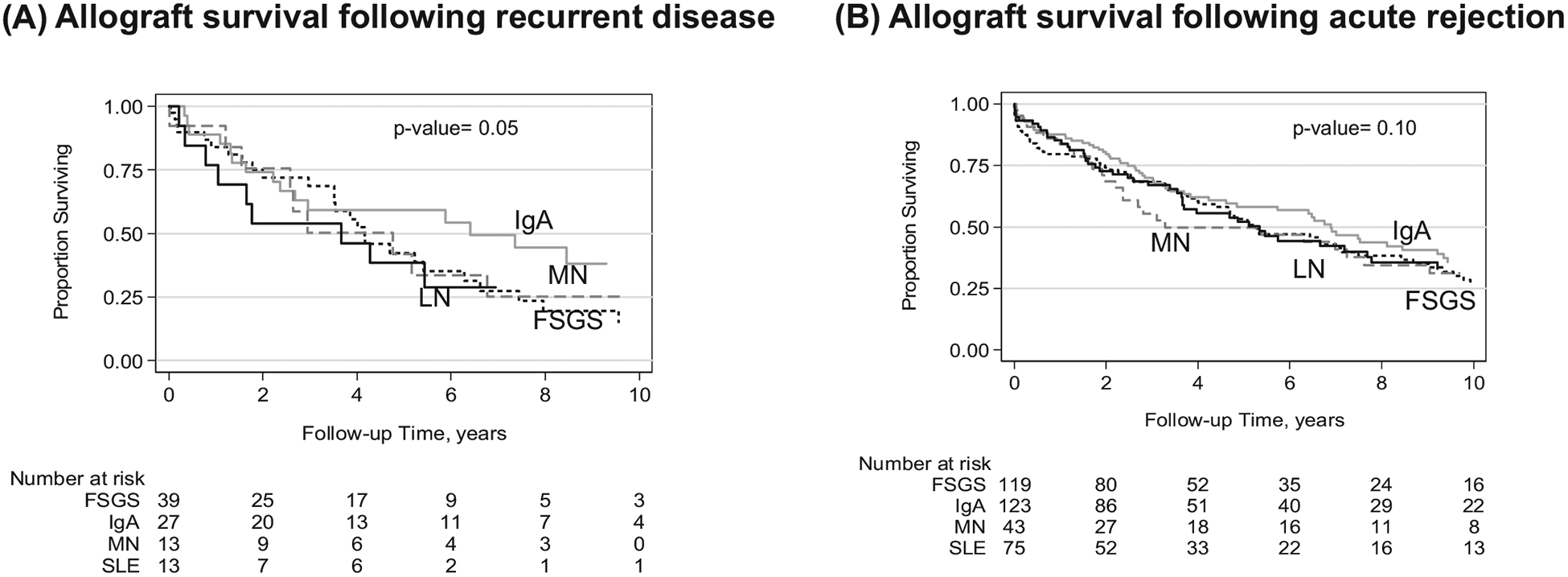
Kaplan-Meier curve of allograft survival for kidney transplant patients stratified by primary glomerular disease diagnosis following recurrent disease (A) or acute rejection (B). Panel A shows allograft survival following biopsy-confirmed recurrent glomerular disease, stratified by primary disease (P = .05 for IgA vs LN, MN, and FSGS). Panel B shows graft survival following biopsy diagnosis of acute rejection, stratified by primary disease (P = .10 for IgA vs LN, MN, and FSGS). IgA (solid gray line), FSGS (dashed black line), MN (dashed gray line), or LN (solid black line)
TABLE 4.
Adjusted hazard ratio for graft survival after acute rejection or recurrent GN with IgA as reference group
| Primary glomerular disease | aHR after rejection | P-interaction | aHR after recurrence | P-interaction |
|---|---|---|---|---|
| FSGS | 1.13 (0.80–1.61) | .48 | 2.33 (0.99–5.52) | .05 |
| MN | 1.04 (0.65–1.68) | .86 | 1.99 (0.91–5.63) | .19 |
| LN | 1.11 (0.73–1.70) | .61 | 2.90 (0.91–9.28) | .07 |
Note: Adjusted for age, sex, race, BMI, dialysis pre-transplant, duration of dialysis pre-transplant PRA, donor age, donor sex, donor race, previous transplant, delayed graft function, CNI use, HLA mismatch.
