Abstract
Aim of the Study:
The purpose of this study is to demonstrate that Chinese herbal medicine is beneficial for survival improvement in patients with multiple myeloma.
Materials and Methods:
We performed a 1:1 propensity score–matched cohort study to analyze patients with multiple myeloma diagnosed between January 1, 2002, and December 31, 2012, through the Taiwanese National Health Insurance Research Database. Patients who received Chinese herbal medicine therapy from the initial date of diagnosis of multiple myeloma to December 31, 2012, were included in the Chinese herbal medicine group. Patients who were not treated with Chinese herbal medicine during the same interval were categorized in the non-Chinese herbal medicine group. A Cox regression model was used to adjust for sex, age, comorbidities, and drug use. Hazard ratios were also compared between the 2 groups.
Results:
A total of 312 patients were identified after 1:1 propensity score matching. The patients had similar basic characteristics. A better survival status was found in the Chinese herbal medicine cohort (log-rank test, P < .0001). Finally, 49 patients in the Chinese herbal medicine cohort and 96 patients in the non-Chinese herbal medicine cohort died (adjusted hazard ratio = 0.35, 95% confidence interval = 0.24-0.51). The effect of survival improvement from Chinese herbal medicine in patients with multiple myeloma could be observed when prescriptions had the duration of ≥30 days.
Conclusions:
Our results showed that patients with multiple myeloma could benefit from Chinese herbal medicine treatment, which could improve the survival rate in Taiwan. The findings offer important ideas for further study.
Keywords: Chinese herbal medicine, multiple myeloma, survival
Introduction
Traditional Chinese medicine (TCM) has been practiced for thousands of years to treat musculoskeletal problems, respiratory diseases, neurological disorders, and gastrointestinal discomfort.1-5 TCM is the mainstay of complementary and alternative medicine therapies and includes Chinese herbal medicine (CHM), acupuncture, and manipulative treatment. In Taiwan, patients use Chinese herbal extractions to treat diseases according to the theory of CHM. In recent years, more and more Taiwanese patients have tried CHM for complementary interventions following a cancer diagnosis, including hematologic malignant diseases.6,7
Multiple myeloma (MM) is a hematologic malignant disease that arises from the abnormal proliferation of plasma cells.8 Males and elderly individuals are more predisposed to MM.9 Bone pain is one of clinical characteristics of MM; nevertheless, clinical doctors still find it difficult to confirm the diagnosis until other specific symptoms can be noted, such as renal failure and hypercalcemia.10 Although duration of survival has been lengthened after chemotherapy, both clinicians and patients are unsatisfied with current treatment results because high-dose chemotherapy is hard to prescribe to elderly patients.11 Furthermore, hematopoietic stem cell transplantation (HSCT) is another powerful intervention for MM patients.12 However, it remains unsuitable for elderly patients.13 The 5-year survival rate of MM is approximately 15% in populations that respond well to chemotherapy, and those who undergo HSCT could add to their lives.14 MM patients seek to overcome the complications from standard therapy and to arrive at better treatment outcomes with complementary therapies. In Taiwan, even hematological doctors in medical centers recommended that their MM patients partake CHM as a complementary intervention.6 Thus, we sought to use the National Health Insurance Research Database (NHIRD) to investigate whether MM patients could benefit from CHM for MM.
The National Health Insurance (NHI) program was enacted by the National Health Insurance Administration in Taiwan in 1995. Currently, the overall NHI coverage in Taiwan is over 99.6%. Information from the Taiwanese NHIRD, therefore, represents almost the entire Taiwanese population. The NHIRD is a large-scale long-term follow-up database for medical interventions often used to avoid sampling biases.15 TCM services have also been reimbursed by the NHI program since 1996.16
However, evidence supporting the idea that CHM is helpful for improving the survival rate in MM patients is limited. In addition, there is no large-scale clinical trial based on large populations of MM patients. Thus, we sought to use the NHIRD to investigate whether MM patients could benefit from CHM.
