Abstract
Purpose
The aim of this study was to explore the use of self-assembling nanoparticles loaded with two chemotherapy drugs for the treatment of multiple myeloma.
Materials and Methods
Nanoparticle systems were developed based on amine polyethylene glycol-polycaprolactone (NH2-PEG-PCL) to encapsulate 5-Aza-2ʹ-deoxycytidine and Bortezomib using the self-assemble method. Morphological changes were observed by transmission electron microscopy (TEM), and the size, drug release, long-term stability, and release of reactive oxygen species (ROS) were analyzed. The MTT assay was used to evaluate the effects of drug-loaded nanoparticles (PEG-PCL-DAC-BTZ) in inhibiting the proliferation of multiple myeloma cells (U266 and LP-1), and the TUNEL assay and Western blotting were used to measure the induction of cell apoptosis.
Results
Based on the diameter of NH2-PEG-PCL and PEG-PCL-DAC-BTZ, the drug-loaded nanoparticles were successfully prepared. TEM revealed that PEG-PCL-DAC-BTZ was spherically shaped. More than 90% of the drugs were released after 72 h, and PEG-PCL-DAC-BTZ maintained a good stability. U266 and LP-1 cells treated with PEG-PCL-DAC-BTZ showed the highest growth inhibition, release of ROS, and cell apoptosis compared to those treated with unloaded nanoparticles and chemotherapy drugs alone.
Conclusion
The drug-loaded nanoparticles are a good foundation for the treatment of multiple myeloma as they showed a slow release profile, good stability, and superior anti-cancer effects in vitro.
Keywords: multiple myeloma, nanoparticle, 5-Aza-2ʹ-deoxycytidine, Bortezomib
Introduction
Multiple myeloma, a neoplasm that originates from plasma cells, accounts for 2% of hematological diseases. It is predominantly prevalent in the elderly population and has a median overall survival of 5 years only.1 Despite the fact that immunomodulatory drugs and proteasome inhibitors have improved its treatment outcome, the frequent relapses still possess a challenge.2,3 Hence, it is urgent to develop new treatment approaches for multiple myeloma.
Combination therapy is considered a promising approach to overcome drug resistance and thereby improve long-term treatment outcomes. Two commonly used drugs for multiple myeloma include 5-Aza-2ʹ-deoxycytidine (DAC) and Bortezomib (BTZ). DAC is a nucleoside analog that incorporates into the genome of highly replicative cancer cells and disrupts their DNA synthesis.4 BTZ exerts its anti-cancer effect by serving as a protease inhibitor that attenuates tumor growth due to the highly replicative nature of tumor cells.5,6 However, the efficacy of combination therapy with DAC and BTZ has not been explored. Additionally, emerging studies have suggested that inefficient delivery of cancer drugs to the cancer cells is the bottleneck of treatment of multiple myeloma, a neoplasm that is highly metastatic and dispersive. Therefore, the development of a drug delivery platform to enhance the specific accumulation of drugs in tumor sites and facilitate the internalization of drugs by cancer cells is warranted.
In previous studies, a variety of nanoparticle systems have been shown to efficiently deliver chemotherapy drugs to multiple myeloma sites.7–11 For example, the use of liposomes with encapsulate carfilzomib and doxorubicin in the treatment of multiple myeloma has been shown to enhance tumor-inhibiting effects and reduce systemic toxicity.12 BTZ delivery using hyaluronic acid shell and disulfide-crosslinked core micelles has also been explored.7 A nanoparticle system based on NH2-PEG-PCL, which self-assembles with hydrophobic drugs into micelles, was previously shown as a potential platform that enhances the delivery and uptake of chemotherapy drugs in breast, ovarian, and gastric cancers.13–15 However, this nanoparticulate system has not been applied to the treatment of multiple myeloma.
Therefore, our study aimed to synthesize a new delivery system for DAC-BTZ using NH2-PEG-PCL, which will serve as a new combination therapy for multiple myeloma and characterize its ability to enhance the antitumor effects of the drugs. The size, drug release profile, and stability of the drug-loaded particles were characterized. On basis of cell survival, ROS release, and apoptosis, cells treated with the drug-loaded particles were compared with cells treated with drugs alone or untreated cells. This finding could potentiate the use of the novel nanoparticle drug delivery system for improving the therapeutic outcome of multiple myeloma.
