Supplemental Digital Content is available in the text.
Keywords: biomarkers; cholesterol; continental population groups; lipids; lipoproteins, HDL; myocardial infarction; risk; stroke
Abstract
Background:
High-density lipoprotein (HDL) cholesterol concentration (HDL-C) is an established atheroprotective marker, in particular for coronary artery disease; however, HDL particle concentration (HDL-P) may better predict risk. The associations of HDL-C and HDL-P with ischemic stroke and myocardial infarction (MI) among women and Blacks have not been well studied. We hypothesized that HDL-P would consistently be associated with MI and stroke among women and Blacks compared with HDL-C.
Methods:
We analyzed individual-level participant data in a pooled cohort of 4 large population studies without baseline atherosclerotic cardiovascular disease: DHS (Dallas Heart Study; n=2535), ARIC (Atherosclerosis Risk in Communities; n=1595), MESA (Multi-Ethnic Study of Atherosclerosis; n=6632), and PREVEND (Prevention of Renal and Vascular Endstage Disease; n=5022). HDL markers were analyzed in adjusted Cox proportional hazard models for MI and ischemic stroke.
Results:
In the overall population (n=15 784), HDL-P was inversely associated with the combined outcome of MI and ischemic stroke, adjusted for cardiometabolic risk factors (hazard ratio [HR] for quartile 4 [Q4] versus quartile 1 [Q1], 0.64 [95% CI, 0.52–0.78]), as was HDL-C (HR for Q4 versus Q1, 0.76 [95% CI, 0.61–0.94]). Adjustment for HDL-C did not attenuate the inverse relationship between HDL-P and atherosclerotic cardiovascular disease, whereas adjustment for HDL-P attenuated all associations between HDL-C and events. HDL-P was inversely associated with the individual end points of MI and ischemic stroke in the overall population, including in women. HDL-P was inversely associated with MI among White participants but not among Black participants (HR for Q4 versus Q1 for Whites, 0.49 [95% CI, 0.35–0.69]; for Blacks, 1.22 [95% CI, 0.76–1.98]; Pinteraction=0.001). Similarly, HDL-C was inversely associated with MI among White participants (HR for Q4 versus Q1, 0.53 [95% CI, 0.36–0.78]) but had a weak direct association with MI among Black participants (HR for Q4 versus Q1, 1.75 [95% CI, 1.08–2.83]; Pinteraction<0.0001).
Conclusions:
Compared with HDL-C, HDL-P was consistently associated with MI and ischemic stroke in the overall population. Differential associations of both HDL-C and HDL-P for MI by Black ethnicity suggest that atherosclerotic cardiovascular disease risk may differ by vascular domain and ethnicity. Future studies should examine individual outcomes separately.
High-density lipoprotein (HDL) cholesterol concentration (HDL-C) is associated with atherosclerotic cardiovascular disease (ASCVD) and remains part of the ASCVD Pooled Cohort Equations and the European SCORE risk charts (Systematic Coronary Risk Evaluation).1,2 However, recent epidemiological studies have suggested that HDL particle concentration (HDL-P) may better associate with ASCVD outcomes, even among those on statin therapy.3 This is underscored by observations showing that drugs that most potently raise HDL-C such as niacin and cholesteryl transfer protein inhibitors do not have consistent effects on HDL-P levels and have not consistently improved ASCVD outcomes.4–8 However, several relevant gaps remain in the role of HDL-P and its association with ASCVD, especially in distinct vascular territories and among women and Black populations.9–12
Most of the studies investigating HDL-P have been performed in single-cohort studies assessing solely coronary heart disease (CHD) or composite outcomes inclusive of different vascular beds.3,13–16 Recent investigations of HDL parameters suggest preserved association of HDL-P with CHD but a lack of association with ischemic cerebrovascular disease.10,16–19 Thus, whether HDL-P is a robust marker for ischemic stroke remains unknown, especially because strokes typically make up relatively few events in any single population-based cohort and not uncommonly include ischemic and nonischemic causes as a combined end point.
Furthermore, whether HDL-P associates with CHD or ischemic stroke among women or Blacks is not well studied. Among cohorts that include women or Black participants, the number of events represented by these groups remains small, limiting the ability to fully assess these relationships.17,20–22 In a previous study, we observed a potential interaction by race on the association between HDL-C but not HDL-P on a composite ASCVD outcome but were limited in exploring interactions for CHD and stroke separately.20
Some reports have suggested that indexing HDL-C to HDL-P or HDL particle size (HDL-size) to HDL-P may capture HDL functionality, with increased ratio of cholesterol size to particle reflecting potential HDL dysfunction.23 Increasing cholesterol content or size per HDL particle may represent HDL particles that are overloaded with cholesterol or larger and potentially dysfunctional and less able to participate in reverse cholesterol transport. Whether these ratios add additional information with respect to risk prediction of incident cardiovascular events remains unknown.
We sought to investigate specific associations between the markers HDL-P, HDL-C, HDL-C/HDL-P, and HDL-size/HDL-P and the outcomes of myocardial infarction (MI) and stroke as well as overall ASCVD. We further assessed whether sex or Black ethnicity modified these associations. To overcome the limitations of previous studies, we conducted an individual participant pooled cohort analysis from 4 separate cohorts: DHS (Dallas Heart Study), MESA (Multi-Ethnic Study of Atherosclerosis), ARIC (Atherosclerosis Risk in Communities Study), and PREVEND (Prevention of Renal and Vascular End-stage Disease).
Methods
Anonymized data and materials for MESA and ARIC have been made publicly available at BIOLINCC and can be accessed at https://biolincc.nhlbi.nih.gov/home/. Data for PREVEND are available on request at https://www.maelstrom-research.org/mica/individual-study/prevend and for DHS at https://www.utsouthwestern.edu/research/translational-medicine/doing-research/dallas-heart/.
For this individual participant pooled cohort analysis, 4 cohorts were included that comprised participants without clinically manifest or self-reported atherosclerotic disease at baseline and that had available HDL data measured by nuclear magnetic resonance (NMR) spectroscopy using the same analytic platform (NMR LipoProfile test; LipoScience [now LabCorp], Raleigh, NC). The DHS is a multiethnic population cohort of Dallas County residents with deliberate oversampling of Black participants.24 From 2000 to 2002, 2782 participants completed detailed in-home surveys, laboratory testing, and imaging studies. MESA is a large, ethnically diverse cohort of 6814 participants 45 to 84 years of age recruited from 6 sites in the United States between 2000 and 2002.25 Data from the MESA study were obtained via the National Heart, Lung, and Blood Institute BIOLINCC repository. ARIC is a population-based cohort to study cardiovascular disease incidence in Black and White adults 45 to 64 years of age from 4 US communities.26 The ARIC Carotid MRI (Magnetic Resonance Imaging) substudy recruited ≈2000 participants with previous carotid ultrasound testing to undergo additional imaging with carotid MRI and advanced lipoprotein analysis with NMR. PREVEND is a prospective cohort based in Groningen, the Netherlands, designed to assess the association of urinary albumin excretion with renal and cardiovascular disease.27 Between 1997 and 1998, participants 28 to 75 years of age were invited to participate, with 8592 subjects (6000 subjects with urinary albumin excretion >10 mg/L and 2592 without) completing the screening program and outpatient visit. For the present analysis, data were used from participants who completed the second screening and had available outcome data, leaving a cohort of 6241 participants with complete information for the present analysis.28 For each cohort, the study was approved by an institutional review committee, and subjects gave informed consent.
Ethnicity, sex, smoking status, and history of ASCVD were self-reported in each cohort. Hypertension was defined uniformly across cohorts as average systolic blood pressure ≥140 mm Hg and average diastolic blood pressure ≥90 mm Hg or use of antihypertensive medication. Diabetes mellitus was also defined uniformly across cohorts as fasting glucose ≥126 mg/dL or 7 mmol/L or use of diabetic medications.