Methods
Data Sources
The Registry for Catastrophic Illness Patients, which records all original claims data, such as clinical visits, demographic characteristics, hospitalizations, diagnosis, medical costs, interventions, and prescriptions, was used as our data source.17 TCM services such as CHM, manipulative therapies, and acupuncture were all covered by the NHI. Diagnosis was coded by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
Study Cohort Identification
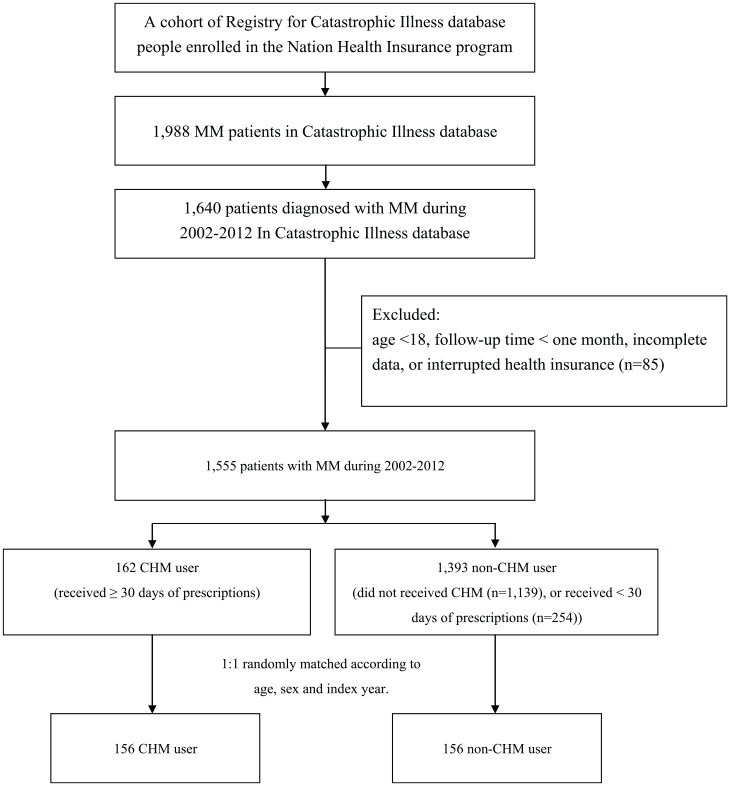
We identified patients who were newly diagnosed with MM from January 1, 2002, to December 31, 2012, and followed them until the end of 2013 (Figure 1). Patients with at least 2 ambulatory or inpatient claims with a diagnosis of ICD-9-CM code 203.00 were included. We excluded patients aged younger than 18 years, those with incomplete data on age or sex, those with interrupted health insurance services during the follow-up period, and those with a follow-up time <1 month. Participants who accepted CHM therapy lasting ≥1 month and from the initial diagnosis of MM to December 31, 2012, were enrolled in the CHM cohort. A propensity score approach was used to minimize confounding factors by the indication of CHM treatment and the caliper value was 0.2. Through multiple logistic regression analysis, a 1:1 propensity score match was performed by age, sex, urbanization level, comorbidities, and standard Western treatment program. No significant difference was found in age, sex, urbanization level, comorbidities, and standard Western treatment program compared with the general population after propensity score matching. Index date was defined as the first date of MM diagnosis. Immortal time was defined as the period from the initial diagnosis of MM to the date of death or end date of study. The most common single herb and herbal formulas prescribed to patients with MM were analyzed. These prescriptions are presented by their phonetic transcriptions and Latin names. Ratios of the ingredients of multiherb products were also included.
Figure 1.
Study population flowchart. A total of 1,988 patients with MM were newly diagnosed from 2002 to 2012. After 1:1 propensity score matching by sex, age, and index date, 156 patients were included in the CHM cohort and non-CHM cohorts, respectively. Abbreviations: MM, multiple myeloma; CHM, Chinese herbal medicine.
Covariate Assessment
Age and sex are sociodemographic factors, and we divided patients into 3 groups according to age (18-49 years, 50-64 years, and ≥65 years). ICD-9-CM codes of comorbidities identified more than one time in the outpatient or inpatient records before the primary diagnosis of MM were taken into consideration, and presented as the Charlson Comorbidity Index (CCI).
Ethical Considerations
There was no informed consent requirement because the secondary data for research was included in the NHIRD. Database users could not identify individuals or caregivers directly. The Research Ethics Committee of Taichung Tzu Chi Hospital approved this study (REC103-43).
Statistical Analyses
Baseline characteristics are presented as the means (standard deviations) or n (%), and the differences between the CHM and non-CHM cohorts were compared using paired t tests and χ2 tests for numeric and categorical variables, respectively. Cox proportional hazards regression was used to estimate crude and adjusted hazard ratios (HRs) of CHM therapy. The difference in the risk of death between the CHM and non-CHM cohorts was estimated using the Kaplan-Meier method with the log-rank test. All data analysis was performed by using commercial statistical software SPSS version 22 (IBM Corp, Released 2013) and SAS 9.3 (SAS Institute). We defined P < .05 in 2-tailed tests as statistically significant.
Results
A total of 1,640 patients were newly diagnosed with MM with at least 2 clinical visits recorded in the NHIRD from January 1, 2002, to December 31, 2012 (Figure 1). We excluded patients aged younger than 18 years, without detailed information, who died before the index date, or who had partaken of a CHM treatment for <30 days. The number of included patients was 1,555; then, we performed 1:1 propensity score matching by age, sex, urbanization level, CCI, and standard anticancer interventions to compare the 2 groups. In the final cohort, 156 patients were included in the non-CHM and CHM cohorts, respectively.
Table 1 shows the baseline characteristics of both cohorts, with similar distributions of age, sex, urbanization level, CCI, bortezomib usage, and HSCT. Male participants were predominant in both cohorts, and the percentages of each age group were the same (54.5%). Most patients came from the lowest urbanization level (>90%) and had a 0 for the CCI (>46%). Bortezomib was used by approximately 44% of patients. Most patients had not undergone HSCT (>98%).
Table 1.
Characteristics of MM Patients According to Use of CHM.
| Variable | Patients of MM | P | |||
|---|---|---|---|---|---|
| CHM | |||||
| No (N = 156) |
Yes (N = 156) |
||||
| n | Percentage | n | Percentage | ||
| Gendera | |||||
| Female | 71 | 45.5 | 71 | 45.5 | 1.000 |
| Male | 85 | 54.5 | 85 | 54.5 | |
| Age groupb (years) | |||||
| 18-49 | 22 | 14.1 | 19 | 12.2 | .846 |
| 50-64 | 73 | 46.8 | 77 | 49.4 | |
| ≥65 | 61 | 39.1 | 60 | 38.5 | |
| Age meanc ± SD (years) | 62.9 ± 10.5 | 62.4 ± 10.6 | .724 | ||
| Urbanization levelb | |||||
| 1 (highest) | 7 | 4.5 | 1 | 0.6 | .054 |
| 2 | 4 | 2.6 | 8 | 5.1 | |
| 3 (lowest) | 145 | 92.9 | 147 | 94.2 | |
| CCI scorea | |||||
| 0 | 73 | 46.8 | 75 | 48.1 | .847 |
| 1 | 51 | 32.7 | 53 | 34.0 | |
| ≥2 | 32 | 20.5 | 28 | 17.9 | |
| Bortezomiba | 62 | 39.7 | 77 | 49.4 | .088 |
| HSCTa | |||||
| No | 153 | 98.1 | 153 | 98.1 | 1.000 |
| Yes | 3 | 1.9 | 3 | 1.9 | |
| Follow-up time (mean, median) | 1.76 (1.46) | 3.01 (2.75) | <.001 | ||
Abbreviations: MM, multiple myeloma; CHM, Chinese herbal medicine; CCI, Charlson Comorbidity Index; HSCT, hematopoietic stem cell transplantation.