Materials and Methods
Materials
Unless stated otherwise, all solvents used for chemistry were purchased from Sigma. The multiple myeloma tumor cell lines (U266 and LP-1) were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA), NH2-PEG-PCL from Ponsure Biotechnology (Shanghai, China), 5-Aza-2ʹ-deoxycytidine from Sigma (Saint Louis, MO, USA), and Bortezomib from Foshan Xinhang Biotechnology, Ltd. (Foshan, China).
Drug Loading
Four milligrams of NH2-PEG-PCL were dissolved in 2 mL N, N-dimethylformamide (DMF). 5-Aza-2ʹ-deoxycytidine (DAC) (6 mg) and Bortezomib (BTZ) (1 mg) were mixed at a molar ratio of 100:1, which was dissolved with 1 mL DMF. The DAC-BTZ mixture was added to NH2-PEG-PCL nanoparticles at a weight ratio of 2:1, followed by stirring for 2 h. The drug-loaded particles were purified by dialysis against ddH2O using a dialysis bag (MWCO=3000 Da) to remove free drugs. After 24 h of dialysis, the nanoparticles were used for further analysis.
The Size of PEG-PCL-DAC-BTZ
The size of the nanoparticles was analyzed by dynamic light scattering (DLS). Briefly, the nanoparticles were diluted with distilled water and put on a copper grid covered with nitrocellulose. Thereafter, the samples were negatively stained with 5% phosphotungstic acid and dried at room temperature. Then, the size of the samples was determined by DLS using Malvern Nano-ZS 90 at room temperature, and each sample was analyzed three times.
Cell Culture
An RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (Gibco, Carlsbad, CA, USA) in a humid chamber maintained at 37°C and 5% CO2. Then, the cells were treated for 4 h with 0.5 μmol/L DAC and 20 ng/mL BTZ.
In vitro Release Ability of PEG-PCL-DAC-BTZ
The lyophilized powder of PEG-PCL-DAC-BTZ was dissolved in deionized water and 5 mL of the solution was put into a dialysis bag (molecular weight cut-off 3000 Da). Then, the bag was immersed in 30 mL of phosphate-buffered saline (PBS, pH 7.4) containing Tween-80 (0.5% w/w) and the medium was stirred at 70 rpm at 37°C. Samples were collected at the 12th, 24th, 36th, 48th, and 72nd h and the same volume of fresh PBS was added to maintain the buffer volume. The concentration of the released DAC-BTZ in the dialysis media was determined by HPLC (LC-10ATvp, Shimadzu) with a C18 column (Symmetry shield TM RP18, 3.9 mm × 150 mm, from Waters) at 25°C. The accumulative release amount of DAC-BTZ was calculated using a calibration curve and expressed as the percentage of released concentration.
MTT Assay
Cells in the logarithmic growth phase (1×104 cells/mL) were seeded in 96-well plates. After culturing for 24 or 48 h, treatment was conducted for 4 h and 200 µL MTT (5 mg/mL) was added to the medium and cultured for another 4 h. Then, the supernatant was discarded and 150 µL of DMSO was added, followed by shaking for 15 min to dissolve the crystal. Absorbance was measured at 570 nm and the following equation was used to calculate the inhibition rate of cell growth: percent survival = (1-ODexperiment/ODcontrol)×100%.
Characterization of ROS Release
The ROS release was quantified using the conversion of non-fluorescent 5, 6-Chloromethyl-2V, 7Vdichlorodihydrofluorescein diacetate (CM-H2DCFDA) to its fluorescent derivative (DCF) by SpectraMax iD5 (Molecular Devices, Sunnyvale, CA, USA). Briefly, U266 or LP-1 cells were treated with PEG-PCL-DAC-BTZ micelles. Then, the media were removed, and the cells were lysed and centrifuged to eliminate debris. The fluorescence in the supernatant was assessed using a fluorometer when excitation wavelength was 500 nm and emission wavelength was 530 nm.