For all cohorts, venous blood was collected in the fasting state. Total cholesterol, triglycerides, and HDL-C were measured enzymatically with standard methods and expressed in milligrams per deciliter or millimoles per liter. Low-density lipoprotein (LDL) cholesterol concentration (LDL-C) levels were calculated with the Friedewald equation. Non–HDL-C was calculated as the difference between total cholesterol and HDL-C. Body mass index was calculated as weight divided by height squared. HDL-P and HDL particle size (HDL-size) were measured on serum or EDTA plasma specimens by NMR LipoProfile testing using a 400-MHz NMR Profiler or Vantera automated analyzer using the LipoProfile-3 (LP3) deconvolution algorithm to obtain uniformity across all cohorts in the measurement of the exposure variables. Spearman rank correlation coefficients between HDL-C measured enzymatically and HDL-C derived by the NMR LipoProfile-3 deconvolution algorithm were 0.92 for ARIC, 0.87 for DHS, 0.96 for MESA, and 0.95 for PREVEND (Figure I in the Data Supplement).
Clinical events were ascertained in each individual cohort. Methods of adjudication of events in DHS have been described previously.24 ARIC used a combination of follow-up phone calls and assessment of hospital discharge information and death certificate information, as well as independent adjudicators, as described on their website (https://sites.cscc.unc.edu/aric/surveillance-manuals). In MESA, events were identified through follow-up phone calls to participants every 9 to 12 months with adjudication committees determining cardiovascular events. Information about cardiovascular end points was obtained from the Dutch Central Bureau for Statistics and the national registry of hospital discharge diagnoses in PREVEND.29 The length of mean follow-up for each cohort was similar, with a range of 8 to 12 years.
The 2 primary outcomes of interest were defined as first fatal and nonfatal MI and fatal and nonfatal ischemic stroke events. For inclusion of ischemic stroke, we excluded all definite or probable hemorrhagic and embolic stroke events in the cohorts. We defined 2 additional outcomes, first fatal and nonfatal MI and ischemic strokes combined and a composite outcome including first fatal and nonfatal MI and ischemic strokes, as well as coronary and peripheral revascularization procedures.
Statistical Analysis
Variables from all cohorts were harmonized and synthesized into 1 large cohort, which was then analyzed in 1 step by using individual patient-level data. Baseline HDL-C, HDL-P, and HDL-size were expressed as medians with interquartile intervals. We tested linearity in Cox models via a supremum test with 1000 bootstrap replications and found that the majority of HDL parameters either were not normally distributed or had nonlinear associations with outcomes other than associations with ischemic stroke. Cox proportional hazards models were used to determine hazard ratios (HRs) per increasing race- and sex-specific quartiles of HDL-C, HDL-P, HDL-size, HDL-C/HDL-P, and HDL-size/HDL-P for time to first events. HRs were reported for quartile 4 (Q4) with quartile 1 (Q1) used as a reference (quartiles for HDL-C and HDL-P are given in Table I in the Data Supplement). For all of the Cox models, we used stratified baseline hazards, allowing a different baseline hazard function for each study. We also used robust standard errors to account for the possible correlation of the same patients within the same cohort. Proportional hazards assumptions were satisfied by checking Schoenfeld residuals. Restricted cubic splines were generated with 5 knots at the 5th, 25th, 50th, 75th, and 95th percentiles.
Models were adjusted for cohort and traditional risk factors such as age, hypertension, diabetes mellitus, smoking, lipid medications, LDL-C, and triglycerides as well as body mass index, waist circumference (centimeters), and high sensitivity C-reactive protein. In addition, for the HDL-C models, adjustments were made for all these covariates and HDL-P. Similarly, independent associations of HDL-P were assessed with adjustments for the same covariates and HDL-C. Data for both models before and after adjustment are reported. No additional adjustment was made in the quartile analysis for race/sex because the quartiles generated were race/sex specific, whereas race and sex were included in continuous spline analyses. Interaction testing was performed by sex and ethnicity (Black versus White) followed by stratified models, with P for interaction ≤0.05 considered a significant interaction. Otherwise, 2-sided values of P<0.05 were considered to indicate statistical significance. No adjustments were made for multiple testing. All analyses were performed with SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
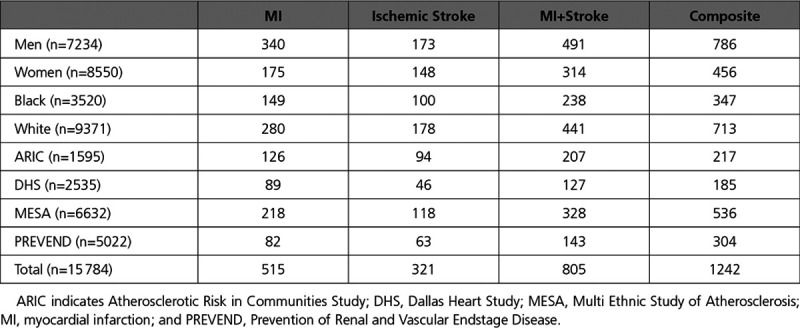
The overall pooled cohort comprised 15 784 participants without baseline atherosclerotic disease. The median age was 56 years; 46% were male; and 22% were Black. Baseline characteristics of the participants by cohort are displayed in Table 1. The median HDL-C was 48 mg/dL; median HDL-P was 32.5 µmol/L; and median HDL-size was 9.1 nm (overall and cohort HDL characteristics are given in Table 2). Over the mean follow-up period of 8 to 12 years across cohorts, there were 515 fatal/nonfatal MI events, 321 fatal/nonfatal ischemic stroke events, and 1242 overall ASCVD events (Table 3). The pooled cohort consisted of 8550 women and 3520 Black participants with variation in the overall number and proportion across cohorts. The numbers of events by ethnicity, sex, and cohort are summarized in Table 3.
Table 1.
Baseline Characteristics of Participants in Individual Cohorts
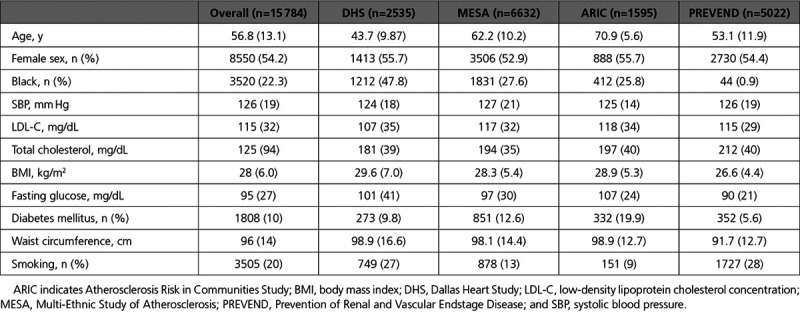
Table 2.
HDL Characteristics of the Overall and Individual Cohorts
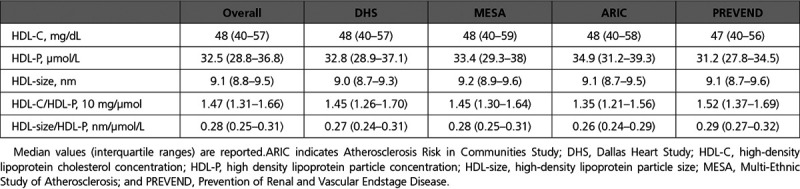
Table 3.
