Abstract
Background:
We previously developed a surface-controlled water-dispersible form of curcumin that we called Theracurmin®. The area under the blood concentration-time curve (AUC) of Theracurmin in humans was 27-fold higher than that of curcumin powder. Previously, we reported on the anti-inflammatory effects of Theracurmin for knee osteoarthritis.
Hypothesis/Purpose:
We determined the clinical effects of orally administered Theracurmin in patients with knee osteoarthritis over a 6-month period.
Study Design:
Open prospective study.
Methods:
Fifty patients Kellgren-Lawrence grade II, III, or IV knee osteoarthritis who were above 40 years old were enrolled in this clinical study. Theracurmin containing 180 mg/day of curcumin was administered orally every day for 6 months. To monitor for adverse events, blood biochemistry analyses were performed before and after 6 months of each intervention. The patients’ knee symptoms were evaluated at 0, 1, 2, 3, 4, 5, and 6 months based on the Japanese Knee Osteoarthritis Measure, the knee pain visual analog scale, and the knee scoring system of the Japanese Orthopedic Association.
Results:
Five cases dropped out during the study, but no cases dropped out because of major problems. No major side effects were observed with Theracurmin treatment, including the blood biochemistry analysis results. The effective group included 34 cases (75.6%), while the not-effective group included 11 cases.
Conclusion:
This study demonstrates the safety and good efficacy of Theracurmin for various types of knee osteoarthritis. Theracurmin shows great potential for the treatment of human knee osteoarthritis.
Keywords: Curcumin, highly bioavailable, knee osteoarthritis, safety, human
Introduction
Hip or knee osteoarthritis, which is also referred to as degenerative joint disease, is a slow destructive process of the joints that affects millions of people worldwide. Although the exact biochemical cause of osteoarthritis remains unknown, the process usually begins with abnormal joint structures or with unusually high stress placed on joint surfaces. Hip, knee, or other types of joint osteoarthritis are long-term conditions mostly treated with analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs); however, these drugs sometimes cause serious gastrointestinal and cardiovascular adverse events, particularly with long-term use.1,2 Thus, there is a need for disease-modifying agents that not only decrease joint pain but also slow the progression of the condition.
Curcumin is a polyphenol extracted from turmeric, which has been safely used in foods, such as curry, for a long time.3 Curcumin is a promising therapeutic food material because of its anti-inflammatory and anti-oxidative functions; it has long been used as an anti-inflammatory treatment in traditional Chinese and Ayurvedic medicine.3 Curcumin regulates various biochemical and molecular pathways by modulating several molecular targets, including transcription factors, cytokines, enzymes, and genes, that regulate cell proliferation or apoptosis.4 The anti-inflammatory effect of curcumin seems comparable with those of steroidal drugs and NSAIDs such as indomethacin and phenylbutazone.5 Some studies have shown that curcumin’s anti-inflammatory properties were related to the suppression of prostaglandin synthesis by its effect on cyclooxygenase (COX),6 a key enzyme responsible for the conversion of arachidonic acid to prostaglandins. Curcumin has also been shown to inhibit proteasome activity and induce apoptosis in human colon cancer cells in vitro and in vivo.7 Moreover, an important mechanism of curcumin is its inhibition of nuclear factor kappa B (NF-kB) activation,8 which is a key event in the long-term inflammatory process. Based on these findings, curcumin is expected to be effective for a range of diseases related to long-term inflammation, including cancer, cardiovascular disease, metabolic syndrome, Alzheimer’s disease, osteoarthritis, and other common diseases and aging conditions.3,4,9,10 Furthermore, curcumin can be a potent inhibitor of the production of inflammatory and catabolic mediators by chondrocytes.9 As osteoarthritis and related osteoarticular conditions of the synovial joints are characterized by inflammation, curcumin’s biological actions in joint tissues may facilitate the development of clinically safe, orally administered therapeutic agents for treating joint diseases.