Cell Apoptosis Assay
Cells seeded in 12-well plates at the density of 1×105cells/mL were cultured for 48 h. Then, 50 µL TUNEL agent (Beyotime, C1088) was added to the cells washed with PBS twice. After incubating for 60 min at 37°C, the cells were washed and resuspended with 250–500 µL PBS. Then, the fluorescence was recorded through the FITC channel of a fluorescence microscope, and intensity of TUNEL signal was quantified using ImageJ.
Western Blot Analysis
After lysing 2×105 cells by culturing them in 6-well plates for 48 h, their proteins were extracted. Equal amounts of protein were loaded in 10% gel and subjected to SDS-PAGE at 120 V. Electrotransferring was performed at 100 V for 120 min on PVDF membranes. After blocking with 5% fat milk for 1 h, primary antibodies against cleaved-caspase-9 (Abcam, ab2324), cleaved-caspase-3 (Abcam, ab2302), or GAPDH (Abcam, ab9485) were added to the membrane and incubated overnight at 4°C. Next, the membrane was washed 3 times with TBST, and the HRP-conjugated secondary antibody was added and incubated for another 1 h. Then, the PVDF membranes were washed three times (15 min each) with TBST, and ECL substrate was visualized using film exposure.
Statistical Analysis
Statistical analysis was performed by SPSS 20.0 using Student’s t-test. Data derived from the three replicates in every experiment were presented as mean ± SD. p values < 0.05 were considered statistically significant.
Results
Drug Loading and Characterization of the Particles
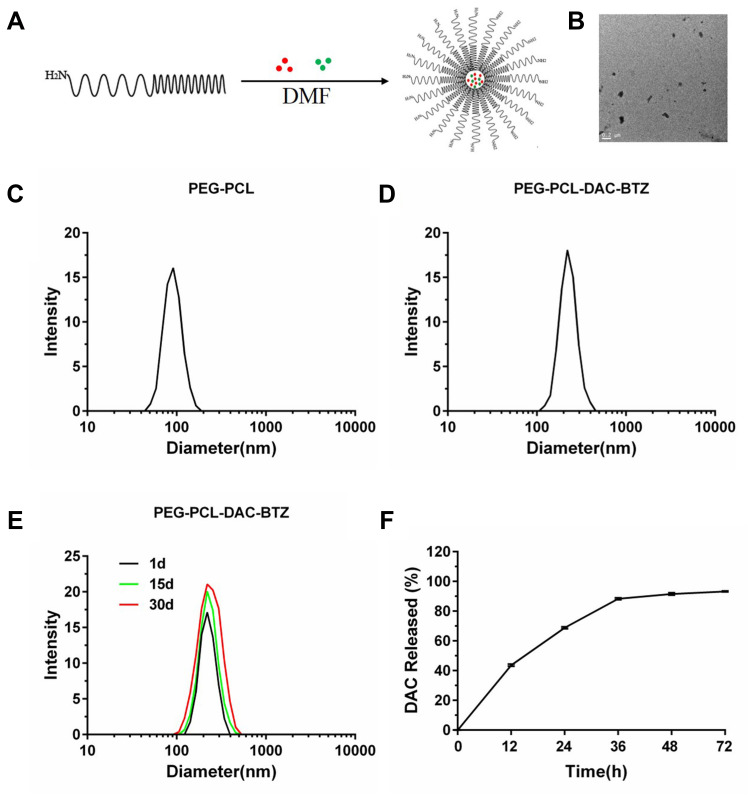
To form a drug-loading system for DAC and BTZ, we employed NH2-PEG-PCL, an amphiphilic nanoparticle system, which encapsulated DAC and BTZ in the core of the particles showing the schematic structure of PEG-PCL-DAC-BTZ (Figure 1A). The transmission electron microscopy (TEM) revealed that the nanoparticle PEG-PCL-DAC-BTZ was spherically shaped (Figure 1B). Dynamic light scattering (DLS) was used to characterize the sizes of the particles before and after drug loading. As shown in (Figure 1C and D), the particle diameter significantly increased after drug loading (220.2 nm) compared with that of NH2-PEG-PCL (91.28 nm), suggesting that the particle formation with the drugs was successful. The drug loading efficacy was 40.87%. To evaluate the stability of the nanoparticle system, particles suspended in PBS for 1, 15, and 30 days were compared for size distribution using DLS (Figure 1E). Drug release was measured by transferring the drug-loaded particles into a dialysis bag and quantifying the drug released into PBS. The drug release profile during 72 h is shown in (Figure 1F). At 72 h, more than 90% of the drug was released from the nanoparticle system, suggesting a slow release profile. Our data suggested that the change of particle size was negligible, indicating that the nanoparticles had a good stability.