χ2 test.
Fischer’s exact test.
t test.
Table 2 presents the related HR of the mortality through a Cox proportional hazards model. After adjusting for age, sex, urbanization level, CCI, and treatment received, the adjusted HR of morbidity in the CHM group was 0.29 (95% confidence interval [CI] = 0.20-0.41; P < .0001), which was significantly different from that of the non-CHM group. Lag time to treatment was defined as the time of diagnosis to the time of the initiation of CHM treatment. Its influence on the improvement of prognosis has not yet been reported because the lag time to treatment may vary.18,19 After including lag time in the analysis, we obtained an adjusted HR of 0.35 (95% CI = 0.24-0.51, P < .0001).
Table 2.
Cox Model With HRs and 95% CIs of Mortality Associated With CHM and Covariates Among MM Patients.
| Variable | Patients of MM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency of death (N = 156) | Crudea |
Adjustedb |
Adjustedc |
|||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
| CHM use | ||||||||||
| Non-CHM | 96 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |||
| CHM | 49 | 0.29 | 0.21-0.42 | <.0001 | 0.29 | 0.20-0.41 | <.0001 | 0.35 | 0.24-0.51 | <.0001 |
Abbreviations: HR, hazard ratio; CI, confidence interval; CHM, Chinese herbal medicine; MM, multiple myeloma; CCI, Charlson Comorbidity Index.
Relative HR.
Represented adjusted HR: mutually adjusted for CHM use, age, gender, urbanization level, and CCI score in Cox proportional hazard regression.
Adjusted for CHM use, age, gender, urbanization level, CCI score, and treatment as well as the lag time for each patient, which was defined as the time from MM diagnosis to initial CHM treatment.
Table 3 reveals the cumulative days of CHM use among MM patients through the Cox proportional hazards model. Comparing with patients from the non-CHM group, patients who used CHM for 30 to 59 days had an adjusted HR of 0.35 (95% CI = 0.21-0.58, P < .001); those who used CHM for 60 to 179 days had an adjusted HR of 0.29 (95% CI = 0.17-0.49, P < .001); and those who used CHM for ≥180 days had an adjusted HR of 0.23 (95% CI = 0.13-0.41, P < .001). The median lag time from the diagnosis of MM to CHM treatment was 100 days in the subgroup of patients with 30 to 59 days of CHM treatment; 173 days in the subgroup with 60 to 179 days of CHM treatment; and 34 days in the subgroup with ≥180 days of CHM treatment.
Table 3.
HRs and 95% CIs of Mortality Risk Associated With Cumulative Use Day of CHM Among MM Patients.
| N | Frequency of death (N = 156) | Median lag time to CHM treatment (day) | HR (95% CI) | ||
|---|---|---|---|---|---|
| Crudea | Adjustedb | ||||
| Non-CHM user (included <30 days) | 156 | 96 | — | 1 (reference) | 1 (reference) |
| CHM user | |||||
| 30-59 days | 47 | 20 | 100 | 0.38 (0.23-0.63)*** | 0.35 (0.21-0.58)*** |
| 60-179 days | 57 | 16 | 173 | 0.30 (0.18-0.51)*** | 0.29 (0.17-0.49)*** |
| ≥180 days | 52 | 13 | 34 | 0.22 (0.12-0.39)*** | 0.23 (0.13-0.41)*** |
Abbreviations: HR, hazard ratio; CI, confidence interval; CHM, Chinese herbal medicine; MM, multiple myeloma; CCI, Charlson Comorbidity Index.
Relative HR.
Represented adjusted HR: mutually adjusted for CHM use, age, gender, urbanization level, CCI score, and treatment in Cox proportional hazard regression.
P < .05. **P < .01. ***P < .001.
Compared with non-CHM group, the CHM group demonstrated better cumulative survival according to the Kaplan-Meier plots in Figure 2 (P < .0001). Figure 3 also shows that compared with the non-CHM group, MM patients with CHM had higher cumulative survival, stratified by treatment duration (P < .0001). Figure 4 reveals that compared with non-CHM patients, the CHM group had had better cumulative survival status after age stratification (P < .0001).
Figure 2.