Number of First Events for Each Primary and Composite Outcome Stratified by Ethnicity, Sex, and Cohort
HDL-P Results
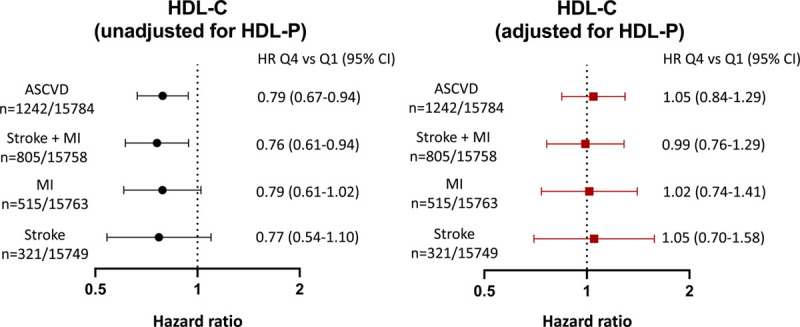
In the pooled cohort, HDL-P was inversely associated with MI+stroke and the individual end points of MI (HR for Q4 versus Q1, 0.63 [95% CI, 0.49–0.81]) and ischemic stroke (HR for Q4 versus Q1, 0.66 [95% CI, 0.48–0.93]) in a model adjusted for established cardiovascular risk factors (Figure 1). The outcome of ischemic stroke met the linearity assumption, and to maximize the power of our analysis, we also examined the relationship between HDL-P and ischemic stroke using continuous HRs. Per 1-SD increase in HDL-P, there was a significant reduction in ischemic stroke risk (HR per 1 SD increase, 0.84 [95% CI, 0.73–0.96]). After additional adjustment for HDL-C, HDL-P remained inversely associated with all outcomes of interest except that the association between HDL-P and ischemic stroke was no longer significant in both the continuous and quartile analyses (Figure 1).
Figure 1.
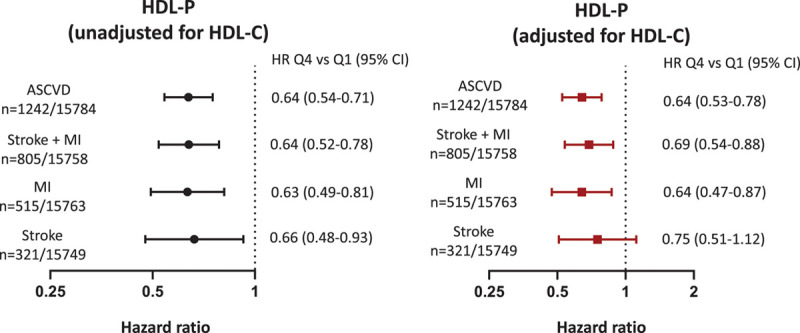
Association of high-density lipoprotein (HDL) particle concentration (HDL-P) with individual and composite atherosclerotic cardiovascular disease (ASCVD) outcomes before and after adjustment for HDL cholesterol concentration (HDL-C). Cox proportional hazards models of sex/ethnicity–adjusted quartile 4 (Q4) vs quartile 1 (Q1) of HDL-P for stroke, myocardial infarction (MI), and composite ASCVD outcomes before and after adjustment for HDL-C. Both models include adjustment for risk factors and cohort. Risk factors adjusted for age, diabetes mellitus, hypertension, smoking, low-density lipoprotein cholesterol, triglycerides, lipid-lowering medications, body mass index, waist circumference, and high-sensitivity C-reactive protein. HR indicates hazard ratio.
Sex did not modify the association between HDL-P and MI+stroke (Pinteraction=0.1; Figure 2). The inverse associations between HDL-P and combined MI+stroke (HR for Q4 versus Q1, 0.50 [95% CI, 0.36–0.69]), MI (HR for Q4 versus Q1, 0.51 [95% CI, 0.34–0.78]), and ischemic stroke (HR for Q4 versus Q1, 0.54 [95% CI, 0.33–0.88]) were also observed in the women in our pooled cohort. After adjustment for HDL-C, the association between HDL-P and composite outcomes remained statistically significant in women (data not shown).
Figure 2.
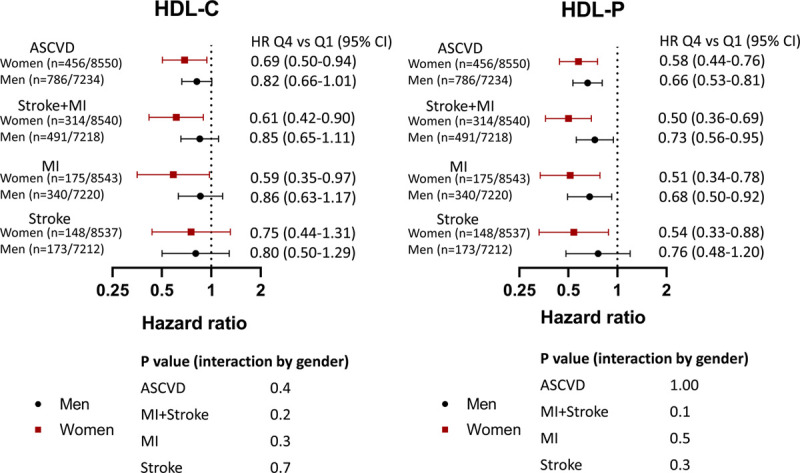
Association of high-density lipoprotein (HDL) cholesterol concentration (HDL-C) with individual and composite atherosclerotic cardiovascular disease (ASCVD) outcomes before and after adjustment for HDL particle concentration (HDL-P). Cox proportional hazards models of sex/ethnicity–adjusted quartile 4 (Q4) vs quartile 1 (Q1) of HDL-C for stroke, myocardial infarction (MI), and composite ASCVD outcomes before and after adjustment for HDL-P. Both models include adjustment for risk factors and cohort. Risk factors adjusted for age, diabetes mellitus, hypertension, smoking, low-density lipoprotein cholesterol, triglycerides, lipid-lowering medications, body mass index, waist circumference, and high-sensitivity C-reactive protein. HR indicates hazard ratio.
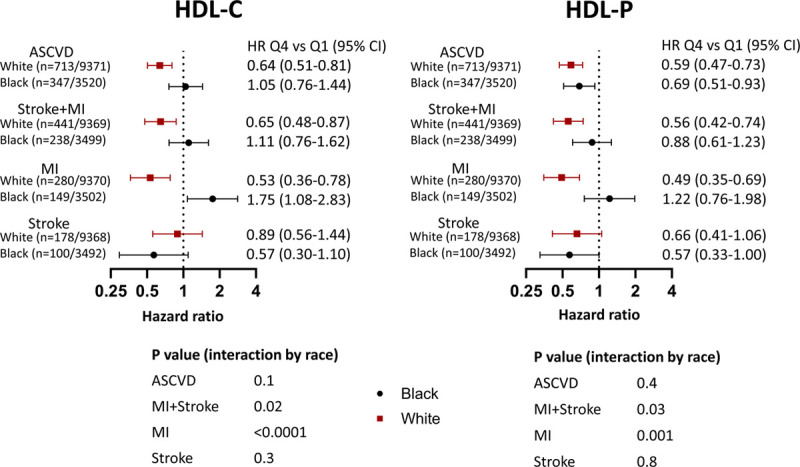
Black ethnicity modified the association between HDL-P and MI+stroke (Pinteraction=0.03; Figure 3). This was driven by the MI end point such that HDL-P was inversely associated with MI among White participants (HR for Q4 versus Q1, 0.49 [95% CI, 0.35–0.69]) but not among Black participants (HR for Q4 versus Q1, 1.22 [95% CI, 0.76–1.98]; Figure 4). Adjustment for HDL-C attenuated the relationship with MI in Whites somewhat but did not attenuate the effect modification by ethnicity (Pinteraction=0.001). Interaction testing by cohort did not modify these results.
Figure 3.
