Abstract
Obesity has become a major risk factor for the development of chronic diseases such as insulin resistance, type 2 diabetes mellitus, and cardiovascular disease. Moreover, obesity induces chronic inflammation in adipose tissue, liver, skeletal muscle, and the vascular system. Quercetin is the major representative of the flavonoid subclass of flavonols, which is ubiquitously contained within natural plants such as green tea, and vegetables, including onions and apples. Researchers have focused greater attention to the beneficial physiological roles of quercetin, which has anti-oxidative, anti-inflammatory, and anti-fibrotic effects on insulin resistance and atherosclerosis in obesity-related diseases. Also, the anti-inflammatory effects of quercetin on intestinal microbiota have been demonstrated in obesity. In addition, there is increasing evidence that quercetin is associated with epigenetic activities in cancer, and in maternal undernutrition during gestation and lactation. In this review, we focus on the chemical properties of quercetin, its dietary sources in obesity, and its anti-inflammatory effects on insulin resistance, atherosclerosis, intestinal microbiota, and maternal under-nutrition with epigenetic activity.
Keywords: quercetin, inflammation, obesity, insulin resistance, atherosclerosis
Introduction
Obesity has become one of the most prevalent health problems globally and represents a major risk factor for the development of chronic diseases such as insulin resistance, type 2 diabetes mellitus, and cardiovascular disease.1 Moreover, obesity induces chronic inflammation in adipose tissue, the liver, skeletal muscle, and the vascular system. Chronic inflammation induces release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and interleukin-6 (IL-6), and immune cell infiltration is closely associated with the development of insulin resistance through interactions with the insulin signaling pathway in adipose tissue and skeletal muscle2,3 and is closely linked to the pathogenesis of atherosclerosis in vessel walls.4
Quercetin, a flavonoid compound, is found in vegetables and plants such as onions, apples, and green tea. For instance, the dominant onion flavonoids have been determined to be quercetin, quercetin-3-O-β-glucoside (Q3G), quercetin-4ʹ-O-β-glucoside (Q4ʹG), and quercetin-3,4ʹ-di- O-β-glucoside (Q3,4ʹG).5 Interestingly, cooking methods affect the final flavonoid content; total quercetin abundance is increased 1.5-fold by microwave heating for 1 min, whereas the levels of Q4ʹG are decreased by boiling.6
To date, researchers have focused more attention on the beneficial physiological roles of quercetin, which has anti-oxidative, anti-inflammatory, and anti-fibrotic effects,7–9 although quercetin has been reported to have its potential pro-oxidative property in addition to its antioxidative property.10,11 More interestingly, there is increasing evidence that quercetin is associated with epigenetic changes in cancer12 and in maternal under-nutrition during gestation and lactation.13 Therefore, studying the physiological roles of quercetin on chronic inflammation would contribute to preventive and therapeutic applications in obesity-related diseases.
In this review, we focused on chemical properties, dietary sources, and anti-inflammatory effects of quercetin on insulin resistance, atherosclerosis, intestinal microbiota, and maternal under-nutrition with epigenetic activity.
Chemical Structure and Dietary Source of Quercetin
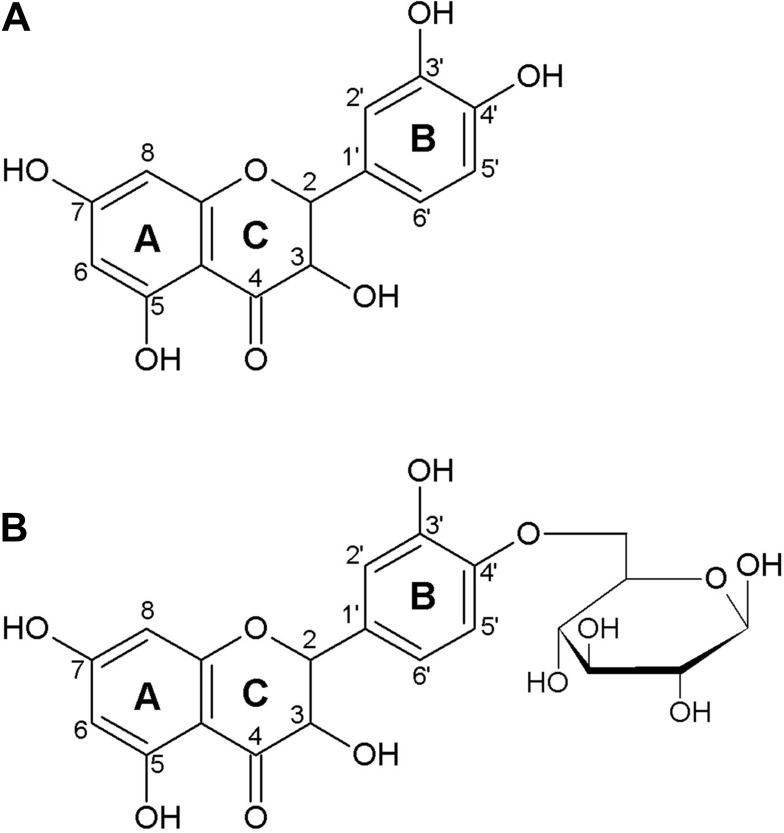
Quercetin is the major representative of the flavonoid subclass of flavonols and is ubiquitously present in plants, fruits, and vegetables. The characteristic structure of flavonoids comprises a basic backbone of flavan, which has a C6-C3-C6 structure in which 2 benzene rings (C6) are bonded by 3 carbons (C3). Flavonols are present in plants as flavonoid-sugar compounds, which are referred to as glycosides in general. Figure 1 illustrates the structures of quercetin aglycone, which lacks a sugar moiety, and of quercetin-β-glucoside, which has been reported to be contained mainly in onions.14,15
Figure 1.
Structure of quercetin aglycon (A), and quercetin-4ʹ-O-β-D-glucoside (B).
The types and amounts of flavonoids are extremely varied among plants. To date, the levels of quercetin in various foodstuffs have been reported in numerous studies.16–20 When foods containing quercetin are consumed, the rate of intestinal absorption is higher for quercetin glycosides than aglycon.21 Hollman et al, who evaluated the bioavailability of quercetin, reported detecting increased concentrations of quercetin in plasma immediately after oral administration of onion and apple supplements.15 Absorbed quercetin is rapidly metabolized in the liver, and circulates as methyl, glucuronide, and sulfate metabolites.22 In human healthy adults, 163 diverse metabolites and quercetin conjugates such as quercetin-3-glucuronide, isorhamnetin-3-glucuronide, quercetin diglucuronide, and quercetin-30-sulphate were measured in the plasma after long-term quercetin-containing supplements; especially the concentrations of conjugates increased at the 1000 mg/day dose for 90 days.23 Therefore, circulating quercetin and its metabolites in peripheral tissues are expected to exhibit bioavailability, resulting in anti-inflammatory effects.