4 |. DISCUSSION
In this study, we found the incidence rate of acute rejection was higher than recurrent glomerular disease, although long-term allograft survival after acute rejection or disease recurrence was similar in transplant recipients with ESRD due to primary GN. The diagnosis of coexisting acute rejection and recurrent GN was rare. When stratified by the primary GN diagnosis, there was no difference in allograft survival following biopsy-confirmed rejection. However, following biopsy-confirmed recurrent disease patients with a history of primary IgA nephropathy had significantly better graft survival than their counterparts with MN, FSGS, or LN.
Briganti et al5 have previously shown that long-term graft survival is worse after GN disease recurrence than acute rejection. However, we found that the long-term graft survival was similar after GN recurrence or acute rejection. The major reason for this difference in outcomes is likely that their data are from 1988 to 1997 that is much older than our data from 1994 to 2013. There are newer immunosuppressants available to treat both acute rejection as well as GN disease recurrence that will affect graft outcome in current era. There are multiple recent registry updates on outcomes of allograft survival in kidney transplant recipients based on primary GN diagnosis.10–13 These registry studies assessed overall allograft survival in transplant recipients with primary glomerular disease, we examined allograft survival in this group following a biopsy-confirmed diagnosis of acute rejection or recurrent glomerular disease. One limitation of registry-based studies is that a biopsy-confirmed diagnosis of GN or rejection often cannot be determined or may not be available for the entire cohort of patients.14 We think that this is an important information for clinicians as well as patients to know the prognosis of graft with ESRD due to GN after the first episode of recurrent disease or rejection.
We observed that acute rejection was more common than recurrent glomerular disease in transplant recipients with ESRD due to primary GN. Acute rejection is an alloimmune response directed at the allograft, while GN recurrence, in many cases, is an autoimmune insult to the allograft. Another interesting observation was that the majority of patients who develop GN recurrence did not develop acute rejection prior to their diagnosis of recurrent glomerular disease, even though acute rejection was more prevalent than glomerular disease recurrence. This suggests the immunosuppressive therapy in the group of transplant recipients with recurrent GN was effective to suppress the alloimmune response, but not the autoimmune response. Reasons for this finding could be because maintenance immunosuppressive regimens after transplant are typically targeted toward T cells, with less suppression of B cells and complement systems. If the activity of B cells and the complement system are left unregulated in patients with a history of primary GN, they can promote immunologic injury and glomerular disease recurrence. More studies are required to understand the effects of immunosuppressive therapies on autoimmune and alloimmune responses and the interplay between them.
We found a graft survival advantage in transplant recipients with primary diagnoses of IgA compared with FSGS, MN, or LN following a biopsy-proven diagnosis of recurrent glomerular disease. The pathogenesis of why this happens is unclear, but we can theorize that patients who develop recurrent disease are patients who had aggressive primary disease. This is evident in patients with a history of primary FSGS who demonstrate an association between the shortest duration of time from transplant to recurrent FSGS and reduced allograft survival.15,16 Previously, we reported patients with MN have higher risk of acute rejection compared with other primary GN.17 In the current study, we found allograft survival following acute rejection in MN patients was similar to other primary GN. However, we observed recurrent LN had a more substantial negative impact on short-term allograft survival than acute rejection but long-term graft survival was similar after recurrent disease or acute rejection. Future studies are needed to diagnose recurrent disease early in this patient group with reduced allograft survival (FSGS, MN, and LN) and to optimize treatment interventions.
We acknowledge that there are limitations in our study. It is a single-center study and hence influenced by the clinical practice trends and patient demographics at our center. However, despite being a single-center study, it does have a sizeable patient cohort to study graft outcomes in this subgroup of patients with native glomerular disease. In addition, we report 10-year allograft survival; many reports in the literature range in follow-up time from 1 year to 15 years. It is important to have a long duration of follow-up in studies of recurrent GN as some recurrent glomerular diseases rarely manifest clinically until after five years or later. Another limitation in our study is that the graft biopsies were done on a for-cause basis of increasing creatinine and/or proteinuria or hematuria and were not performed on a protocol basis.