Association of high-density lipoprotein (HDL) cholesterol concentration (HDL-C) and HDL particle concentration (HDL-P) with outcomes stratified by sex. Cox proportional hazards models of sex/ethnicity-adjusted quartile 4 (Q4) quartile 1 (Q1) of HDL-C and HDL-P for stroke, myocardial infarction (MI), and composite atherosclerotic cardiovascular disease (ASCVD) outcomes in men and women. Both models include adjustment for risk factors (age, diabetes mellitus, hypertension, smoking, low-density lipoprotein cholesterol, triglycerides, lipid-lowering medications, body mass index, waist circumference, and high-sensitivity C-reactive protein) and cohort. HR indicates hazard ratio.
Figure 4.
Association of high-density lipoprotein (HDL) cholesterol concentration (HDL-C) and HDL particle concentration (HDL-P) with outcomes stratified by ethnicity. Cox proportional hazards models of sex/ethnicity-adjusted quartile 4 (Q4) vs quartile 1 (Q1) of HDL-C and HDL-P for stroke, myocardial infarction (MI), and composite atherosclerotic cardiovascular disease (ASCVD) outcomes stratified by Black vs White participants. Both models include adjustment for risk factors (age, diabetes mellitus, hypertension, smoking, low-density lipoprotein cholesterol, triglycerides, lipid-lowering medications, body mass index, waist circumference, and high-sensitivity C-reactive protein) and cohort. In this model, no additional adjustment for HDL-P or HDL-C was made. HR indicates hazard ratio.
HDL-C Results
In the overall pooled cohort, HDL-C was inversely associated with MI+stroke (HR for Q4 versus Q1, 0.76 [95% CI, 0.61–0.94]) in a model adjusted for the same cardiovascular risk factors as above (Figure 4). The associations between HDL-C and the individual end points of MI (HR for Q4 versus Q1, 0.79 [95% CI, 0.61–1.02]) and ischemic stroke (HR for Q4 versus Q1, 0.77 [95% CI, 0.54–1.10]) were not statistically significant. When ischemic stroke was analyzed as a continuous variable to maximize power, there was a significant reduction in ischemic stroke risk per 1-SD increase in HDL-C (HR per 1-SD increase, 0.85 [95% CI, 0.75–0.97]). However, after additional adjustment for HDL-P, there was no remaining association between HDL-C and combined MI and stroke (HR for Q4 versus Q1, 0.99 [95% CI, 0.76–1.29]) or individual MI and ischemic stroke (Figure 4).
Sex did not modify these associations, with no significant interaction for combined or individual end points. The inverse associations between HDL-C and combined MI+stroke (HR for Q4 versus Q1, 0.61 [95% CI, 0.42–0.90]) and MI (HR for Q4 versus Q1, 0.59 [95% CI, 0.35–0.97]) were preserved in women (Figure 2). HDL-C was not associated with ischemic stroke in women (HR Q4 versus Q1, 0.75 [95% CI, 0.44–1.31]). After adjustment for HDL-P, all associations between HDL-C and outcomes in women were no longer statistically significant (data not shown).
Similar to the results for HDL-P, Black ethnicity modified the associations between HDL-C and events, driven in particular by MI (Figure 3). HDL-C was inversely associated with the combined hard end point of MI and ischemic stroke and the composite end point among White participants but had no association in Black participants (Pinteraction=0.02). Whereas HDL-C was inversely associated with MI among White participants (HR Q4 versus Q1, 0.53 [95% CI, 0.36–0.78]), this was not observed among Black participants (HR Q4 versus Q1, 1.75 [95% CI, 1.08–2.83]; Pinteraction<0.0001; Figure 3). No relationship was evident between HDL-C and ischemic stroke among either Black or White participants (Figure 3). Using HDL-C values obtained from the LipoProfile-3 algorithm did not change our results (data not shown). Interaction testing by cohort revealed a modification of the results by inclusion of participants from the PREVEND cohort (Pinteraction=0.001).
Quartiles defining values of HDL-C and HDL-P by ethnicity and sex are displayed in Tables II and III in the Data Supplement.
Effect Modification by Ethnicity for MI
Black ethnicity modified the association with MI events for both HDL-C and HDL-P in our pooled cohort. To examine this further, we stratified our results by ethnicity in individual cohorts (Figure 5). Given the small sample size of Black participants in PREVEND (2 MI events in a total of 44 Black participants), HRs were reported for White but not for Black participants in this cohort. The relationship between MI and each HDL parameter in each individual cohort paralleled the different results by ethnicity observed in our pooled cohort (Figure 5). Spline curves demonstrating the differences in the curves between HDL-C and HDL-P with MI by Black and White participants are shown in Figure 6. Adjustment of HDL-C for HDL-P did not attenuate the effect modification by ethnicity for MI or combined end points (Pinteraction for MI <0.0001).
Figure 5.
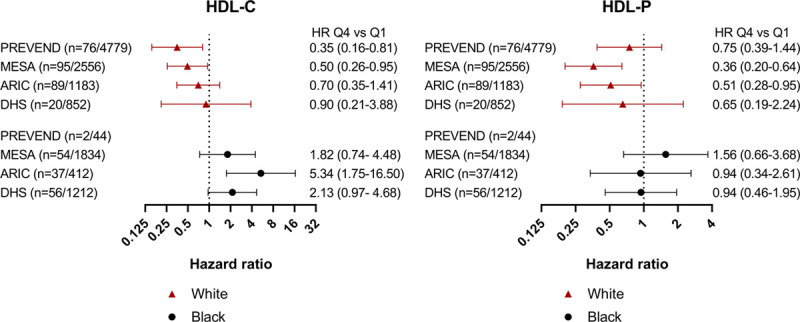
Association of high-density lipoprotein (HDL) cholesterol concentration (HDL-C) and HDL particle concentration (HDL-P) with myocardial infarction (MI) stratified by race and cohort. Cox proportional hazards models of sex/ethnicity-adjusted quartile 4 (Q4) vs quartile 1 (Q1) of HDL-C and HDL-P for fatal/nonfatal MI outcomes stratified by race and cohort. The number of Black participants in each cohort is specified. This model is adjusted for risk factors (age, diabetes mellitus, hypertension, smoking, low-density lipoprotein cholesterol, triglycerides, lipid-lowering medications, body mass index, waist circumference, and high-sensitivity C-reactive protein). No additional adjustment for HDL-P or HDL-C was made in this model. ARIC indicates Atherosclerosis Risk in Communities; DHS, Dallas Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis; and PREVEND, Prevention of Renal and Vascular Endstage Disease.
Figure 6.