Anti-Inflammatory Effects of Quercetin on Insulin Resistance
Insulin is well known to induce glucose uptake by binding to its receptors on the cell membrane of target organs such as the liver, skeletal muscle, and adipose tissue. When the insulin receptor (IR) is phosphorylated, the phosphorylation of insulin receptor substances (IRS) is increased. IRS activate the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is largely responsible for the metabolic action of insulin, as well as the Ras/mitogen-activated protein kinase (MAPK) pathway, which mediates gene expression for insulin’s effect.24 When the PI3K/Akt pathway is activated, glucose transporter 4 (Glut4) expression and translocation are upregulated, promoting the uptake of glucose into cells.25,26 Insulin resistance is one of the main factors responsible for the onset and progression of diseases such as obesity, diabetes, and atherosclerosis. Insulin resistance is involved in impairment of the PI3K/Akt pathway in target organs such as adipose tissue and skeletal muscle, leading to the downregulation of Glut4 expression and its translocation. In obesity, excessive accumulation of fat in the visceral adipose tissue induces hypertrophy and dysfunction of adipocytes.27,28 The excessive amount of free fatty acids (FFAs) released from visceral adipose tissue is associated with interference in insulin’s action.29
Insulin resistance is closely associated with chronic low-grade inflammation through interactions with the insulin signaling pathway in the liver and adipose tissue.2,3 For instance, elevated levels of proinflammatory cytokines such as TNF-α, MCP-1, and IL-6, and of proinflammatory enzymes such as cyclooxygenases (COXs) and inducible nitric oxide synthase (iNOS) in adipose tissue, skeletal muscle, and neuronal systems, have been demonstrated to lead to the development of insulin resistance.30,31 In particular, TNF-α is one of the most important pro-inflammatory mediators which is involved in the development of insulin resistance.32
Regarding the anti-inflammatory effects of quercetin on proinflammatory cytokine production, in macrophages and adipocytes, it decreases the expression levels of the inflammatory genes TNF-α, IL-6, IL-1β, and COX-2, suppressing the activation of nuclear factor (NF-κB) and c-Jun N-terminal kinase (JNK).33 Dietary quercetin attenuates adipose tissue expansion and decreases the levels of serum IL-6 and MCP-1 mRNA in white adipose tissue in high fat diet (HFD)-induced obese mice.34 In the hypothalamus, quercetin reduces the mRNA expression levels of TNF-α, MCP-1, and IL-1β in HFD-fed obese mice.35 Moreover, dietary quercetin and the combination of quercetin and catechin reduce metabolic parameters such as insulin concentrations and insulin resistance (HOMA-IR), and also levels of adipocytokine proteins such as TNF-α, visfatin, and resistin in adipose tissue in HFD-fed mice.36 The effects of quercetin on proinflammatory enzymes have been investigated in several studies. For example, quercetin inhibits nitric oxide (NO) production and iNOS expression in PC cells treated with dopaminergic neurotoxin 6-hydrooxydopamine, which induces neural damage.37 In lipopolysaccharide-treated colon epithelial cells, the quercetin 3,7-O-dimethyl ether isolated from herbs downregulates the expression of iNOS and COX-2 proteins.38 Intraperitoneal treatment with quercetin suppresses increased COX-2 expression in rats with unilateral ureteral obstruction, which is associated with increased inflammation and oxidative stress.39 Importantly, quercetin may improve insulin resistance through inhibiting the production and expression of proinflammatory cytokines and/or enzymes.
Toll-like receptors (TLRs) are one of the key elements of the immune system, which play central roles in host cell recognition and responses to microbial pathogens.40 Also, TLRs are known to regulate obesity-related inflammation and insulin resistance.41 The activation of TLR pathways encourages the production of proinflammatory cytokines through the upregulation of transcription factors such as NF-κB and activated protein 1 (AP-1).41,42 FAAs released from adipose tissue increases proinflammatory reactions and polarization of M1 macrophages (classically activated macrophages) through upregulation of the TLR4 pathway.43 In addition, age-associated adipose tissue inflammation is reported to be reduced in TLR4-deficient mice fed HFD.44 There is evidence to demonstrate that quercetin has potential to regulate inflammation through the TLR4/NF-κB signaling pathway. Interestingly, quercetin treatment decreases cortical inflammation by inhibiting the TLR4/NF-κB signaling pathway in hypoxia-ischemia-induced brain injury in neonatal rats.45 In addition, the combined treatment of quercetin and catechin inhibits increased levels of proinflammatory mediators, including TNF-α, IL-1β, and COX-2 in lipopolysaccharide (LPS)-stimulated macrophage RAW 264.7 cells, suggesting that the combined treatment may suppress the activation of TLR4-mediated NF-κB and MAPK signaling pathways.46 Therefore, quercetin contributes to the suppression of proinflammatory mediators through inhibition of the TLR4/NF-κB signaling pathway.
Increased activation of AMP-activated protein kinase (AMPK) may play a role in suppression of proinflammatory mediator expression by inhibiting NF-κB activation.47 In addition, metformin, which is widely used as an anti-diabetic drug, ameliorates chronic low-grade inflammation and modulates macrophage polarization through the activation of AMPK in HFD-fed mice.48 Moreover, AMPK is closely linked to fibrosis-promoting pathways, as well as inflammation.49 AMPK activation reduces glomerular TGF-β, collagen, and fibronectin accumulation in several murine models of diabetic kidney disease.50
Several studies have revealed that polyphenolic compounds, such as quercetin, resveratrol, and curcumin, enhance the phosphorylation of AMPK.51 For example, quercetin treatment showed anti-adipogenesis activity through the upregulation of AMPK and acetyl-CoA carboxylase (ACC) phosphorylation in 3T3-L1 preadipocytes.52 The expression of uncoupling protein 1 (UCP1), which plays an important role in increasing energy expenditure, increased in 3T3-L1 adipocytes treated with quercetin.53 The authors suggested that quercetin increased the level of UCP1 in adipose tissues, accompanied by AMPK activation, and may lead to the prevention of obesity.53 In C2C12 myotubes, quercetin ameliorated insulin resistance under inflammatory conditions with activation of AMPK.54 Quercetin suppresses inflammation by modulating the AMPK pathway as well as the silent information regulator (Sirt) 1.55–57 In addition, quercetin reduces macrophage infiltration of adipose tissues, lowers the levels of proinflammatory mediators, and upregulates AMPK phosphorylation and Sirt1 expression in adipose tissues of HFD-fed mice.55 In a renal ischemia/reperfusion (I/R) animal model, quercetin improves renal I/R injury through upregulation of AMPK phosphorylation and activates autophagy during I/R.58 When HFD-fed ApoE-/- mice, an animal atherosclerosis model, received quercetin, the levels of TNF-α, IL-1β, and IL-18 and ratios of microtubule-associated protein light chain 3A (LC3) II/I were restored,59 suggesting that the attenuation of atherosclerosis by quercetin is associated with enhancement of autophagy. Therefore, quercetin may be involved in the resolution of inflammation through upregulation of autophagy activation.