Figure 1.
Drug loading and characterization of the particles. (A) The schematic structure of PEG-PCL-DAC-BTZ prepared in this study; (B) the morphology of PEG-PCL-DAC-BTZ by transmission electron microscopy (TEM); (C and D) dynamic light scattering characterization of unloaded particles, PEG-PCL, and drug-loaded particles, PEG-PCL-DAC-BTZ; and (E) stability characterization using dynamic light scattering. Particles were placed in dialysis bags against PBS for 1, 15, and 30 days. (F) DAC release profile of the PEG-PCL-DAC-BTZ nanoparticle system.
PEG-PCL-DAC-BTZ Inhibits Multiple Myeloma by Promoting Cell Apoptosis
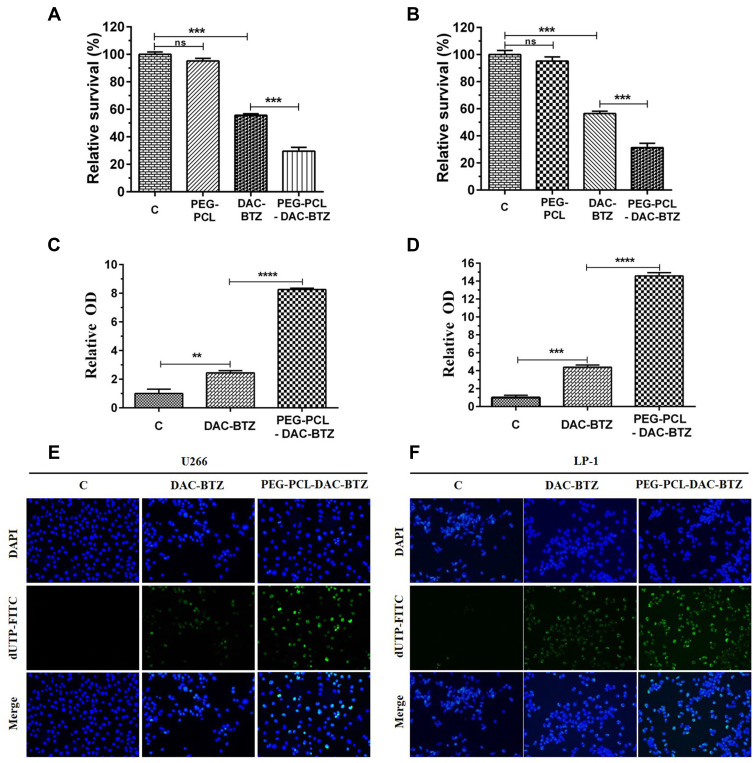
The cytotoxicity effects of the drug-loaded nanoparticles were first tested using MTT assay. The multiple myeloma cells were incubated for 4 h with the unloaded nanoparticles (NH2-PEG-PCL), the mixture of two drugs (DAC-BTZ), and the drug-loaded particles (PEG-PCL-DAC-BTZ) at the dose of 0.5 μmol/L and 20 ng/mL for DAC and BTZ, respectively, and the cell survival was evaluated. The untreated cells were used as control. As shown in Figure 2A and B, cells treated with PEG-PCL-DAC-BTZ exhibited the lowest survival among other groups, and their survival was approximately 40% lower (p < 0.001) than that of cells treated with DAC-BTZ or control. To explain the mechanism of the cytotoxic effects, the ROS levels of cells treated with DAC-BTZ or PEG-PCL-DAC-BTZ were measured. As shown in (Figure 2C and D), the ROS level in the multiple myeloma cells treated with PEG-PCL-DAC-BTZ was dramatically higher than that in the cells treated with DAC-BTZ (p < 0.0001). In agreement to this, TUNEL assay also suggested that PEG-PCL-DAC-BTZ treatment let to prominently higher apoptosis in cells, which was evidenced by apparently stronger fluorescence in cells with PEG-PCL-DAC-BTZ treatment (Figure 2E and F), compared to those treated with DAC-BTZ and control.