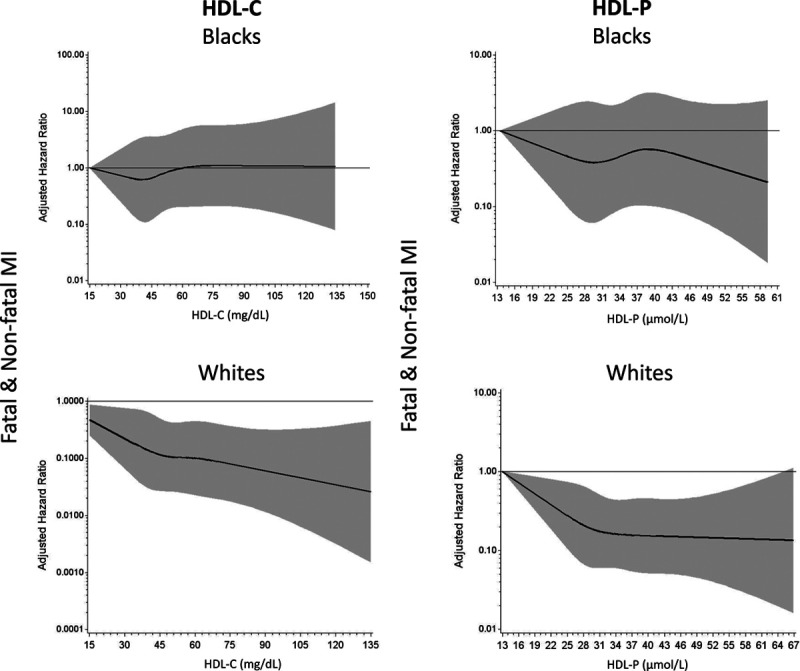
Spline curves demonstrating the relationship between high-density lipoprotein (HDL) cholesterol concentration (HDL-C) and HDL particle concentration (HDL-P) with myocardial infarction (MI) by Black vs White participants. Spline curves of adjusted hazard ratios for the association between HDL-C and HDL-P with MI in Black and White populations in our pooled cohort. This model is adjusted for risk factors (age, sex, race/ethnicity, diabetes mellitus, hypertension, smoking, low-density lipoprotein cholesterol, triglycerides, lipid-lowering medications, body mass index, waist circumference, and high-sensitivity C-reactive protein). No additional adjustment for HDL-P or HDL-C was made in this model. Shaded area around the spline curves represents 95% CI.
Additional HDL Parameters
Associations between outcomes and ratios of HDL-C and cholesterol size indexed to particle number were also explored. The HDL-C/HDL-P ratio was not associated with either individual events or overall ASCVD in adjusted models (Table IV in the Data Supplement). In contrast, increasing HDL-size/HDL-P was associated with both individual and composite outcomes (HR for composite Q4 versus Q1, 1.21 [95% CI, 1.13–1.29]) even after adjustment for risk factors (Table V in the Data Supplement). However, the point estimates and CIs for the inverse ratio of HDL-P (1/HDL-P) were similar to those of HDL-size/HDL-P (Table V in the Data Supplement). The results were similar for the subgroups of ethnicity and sex (data not shown).
HDL-size alone was not significantly associated with ASCVD after adjustment for cardiovascular risk factors (HR for composite outcome Q4 versus Q1, 0.91 [95% CI, 0.77–1.09]), as shown in Table IV in the Data Supplement. These results were unchanged when stratified by ethnicity or sex (data not shown).
Discussion
In this pooled cohort analysis of individual participants free of CVD across 4 cohorts, increasing HDL-P inversely correlated with both MI and ischemic stroke, whereas the relationship of HDL-C with these end points was more modest and not statistically significant. In contrast, increasing HDL-C was associated only with reduced ASCVD risk among White participants. The association of both HDL-C and HDL-P with the individual end point of MI was significantly modified by ethnicity, with no association between either HDL marker and MI in the Black population. With a relatively large number of ischemic stroke events in this combined cohort analysis, we were able to demonstrate an inverse association between both HDL-C and HDL-P and stroke. HDL-P attenuated all associations between HDL-C and events, whereas HDL-C had negligible effects on associations between HDL-P and events in the overall population.
Although traditional analyses have focused on the cholesterol content of lipoprotein particles (LDL-C and HDL-C), recent studies have elucidated the concept that lipoprotein particle concentration may have a stronger association with ASCVD risk compared with cholesterol content. In the case of LDL-C, when concentrations are in agreement (concordant) with LDL-P, there is a reliable, graded relationship with ASCVD risk and response to therapy. However, discordances between LDL-C and LDL-P can occur within the milieu of marked dyslipidemia and insulin resistance and with certain lipid-modifying therapies such as cholesterylester transfer protein inhibitors.30,31 In these situations, LDL-P typically is linked more strongly to risk and better reflects treatment efficacy.32 Thus, the hypothesis that HDL-P may also provide better risk prediction compared with HDL-C is justified, despite the fact that HDL-C remains a key and easily measured lipid marker in guideline-recommended risk score algorithms.31 HDL-C is also required to calculate non–HDL-C, which captures cholesterol in all apolipoprotein B–containing lipoproteins and is proven to predict risk ASCVD risk in all age categories of men and women.33 However, in predominantly White cohorts, the inverse association between apolipoprotein AI with coronary events remained significant, whereas HDL-C had no association with coronary events after adjustment for apolipoprotein AI.34 Furthermore, the most potent HDL-C–raising therapies such as niacin and cholesterylester transfer protein inhibitors have not improved ASCVD outcomes.8,35–37
In this regard, our pooled cohort analysis confirms that HDL-P more consistently associates with ASCVD compared with HDL-C and essentially attenuates all associations between HDL-C and individual and combined ASCVD outcomes. We aimed to extend these observations to events by specific vascular domains, namely MI and ischemic stroke, and to events in specific populations that have been underrepresented in most longitudinal cohort studies of HDL markers, namely women and Blacks. Our strategy to use a pooled cohort study design specifically addressed the key limitations of previous single-cohort studies: limited numbers of events and subsequent reduced statistical power in investigating these relationships.
Analysis of MI and ischemic stroke end points in this pooled cohort analysis revealed complex interactions for both HDL-C and HDL-P. Among women, HDL-C was inversely associated with MI, but the association with ischemic stroke was not significant. These inconsistent relationships by sex and vascular domain have not been reported thus far for HDL-C and highlight its further limitations as an overall ASCVD risk marker. In contrast, HDL-P was consistently associated with both MI and ischemic stroke among women. Most previous analysis in single cohorts such as MESA and ARIC revealed inconsistent associations between HDL-C and HDL-P with stroke or examined subclinical end points of cerebrovascular disease.12,18,19,38,39 Our current pooled cohort analysis includes the largest number of ischemic strokes in a multiethnic cohort analyzed for HDL parameters and strongly suggests that HDL-P is inversely related to ischemic stroke risk. We demonstrate that HDL-P was inversely associated with ischemic stroke not just in our overall cohort but also in women. This is contrasted with a lack of association in MESA with total strokes (n=176 total and 150 ischemic strokes), likely as a result of limited power and a lack of association with ischemic strokes in the Heart Protection Study, which was high risk and predominantly European.16,39 Neither explored the impact of sex on these associations. Therefore, our cohort is one of the first studies to demonstrate inverse associations between HDL-P and hard cerebrovascular events in women. Furthermore, the lack of association between HDL-C and ischemic stroke overall and in women in our large multiethnic pooled cohort contrasts with previous reports with fewer events and less ethnic diversity.40,41 This suggests the need to examine HDL-P as a risk marker for ischemic stroke overall and global ASCVD among women in further studies. Although not assessed in this analysis, cholesterol efflux, a primary antiatherosclerotic function of HDL, inversely associated with incident CHD in both the MESA and PREVEND cohorts; however, it did not associate with carotid plaque progression or with incident ischemic stroke in the MESA cohort.42,43 Thus, parameters reflecting different aspects of HDL metabolism, from cholesterol content to particle concentration to function, appear to contain heterogeneous information on atherosclerotic risk. Of all these measures, HDL-P most consistently associates with risk for both MI and ischemic stroke in the overall population.