However, curcumin’s poor bioavailability has been an obstacle to realizing its beneficial health effects because only a small amount of curcumin is absorbed via oral administration.11 To overcome the bioavailability problem, we previously developed a surface-controlled water-dispersible curcumin that we named it Theracurmin® (Theracurmin: Theravalues, Tokyo, Japan).12 The absorption efficacy of Theracurmin was investigated and compared with that of curcumin powder. In rats, the area under the blood concentration-time curve (AUC) after the oral administration of Theracurmin was more than 40-fold higher than that of curcumin powder. In healthy human volunteers, the AUC of Theracurmin was 27-fold higher than that of curcumin powder. These findings demonstrate Theracurmin’s significantly higher bioavailability compared to currently available curcumin preparations. Thus, Theracurmin is believed to be useful for providing the clinical benefits of curcumin in humans.
In our previous study, we conducted a randomized, double-blinded, placebo-controlled, prospective clinical study of the efficacy of Theracurmin, which is a highly bioavailable form of curcumin, in patients with osteoarthritis.13 The knee pain visual analog scale (VAS) scores were significantly lower in the patients treated with Theracurmin than in those treated with placebo at 8 weeks among those who had initial VAS scores >0.15. The same tendency was displayed in the total Japanese Knee Osteoarthritis Measure (JKOM)14 score and its subcategory scores, including pain and stiffness in the knees, conditions in daily life, general activities, and health conditions. Moreover, Theracurmin significantly lowered celecoxib dependence compared to placebo. These results suggest that Theracurmin may decrease knee osteoarthritis pain and discomfort and may improve patients’ general condition and quality of daily life. As osteoarthritis is a slowly progressive and long-term condition that decreases quality of life, agents such as Theracurmin, which is also a common food ingredient that is mildly effective with no major side effects, have modest potential for the treatment of human knee osteoarthritis.
The purpose of this study was to determine the clinical efficacy and safety of orally administered Theracurmin in patients with knee osteoarthritis over 6 months of treatment. We hypothesized that Theracurmin ingestion for 6 months would improve the symptoms and functional abilities of patients with knee osteoarthritis with no major side effects.
Materials and Methods
This was an open prospective study. A total of 50 patients with knee osteoarthritis confirmed by radiographic analysis were selected and enrolled in this study. Written informed consent was obtained from all subjects before participation. All procedures were reviewed and approved by the research ethics committee of our hospital, and this study was performed in accordance with the World Medical Association’s Declaration of Helsinki.
The inclusion criteria were knee osteoarthritis patients with primary medial or lateral type in the femorotibial joints or patellofemoral joints, above 40 years of age, and with Kellgren-Lawrence (KL) grade II, III, or IV osteoarthritis with radiographic classification. The other combined therapies allowed during the study were NSAIDs, pain relief patches, and hyaluronic acid knee injection treatment. The exclusion criteria were knee surgeries during the study, knee steroid injections within 2 months before the study, or other steroid administrations within 4 weeks before the study. We examined the previous treatments before enrolment of patients in this study but did not assess them except the above exclusion criteria. The flow chart of this study was shown in Figure 1.
Figure 1.
Flow chart in this study.
Theracurmin (Theracurmin: Theravalues, Tokyo, Japan) was administered orally twice a day for 6 months. Theracurmin consisted of 30% curcumin, 6% undisclosed components (curcumin derivatives), 0.7% citric acid, 8.7% dextrin, 14.6% gum ghatti, and 40% maltose.15 Subjects in the Theracurmin group took 6 capsules of Theracurmin per day, corresponding to a total daily dose of 180 mg of curcumin. Its dose was same as Nakagawa’s study.13 The subjects were requested to report the number of remaining capsules at their 1, 2, 3, 4, 5, and 6-month visits at our outpatient clinic to assess compliance.