In addition, insulin resistance is closely associated with inflammation and atrophy in skeletal muscle in the obese. In insulin resistance, the production of proinflammatory mediators such as TNF-α, IL-1, and IL-6 is upregulated in skeletal muscle and adipose tissue,60,61 which consequently leads to impaired insulin action and muscle fiber metabolism.62,63 Treatment with quercetin suppresses the upregulation of TNF-α and iNOS expression levels and restores the reduction of glucose uptake by L6 myotubes treated with palmitate.64 In an obese animal model, quercetin suppresses obesity-related skeletal muscle atrophic factors such as MAFbx/atrogin-1 and muscle ring-finger protein 1 (MuRF1), accompanied by increases in heme oxygenase 1 (HO-1) levels, and inactivation of NF-κB.65 In addition, quercetin reduces macrophage accumulation and levels of inflammatory cytokines (eg, TNF-α and MCP-1) and the mRNA levels of MAFbx/atrogin-1 and MuRF1 in skeletal muscle of HFD-fed obese mice, suggesting that quercetin may prevent obesity-induced skeletal muscle atrophy through suppressing inflammatory responses.66 Thus, quercetin could contribute to protect against obesity-related skeletal muscle atrophy by suppressing inflammatory mediators and macrophage infiltration in skeletal muscle, leading to improved insulin resistance.
Anti-Inflammatory Effects of Quercetin on Atherosclerosis
Inflammation is closely linked to the pathogenesis of atherosclerosis.4 Atherosclerosis is known as an inflammatory condition of vessel walls, characterized by infiltration involving mainly macrophage and T-cells; macrophages play key roles in the onset and development of atherosclerosis. As stimulated by lipid deposition, hypertension, and oxidative stress during the early stages of atherosclerosis, monocytes and T-cells migrate from the circulation into the vascular intima to differentiate into macrophages. Macrophages phagocytose oxidized low-density lipoprotein (ox-LDL), and the number of cells is increased. Thereafter, macrophages are transformed into foam cells, which take up excessive ox-LDL. These conditions promote the development of atherosclerosis.67 Furthermore, macrophages produce pro- and anti-inflammatory cytokines, including IL-1β, and TNF-α, and emerge as a key mediator in the pathogenesis of atherosclerosis.
Quercetin suppresses the formation of ox-LDL-induced RAW264.7 macrophage foam cells, which is a foam cell model, reducing cellular lipid accumulation.68 These phenomena can be interpreted; quercetin increases autophagy and decreases mammalian Ste20-like kinase 1 (MST1) during atherosclerotic progression.69 Quercetin improves glucosamine-induced apoptosis and inflammation in human umbilical vein endothelial cells (HUVECs), which represent a model of vascular endothelial injury in the initial stages of atherosclerosis.70 The combination of quercetin and docosahexaenoic acid (DHA) suppresses mRNA expression and phosphorylation of NF-κB subunits p50 and p65, and ERK1/2 and JNK1/2 in LPS-stimulated RAW264.7 macrophage cells.71 Cao et al revealed that quercetin reduces otherwise elevated levels of TNF-α, IL-1β, and IL-18, and enhances lowered autophagy activity in HFD-fed ApoE-/- mice.59 In addition, quercetin reduces the elevated mRNA expression of TLRs and TNF-α in high cholesterol diet-fed atherosclerotic rats, and quercetin also inhibits the nuclear translocation of NF-κB and release of cytokines in ox-LDL-stimulated human peripheral blood mononuclear cells in vitro, suggesting that quercetin may be a promising agent for the prevention of atherosclerosis.72 Interestingly, quercetin treatment suppresses the progression of atherosclerosis in ApoE-/- mice by inhibiting dendritic cell activation associated with the development of atherosclerosis through downregulation of CD80, CD86, MHC-II, IL-6, and IL-12 levels.73
Clinical Studies Involving Quercetin Supplementation
In randomized clinical trials, participants who were overweight-to-obese patients with pre- and stage 1 hypertension were randomized to receive a dose of 162 mg/day quercetin from onion skin extract for 6 weeks. As a result, no significant effects were observed in terms of serum C-reactive protein and TNF-α, as well as the levels of glucose, insulin, and HOMA-IR compared to a placebo group.74 When healthy (pre) hypertensive men and women received quercetin-3-glucoside (160 mg/day) for 4 weeks, the levels of soluble endothelial selectin (p = 0.03) and IL-1β (p = 0.009), and the z score for inflammation (p = 0.02) were lower compared to placebo.75 No effects of supplementation of four quercetin capsules per day containing 100 mg quercetin dihydrate (100 mg/day for 10 weeks) were observed for the inflammatory markers, IL-6 and soluble vascular cell adhesion molecule-1, in healthy male smokers, although quercetin reduced the serum levels of total cholesterol and LDL-cholesterol compared to a placebo group.76 Women with type 2 diabetes receiving quercetin (500 mg/day for 10 weeks) exhibited reduced systolic blood pressure. However, there were no effects on serum levels of IL-6, TNF-α, and C-reactive protein in comparing the quercetin and placebo groups.77 In addition, women with polycystic ovary syndrome were assigned to 2 groups of quercetin treatment: 1 g/day (two 500 mg capsules) daily for 12 weeks, and placebo. Quercetin reduced HOMA-IR levels (p < 0.001) and slightly increased serum adiponectin compared to a placebo group, the authors suggesting that quercetin supplementation may improve adiponectin-mediated insulin resistance.78 Furthermore, women with rheumatoid arthritis (RA) were assigned into quercetin (500 mg/day) or placebo groups for 8 weeks. The study’s results indicated that there were no effects of quercetin on plasma oxidative and inflammatory status, or systolic and diastolic blood pressure in patients with RA.79 On the other hand, Javadi et al demonstrated that plasma TNFα levels were significantly decreased in a quercetin group compared to placebo in women with RA allocated into a quercetin (500 mg/day) or placebo group for 8 weeks.80 Based on previous clinical studies, the effects of quercetin remain unclear. Further studies with various design and sample sizes, and with different quercetin doses, are needed, considering the beneficial effects of quercetin observed in previous animal and cell investigations.