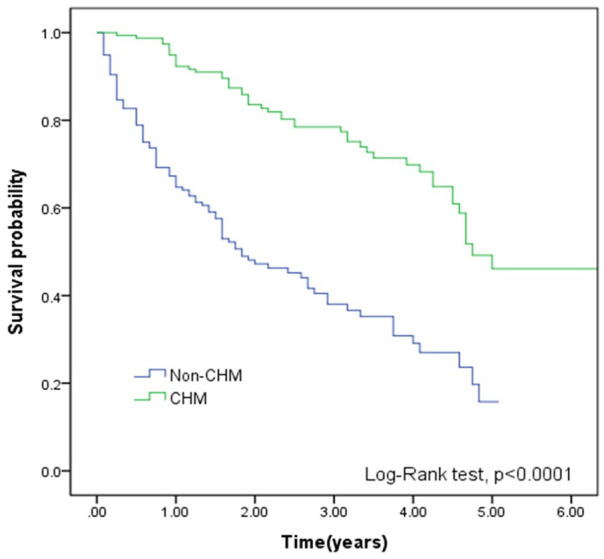
Kaplan-Meier curves of overall survival of MM patients according to treatment duration of CHM (green line) and non-CHM user (blue line). Patients in the CHM cohort had a higher cumulative survival rate (log-rank test, P < .0001). Abbreviations: MM, multiple myeloma; CHM, Chinese herbal medicine.
Figure 3.
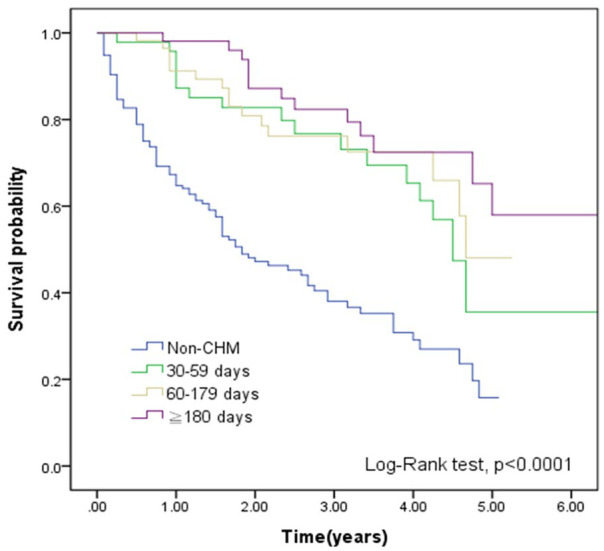
Kaplan-Meier curves of overall survival in MM patients according to CHM use, stratified by treatment duration. Highest cumulative survival rate could be noted in treatment duration ≥180 days (log-rank test, P < .0001). Abbreviations: MM, multiple myeloma; CHM, Chinese herbal medicine.
Figure 4.
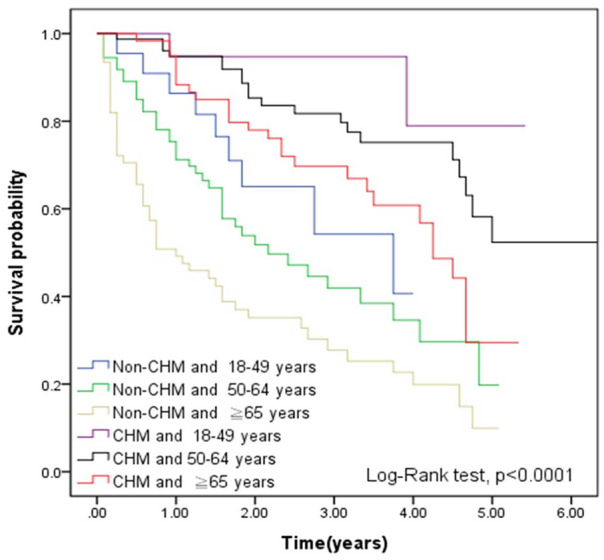
Kaplan-Meier curves of overall survival in MM patients according to CHM use, stratified by age (log-rank test, P < .0001). Abbreviations: MM, multiple myeloma; CHM, Chinese herbal medicine.
Table 4 reveals the Chinese herbal formulas and single herbs most commonly prescribed to patients with MM. The 3 most frequently prescribed Chinese herbal formulas and single herbs were Shu-Jing-Huo-Xue-Tang, Ban-Xia-Xie-Xin-Tang and Du-Huo-Ji-Sheng-Tang, and Tian-Hua-Fen (Trichosanthes kirilowii Maxim.), Da-Huang (Rheum officinale Baill.) and E-Zhu (Curcuma kwangsiensis S.G.Lee & C.F.Liang), respectively. The analyses of most of these prescriptions resulted in a P value < .001.
Table 4.
HRs and 95% CIs of Mortality Risk Associated With the Most-Used Herbal Products Among MM Patients.