Blood biochemistry analyses (including high-sensitivity C-reactive protein: hs-CRP) were performed before the study and after 6 months of each intervention. The patients’ knee symptoms were evaluated at 0, 1, 2, 3, 4, 5, and 6 months according to the following criteria: the JKOM,14 the knee pain VAS included in the JKOM, and the knee scoring system of the Japanese Orthopedic Association (JOA).13 The VAS has a minimum score of 0 and a maximum score of 1. The JKOM consists of 25 questions divided into 4 subcategories: pain and stiffness, condition in daily life, general activities, and health conditions. The JKOM is used for patient self-assessment and is based on the World Health Organization’s International Classification of Functioning, Disability, and Health; it is validated in the same manner as the Western Ontario and McMaster Universities’ Arthritis Index (WOMAC). The JOA scale evaluates 4 items: ability to walk (30 points), ability to climb up and down stairs (25 points), range of motion (35 points), and joint swelling (10 points). Each knee joint can achieve a maximum score of 100 points on the JOA scale. The JOA score was significantly correlated with validated patient-related outcome measures (JKOM and the Medical Outcome Study Short-Form 36-Item Health Survey), indicating the concurrent validity of the JOA.16 We evaluated adverse events and the combined therapies that the subjects required during the 6-month period.
In this study, we defined effective cases as follows: patients in the effective group felt that the treatment was effective and the VAS, JKOM, or JOA scores improved between 0 and 6 months at a minimum of 1 assessment. We examined the proportion of effective cases according to the KL classification and type of knee osteoarthritis. Thirteen patients were treated with only Theracurmin without combined therapy because the patients desired this strategy. We also examined the proportion of effective cases in the Theracurmin only group.
To determine the clinical and chronological effects of orally administered Theracurmin in patients with knee osteoarthritis over the 6 months treatment period, we calculated the differences in VAS, JKOM, and JOA scores between the assessment and pretreatment periods. We then compared the data from the assessment periods with the pretreatment period; we also compared the data between each assessment period.
The Mann-Whitney U-test was used to statistically analyze the VAS, JKOM, and JOA scores. A paired t-test was used to statistically analyze the VAS, JKOM, and JOA scores of each patient between the pretreatment period and 6 months after the treatment. A P value of <.05 was considered statistically significant.
Results
Five cases dropped out of this study (Figure 1), and 1 subject did not attend her 1 month follow-up visit. One subject dropped out at 1 month because the patient felt that her personal costs for the Theracurmin treatment were expensive (7500 yen per month). The other 3 patients discontinued participation in the study because of minor side effects. One patient had a decreased appetite at 22 weeks, and another patient had diarrhea on Day 6. In addition, one patient felt increased joint pain at 2 months. Therefore, we included 45 patients (11 men and 34 women) for further analysis. The mean therapeutic age was 67.2 (range: 42-85) years.
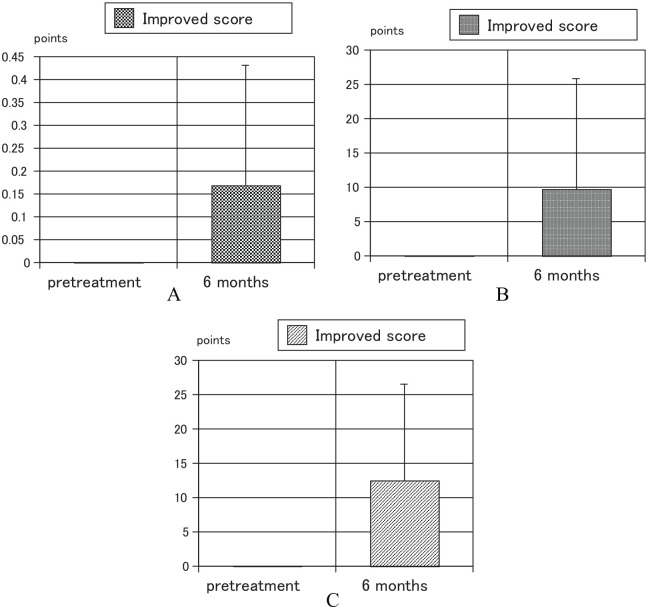
Using a paired t-test, the VAS, JKOM, and JOA scores at 6 months after the treatment were significantly better than those at the pretreatment period, of all 45 patients (Table 1). Of the Theracurmin only group, the JOA score at 6 months after the treatment was significantly better than that at the pretreatment period (Table 2). Improved scores of VAS, JKOM, and JOA between pretreatment period and 6 months after treatment for all 45 patients were significant (Figure 2) and also improved score of JOA between them for Theracurmin only group was significant (Figure 3).