Anti-Inflammatory Effects of Quercetin via Intestinal Microbiota in Obesity
Intestinal microbiota, which exist with a certain diversity in the human gastrointestinal lumen, are also involved in obesity. For instance, it has been revealed in humans and in animal models that obesity is associated with changes in the relative abundance of the two dominant bacterial divisions, the Bacteroidetes and the Firmicutes, with increased levels of Actinobacteria.81,82 Turnbaugh et al demonstrated that colonization of germ-free mice with microbiota from obese animals resulted in significantly greater increases in total body fat than colonization with a non-obese “lean” microbiota.83 Although there are some studies indicating that the proportions of intestinal microbiota are different among populations84 or are not involved in obesity,85 imbalances and alterations in composition and/or function of intestinal microbiota, so-called “dysbiosis”, have been identified to be related to onset and/or development of obesity. In addition, the gut microbiota may be associated with the onset and/or development of atherosclerosis including inflammation and lipid metabolism.86 For example, berberine isolated from various medicinal plants showed the anti-atherosclerotic effect with the changes in composition and functions of gut microbiota, which is associated with anti-inflammatory and glucose and lipid metabolisms.87
In recent years, attention has been focused on the effects of food components such as quercetin on intestinal microbiota in obesity. Etxeberria et al found that quercetin administration in rats effectively alleviates intestinal dysbiosis induced by a high-fat sucrose diet.88 Quercetin supplementation attenuates the Firmicutes/Bacteroidetes ratio and inhibits the growth of bacterial species associated with diet-induced obesity. In another study, a combination of quercetin and resveratrol was able to ameliorate obesity and reverse the gut microbiota dysbiosis in HFD-fed rats.89 Moreover, there are recent reports indicating that the relationship between quercetin and gut microbiota is associated with an anti-inflammatory status. Citrobacter rodentium-induced colitis in mice is well documented as an animal model of inflammatory bowel disease (IBD). Interestingly, pre-administered quercetin could alleviate Citrobacter rodentium-induced colitis, due to quercetin’s ability to suppress pro-inflammatory cytokines such as IL-6 and TNF-α, and/or to modify gut microbiota. That is, pre-administration quercetin may enhance population numbers of Bifidobacterium, Bacteroidetes, and Lactobacillus, and may reduce those of Fusobacterium and Enterococcus.90 In in vitro studies, quercetin reduces the levels of inflammatory mediators in LPS-stimulated macrophages by enhancing secretion of anti-inflammatory substances by Bifidobacterium adolescentis.91 Stearic acid is tentatively identified as the anti-inflammatory molecule from B. adolescentis stimulated by quercetin.92
From these findings of the relationships between quercetin and microbiota, quercetin is expected to play a role in moderating intestinal inflammation in obesity. Indeed, a recent study using a mouse model of nonalcoholic fatty liver disease (NAFLD) associated with obesity demonstrates that quercetin can revert gut dysbiosis and related endotoxemia-mediated TLR4 pathway induction, with subsequent inhibition of inflammasome responses.93 In addition, it is reported that the atherosclerotic lesions and size of plaques were reduced in mice fed HFD diets with oral quercetin treatment, by the alternation of the composition of the gut microbiota.94 Quercetin treatment may contribute to mitigate the onset and/or development of atherosclerosis by modulating intestinal microbiota balance. In order to elucidate the anti-inflammatory effects of quercetin in obesity, including atherosclerosis, further investigations regarding intestinal microbiota are needed.
Epigenetic Activities of Quercetin During Inflammation
Epigenetics is referred to as heritable phenotypic alterations in gene expression that are independent of DNA sequence changes.95,96 Common epigenetic modifications in mammalian cells include changes in DNA methylation, histone modification, and expression of various non-coding microRNAs (miRNAs). DNA methylation is catalyzed by DNA methyltransferases (DNMTs) and is thought to act at promoters so as to induce gene silencing. Histone modifications to specific amino acid residues modulate chromatin structure and gene expression. For example, the histone acetylation state is thought to be adjusted by histone acetyltransferases (HATs) and histone deacetylases (HDACs). miRNAs are known to regulate gene expression post-transcriptionally and function in RNA-silencing.97
Studies of epigenetic mechanisms have allowed advances in the understanding of cancer.98,99 Interestingly, polyphenolic compounds derived from plants seem to exert anti-tumor effects through epigenetic activities.100,101 Treatment with quercetin upregulates miR-503-5p and miR-6867-5p expression and exhibits the potential for anti-proliferative and anti-inflammatory actions in endometriosis implanted mouse models.102 Treatment with quercetin decreases global DNA methylation levels and the activity of DNMTs, HDACs, and histone methyltransferases (HMTs) in quercetin-treated HeLa cells, a human cervical cancer cell line.12 A combination of quercetin and butyrate with chemopreventive activity suppresses human esophageal cancer cell growth and downregulates the expression of DNMT1, NF-κBp65, HDAC1, and Cyclin D1.103 In addition, quercetin enhances apoptosis by increasing the expression level of Fas ligand through the upregulation of HAT activity in human leukemia HL-60 cells.104 Thus, quercetin is expected to act as a candidate natural therapeutic agent to prevent cancer through epigenetics activity. The HFD-fed mice showed hypermethylation in the peroxisome proliferator activated-receptor gamma coactivator 1 alpha (PGC-1α) promoter and Pgc-1α mRNA expression, which is a transcriptional coactivator, in skeletal muscle. Conversely, quercetin supplementation reduced the increases in DNA methylation and PGC-1α expression,105 suggesting that quercetin may regulate PGC-1α expression through DNA methylation in obesity. Moreover, the treatment of quercetin inhibited inflammation in livers of nickel-treated mice by modulating nuclear factor-E2 related factor 2 (Nrf2) nuclear translocation and HO-1 activity and decreased DNMTs activity and DNA methylation level of the Nrf2 DNA.106 Therefore, quercetin may epigenetically regulate the obesity and inflammation.
There is growing evidence that nutrients may modify epigenetic programs, thus regulating gene expression. For example, maternal under-nutrition or restriction of dietary protein during pregnancy leads to many diseases, including obesity, diabetes, and renal disease in adult offspring.107–110 Such diseases are closely associated with the development of chronic inflammation. Maternal over-nutrition in utero results in developmental programming of genes involved in obesity, inflammation, and pro-fibrogenic genes in the liver of the offspring.111 The unfolding pattern of histone H3 lysine 4 trimethylation in children and mothers was associated with human undernutrition.112 Although quercetin exerts anti-inflammatory action with a wide range of mechanisms of actions, few reports have addressed mechanisms by which quercetin modulates inflammation by regulating epigenetic pathways, which is linked to maternal under-nutrition.