| CHM type | Ingredients and ratio | Accumulated person-days | n | Frequency of mortality | HR (95% CI) | |
|---|---|---|---|---|---|---|
| Crudea | Adjustedb | |||||
| Non-Chinese herbal medicine group | 156 | 96 | 1 (reference) | 1 (reference) | ||
| Single-herb products | ||||||
| Tian-Hua-Fen | Trichosanthes kirilowii Maxim. | 8,703 | 105 | 32 | 0.29 (0.19-0.43)*** | 0.29 (0.20-0.44)*** |
| Da-Huang | Rheum officinale Baill. | 2,748 | 42 | 13 | 0.33 (0.18-0.58)*** | 0.35 (0.19-0.62)*** |
| E-Zhu | Curcuma kwangsiensis S.G.Lee & C.F.Liang | 2,424 | 10 | 5 | 0.43 (0.17-1.06) | 0.51 (0.21-1.28) |
| Ji-Xue-Teng | Spatholobus suberectus Dunn | 2,294 | 29 | 7 | 0.21 (0.10-0.46)*** | 0.19 (0.09-0.42)*** |
| Dan-Shan | Salvia miltiorrhiza Bunge | 1,695 | 33 | 7 | 0.17 (0.08-0.38)** | 0.17 (0.08-0.37)*** |
| Du-Zhong | Eucommia ulmoides Oliv. | 1,556 | 31 | 10 | 0.30 (0.16-0.58)*** | 0.32 (0.16-0.61)*** |
| Pu-Gong-Ying | Taraxacum mongolicum Hand.-Mazz. | 1,466 | 24 | 9 | 0.36 (0.18-0.72)** | 0.34 (0.17-0.68)** |
| Yan-Hu-Suo | Corydalis yanhusuo (Y.H.Chou & Chun C.Hsu) W.T.Wang ex Z.Y.Su & C.Y.Wu | 1,377 | 33 | 6 | 0.16 (0.07-0.36)*** | 0.18 (0.08-0.41)*** |
| Niu-Xi | Achyranthes bidentata Blume | 1,327 | 29 | 5 | 0.15 (0.06-0.36)*** | 0.14 (0.06-0.35)*** |
| Jie-Geng | Platycodon grandifloras (Jacq.) A.DC. | 1,162 | 41 | 13 | 0.26 (0.15-0.48)*** | 0.29 (0.16-0.53)*** |
| Multiherb products | ||||||
| Shu-Jing-Huo-Xue-Tan | Zhi-Gan-Cao (Glycyrrhiza glabra L.), Dang-Gui (Angelica sinensis (Oliv.) Diels), Bai-Shao (Paeonia sterniana H.R.Fletcher), Sheng-Di-Huang (Rehmannia glutinosa Libosch.), Cang-Zhu (Atractylodes lancea (Thunb.) DC.), Niu-Xi (Achyranthes bidentata Blume), Chen-Pi (Citrus reticulata Blanco), Tao-Ren (Prunus persica (L.) Batsch), Wei-Ling-Xian (Clematis chinensis Osbeck), Chuan-Xiong (Ligusticum chuanxiong Hort.), Fan-Gji (Stephania tetrandra S. Moore), Qiang-Huo (Notopterygium incisum Ting ex H. T. Chang), Fang-Feng (Saposhnikovia divaricata (Turcz.) Schischk), Bai-Zhi (Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f. var. formosana (Boiss.) Shan et Yuan), Long-Dan (Gentiana manshurica Kitag), Fu-Ling (Poria cocos (Schw.) Wolff), Shen-Gjiang (Zingiber officinale (Willd.) Rosc.) 1:2:2.5:2:2:2:2:2:2:1:1:1:1:1:1:1:3 |
2,231 | 36 | 11 | 0.28 (0.14-0.53)*** | 0.29 (0.15-0.56)*** |
| Ban-Xia-Xie-Xin-Tang | Ban-Xia (Pinellia ternata (Thunb.) Makino); Huang-Qin (Scutellaria baicalensis Georgi), Gan-Jiang (Zingiber officinale Roscoe), Ren-Shen (Panax ginseng C.A.Mey.), Huang-Lian (Coptis chinensis Franch.), Da-Zao (Ziziphus jujuba Mill.), Zhi-Gan-Cao (Glycyrrhiza glabra L.) 7.5:4.5:4.5:4.5:1.5:3:4.5 |
2,181 | 33 | 5 | 0.14 (0.06-0.33)*** | 0.14 (0.06-0.34)*** |
| Du-Huo-Ji-Sheng-Tang | Du-Huo (Angelica pubescens Maxim.), Ji-Sheng (Taxillus chinensis (DC.) Danser), Du-Zhong (Eucommia ulmoides Oliv.), Niu-Xi (Achyranthes bidentata Blume), Xi-Xin (Asarum heterotropoides f. mandshuricum (Maxim.) Kitag.), Qin-Jiao (Gentiana macrophylla Pall.), Fu-Ling (Poria cocos (Schw.) Wolff), Rou-Gui (Cinnamomun cassia Presl), Fang-Feng (Saposhnikovia divaricata (Turcz.) Schischk), Chuan-Qiong (Ligusticum chuanxiong Hortorum), Ren-Shan (Panax ginseng C.A.Mey.), Zhi-Gan-Cao (Glycyrrhiza glabra L.), Dang-Gui (Angelica sinensis (Oliv.) Diels), Bai-Shao (Paeonia sterniana H.R.Fletcher), Shou-Di-Huang (Rehmannia glutinosa Libosch.) 3:2:2:2:2:2:2:2:2:2:2:2:2:2:2 |
1,764 | 31 | 8 | 0.24 (0.11-0.49)*** | 0.23 (0.11-0.47)*** |
| Xiao-Chai-Hu-Tang | Chai-Hu (Bupleurum chinense DC.), Huang-Qin (Scutellaria baicalensis Georgi), Ren-Shan (Panax ginseng C.A.Mey.), Ban-Xia (Pinellia Ternate (Thunb.) Makino), Zhi-Gan-Cao (Glycyrrhiza glabra L.), Sheng-Jiang (Zingiber officinale Roscoe), Da-Zao (Ziziphus jujuba Mill.) 8:3:3:3:5:3:2 |
1,595 | 28 | 9 | 0.35 (0.18-0.70)** | 0.33 (0.17-0.66)** |