To conclude, we observed that recurrent GN had similar impact on long-term graft survival as acute rejection in patients with ESRD due to GN. The group of transplant recipients with diagnoses of primary FSGS, MN, or LN had the lowest allograft survival and this appears to be driven by recurrent primary disease. The analysis of transplant recipient outcomes based on underlying disease marks a step forward in understanding the pathophysiology of graft loss in this segment of the transplant population and makes progress toward a personalized medicine approach.
ACKNOWLEDGEMENTS
We thank Dana Clark, MA for her copy editing assistance.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Saran R, Robinson B, Abbott KC, et al. US renal data system 2016 annual data report: epidemiology of kidney disease in the united states. Am J Kidney Dis. 2017;69(3 Suppl 1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9(3):527–535. [DOI] [PubMed] [Google Scholar]
- 3.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of anti-body-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–399. [DOI] [PubMed] [Google Scholar]
- 4.Hariharan S, Adams MB, Brennan DC, et al. Recurrent and de novo glomerular disease after renal transplantation: a report from renal allograft disease registry (RADR). Transplantation. 1999;68(5):635–641. [DOI] [PubMed] [Google Scholar]
- 5.Briganti EM, Russ GR, McNeil JJ, et al. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347(2):103–109. [DOI] [PubMed] [Google Scholar]
- 6.Chailimpamontree W, Dmitrienko S, Li G, et al. Probability, predictors, and prognosis of posttransplantation glomerulonephritis. J Am Soc Nephrol. 2009;20(4):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moroni G,Longhi S, Quaglini S, et al. The impact of recurrence of primary glomerulonephritis on renal allograft outcome. Clin Transplant. 2014;28(3):368–376. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim H, Rogers T, Casingal V, et al. Graft loss from recurrent glomerulonephritis is not increased with a rapid steroid discontinuation protocol. Transplantation. 2006;81(2):214–219. [DOI] [PubMed] [Google Scholar]
- 9.Kukla A, Chen E, Spong R, et al. Recurrent glomerulonephritis under rapid discontinuation of steroids. Transplantation. 2011;91(12):1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruthi R, McClure M, Casula A, et al. Long-term graft outcomes and patient survival are lower posttransplant in patients with a primary renal diagnosis of glomerulonephritis. Kidney Int. 2016;89(4):918–926. [DOI] [PubMed] [Google Scholar]
- 11.Pippias M,Stel VS, Aresté-Fosalba N, et al. Long-term kidney transplant outcomes in primary glomerulonephritis: analysis from the ERA-EDTA registry. Transplantation. 2016;100(9):1955–1962. [DOI] [PubMed] [Google Scholar]
- 12.O’Shaughnessy MM, Liu S, Montez-Rath ME, et al. Kidney transplantation outcomes across GN subtypes in the United States. J Am Soc Nephrol. 2017;28(2):632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosio FG, Cattran DC. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int. 2017;91(2):304–314. [DOI] [PubMed] [Google Scholar]
- 14.Layton JB, Hogan SL, Jennette CE, et al. Discrepancy between medical evidence form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol. 2010;5(11):2046–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardon A, Audard V, Caillard S, et al. Risk factors and outcome of focal and segmental glomerulosclerosis recurrence in adult renal transplant recipients. Nephrol Dial Transplant. 2006;21(4):1053–1059. [DOI] [PubMed] [Google Scholar]
- 16.Francis A, Trnka P, McTaggart SJ. Long-term outcome of kidney transplantation in recipients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2016;11(11):2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh T, Astor B, Zhong W, et al. Kidney transplant recipients with primary membranous glomerulonephritis have a higher risk of acute rejection compared with other primary glomerulonephritides. Transplant Direct. 2017;3(11):e223. [DOI] [PMC free article] [PubMed] [Google Scholar]