Table 1.
Comparison of the clinical scores between the pretreatment period and 6 months after the treatment of all 45 patients using a paired t-test.
| Pretreatment | 6 months | P value | |
|---|---|---|---|
| VAS (point) | 0.514 + 0.242 | 0.345 + 0.257 | <.0001 |
| JKOM (point) | 32.71 + 15.01 | 23.27 + 15.79 | .0003 |
| JOA (point) | 71.43 + 13.86 | 83.94 + 11.52 | <.0001 |
Abbreviations: JKOM, Japanese Knee Osteoarthritis Measure; JOA, Japanese Orthopedic Association; VAS, visual analog scale.
Mean value + standard deviation.
Table 2.
Comparison of the clinical scores between the pretreatment period and 6 months after the treatment of 13 patients who were treated Theracurmin only using a paired t-test.
| Pretreatment | 6 months | P value | |
|---|---|---|---|
| VAS (point) | 0.372 + 0.190 | 0.263 + 0.25 | .1739 |
| JKOM (point) | 27.69 + 10.86 | 20.46 + 14.80 | .1854 |
| JOA (point) | 75.77 + 16.09 | 89.04 + 8.51 | .0203 |
Abbreviations: JKOM, Japanese Knee Osteoarthritis Measure; JOA, Japanese Orthopedic Association; VAS, visual analog scale.
Mean value + standard deviation.
Figure 2.
Improved scores of VAS, JKOM, and JOA between pretreatment period and 6 months after treatment for all 45 patients: (A) VAS P < .0001, (B) JKOM P = .0003, and (C) JOA P < .0001.
JKOM indicates Japanese Knee Osteoarthritis Measure; JOA, Japanese Orthopedic Association; VAS, visual analog scale.
Figure 3.

Improved score of JOA between pretreatment period and 6 months after treatment for Theracurmin only group. P = .0203.
JOA indicates Japanese Orthopedic Association.
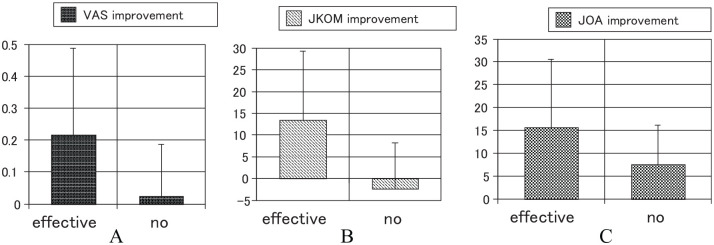
Of the 45 patients, the 34 were effective cases (75.6 %) and 11 were not effective cases; the latter group included 8 patients who did not feel the effectiveness of Theracurmin. The improvements in the VAS (Figure 4A), JKOM (Figure 4B), and JOA scores (Figure 4C) between 6-month treatment and pretreatment periods are shown in Figure 4. The proportion of effective cases for each KL classification grade is shown in Table 3. The proportion of effectiveness ranged from 65% to 88%. There were no significant differences in effectiveness between different grades of KL classification. The proportions of effective cases for each type of knee osteoarthritis are shown in Table 4. The proportion of effectiveness ranged from 71% to 100%. There were no significant differences in effectiveness between various types of knee osteoarthritis.
Figure 4.
Improvements in the clinical scores: (A) VAS scores improved by .216 points in the effective group, and .025 in the not-effective group. This difference was significant (P = .0332). (B) JKOM scores improved by 13.5 points in the effective group, and −2.3 in the not-effective group. This difference was significant (P = .0036). (C) JOA scores improved by 15.7 points in the effective group, and 7.5 in the not-effective group. This difference was no significant (P = .0900).
JKOM indicates Japanese Knee Osteoarthritis Measure; JOA, Japanese Orthopedic Association; VAS, visual analog scale.