Figure 2.
PEG-PCL-DAC-BTZ inhibits multiple myeloma by promoting cell apoptosis. (A and B) MTT assay to study the survival of PEG-PCL-DAC-BTZ treated multiple myeloma cell lines (U266 and LP-1) compared to the control (untreated), PEG-PCL, or DAC-BTZ treated cells. (C and D) ROS release characterization. U266 cells and LP-1 cells treated with PEG-PCL-DAC-BTZ showed the highest ROS release. (E and F) TUNEL assay of multiple myeloma cell lines (U266 and LP-1) treated with DAC-BTZ or PEG-PCL-DAC-BTZ shows that PEG-PCL-DAC-BTZ induced the highest apoptosis (**p < 0.01, ***p < 0.001, ****p < 0.0001).
PEG-PCL-DAC-BTZ Up-Regulates the Expression of Cell Apoptosis Markers
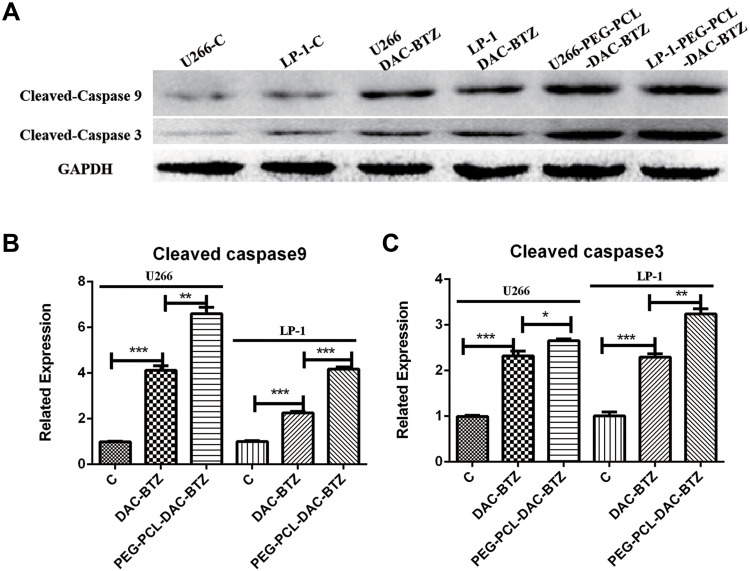
Western blot assay (Figure 3) was performed to detect the expression of the apoptosis markers cleaved-caspase-3 and cleaved-caspase-9. Compared to untreated cells, DAC-BTZ treated cells demonstrated a higher expression of cleaved-caspase-3 (p = 0.0005) and cleaved-caspase-9 (p = 0.0006), suggesting that DAC-BTZ has pro-apoptotic ability. Notably, PEG-PCL-DAC-BTZ treatment led to an even higher up-regulation of cleaved-caspase-3 (p < 0.0001) and cleaved-caspase-9 (p = 0.0001) compared to DAC-BTZ treatment, indicating a higher apoptosis rate in PEG-PCL-DAC-BTZ treated cells.
Figure 3.
PEG-PCL-DAC-BTZ up-regulates the expression of cell apoptosis markers. (A) Western blot assay of cleaved-caspase-9 and cleaved caspase-3 to quantify the levels of cell apoptosis in multiple myeloma cell lines (U266 and LP-1) treated with DAC-BTZ or PEG-PCL-DAC-BTZ. (B and C). Quantification of cleaved-caspase-3 and cleaved-caspase-9. GAPDH was used as loading control (*p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
Bortezomib (BTZ) is one of the most effective drugs used in treating multiple myeloma. However, most multiple myeloma patients who receive BTZ develop drug resistance which leads to recurrence and even poor prognosis.16,17 To overcome this, novel reagents that treat multiple myeloma need to be urgently developed. Decitabine (DAC) not only enhances myeloma cell sensitivity to BTZ, but also depletes myeloid-derived suppressor cells (MDSCs) in the multiple myeloma microenvironment. MDSCs are essential for myeloma cell survival and immune escape in the multiple myeloma microenvironment.18 To sum up, we deduced that novel and potential strategies for multiple myeloma patients after relapse can likely be developed by adding DAC to currently available and effective anti-myeloma therapies.