We previously reported that the feeding of quercetin to protein-restricted dams during lactation upregulates AMPK activation in the liver of 23 week-old adult offspring.113 Significant increases in AMPK-associated phosphorylated ACC and endothelial nitric oxide synthase (eNOS) levels are found in the liver of such adult offspring. Quercetin treatment during lactation may lead to long-term alterations to the AMPK pathway in the liver of adult offspring of protein-restricted dams. However, whether quercetin treatment during lactation directly activated AMPK in adult offspring remain unclear. The decreased levels of histone acetylation and increased levels of promoter methylation of PGC-1α promoter methylation were correlated with the activity of AMPK in human placenta of diabetic mothers. When diabetic placental explant was treated with metformin, an anti-diabetic drug, AMPK was activated, concomitant with increased H3K27 acetylation and decreased PGC-1α promoter methylation.114 Thus, because metformin as well as quercetin upregulate the AMPK activation,51 we hypothesized that quercetin treatment during lactation may activate AMPK via epigenetic regulation.
More interestingly, maternal quercetin intake during lactation may cause long-term alterations to inflammation and autophagy flux in the kidneys of high-fructose-diet fed adult female rat offspring.13 Maternal quercetin intake during lactation decreases the number of infiltrating macrophages and depresses IL-6 mRNA levels in the kidneys of adult female offspring fed a high-fructose diet after birth. After inducing obesity in female rats fed a HFD, maternal quercetin treatment improved glucose metabolism, insulin sensitivity, hepatic inflammation, and adipose tissue deposition in the adult offspring of obese dams.115 On the other hand, maternal quercetin treatment, starting from 3 days before conception until the end of gestation, resulted in increased iron storage and decreased 8-oxo-dG levels in the liver of 12-week old adult murine offspring.116 In addition, the authors indicated that maternal quercetin treatment increases IL-1β, IL-6, and IL-10 levels in the liver of the adult offspring. Importantly, maternal quercetin intake during gestation and/or lactation may modulate long-term alterations, including inflammatory responses in adult offspring. However, further experiments are required to clarify whether quercetin treatment during gestation and/or lactation contributes to the regulation of epigenetic pathways after birth.
Conclusions and Perspectives
This review provided recent evidence of the anti-inflammatory effects of quercetin. First, quercetin is involved in the attenuation of insulin resistance and atherosclerosis in obesity-related diseases. Insulin resistance is closely associated with the development of chronic low-grade inflammation. Quercetin may improve insulin resistance through inhibiting the production and expression of proinflammatory cytokines and/or enzymes. Importantly, quercetin is associated with inhibition of the TLR4/NF-κB signaling pathway. Based on previous cell culture studies and animal experiments, quercetin treatment is clearly required for preventative and therapeutic applications. However, different studies may also show the effects of quercetin in clinical studies. In this regard, the bioavailability of quercetin in organisms may be variable. It is necessary to establish more comprehensive studies that help to guide clinical studies. Second, some studies have shown the anti-inflammatory effects of quercetin on intestinal microbiota in obesity.
Prebiotics alter the intestinal microbiota and reduce serum levels of IL-6 in children with overweight or obesity.117 In addition, quercetin treatment may mitigate the onset and/or development of atherosclerosis.94 Not only quercetin but also a combination of quercetin and prebiotic treatment may contribute to improve chronic inflammation in obesity-related diseases. Third, we summarized that maternal quercetin intake during lactation may exert long-term alterations in inflammation. Several studies have investigated epigenetic modulation mediated by quercetin. Quercetin is thought to act as a candidate therapeutic agent to prevent cancer through epigenetic activities. On the other hand, few reports have addressed suggestions that maternal quercetin exerts anti-inflammatory effects in adult offspring programmed by maternal under-nutrition and over-nutrition through modulation of epigenetic pathways. Further investigations are required to advance our understanding of the effects of maternal quercetin intake during gestation and/or lactation on anti-inflammatory activity in obesity- and age-related diseases.
Acknowledgment
The authors thank Keiko Tamakuma for her generous support.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114(2):147–152. doi: 10.1172/JCI22422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. doi: 10.2337/db08-0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. doi: 10.1038/nm.2627 [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- 5.Zielinska D, Wiczkowski W, Piskula MK. Determination of the relative contribution of quercetin and its glucosides to the antioxidant capacity of onion by cyclic voltammetry and spectrophotometric methods. J Agric Food Chem. 2008;56(10):3524–3531. doi: 10.1021/jf073521f [DOI] [PubMed] [Google Scholar]
- 6.Ioku K, Aoyama Y, Tokuno A, et al. Various cooking methods and the flavonoid content in onion. J Nutr Sci Vitaminol. 2001;47(1):78–83. doi: 10.3177/jnsv.47.78 [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients. 2016;8(3):167. doi: 10.3390/nu8030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcolin E, San-Miguel B, Vallejo D, et al. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis. J Nutr. 2012;142(10):1821–1828. doi: 10.3945/jn.112.165274 [DOI] [PubMed] [Google Scholar]
- 9.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585(2–3):325–337. doi: 10.1016/j.ejphar.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 10.Yen GC, Duh PD, Tsai HL, Huang SL. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci Biotechnol Biochem. 2003;67(6):1215–1222. doi: 10.1271/bbb.67.1215 [DOI] [PubMed] [Google Scholar]
- 11.Dajas F, Abin-Carriquiry JA, Arredondo F, et al. Quercetin in brain diseases: potential and limits. Neurochem Int. 2015;89:140–148. doi: 10.1016/j.neuint.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 12.Kedhari Sundaram M, Hussain A, Haque S, et al. Quercetin modifies 5ʹCpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J Cell Biochem. 2019;120(10):18357–18369. doi: 10.1002/jcb.29147 [DOI] [PubMed] [Google Scholar]
- 13.Sato S, Norikura T, Mukai Y. Maternal quercetin intake during lactation attenuates renal inflammation and modulates autophagy flux in high-fructose-diet-fed female rat offspring exposed to maternal malnutrition. Food Funct. 2019;10(8):5018–5031. doi: 10.1039/c9fo01134j [DOI] [PubMed] [Google Scholar]