The most striking and unexpected finding was an effect modification by Black race/ethnicity for both HDL-C and HDL-P and risk of MI. Among White participants, HDL-C and HDL-P were inversely associated with incident MI. Initial epidemiological studies, which were done primarily in predominantly White cohorts, consistently show this association with HDL-C, leading to its inclusion as a major risk biomarker for heart disease. It is also consistent with more contemporary studies in exclusively White or predominantly White cohorts such as EPIC (European Prospective Investigation into Cancer)–Norfolk and PREVEND.44,45 In contrast, among Black participants in our pooled cohort, HDL-C and HDL-P did not have an inverse association with MI. This is suggested in the Pooled Cohort Equation, in which the β coefficients for HDL-C and overall ASCVD risk are much weaker in Black (−0.307) compared with White men (−13.578), although they do not capture differences in vascular domains of coronary versus cerebrovascular disease. Previous studies from multiethnic cohorts such as MESA did not reveal significant effect modifications of HDL-C by race/ethnicity for combined ASCVD end points but similarly did not parse out MI separately from stroke or other ASCVD end points and were likely not powered to test for interactions by race/ethnicity.15 A previous study in the DHS suggested effect modification by Black race/ethnicity for composite ASCVD but did not parse out MI versus ischemic stroke because of small numbers of events.20 However, our results parallel the findings from a meta-analysis of the Jackson Heart Study with 4114 Black participants and the Framingham Offspring Cohort, which was predominantly White. Although other risk factors such as age, diabetes mellitus, body mass index, and triglycerides were significantly different among Black participants with and without CHD, HDL-C was not significantly different. HDL-C was not associated with CHD among the Black participants in adjusted models in this study, similar to our findings.46 Our pooled cohort had a higher number of MI events (n=166) among a similar number of Black participants compared with the Jackson Heart Study. Increasing HDL-C was not associated with fewer coronary events among the Black population in the ARIC-Carotid MRI substudy we examined, which could explain the difference in association compared with previous analyses of ARIC, which served as one of the cohorts for validation of Framingham CHD prediction. However, even with exclusion of participants from ARIC-Carotid MRI, there was no inverse association between HDL-C and MI in the Black population among the remaining cohorts, challenging traditional notions of HDL-C as a biomarker of inverse risk in this ethnic group. A recently published analysis from the REGARDS cohort (Reasons for Geographical and Racial Differences in Stroke) identified an HDL paradox with lower risk of CHD at an HDL-C range of 30 to <40 mg/dL among the Black population, consistent with our findings that higher HDL-C did not translate into lower MI risk.47 In our pooled cohort analyses, another novel observation was the lack of association between HDL-P and MI in Black participants, suggesting that both HDL-C and HDL-P have distinct associations with MI among Blacks compared with Whites.
There may be some possible explanations for ethnic differences in HDL biology. In general, Blacks have higher HDL-C and lower triglyceride levels compared with Whites, but these characteristics do not necessarily translate into a lower risk of CHD.48–51 According to our analysis of participants by race/ethnicity in individual cohorts, the surprising observation that higher HDL-C may even be directly associated with MI among Blacks may be partly explained not only by differences in HDL subclass composition but also by different relationships between HDL2-C and HDL3-C levels and the risk of coronary disease in White and Black populations.46 Studies examining HDL functionality have found that HDL in Black populations had lower antioxidant and anti-inflammatory activity compared with White populations, which may be one explanation of this paradoxical result. Although known genetic polymorphisms in hepatic lipase activity may partly explain the higher HDL levels observed in Blacks, data also suggest that these higher HDL levels may not be antiatherogenic. Blacks also have higher lipoprotein(a) levels compared with Whites, but the direct associations with ischemic/thrombotic events are similar.52
Last, with respect to ischemic stroke, although there was no effect modification by ethnicity, HDL-C was not associated with ischemic stroke among White or Black participants. By way of comparison, Black race/ethnicity modified the inverse associations between HDL-P and MI but not between HDL-P and ischemic stroke. Overall, HDL-P is a more consistent risk marker compared with HDL-C, except that Black race/ethnicity seems to modify risk associations between HDL-related markers and MI.
We also explored the concept that cholesterol-overloaded HDL may be dysfunctional and impart increased risk. Previous studies have suggested that varying metrics of overloaded HDL such as HDL-size or increased HDL-C to HDL-P ratios may be cross-sectionally associated with increased atherosclerotic disease.23,34 In our study, although HDL-C indexed to HDL-P was not linked to any outcomes, HDL-size/HDL-P did not impart any additional information beyond HDL-P alone. Theoretically, the cholesterol-overloaded HDL particle may be less efficient at cholesterol uptake and reverse cholesterol transport, but simple ratios of overall HDL concentration and size to particle number may be too crude to reflect this dynamic process.
Our analysis had several limitations. Although the diverse ethnic and geographic makeup of our pooled cohort improves overall generalizability, the significant heterogeneity of the populations recruited in the individual cohorts could have biased our results. Geographic or environmental factors that were not adjusted for could have affected our analysis, especially with respect to the differences in outcomes by race that have not been reported in previous epidemiological studies. The PREVEND cohort was enriched with participants with albuminuria, which is a known risk factor for increased metabolic abnormalities and cardiovascular morbidity and mortality, which we attempted to account for by adjustment for the cohort in our analyses.29,53–55 Although there is a more consistent association between HDL-P and ASCVD events, our study does not address whether HDL-P would improve clinical risk stratification for ASCVD over HDL-C as it stands in current risk prediction models. We did not see effect modification by sex in the overall population, but whether there is a difference between sexes within the racial subgroups was not addressed by our analysis. Given the overall healthy baseline cohorts and our goal to examine outcomes for MI and ischemic stroke, we may not have sufficient power to examine these differences. All 4 cohorts in our study used the identical proprietary NMR algorithm to measure HDL-P, which is critical because there is significant variation between the absolute measurements of HDL-P derived by different methodologies.56 It is unknown whether measurement of HDL-P by alternative methods such as calibrated ion mobility would have altered our primary findings, although it is important to note that even with different methodologies, the inverse association between HDL-P and atherosclerotic disease has been consistently present.57–59
Conclusions
Our study suggests that HDL-C may not be as consistent a marker for ASCVD as previously thought, especially for ischemic stroke. Our large pooled cohort demonstrated that HDL-P is more consistent than HDL-C in associating with MI and ischemic stroke in the general population and in women. An important exception was that neither HDL-C nor HDL-P was associated with MI in the Black population, suggesting that ethnicity differentially affects the association between HDL parameters and atherosclerotic disease in different vascular beds. Future refinements of risk prediction algorithms should more precisely parse out ischemic end points by race/ethnicity if HDL-C is to remain a risk factor in these equations for the Black population. An important next step is examining whether HDL particle composition imparts additional risk prediction information.
Acknowledgments
This manuscript was prepared using MESA Research Materials from the National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
This study was supported by American Heart Association grant 17UNPG33840006 (to Dr Rohatgi). Dr Rohatgi is supported by National Institutes of Health/National Heart, Lung and Blood Institute R01HL136724 and National Institutes of Health/National Heart, Lung and Blood Institute K24HL146838. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1TR001105. The ARIC study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I.
Disclosures
Drs Singh and Sperry report grants from American Heart Association. Dr Joshi reports grants from the American Heart Association, National Aeronautics and Space Administration, NovoNordisk, AstraZeneca, GlaxoSmithKline, and Sanofi; personal fees from Regeneron and Bayer; and other from G3 Therapeutics. Dr Virani reports grants from the Department of Veterans Affairs, World Heart Federation, and Tahir and Jooma Family, as well as other from the American College of Cardiology and Steering Committee. Dr Ballantyne reports grants from National Institutes of Health. Dr Otvos reports other from LabCorp. Dr Connelly reports being a salaried employee of LabCorp. Dr Ayers reports personal fees from the National Institutes of Health. Dr Rohatgi reports grants from the American Heart Association and Merck and personal fees from CSL Ltd and HDL Diagnostics. The other authors report no conflicts.
Supplemental Material
Data Supplement Figure I
Data Supplement Tables I–V
Supplementary Material
Footnotes
Sources of Funding, see page 667
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.045713.
This manuscript was sent to Dr Michael Miller, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Clinical Perspective
What Is New?
High-density lipoprotein (HDL) particle concentration is inversely associated with the specific end point of ischemic stroke overall and among women, whereas HDL cholesterol concentration is not associated with ischemic stroke.