| Gan-Lu-Yin | Shou-Di-Huang (Rehmannia glutinosa Libosch.), Mai-Men-Dong (Ophiopogon japonicus (Thunb.) Ker Gawl.), Zhi-Ke (Citrus × aurantium L.), Chi-Shao (red Paeonia lactiflora Pall.), Zhi-Gan-Cao (Glycyrrhiza glabra L.), Yin-Chen-Hao (Artemisia capillaris Thunb.), Pi-Pa-Ye (Eriobotrya japonica (Thunb.) Lindl.), Shi-Hu (Dendrobium nobile Lindl.), Huang-Qin (Scutellaria baicalensis Georgi), Sheng-Di-Huang (Rehmannia glutinosa Libosch.), Tian-Men-Dong (Asparagus cochinchinensis (Lour.)Merr.) 2.5:2.5:2.5:2.5:2.5:2.5:2.5:2.5:2.5:2.5:2.5 |
1,552 | 31 | 3 | 0.09 (0.03-0.28)*** | 0.10 (0.03-0.30)*** |
| Ma-Zi-Ren-Wan | Huo-Ma-Ren (Cannabis sativa L.), Bai-Shao(Paeonia sterniana H.R.Fletcher), Zhi-Shi (Citrus × aurantium L.), Da-Hunag (Rheum officinale Baill.), Hou-Po (Magnolia officinalis Rehder & E.H. Wilson), Xing-Ren (Prunus armeniaca L.) 7.5:2.5:2.5:5.0:2.5:2.5 |
1,439 | 22 | 3 | 0.15 (0.05-0.47)*** | 0.15 (0.05-0.48)*** |
| Ji-Sheng-Shen-Qi-Wan | Shou-Di-Huang (Rehmannia glutinosa Libosch.), Shan-Zhu-Yu (Cornus officinalis Siebold & Zucc.), Shan-Yao (Dioscorea opposita Thunb.), Fu-Ling (Poria cocos (Schw.) Wolff), Mu-Dan-Pi (Paeonia × suffruticosa Andrews), Ze-Xie (Alisma orientale (Sam.) Juz.), Fu-Zi (Aconitum carmichaelii Debeaux), Rou-Gui (Cinnamomun cassia Presl), Niu-Xi (Achyranthes bidentata Blume), Che-Qian-Zi (Plantago asiatica L.) 8:4:4:6:3:3:1:1:2:2 |
1,364 | 24 | 7 | 0.29 (0.13-0.62)*** | 0.28 (0.13-0.60)*** |
| Tian-Wang-Bu-Xin-Dan | Tian-Men-Dong (Asparagus cochinchinensis (Lour.) Merr.), Ren-Shen (Panax ginseng C.A.Mey.), Fu-Ling (Poria cocos (Schw.) Wolff), Xuan-Shen (Scrophularia ningpoensis Hemsl.), Dan-Shen (Salvia miltiorrhiza Bunge), Yuan-Zhi (Polygala tenuifolia Willd.), Jie-Geng (Platycodon grandifloras (Jacq.) A.DC.), Dang-Gui (Angelica sinensis (Oliv.) Diels), Wu-Wei-Zi (Schisandra chinensis (Turcz.) Baill.), Mai-Dong (Ophiopogon japonicus (Thunb.) Ker-Gawl.), Bai-Zi-Ren (Platycladus orientalis (L.) Franco), Suan-Zao-Ren (Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou), Sheng-Di-Huang (Rehmannia glutinosa Libosch.) 2:1:1:1:1:1:1:2:2:2:2:2:8 |
1,338 | 22 | 7 | 0.31 (0.14-0.66)** | 0.28 (0.13-0.61)** |
| Shao-Yao-Gan-Cao-Tang | Bai-Shao(Paeonia sterniana H.R.Fletcher), Zhi-Gan-Cao (Glycyrrhiza glabra L.) 12:12 |
1,298 | 37 | 12 | 0.29 (0.16-0.52)*** | 0.30 (0.16-0.56)*** |
| Zhi-Bai-Di-Huang-Wan | Shou-Di-Huang (Rehmannia glutinosa Libosch.), Shan-Zhu-Yu (Cornus officinalis Siebold & Zucc.), Fu-Ling (Poria cocos (Schw.) Wolff), Shan-Yao (Dioscorea opposita Thunb.), Mu-Dan-Pi (Paeonia × suffruticosa Andrews), Ze-Xie (Alisma orientale (Sam.) Juz.), Zhi-Mu (Anemarrhena asphodeloides Bge.), Huang-Bai (Phellodendron chinense Schneid.) 8:4:3:4:3:3:2:2 |
1,052 | 28 | 9 | 0.28 (0.14-0.56)*** | 0.29 (0.15-0.59)*** |
Abbreviations: HR, hazard ratio; CHM, Chinese herbal medicine; MM, multiple myeloma.
Relative HR.
Represented adjusted HR: mutually adjusted for CHM use, age, gender, urbanization level, and Charlson Comorbidity Index score in Cox proportional hazard regression.
P < .05. **P < .01. ***P < .001.
To understand the safety of CHM, we compared the incidences of chronic kidney disease (CKD) and chronic hepatitis in our cohorts. During the follow-up period, the incidence of CKD has no significant difference in the CHM group and non-CHM group (6.09 vs 6.42 per 100 person-years, 95% CI = 0.46-1.95); the incidence of chronic hepatitis has no significant difference in the CHM group and non-CHM group (10.81 vs 4.42 per 1,000 person-years, 95% CI = 0.27-21.86). Additionally, the cumulative incidences of CKD and chronic hepatitis, as calculated by the Kaplan–Meier method, revealed no significant difference between the CHM cohort and the non-CHM cohort in patients with MM (P value = .969 and .324, respectively).