Table 3.
The proportion of effective cases according to KL classification.
| KL classification | Effective group | Not-effective group |
|---|---|---|
| II | 12 cases (85.7%) | 2 cases |
| III | 15 cases (65.2%) | 8 cases |
| IV | 7 cases (87.5%) | 1 case |
Abbreviation: KL, Kellgren-Lawrence.
Table 4.
The proportion of effective cases according to the various types of knee osteoarthritis.
| Type of knee OA | Effective group | No effective group |
|---|---|---|
| FT lateral | 6 cases (100 %) | 0 cases |
| FT medial | 25 cases (71.4 %) | 10 cases |
| Patello femoral joint | 3 cases (75.0 %) | 1 case |
Abbreviations: FT, femorotibial joint; OA, osteoarthritis.
Five patients felt itchy. There were a slight increase in neutral fat (6 cases), and hepatic enzymes (4 cases) in the blood analyses during the study. However, no major side effects were observed with Theracurmin treatment, including in the blood biochemistry analyses performed during this study. There was no significant difference in the improvement of sensitive CRP values between the effective group and the not-effective group.
The Theracurmin only group, included 10 effective cases (76.9 %) and 3 not-effective cases; the latter group included 2 patients who did not feel the effectiveness of Theracurmin.
The chronological changes of the clinical scores in the effective group are shown in Tables 5 to 7. The P values for improvement in VAS (Table 5), JKOM (Table 6), and JOA (Table 7) scores during each assessment period are indicated. In the effective group, 2 months of Theracurmin treatment resulted in significant clinical effects; these positive effects were maintained for 6 months according to the VAS and JOA scores. One month of Theracurmin treatment also resulted significant clinical effects that lasted for 6 months according to the JKOM scores.
Table 5.
The chronological changes of the clinical scores in VAS scores.
| 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
|---|---|---|---|---|---|---|
| 0 month | .1062 | .0047 | .0177 | .0014 | .0007 | <.0001 |
| 1 month | .2825 | .3367 | .0383 | .0112 | .0005 | |
| 2 months | .7910 | .1969 | .1189 | .0068 | ||
| 3 months | .3195 | .1464 | .0210 | |||
| 4 months | .4533 | .0210 | ||||
| 5 months | .1100 |
Abbreviation: VAS, visual analog scale.
The P values between the assessment periods for improvement in VAS scores.
Bold numbers indicate a significant difference.
Table 7.
The chronological changes of the clinical scores in JOA scores.
| 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
|---|---|---|---|---|---|---|
| 0 month | .1270 | .0052 | <.0001 | .0114 | .0019 | .0062 |
| 1 month | .0022 | .0053 | .0258 | .0038 | .0032 | |
| 2 months | .2903 | .6480 | .561 | .3342 | ||
| 3 months | .0021 | .0742 | .0601 | |||
| 4 months | .8247 | .5551 | ||||
| 5 months | .4740 |
Abbreviation: JOA, Japanese Orthopedic Association.
The P values between the assessment periods for improvement in JOA scores.
Bold numbers indicate a significant difference.
Table 6.
The chronological changes of the clinical scores in JKOM scores.
| 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
|---|---|---|---|---|---|---|
| 0 month | <.0001 | <.0001 | .0011 | .0006 | .0054 | <.0001 |
| 1 month | .3503 | .3896 | .2930 | .7304 | .0080 | |
| 2 months | .8112 | .7960 | .6405 | .253 | ||
| 3 months | .9706 | .4049 | .2938 | |||
| 4 months | .2424 | .1850 | ||||
| 5 months | .0031 |
Abbreviation: JKOM, Japanese Knee Osteoarthritis Measure.
The P values between the assessment periods for improvement in JKOM scores.
Bold numbers indicate a significant difference.