Neither HDL particle concentration nor HDL cholesterol concentration is associated with myocardial infarction in Blacks.
What Are the Clinical Implications?
HDL particle concentration but not HDL cholesterol concentration may be a useful risk marker for ischemic stroke.
HDL particle concentration may be a useful risk marker for both myocardial infarction and ischemic stroke among women.
There is likely minimal utility of HDL markers for risk prediction of myocardial infarction in the Black population.
References
- 1.Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, et al. ; SCORE Project Group Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 200324987–1003doi: 10.1016/s0195-668x(03)00114-3 [DOI] [PubMed] [Google Scholar]
- 2.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 201974e177–e232doi: 10.1016/j.jacc.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khera AV, Demler OV, Adelman SJ, Collins HL, Glynn RJ, Ridker PM, Rader DJ, Mora S. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 20171352494–2504doi: 10.1161/CIRCULATIONAHA.116.025678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, et al. ; ILLUMINATE Investigators Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 20073572109–2122doi: 10.1056/NEJMoa0706628 [DOI] [PubMed] [Google Scholar]
- 5.AIM-HIGH Investigators Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 20113652255–2267 [DOI] [PubMed] [Google Scholar]
- 6.Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, et al. ; ACCELERATE Investigators Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 20173761933–1942doi: 10.1056/NEJMoa1609581 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, et al. ; dal-OUTCOMES Investigators Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 20123672089–2099doi: 10.1056/NEJMoa1206797 [DOI] [PubMed] [Google Scholar]
- 8.Otvos JD, Guyton JR, Connelly MA, Akapame S, Bittner V, Kopecky SL, Lacy M, Marcovina SM, Muhlestein JB, Boden WE. Relations of GlycA and lipoprotein particle subspecies with cardiovascular events and mortality: a post hoc analysis of the AIM-HIGH trial. J Clin Lipidol 201812348–355.e2doi: 10.1016/j.jacl.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackey RH, McTigue KM, Chang YF, Barinas-Mitchell E, Evans RW, Tinker LF, Lewis CE, Manson JE, Stefanick ML, Howard BV, et al. Lipoprotein particles and size, total and high molecular weight adiponectin, and leptin in relation to incident coronary heart disease among severely obese postmenopausal women: the Women’s Health Initiative observational study. BBA Clin 20153243–250doi: 10.1016/j.bbacli.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bots ML, Elwood PC, Nikitin Y, Salonen JT, Freire de Concalves A, Inzitari D, Sivenius J, Benetou V, Tuomilehto J, Koudstaal PJ, et al. Total and HDL cholesterol and risk of stroke: EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health 200256suppl 1i19–i24doi: 10.1136/jech.56.suppl_1.i19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Total and high-density lipoprotein cholesterol and stroke risk. Stroke 2012431768–1774.doi: 10.1161/STROKEAHA.111.646778 [DOI] [PubMed] [Google Scholar]
- 12.Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology 200768556–562doi: 10.1212/01.wnl.0000254472.41810.0d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation 20131281189–1197doi: 10.1161/CIRCULATIONAHA.113.002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenson RS, Brewer HB, Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR, Webb NR. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 20161348–60doi: 10.1038/nrcardio.2015.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackey RH, Greenland P, Goff DC, Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 201260508–516doi: 10.1016/j.jacc.2012.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R; Heart Protection Study Collaborative Group Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation 20121252469–2478doi: 10.1161/CIRCULATIONAHA.111.073684 [DOI] [PubMed] [Google Scholar]
- 17.Amarenco P, Labreuche J, Touboul PJ. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis 2008196489–496doi: 10.1016/j.atherosclerosis.2007.07.033 [DOI] [PubMed] [Google Scholar]
- 18.Bowman TS, Sesso HD, Ma J, Kurth T, Kase CS, Stampfer MJ, Gaziano JM. Cholesterol and the risk of ischemic stroke. Stroke 2003342930–2934doi: 10.1161/01.STR.0000102171.91292.DC [DOI] [PubMed] [Google Scholar]
- 19.Virani SS, Catellier DJ, Pompeii LA, Nambi V, Hoogeveen RC, Wasserman BA, Coresh J, Mosley TH, Otvos JD, Sharrett AR, et al. Relation of cholesterol and lipoprotein parameters with carotid artery plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Atherosclerosis 2011219596–602doi: 10.1016/j.atherosclerosis.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra A, Neeland IJ, Das SR, Khera A, Turer AT, Ayers CR, McGuire DK, Rohatgi A. Relation of black race between high density lipoprotein cholesterol content, high density lipoprotein particles and coronary events (from the Dallas Heart Study). Am J Cardiol 2015115890–894doi: 10.1016/j.amjcard.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agerholm-Larsen B, Nordestgaard BG, Steffensen R, Jensen G, Tybjaerg-Hansen A. Elevated HDL cholesterol is a risk factor for ischemic heart disease in white women when caused by a common mutation in the cholesteryl ester transfer protein gene. Circulation 20001011907–1912doi: 10.1161/01.cir.101.16.1907 [DOI] [PubMed] [Google Scholar]
- 22.Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: the Los Angeles Atherosclerosis Study. Atherosclerosis 2007195e191–e196doi: 10.1016/j.atherosclerosis.2007.03.045 [DOI] [PubMed] [Google Scholar]
- 23.Qi Y, Fan J, Liu J, Wang W, Wang M, Sun J, Liu J, Xie W, Zhao F, Li Y, et al. Cholesterol-overloaded HDL particles are independently associated with progression of carotid atherosclerosis in a cardiovascular disease-free population: a community-based cohort study. J Am Coll Cardiol 201565355–363doi: 10.1016/j.jacc.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 24.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, et al. ; Dallas Heart Study Investigators The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004931473–1480doi: 10.1016/j.amjcard.2004.02.058 [DOI] [PubMed] [Google Scholar]
- 25.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002156871–881doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 26.ARIC Study Investigators Design and objectives: the ARIC Investigators. Am J Epidemiol 1989129687–702 [PubMed] [Google Scholar]
- 27.Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, Van Gilst WH, De Zeeuw D, De Jong PE; PREVEND Study Group Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 2001249519–526doi: 10.1046/j.1365-2796.2001.00833.x [DOI] [PubMed] [Google Scholar]
- 28.Gruppen EG, Riphagen IJ, Connelly MA, Otvos JD, Bakker SJ, Dullaart RP. GlycA, a pro-inflammatory glycoprotein biomarker, and incident cardiovascular disease: relationship with C-reactive protein and renal function. PLoS One. 2015;10:e0139057. doi: 10.1371/journal.pone.0139057. doi: 10.1371/journal.pone.0139057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smink PA, Lambers Heerspink HJ, Gansevoort RT, de Jong PE, Hillege HL, Bakker SJ, de Zeeuw D. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 201260804–811doi: 10.1053/j.ajkd.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 30.Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation 2014129553–561doi: 10.1161/CIRCULATIONAHA.113.005873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC., Jr. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol 20115105–113doi: 10.1016/j.jacl.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, Wilson PW, D’Agostino RB. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study: implications for LDL management. J Clin Lipidol 20071583–592doi: 10.1016/j.jacl.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunner FJ, Waldeyer C, Ojeda F, Salomaa V, Kee F, Sans S, Thorand B, Giampaoli S, Brambilla P, Tunstall-Pedoe H, et al. ; Multinational Cardiovascular Risk Consortium Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. Lancet 20193942173–2183doi: 10.1016/S0140-6736(19)32519-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Lindahl C, Stroes ES, Tikkanen MJ, Wareham NJ, Faergeman O, Olsson AG, et al. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J Am Coll Cardiol 200851634–642doi: 10.1016/j.jacc.2007.09.060 [DOI] [PubMed] [Google Scholar]