- 14.Khiari Z, Makris DP. Stability and transformation of major flavonols in onion (Allium cepa) solid wastes. J Food Sci Technol. 2012;49(4):489–494. doi: 10.1007/s13197-010-0201-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollman PC, van Trijp JM, Buysman MN, et al. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418(1–2):152–156. doi: 10.1016/s0014-5793(97)01367-7 [DOI] [PubMed] [Google Scholar]
- 16.Nishimuro H, Ohnishi H, Sato M, et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients. 2015;7(4):2345–2358. doi: 10.3390/nu7042345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu X, Ross CF, Powers JR, Rasco BA. Determination of quercetins in onion (Allium cepa) using infrared spectroscopy. J Agric Food Chem. 2011;59(12):6376–6382. doi: 10.1021/jf200953z [DOI] [PubMed] [Google Scholar]
- 18.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49(6):3106–3112. doi: 10.1021/jf000892m [DOI] [PubMed] [Google Scholar]
- 19.Andarwulan N, Batari R, Sandrasari DA, et al. Flavonoid content and antioxidant activity of vegetables from Indonesia. Food Chem. 2010;121(4):1231–1235. doi: 10.1016/j.foodchem.2010.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang RY, Lin S, Kuo G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac J Clin Nutr. 2008;17(Suppl 1):275–279. [PubMed] [Google Scholar]
- 21.Hollman PC, de Vries JH, van Leeuwen SD, et al. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62(6):1276–1282. doi: 10.1093/ajcn/62.6.1276 [DOI] [PubMed] [Google Scholar]
- 22.Lotito SB, Zhang WJ, Yang CS, et al. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic Biol Med. 2011;51(2):454–463. doi: 10.1016/j.freeradbiomed.2011.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cialdella-Kam L, Nieman DC, Sha W, et al. Dose-response to 3 months of quercetin-containing supplements on metabolite and quercetin conjugate profile in adults. Br J Nutr. 2013;109(11):1923–1933. doi: 10.1017/S0007114512003972 [DOI] [PubMed] [Google Scholar]
- 24.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582(1):97–105. doi: 10.1016/j.febslet.2007.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell RR 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277(2):H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643 [DOI] [PubMed] [Google Scholar]
- 26.Mackenzie RW, Elliott BT. Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:55–64. doi: 10.2147/DMSO.S48260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314(1):1–16. doi: 10.1016/j.mce.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 28.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059 [DOI] [PubMed] [Google Scholar]
- 29.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18(2):139–143. doi: 10.1097/MED.0b013e3283444b09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–645. doi: 10.1016/j.cmet.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spagnuolo C, Moccia S, Russo GL. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem. 2018;153:105–115. doi: 10.1016/j.ejmech.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 32.Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2018;119(1):105–110. doi: 10.1002/jcb.26174 [DOI] [PubMed] [Google Scholar]
- 33.Overman A, Chuang CC, McIntosh M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int J Obes. 2011;35(9):1165–1172. doi: 10.1038/ijo.2010.272 [DOI] [PubMed] [Google Scholar]
- 34.Forney LA, Lenard NR, Stewart LK, Henagan TM. Dietary quercetin attenuates adipose tissue expansion and inflammation and alters adipocyte morphology in a tissue-specific manner. Int J Mol Sci. 2018;19(3):895. doi: 10.3390/ijms19030895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Kim CS, Tu TH, et al. Quercetin protects obesity-induced hypothalamic inflammation by reducing microglia-mediated inflammatory responses via HO-1 induction. Nutrients. 2017;9(7):650. doi: 10.3390/nu9070650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vazquez Prieto MA, Bettaieb A, Rodriguez Lanzi C, et al. Catechin and quercetin attenuate adipose inflammation in fructose-fed rats and 3T3-L1 adipocytes. Mol Nutr Food Res. 2015;59(4):622–633. doi: 10.1002/mnfr.201400631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang ZJ, Cheang LC, Wang MW, Lee SM. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. Int J Mol Med. 2011;27(2):195–203. doi: 10.3892/ijmm.2010.571 [DOI] [PubMed] [Google Scholar]
- 38.Lee SG, Kim M, Kim CE, et al. Quercetin 3,7-O-dimethyl ether from Siegesbeckia pubescens suppress the production of inflammatory mediators in lipopolysaccharide-induced macrophages and colon epithelial cells. Biosci Biotechnol Biochem. 2016;80(11):2080–2086. doi: 10.1080/09168451.2016.1204219 [DOI] [PubMed] [Google Scholar]
- 39.Carlsen I, Frokiaer J, Norregaard R. Quercetin attenuates cyclooxygenase-2 expression in response to acute ureteral obstruction. Am J Physiol Renal Physiol. 2015;308(11):F1297–F1305. doi: 10.1152/ajprenal.00514.2014 [DOI] [PubMed] [Google Scholar]
- 40.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 41.Jia L, Vianna CR, Fukuda M, et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:3878. doi: 10.1038/ncomms4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27(1):84–91. doi: 10.1161/01.ATV.0000251608.09329.9a [DOI] [PubMed] [Google Scholar]
- 44.Ghosh AK, O’Brien M, Mau T, Yung R. Toll-like receptor 4 (TLR4) deficient mice are protected from adipose tissue inflammation in aging. Aging (Albany NY). 2017;9(9):1971–1982. doi: 10.18632/aging.101288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu M, Liu F, Guo Q. Quercetin attenuates hypoxia-ischemia induced brain injury in neonatal rats by inhibiting TLR4/NF-kappaB signaling pathway. Int Immunopharmacol. 2019;74:105704. doi: 10.1016/j.intimp.2019.105704 [DOI] [PubMed] [Google Scholar]
- 46.Li T, Li F, Liu X, et al. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4-MyD88-mediated NF-kappaB and MAPK signaling pathways. Phytother Res. 2019;33(3):756–767. doi: 10.1002/ptr.6268 [DOI] [PubMed] [Google Scholar]
- 47.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89(7):667–676. doi: 10.1007/s00109-011-0748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jing Y, Wu F, Li D, et al. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol Cell Endocrinol. 2018;461:256–264. doi: 10.1016/j.mce.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 49.Sharma K. Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int Suppl. 2014;4(1):113–117. doi: 10.1038/kisup.2014.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dugan LL, You YH, Ali SS, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123(11):4888–4899. doi: 10.1172/JCI66218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, Moustaid-Moussa N, Chen L, et al. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014;25(1):1–18. doi: 10.1016/j.jnutbio.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn J, Lee H, Kim S, et al. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373(4):545–549. doi: 10.1016/j.bbrc.2008.06.077 [DOI] [PubMed] [Google Scholar]