Discussion
A large-scale, long-term follow-up, comprehensive database such as the NHIRD in Taiwan is helpful in decreasing participation bias and selection bias because of its large sample size.15 The basic characteristics of the enrolled patients with MM in this study were compatible with those observed in clinical practice, such as the use of bortezomib and HSCT, and were variable factors in this study.20 We noted similar characteristics between the 2 cohorts after 1:1 propensity score matching. To the best of our knowledge, this is the first study to reveal the benefit of the risk control of survival resulting from CHM treatment in individuals with MM. The survival rate from targeted therapies such as bortezomib may not convince patients, leading them to seek integrative therapies for better outcomes.21 Currently, clinical doctors are greatly interested in CHM treatment for MM because of its popularity as an integrative therapy in Taiwan.22 The therapeutic results of CHM in MM patients had previously been reported in the form of quality of life.23 However, whether MM patients have a higher survival rate after CHM therapy has never been explored. Our results showed that the benefit of CHM intervention was independent of sex, age, comorbidities, and standard Western medical treatment, such as bortezomib or HSCT.
The incidence of MM throughout the world is very different, although age and genetic environmental and occupational factors have been associated with the pathogenesis of MM. Caucasian populations from the United States and Europe have a higher incidence of MM than individuals of Chinese descent in China and Singapore.24-26 However, the incidence of MM has shown an increasing trend in the past decades throughout the world. There are no definitive reasons for this phenomenon.27-30 The incidence per 10,000 individuals of MM is 0.75 among Taiwanese adults and 5.2 in Taiwanese elderly individuals.31 Approximately 50% of MM patients respond to traditional chemotherapy, but most elderly individuals cannot tolerate it.32 Taiwanese patients still seek to improve the treatment effect and minimize the severity of complications from chemotherapy or targeted therapy through integrative interventions, and CHM is always their first choice.
Our results demonstrated that CHM treatment was associated with better survival rates in MM patients. Compared with the non-CHM group, the CHM group had a lower adjusted HR of 0.68 (95% CI = 0.24-0.51). Furthermore, patients with longer CHM treatment courses tended to have better survival status. The dose-dependence relationship showed the possibility of an effect of CHM in MM treatment. The association between the lag time to treatment and outcome is a well-discussed topic among leukemia patients. Analysis regarding the median lag time to treatment for subgroups stratified by treatment duration is presented in Table 3. Although patients with the longest treatment duration had the shortest medical lag time, those who had treatment duration of 60 to 179 days presented with the longest medical lag time. This result may not support the role of lag time as a significant factor in the outcome of the treatment. HSCT has been suggested to MM patients for long-term control. Prior to the analysis, the percentage of patients with HSCT in the 2 cohorts was almost identical. This suggests that whether patients accepted HSCT did not change the final result of CHM intervention.
The Kaplan-Meier curves stratified by age are presented in Figure 3. The correlation between age and survival rate can be noted, which matches the clinical situation in the real world. In each of the age subgroups, we can find that CHM truly improves the survival rate. Currently, the treatment of elderly MM patient remains a great challenge because they are unable to submit to full-dose chemotherapy due to their physical characteristics.33 In other words, if the benefit of survival improvement did not come from CHM, we would have noted as such in Figure 4.
Herbal safety is an important topic for clinical doctors and patients taking CHM. We investigated the incidences of developing CKD and chronic hepatitis in CHM and non-CHM groups during follow-up period. The results showed no significant difference between these 2 groups, which represent that CHM treatment is a safe intervention or, at least, does no harm to MM patients.
Chinese herbal medicine has been investigated with regard to its effect in controlling complications from chemotherapy or radiation therapy in cancer patients. Some clinical human trials have also shown that CHM is of benefit for the treatment of cancer.34 However, there is little evidence of the benefit of CHM in treating individuals with malignant hematologic diseases. Therefore, we disclosed the top 10 single and combinations of herbal products used to treat MM in Taiwan in the hope that they will be further candidates for a potential treatment choice.
Conclusions
Our study shows that CHM treatment was beneficial for MM patients. Although it is difficult to determine whether this phenomenon could be attributed to CHM alone, a synergistic effect or to an unrelated relieving of complications, this study nevertheless provides important ideas for more comprehensive investigations in the future.
Footnotes
Author Contributions: Chia-Yu Huang designed, performed the research, and helped writing the manuscript. Mei-Yao Wu designed the study. Mei-Yao Wu designed the study. Yu-Hung Kuo analyzed the data. Hung-Rong Yen and Sio-Ian Tou helped writing the manuscript and revising the manuscript. Sio-Ian Tou and Hung-Rong Yen contributed equally to this work. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the “Chinese Medicine Research Center, China Medical University” from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (CMRC-CHM-1). This study was also supported in part by China Medical University Hospital (DMR-107-011, DMR-108-115 and DMR-108-116), China Medical University (CMU105-BC-1-1), National Research Institute of Chinese Medicine, Ministry of Health and Welfare (MOHW109-NRICM-M-124-000002), Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), and health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence, Ministry of Health and Welfare (MOHW109-TDU-B-212-134024), Taiwan. None of the funders and institutions listed had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ORCID iD: Sio-Ian Tou  https://orcid.org/0000-0003-2868-4881