- 35.Morgan J, Carey C, Lincoff A, Capuzzi D. High-density lipoprotein subfractions and risk of coronary artery disease. Curr Atheroscler Rep 20046359–365doi: 10.1007/s11883-004-0047-0 [DOI] [PubMed] [Google Scholar]
- 36.Jafri H, Alsheikh-Ali AA, Mooney P, Kimmelstiel CD, Karas RH, Kuvin JT. Extended-release niacin reduces LDL particle number without changing total LDL cholesterol in patients with stable CAD. J Clin Lipidol 2009345–50doi: 10.1016/j.jacl.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 37.Otvos JD. The surprising AIM-HIGH results are not surprising when viewed through a particle lens. J Clin Lipidol 20115368–370doi: 10.1016/j.jacl.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 38.Zaid M, Fujiyoshi A, Miura K, Abbott RD, Okamura T, Takashima N, Torii S, Saito Y, Hisamatsu T, Miyagawa N, et al. ; SESSA Research group High-density lipoprotein particle concentration and subclinical atherosclerosis of the carotid arteries in Japanese men. Atherosclerosis 2015239444–450doi: 10.1016/j.atherosclerosis.2015.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reina SA, Llabre MM, Allison MA, Wilkins JT, Mendez AJ, Arnan MK, Schneiderman N, Sacco RL, Carnethon M, Delaney JA. HDL cholesterol and stroke risk: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2015243314–319doi: 10.1016/j.atherosclerosis.2015.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everett BM, Kurth T, Buring JE, Ridker PM. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol 2006482235–2242doi: 10.1016/j.jacc.2006.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soyama Y, Miura K, Morikawa Y, Nishijo M, Nakanishi Y, Naruse Y, Kagamimori S, Nakagawa H; Oyabe Study High-density lipoprotein cholesterol and risk of stroke in Japanese men and women: the Oyabe Study. Stroke 200334863–868doi: 10.1161/01.STR.0000060869.34009.38 [DOI] [PubMed] [Google Scholar]
- 42.Shea S, Stein JH, Jorgensen NW, McClelland RL, Tascau L, Shrager S, Heinecke JW, Yvan-Charvet L, Tall AR. Cholesterol mass efflux capacity, incident cardiovascular disease, and progression of carotid plaque. Arterioscler Thromb Vasc Biol 20193989–96doi: 10.1161/ATVBAHA.118.311366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebtehaj S, Gruppen EG, Bakker SJL, Dullaart RPF, Tietge UJF. HDL (high-density lipoprotein) cholesterol efflux capacity is associated with incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol 2019391874–1883doi: 10.1161/ATVBAHA.119.312645 [DOI] [PubMed] [Google Scholar]
- 44.Borggreve SE, Hillege HL, Wolffenbuttel BH, de Jong PE, Zuurman MW, van der Steege G, van Tol A, Dullaart RP; PREVEND Study Group An increased coronary risk is paradoxically associated with common cholesteryl ester transfer protein gene variations that relate to higher high-density lipoprotein cholesterol: a population-based study. J Clin Endocrinol Metab 2006913382–3388doi: 10.1210/jc.2005-2322 [DOI] [PubMed] [Google Scholar]
- 45.Kappelle PJ, Gansevoort RT, Hillege JL, Wolffenbuttel BH, Dullaart RP; PREVEND Study Group Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J Intern Med 2011269232–242doi: 10.1111/j.1365-2796.2010.02323.x [DOI] [PubMed] [Google Scholar]
- 46.Joshi PH, Toth PP, Lirette ST, Griswold ME, Massaro JM, Martin SS, Blaha MJ, Kulkarni KR, Khokhar AA, Correa A, et al. ; Lipoprotein Investigators Collaborative (LIC) Study Group Association of high-density lipoprotein subclasses and incident coronary heart disease: the Jackson Heart and Framingham offspring cohort studies. Eur J Prev Cardiol 20162341–49doi: 10.1177/2047487314543890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Penson P, Long DL, Howard G, Howard VJ, Jones SR, Martin SS, Mikhailidis DP, Muntner P, Rizzo M, Rader DJ, et al. Associations between cardiovascular disease, cancer, and very low high-density lipoprotein cholesterol in the REasons for Geographical and Racial Differences in Stroke (REGARDS) study. Cardiovasc Res 2019115204–212doi: 10.1093/cvr/cvy198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bravo K, Velarde GP. Ethnicity and coronary artery disease: the role of high-density lipoprotein: a change in paradigm. Expert Rev Cardiovasc Ther 201513923–931doi: 10.1586/14779072.2015.1065178 [DOI] [PubMed] [Google Scholar]
- 49.Tillin T, Hughes AD, Mayet J, Whincup P, Sattar N, Forouhi NG, McKeigue PM, Chaturvedi N. The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans: SABRE (Southall and Brent Revisited): a prospective population-based study. J Am Coll Cardiol 2013611777–1786doi: 10.1016/j.jacc.2012.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bentley AR, Rotimi CN. Interethnic differences in serum lipids and implications for cardiometabolic disease risk in African ancestry populations. Glob Heart 201712141–150doi: 10.1016/j.gheart.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osei K, Gaillard T. Disparities in cardiovascular disease and type 2 diabetes risk factors in Blacks and Whites: dissecting racial paradox of metabolic syndrome. Front Endocrinol (Lausanne) 2017;8:204. doi: 10.3389/fendo.2017.00204. doi: 10.3389/fendo.2017.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in Black and White subjects: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2012125241–249doi: 10.1161/CIRCULATIONAHA.111.045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambers Heerspink HJ, Brantsma AH, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort RT; PREVEND Study Group Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol 2008168897–905doi: 10.1093/aje/kwn209 [DOI] [PubMed] [Google Scholar]
- 54.Lee SH, Kim DH, Kim YH, Roh YK, Ju SY, Nam HY, Nam GE, Choi JS, Lee JE, Sang JE, et al. Relationship between dyslipidemia and albuminuria in hypertensive adults: a nationwide population-based study. Medicine (Baltimore) 2016;95:e3224. doi: 10.1097/MD.0000000000003224. doi: 10.1097/MD.0000000000003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corsetti JP, Gansevoort RT, Bakker SJ, Sparks CE, Vart P, Dullaart RP. Apolipoprotein B attenuates albuminuria-associated cardiovascular disease in Prevention of Renal and Vascular Endstage Disease (PREVEND) participants. J Am Soc Nephrol 2014252906–2915doi: 10.1681/ASN.2013121256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matyus SP, Braun PJ, Wolak-Dinsmore J, Saenger AK, Jeyarajah EJ, Shalaurova I, Warner SM, Fischer TJ, Connelly MA. HDL particle number measured on the Vantera®, the first clinical NMR analyzer. Clin Biochem 201548148–155doi: 10.1016/j.clinbiochem.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 57.Matera R, Horvath KV, Nair H, Schaefer EJ, Asztalos BF. HDL particle measurement: comparison of 5 methods. Clin Chem 201864492–500doi: 10.1373/clinchem.2017.277632 [DOI] [PubMed] [Google Scholar]
- 58.Davidson WS. HDL-C vs HDL-P: how changing one letter could make a difference in understanding the role of high-density lipoprotein in disease. Clin Chem 201460e1–e3doi: 10.1373/clinchem.2014.232769 [DOI] [PubMed] [Google Scholar]
- 59.Hutchins PM, Ronsein GE, Monette JS, Pamir N, Wimberger J, He Y, Anantharamaiah GM, Kim DS, Ranchalis JE, Jarvik GP, et al. Quantification of HDL particle concentration by calibrated ion mobility analysis. Clin Chem 2014601393–1401doi: 10.1373/clinchem.2014.228114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