The recent development of drug-delivery systems based on nanoparticles has attested that this strategy has great potential in enhancing cancer treatment outcome. Here, we characterized the anti-cancer effects of nanoparticles self-assembled with DAC and BTZ in vitro, which showed that these nanoparticles significantly inhibited cell survival and induced ROS release and apoptosis. The slow-release profile could also increase the amounts of drugs metabolized by the cells, as high-concentration drugs could easily be execrated by cells as a defensive system.
In this present study, transmission electron microscopy (TEM) revealed that the nanoparticle PEG-PCL-DAC-BTZ, which was prepared in the study, was spherically shaped. Our data suggested that the size of nanoparticles after drug-loading (220.2 nm in diameter) was drastically higher than that before drug-loading (91.28 nm in diameter), which confirmed that the drug-loading micelle was successfully prepared. The size of the nanoparticles was not influenced by drug release. The slow-release profile of the drug-load nanoparticles in this study is similar to that of nanoparticles in another study,14 which showed the release of PTX from NH2-PEG-PCL-based particles. These characteristics, along with the high stability of the nanoparticles, are desirable traits for in vivo delivery of drugs to tumors. Although we have not tested the in vivo delivery efficacy of the system, it is expected that the nanoparticles would demonstrate a higher efficacy in smuggling drugs into tumors due to the enhanced perfusion and retention (EPR) effect of tumors.
The nanoparticles were programmed to simultaneously deliver a combination of DAC and BTZ to multiple myeloma. Recently, this combinatory approach is gaining increasing attention due to its enhanced efficacy in relapsed cases.19–21 For example, the immunotherapy drug, lenalidomide, has been used in combination with dexamethasone as a standard regimen in patients with relapsed or refractory multiple myeloma.22 However, caution has to be taken to avoid increased toxicity due to the use of multiple drugs. In this scenario, the enhanced tumor uptake and decreased systemic toxicity of the nanoparticle delivery system make it a desirable platform. Here, we showed that the DAC-BTZ combination therapy remarkably attenuated proliferation and induced ROS release and apoptosis in multiple myeloma cells. Also, the DAC-BTZ-loaded nanoparticles exerted higher anti-cancer effects. Further in vivo testing of this system is warranted. In addition, the current system can be improved by modifying the nanoparticles with tumor-targeting ligand, as it has been explored by previous studies.7,14 In the present study, the properties and in vitro anti-tumor effect of the drug-loaded nanoparticle have been preliminarily studied. Additionally, the anti-tumor mechanism and in vivo anti-cancer activity of the nanoparticle needs to be further studied. Meanwhile, enhancing the targeting of the drug-loaded nanoparticle is an important content and a challenge for follow-up research.
Conclusion
Summarily, we exploited the self-assemble NH2-PEG-PCL nanoparticle system to encapsulate two chemotherapy drugs, DAC and BTZ, for the treatment of multiple myeloma. First, our results indicated that the PEG-PCL-DAC-BTZ nanoparticle exhibited properties with a slow release and good stability. Then, we verified the enhanced efficacy of the drug-loaded nanoparticles in inhibiting multiple myeloma cell growth and inducing ROS release and apoptosis, which has paved the way for further development of the system for the treatment of multiple myeloma in vivo.
Acknowledgment
Many thanks go to Medical Research Youth Innovation Project of Sichuan Province.
Funding Statement
This work was supported by grants from Medical Research Youth Innovation Project of Sichuan Province (Q18017).
Disclosure
Non-financial competing interests. The authors report no conflicts of interest in this work.