- 53.Choi H, Kim CS, Yu R. Quercetin upregulates uncoupling protein 1 in white/brown adipose tissues through sympathetic stimulation. J Obes Metab Syndr. 2018;27(2):102–109. doi: 10.7570/jomes.2018.27.2.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu K, Mei F, Wang Y, et al. Quercetin oppositely regulates insulin-mediated glucose disposal in skeletal muscle under normal and inflammatory conditions: the dual roles of AMPK activation. Mol Nutr Food Res. 2016;60(3):551–565. doi: 10.1002/mnfr.201500509 [DOI] [PubMed] [Google Scholar]
- 55.Dong J, Zhang X, Zhang L, et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKalpha1/SIRT1. J Lipid Res. 2014;55(3):363–374. doi: 10.1194/jlr.M038786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hung CH, Chan SH, Chu PM, Tsai KL. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol Nutr Food Res. 2015;59(10):1905–1917. doi: 10.1002/mnfr.201500144 [DOI] [PubMed] [Google Scholar]
- 57.Qiu L, Luo Y, Chen X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed Pharmacother. 2018;103:1585–1591. doi: 10.1016/j.biopha.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 58.Chen BL, Wang LT, Huang KH, et al. Quercetin attenuates renal ischemia/reperfusion injury via an activation of AMP-activated protein kinase-regulated autophagy pathway. J Nutr Biochem. 2014;25(11):1226–1234. doi: 10.1016/j.jnutbio.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 59.Cao H, Jia Q, Shen D, et al. Quercetin has a protective effect on atherosclerosis via enhancement of autophagy in ApoE(-/-) mice. Exp Ther Med. 2019;18(4):2451–2458. doi: 10.3892/etm.2019.7851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–614. doi: 10.1038/39335 [DOI] [PubMed] [Google Scholar]
- 61.Furuya DT, Poletto AC, Favaro RR, et al. Anti-inflammatory effect of atorvastatin ameliorates insulin resistance in monosodium glutamate-treated obese mice. Metabolism. 2010;59(3):395–399. doi: 10.1016/j.metabol.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 62.Thrush AB, Heigenhauser GJ, Mullen KL, et al. Palmitate acutely induces insulin resistance in isolated muscle from obese but not lean humans. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1205–R1212. doi: 10.1152/ajpregu.00909.2007 [DOI] [PubMed] [Google Scholar]
- 63.Thrush AB, Antoun G, Nikpay M, et al. Diet-resistant obesity is characterized by a distinct plasma proteomic signature and impaired muscle fiber metabolism. Int J Obes. 2018;42(3):353–362. doi: 10.1038/ijo.2017.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anhe GF, Okamoto MM, Kinote A, et al. Quercetin decreases inflammatory response and increases insulin action in skeletal muscle of ob/ob mice and in L6 myotubes. Eur J Pharmacol. 2012;689(1–3):285–293. doi: 10.1016/j.ejphar.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 65.Kim Y, Kim CS, Joe Y, Chung HT, Ha TY, Yu R. Quercetin reduces tumor necrosis factor alpha-induced muscle atrophy by upregulation of heme oxygenase-1. J Med Food. 2018;21(6):551–559. doi: 10.1089/jmf.2017.4108 [DOI] [PubMed] [Google Scholar]
- 66.Le NH, Kim CS, Park T, et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediators Inflamm. 2014;2014:834294. doi: 10.1155/2014/834294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taleb S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. 2016;109(12):708–715. doi: 10.1016/j.acvd.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 68.Cao H, Jia Q, Yan L, Chen C, Xing S, Shen D. Quercetin suppresses the progression of atherosclerosis by regulating MST1-mediated autophagy in ox-LDL-induced RAW264.7 macrophage foam cells. Int J Mol Sci. 2019;20(23):6093. doi: 10.3390/ijms20236093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang T, Zhang L, Hu J, et al. Mst1 participates in the atherosclerosis progression through macrophage autophagy inhibition and macrophage apoptosis enhancement. J Mol Cell Cardiol. 2016;98:108–116. doi: 10.1016/j.yjmcc.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 70.Cai X, Bao L, Ding Y, et al. Quercetin alleviates cell apoptosis and inflammation via the ER stress pathway in vascular endothelial cells cultured in high concentrations of glucosamine. Mol Med Rep. 2017;15(2):825–832. doi: 10.3892/mmr.2016.6054 [DOI] [PubMed] [Google Scholar]
- 71.Si TL, Liu Q, Ren YF, et al. Enhanced anti-inflammatory effects of DHA and quercetin in lipopolysaccharide-induced RAW264.7 macrophages by inhibiting NF-kappaB and MAPK activation. Mol Med Rep. 2016;14(1):499–508. doi: 10.3892/mmr.2016.5259 [DOI] [PubMed] [Google Scholar]
- 72.Bhaskar S, Helen A. Quercetin modulates toll-like receptor-mediated protein kinase signaling pathways in oxLDL-challenged human PBMCs and regulates TLR-activated atherosclerotic inflammation in hypercholesterolemic rats. Mol Cell Biochem. 2016;423(1–2):53–65. doi: 10.1007/s11010-016-2824-9 [DOI] [PubMed] [Google Scholar]
- 73.Lin W, Wang W, Wang D, Ling W. Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol Nutr Food Res. 2017;61(9):1700031. doi: 10.1002/mnfr.201700031 [DOI] [PubMed] [Google Scholar]
- 74.Brull V, Burak C, Stoffel-Wagner B, et al. No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in overweight-to-obese patients with (pre-)hypertension: a randomized double-blinded, placebo-controlled crossover trial. Eur J Nutr. 2017;56(7):2265–2275. doi: 10.1007/s00394-016-1267-0 [DOI] [PubMed] [Google Scholar]
- 75.Dower JI, Geleijnse JM, Gijsbers L, et al. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: a randomized double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145(7):1459–1463. doi: 10.3945/jn.115.211888 [DOI] [PubMed] [Google Scholar]
- 76.Lee KH, Park E, Lee HJ, et al. Effects of daily quercetin-rich supplementation on cardiometabolic risks in male smokers. Nutr Res Pract. 2011;5(1):28–33. doi: 10.4162/nrp.2011.5.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zahedi M, Ghiasvand R, Feizi A, et al. Does quercetin improve cardiovascular risk factors and inflammatory biomarkers in women with type 2 diabetes: a double-blind randomized controlled clinical trial. Int J Prev Med. 2013;4(7):777–785. [PMC free article] [PubMed] [Google Scholar]
- 78.Rezvan N, Moini A, Janani L, et al. Effects of quercetin on adiponectin-mediated insulin sensitivity in polycystic ovary syndrome: a randomized placebo-controlled double-blind clinical trial. Horm Metab Res. 2017;49(2):115–121. doi: 10.1055/s-0042-118705 [DOI] [PubMed] [Google Scholar]