https://orcid.org/0000-0003-2868-4881
References
- 1. Huang MC, Pai FT, Lin CC, et al. Characteristics of traditional Chinese medicine use in patients with rheumatoid arthritis in Taiwan: a nationwide population-based study. J Ethnopharmacol. 2015;176:9-16. [DOI] [PubMed] [Google Scholar]
- 2. Liao HH, Yeh CC, Lin CC, et al. Prescription patterns of Chinese herbal products for patients with fractures in Taiwan: a nationwide population-based study. J Ethnopharmacol. 2015;173:11-19. [DOI] [PubMed] [Google Scholar]
- 3. Huang TP, Liu PH, Lien AY, Yang SL, Chang HH, Yen HR. Characteristics of traditional Chinese medicine use in children with asthma: a nationwide population-based study. Allergy. 2013;68:1610-1613. [DOI] [PubMed] [Google Scholar]
- 4. Liao HH, Yen HR, Muo CH, et al. Complementary traditional Chinese medicine use in children with cerebral palsy: a nationwide retrospective cohort study in Taiwan. BMC Complement Altern Med. 2017;17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang CY, Lai WY, Sun MF, et al. Prescription patterns of traditional Chinese medicine for peptic ulcer disease in Taiwan: a nationwide population-based study. J Ethnopharmacol. 2015;176:311-320. [DOI] [PubMed] [Google Scholar]
- 6. Kuo YT, Chang TT, Muo CH, et al. Use of complementary traditional Chinese medicines by adult cancer patients in Taiwan: a nationwide population-based study. Integr Cancer Ther. 2018;17:531-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fleischer T, Chang TT, Chiang JH, Yen HR. A controlled trial of Sheng-Yu-Tang for post–hematopoietic stem cell transplantation leukemia patients: a proposed protocol and insights from a preliminary pilot study. Integr Cancer Ther. 2018;17:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585-598. [DOI] [PubMed] [Google Scholar]
- 9. Cohen HJ, Crawford J, Rao MK, Pieper CF, Currie MS. Racial differences in the prevalence of monoclonal gammopathy in a community-based sample of the elderly. Am J Med. 1998;104:439-444. [DOI] [PubMed] [Google Scholar]
- 10. Kyle RA, Rajkumar SV. Treatment of multiple myeloma: a comprehensive review. Clin Lymphoma Myeloma. 2009;9:278-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ludwig H, Hajek R, Tóthová E, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113:3435-3442. [DOI] [PubMed] [Google Scholar]
- 12. Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875-1883. [DOI] [PubMed] [Google Scholar]
- 13. Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825-831. [DOI] [PubMed] [Google Scholar]
- 14. Brenner H, Gondos A, Pulte D. Expected long-term survival of patients diagnosed with multiple myeloma in 2006-2010. Haematologica. 2009;94:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan national health insurance research database. JAMA Intern Med. 2015;175:1527-1529. [DOI] [PubMed] [Google Scholar]
- 16. Chang CC, Lee YC, Lin CC, et al. Characteristics of traditional Chinese medicine usage in patients with stroke in Taiwan: a nationwide population-based study. J Ethnopharmacol. 2016;186:311-321. [DOI] [PubMed] [Google Scholar]
- 17. Lin LY, Warren-Gash C, Smeeth L, Chen PC. Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health. 2018;40:e2018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013;121:2618-2626. [DOI] [PubMed] [Google Scholar]
- 19. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barlogie B, Shaughnessy J, Tricot G, et al. Treatment of multiple myeloma. Blood. 2004;103:20-32. [DOI] [PubMed] [Google Scholar]
- 21. Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745-4753. [DOI] [PubMed] [Google Scholar]
- 22. Shih CC, Huang LH, Yeh CC, et al. The prevalence, characteristics, and factors associated with purchasing Chinese herbal medicine among adults in Taiwan. BMC Complement Altern Med. 2017;17:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang M. Effect of Chinese medicine combined with improved VAD chemotherapy on quality of life in multiple myeloma. J Guangzhou Univ Tradit Chin Med. 2004;6:427-429. [Google Scholar]
- 24. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am.1992;6:225-247. [PubMed] [Google Scholar]
- 25. Phekoo KJ, Schey SA, Richards MA, et al. A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol. 2004;127:299-304. [DOI] [PubMed] [Google Scholar]
- 26. Cartwright RA, Gilman EA, Gurney KJ. Time trends in incidence of haematological malignancies and related conditions. Br J Haematol. 1999;106:281-295. [DOI] [PubMed] [Google Scholar]
- 27. Crocetti E, Capocaccia R, Casella C, et al. Population-based incidence and mortality cancer trends (1986-1997) from the network of Italian cancer registries. Eur J Cancer Prev. 2004;13:287-295. [DOI] [PubMed] [Google Scholar]
- 28. Davis DL, Hoel D, Fox J, Lopez A. International trends in cancer mortality in France, West Germany, Italy, Japan, England and Wales, and the USA. Lancet. 1990;336:474-481. [DOI] [PubMed] [Google Scholar]
- 29. Turesson I, Zettervall O, Cuzick J, Waldenstrom JG, Velez R. Comparison of trends in the incidence of multiple myeloma in Malmö, Sweden, and other countries, 1950-1979. N Engl J Med. 1984;310:421-424. [DOI] [PubMed] [Google Scholar]
- 30. Yoshimi I, Kaneko S. Mortality trend of hematologic and lymphatic malignancy (lymphoma, multiple myeloma and leukemia) in Japan: 1960-2000. Jpn J Clin Oncol. 2004;34:218-225. [PubMed] [Google Scholar]
- 31. Huang SY, Yao M, Tang JL, et al. Epidemiology of multiple myeloma in Taiwan: increasing incidence for the past 25 years and higher prevalence of extramedullary myeloma in patients younger than 55 years. Cancer. 2007;110:896-905. [DOI] [PubMed] [Google Scholar]
- 32. Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bladé J, Muñoz M, Fontanillas M, et al. Treatment of multiple myeloma in elderly people: long-term results in 178 patients. Age Ageing. 1996;25:357-361. [DOI] [PubMed] [Google Scholar]
- 34. Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med. 2013;2013:302426. [DOI] [PMC free article] [PubMed] [Google Scholar]