Discussion
This study showed that Theracurmin was effective for treating knee osteoarthritis in 75.6% of the total population (45 patients) and in 76.9% of the Theracurmin only patients (13 patients). There were no significant differences in effectiveness between different KL classification grades or various types of knee osteoarthritis. We also evaluated the safety of Theracurmin for knee osteoarthritis; no major side effects were observed with Theracurmin treatment, including in the blood biochemistry analyses performed during this study. However, there was no significant difference in the improvement of sensitive CRP levels between the effective group and the not-effective group. In the effective group, 1 or 2 months of Theracurmin treatment resulted in significant clinical effects that could be maintained for 6 months.
There have been several reports on the effectiveness of curcumin to treat knee osteoarthritis. Nakagawa reported a trial involving 50 knee OA patients with a KL grade II or III classification; a significant reduction in pain was observed after an 8-week administration of a surface-controlled water-dispersible form of curcumin in a randomized, double-blinded, placebo-controlled study.13 The authors also observed a more pronounced lowering of celecoxib dependence in the group of patients treated with curcumin than in the placebo group. Three other randomized, double-blinded, placebo-controlled trials have been conducted. Treatment of knee OA with curcuminoids plus glycosaminoglycans, combined with physical therapy, improves VAS scores at motion and Lequesne Index scores.17 Treatment with curcuminoids was associated with significantly greater reductions in WOMAC, VAS, and Lequesne’s pain function index scores compared to placebo.18 In addition, the 12-week use of curcumin complex or its combination with boswellic acid reduces pain-related symptoms in patients with OA.19 In a multicenter study, Curcuma domestica extracts were shown to be effective as ibuprofen for the treatment of knee osteoarthritis.20 This study showed that Theracurmin were effective for treating knee osteoarthritis in 75.6% of the total population of 45 cases.
The use of curcumin is limited by its bioavailability. To overcome these obstacles, Nakagawa reported that the blood concentration-time curve of Theracurmin was 27-fold higher than that of curcumin powder with healthy human volunteers.13 Appelboom reported that the administration of 42 mg of Flexofytol was equivalent to the ingestion of 57 g of native curcumin.21 Belcaro reported that Meriva was a lecithin delivery form of curcumin,22 and Panahi reported that curcuminoids were co-administered with piperine to improve bioavailability.23 In these 4 studies, curcumin was more effective for the treatment of knee osteoarthritis. In this study, the proportion of effective cases was 76.9% in the Theracurmin only group.
Clinical studies on the efficacy of curcumin for the treatment of knee osteoarthritis have reported on specific biomarkers. There was significant elevation in serum superoxide dismutase activities, a borderline significant elevation in glutathione concentrations, and a significant reduction in malonedialdehyde concentrations in the curcuminoids compared with the placebo group.22 Curcumin significantly reduced the serum levels of Coll2-1 and tended to decrease CRP levels in Flexofytol for 3 months.23 Belcaro reported that the treatment group experienced a greater decrease in CRP levels compared to the control group.24 Unfortunately, in our study, there was no significant difference in the improvement of sensitive CRP levels between the effective group and the not-effective group, similar to another study.17 Chin reported that the extensive clinical trials on the effects of curcumin in osteoarthritic patients reported reduced pain and improved functionality of the patients treated with curcumin. These effects could be achieved as early as 4 weeks.25 Similarly, 1 or 2 months of Theracurmin treatment resulted in significant clinical effects that could be maintained for 6 months in this study.
Several reports have discussed the effectiveness of curcumin for cartilage degeneration in animal models and cell cultures. Curcumin treatment led to reduced proteoglycan loss and cartilage erosion in a posttraumatic osteoarthritis mouse model.26 Curcumin treatment enhanced autophagy and reduced apoptosis, and cartilage loss in a spontaneous and surgically induced osteoarthritis mice model.27 Curcumin prevented the deterioration of articular cartilage compared to the control group in estrogen-deficient rats.28 In cell culture studies, noncytotoxic concentrations of curcumin exerted anticatabolic and anti-inflammatory effects in cartilage explants.29 Curcumin was demonstrated to inhibit the interleukin (IL)-1b-induced activation of NF-kB by suppressing IkBa phosphorylation.30 Curcumin inhibited chondrocyte hypertrophy through Indian hedgehog homolog and notch signaling. Curcumin was a potential agent in modulating cartilage homeostasis and maintaining the chondrocyte phenotype.31 However, although this study was unable to demonstrate improvements in patient knee cartilage, the above findings suggest that curcumin might improve the knee cartilage of such patients.