- 79.Javadi F, Eghtesadi S, Ahmadzadeh A, et al. The effect of quercetin on plasma oxidative status, C-reactive protein and blood pressure in women with rheumatoid arthritis. Int J Prev Med. 2014;5(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- 80.Javadi F, Ahmadzadeh A, Eghtesadi S, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J Am Coll Nutr. 2017;36(1):9–15. doi: 10.1080/07315724.2016.1140093 [DOI] [PubMed] [Google Scholar]
- 81.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 82.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 84.Andoh A, Nishida A, Takahashi K, et al. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J Clin Biochem Nutr. 2016;59(1):65–70. doi: 10.3164/jcbn.15-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14(2):79–87. doi: 10.1038/nrcardio.2016.183 [DOI] [PubMed] [Google Scholar]
- 87.Wu M, Yang S, Wang S, et al. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE-/- mice. Front Pharmacol. 2020;11:223. doi: 10.3389/fphar.2020.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Etxeberria U, Arias N, Boque N, et al. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem. 2015;26(6):651–660. doi: 10.1016/j.jnutbio.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 89.Zhao L, Zhang Q, Ma W, et al. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017;8(12):4644–4656. doi: 10.1039/c7fo01383c [DOI] [PubMed] [Google Scholar]
- 90.Lin R, Piao M, Song Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in Citrobacter rodentium-infected mice. Front Microbiol. 2019;10:1092. doi: 10.3389/fmicb.2019.01092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawabata K, Sugiyama Y, Sakano T, Ohigashi H. Flavonols enhanced production of anti-inflammatory substance(s) by Bifidobacterium adolescentis: prebiotic actions of galangin, quercetin, and fisetin. Biofactors. 2013;39(4):422–429. doi: 10.1002/biof.1081 [DOI] [PubMed] [Google Scholar]
- 92.Kawabata K, Baba N, Sakano T, et al. Functional properties of anti-inflammatory substances from quercetin-treated Bifidobacterium adolescentis. Biosci Biotechnol Biochem. 2018;82(4):689–697. doi: 10.1080/09168451.2017.1401916 [DOI] [PubMed] [Google Scholar]
- 93.Porras D, Nistal E, Martinez-Florez S, et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 94.Nie J, Zhang L, Zhao G, Du X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J Appl Microbiol. 2019;127(6):1824–1834. doi: 10.1111/jam.14441 [DOI] [PubMed] [Google Scholar]
- 95.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913 [DOI] [PubMed] [Google Scholar]
- 96.Nagase H, Ghosh S. Epigenetics: differential DNA methylation in mammalian somatic tissues. FEBS J. 2008;275(8):1617–1623. doi: 10.1111/j.1742-4658.2008.06330.x [DOI] [PubMed] [Google Scholar]
- 97.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 98.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–428. doi: 10.1038/nrg816 [DOI] [PubMed] [Google Scholar]
- 99.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Busch C, Burkard M, Leischner C, et al. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin Epigenetics. 2015;7:64. doi: 10.1186/s13148-015-0095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carlos-Reyes A, Lopez-Gonzalez JS, Meneses-Flores M, et al. Dietary compounds as epigenetic modulating agents in cancer. Front Genet. 2019;10:79. doi: 10.3389/fgene.2019.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park S, Lim W, Bazer FW, et al. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J Nutr Biochem. 2019;63:87–100. doi: 10.1016/j.jnutbio.2018.09.024 [DOI] [PubMed] [Google Scholar]
- 103.Zheng NG, Wang JL, Yang SL, Wu JL. Aberrant epigenetic alteration in Eca9706 cells modulated by nanoliposomal quercetin combined with butyrate mediated via epigenetic-NF-kappaB signaling. Asian Pac J Cancer Prev. 2014;15(11):4539–4543. doi: 10.7314/apjcp.2014.15.11.4539 [DOI] [PubMed] [Google Scholar]
- 104.Lee WJ, Chen YR, Tseng TH. Quercetin induces FasL-related apoptosis, in part, through promotion of histone H3 acetylation in human leukemia HL-60 cells. Oncol Rep. 2011;25(2):583–591. doi: 10.3892/or.2010.1097 [DOI] [PubMed] [Google Scholar]
- 105.Devarshi PP, Jones AD, Taylor EM, et al. Quercetin and quercetin-rich red onion extract alter Pgc-1alpha promoter methylation and splice variant expression. PPAR Res. 2017;2017:3235693. doi: 10.1155/2017/3235693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu CM, Ma JQ, Xie WR, et al. Quercetin protects mouse liver against nickel-induced DNA methylation and inflammation associated with the Nrf2/HO-1 and p38/STAT1/NF-kappaB pathway. Food Chem Toxicol. 2015;82:19–26. doi: 10.1016/j.fct.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 107.Bol VV, Reusens BM, Remacle CA. Postnatal catch-up growth after fetal protein restriction programs proliferation of rat preadipocytes. Obesity. 2008;16(12):2760–2763. doi: 10.1038/oby.2008.417 [DOI] [PubMed] [Google Scholar]
- 108.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharkey D, Gardner DS, Fainberg HP, et al. Maternal nutrient restriction during pregnancy differentially alters the unfolded protein response in adipose and renal tissue of obese juvenile offspring. FASEB J. 2009;23(5):1314–1324. doi: 10.1096/fj.08-114330 [DOI] [PubMed] [Google Scholar]
- 110.Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes–a global concern. Nat Rev Nephrol. 2015;11(3):135–149. doi: 10.1038/nrneph.2014.251 [DOI] [PubMed] [Google Scholar]
- 111.Wankhade UD, Zhong Y, Kang P, et al. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS One. 2017;12(4):e0175675. doi: 10.1371/journal.pone.0175675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Uchiyama R, Kupkova K, Shetty SJ, et al. Histone H3 lysine 4 methylation signature associated with human undernutrition. Proc Natl Acad Sci USA. 2018;115(48):E11264–E11273. doi: 10.1073/pnas.1722125115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sato S, Mukai Y, Saito T. Quercetin intake during lactation modulates the AMP-activated protein kinase pathway in the livers of adult male rat offspring programmed by maternal protein restriction. J Nutr Biochem. 2013;24(1):118–123. doi: 10.1016/j.jnutbio.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 114.Jiang S, Teague AM, Tryggestad JB, et al. Role of metformin in epigenetic regulation of placental mitochondrial biogenesis in maternal diabetes. Sci Rep. 2020;10(1):8314. doi: 10.1038/s41598-020-65415-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu Z, Zhao J, Xu H, et al. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur J Nutr. 2014;53(8):1669–1683. doi: 10.1007/s00394-014-0673-4 [DOI] [PubMed] [Google Scholar]
- 116.Vanhees K, Godschalk RW, Sanders A, et al. Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology. 2011;290(2–3):350–358. doi: 10.1016/j.tox.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 117.Nicolucci AC, Hume MP, Martinez I, et al. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153(3):711–722. doi: 10.1053/j.gastro.2017.05.055 [DOI] [PubMed] [Google Scholar]