References
- 1.Kumar SK, Rajkumar SV. The multiple myelomas – current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol. 2018;15(7):409–421. doi: 10.1038/s41571-018-0018-y [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;1(91):101–119. doi: 10.1016/j.mayocp.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149. doi: 10.1038/leu.2011.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heller G, Schmidt WM, Ziegler B, et al. Genome-wide transcriptional response to 5-aza-2′-deoxycytidine and trichostatin a in multiple myeloma cells. Cancer Res. 2008;68(1):44–54. doi: 10.1158/0008-5472.CAN-07-2531 [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038 [DOI] [PubMed] [Google Scholar]
- 6.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479 [DOI] [PubMed] [Google Scholar]
- 7.Gu ZX, Wang XX, Cheng R, et al. Hyaluronic acid shell and disulfide-crosslinked core micelles for in vivo targeted delivery of bortezomib for the treatment of multiple myeloma. Acta Biomater. 2018;80:288–295. doi: 10.1016/j.actbio.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 8.Hu QY, Qian CG, Sun WJ, et al. Engineered nanoplatelets for enhanced treatment of multiple myeloma and thrombus. Adv Mater. 2016;28(43):9573-+. doi: 10.1002/adma.201603463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiziltepe T, Ashley J, Stefanick J, et al. Rationally engineered nanoparticles target multiple myeloma cells, overcome cell-adhesion-mediated drug resistance, and show enhanced efficacy in vivo. Blood Cancer J. 2012;2(4):e64. doi: 10.1038/bcj.2012.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel P, Misra S, Rodriguez NS, et al. Combined nanoparticle delivery of PARP and DNA-PK inhibition for multiple myeloma. Am Soc Hematol. 2017;130:1809. [Google Scholar]
- 11.de la Puente P, Luderer MJ, Federico C, et al. Enhancing proteasome-inhibitory activity and specificity of bortezomib by CD38 targeted nanoparticles in multiple myeloma. J Control Release. 2018;270:158–176. doi: 10.1016/j.jconrel.2017.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashley JD, Quinlan CJ, Schroeder VA, et al. Dual carfilzomib and doxorubicin-loaded liposomal nanoparticles for synergistic efficacy in multiple myeloma. Mol Cancer Ther. 2016;15(7):1452–1459. doi: 10.1158/1535-7163.MCT-15-0867 [DOI] [PubMed] [Google Scholar]
- 13.Ding J, Feng M, Zhi W, et al. [Tracing study of nanometer microspheres labeled with fluorescent dye on gastric cancer cells]. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18(2):171–176. Chinese. [PubMed] [Google Scholar]
- 14.Wang Q, Wang D, Li D, et al. Folate modified nanoparticles for targeted co-delivery chemotherapeutic drugs and imaging probes for ovarian cancer. Biomed Phys Eng Express. 2015;1(4):045009. [Google Scholar]
- 15.Youm I, Agrahari V, Murowchick JB, et al. Uptake and cytotoxicity of docetaxel-loaded hyaluronic acid-grafted oily core nanocapsules in MDA-MB 231 cancer cells. Pharm Res. 2014;31(9):2439–2452. doi: 10.1007/s11095-014-1339-x [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Gu H, Zhang E, et al. The NEDD4-1 E3 ubiquitin ligase: a potential molecular target for bortezomib sensitivity in multiple myeloma. Int J Cancer. 2020;146(7):1963–1978. doi: 10.1002/ijc.32615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Z, Fan S, Zheng J, et al. Inhibition of thioredoxin activates mitophagy and overcomes adaptive bortezomib resistance in multiple myeloma. J Hematol Oncol. 2018;11:29. doi: 10.1186/s13045-018-0575-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ning L, Liu L, Xiang P, et al. Addition of low-dose decitabine to bortezomib and dexamethasone as second-line therapy in multiple myeloma. Br J Haematol. 2020. [DOI] [PubMed] [Google Scholar]
- 19.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.San-Miguel JF, Hungria VT, Yoon -S-S, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind Phase 3 trial. Lancet Oncol. 2014;15(11):1195–1206. doi: 10.1016/S1470-2045(14)70440-1 [DOI] [PubMed] [Google Scholar]
- 21.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. doi: 10.1056/NEJMoa1411321 [DOI] [PubMed] [Google Scholar]
- 22.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594 [DOI] [PubMed] [Google Scholar]