In this study, 5 patients felt itchy. There were a slight increase in neutral fat (6 cases) and hepatic enzymes (4 cases) in the blood analyses during the study. However, no major side effects were observed with Theracurmin treatment including in the blood biochemistry analyses performed during this study. Likewise, there were no considerable adverse effects in curcumin groups in other clinical studies.17,18,19,21 Curcuma domestica extracts were noninferior to ibuprofen for the treatment of knee osteoarthritis, and the Curcuma domestica extracts group had a similar incidence of adverse events but with gastrointestinal adverse events.20 Daily reported that turmeric preparations and curcumin were considered safe at doses not exceeding 1200 mg/day for up to 4 months.32 A study on the safety of Theracurmin revealed that a dose of up to 400 mg for 9 months was safe in cancer patients receiving chemotherapy.33 In this study, the dosage of curcumin was 180 mg/day, similar to Nakagawa study.13 This was the smallest dose used in the published clinical studies.34 The safety of curcumin was demonstrated in this study, and the dosage of curcumin used in our study was safe.
There are 6 main limitations in this study: there was a short follow-up period (6 months), the sample size was small (50 cases), the lack of other treatment adjustment in the analysis, the placebo effects could not be eliminated, and this was an open study. Also, it is a potential selection bias that the patients are paying for their treatment from the start.
Conclusion
Fifty patients with knee osteoarthritis took 6 capsules of Theracurmin per day, corresponding to a total daily dose of 180 mg of curcumin, for 6 months. Five cases dropped out because of minor side effects. Therefore, we included 45 patients (11 men and 34 women) in the further analyses. This study showed that Theracurmin was effective for treating knee osteoarthritis in 75.6% of all cases and in 76.9 % of cases in the Theracurmin only group. Of the Theracurmin-only group, the JOA score at 6 months after the treatment was significantly better than that at the pretreatment period using the paired t-test. In our evaluation of the safety of Theracurmin for knee osteoarthritis, no major side effects were observed with Theracurmin treatment, including in the blood biochemistry analyses performed during this study. Theracurmin showed great potential for the treatment of human knee osteoarthritis based on efficacy and safety findings.
Acknowledgments
The authors thank Mrs Kaori Akiyoshi for technical assistance in this study.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was financially supported by Theravalues Corporation (Tokyo, Japan).
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Study design and data interpretation were by Y.N. and S.Y. Study implementation and drafting the manuscript were by Y.N. and S.M. Data collection was by Y.N., S.M., H.Y., and T.M. Data analysis was by Y.N. and S.A.
Ethics Approval: Ethical approval for this study was obtained from the institutional review board (approval no. KMC 13-05).
Informed Consent: Written informed consent was obtained from all subjects before conducting this study.
ORCID iD: Yasuaki Nakagawa  https://orcid.org/0000-0002-5664-963X
https://orcid.org/0000-0002-5664-963X
References
- 1. Felson DT, Lawrence RC, Hochberg MC, et al. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med. 2000;133:726-737. [DOI] [PubMed] [Google Scholar]
- 2. Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol. 1995;141:539-545. [DOI] [PubMed] [Google Scholar]
- 3. Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787-809. [DOI] [PubMed] [Google Scholar]
- 4. Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105-125. [DOI] [PubMed] [Google Scholar]
- 6. Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol. 2007;595:213-226. [DOI] [PubMed] [Google Scholar]
- 7. Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumbar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283-7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Curcumin mediated suppression of nuclear factor-kB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res Ther. 2010;12:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58:899-908. [DOI] [PubMed] [Google Scholar]
- 10. Wongcharoen W, Phrommintikul A. The protective role of curcumin in cardiovascular diseases. Int J Cardio. 2009;133:145-151. [DOI] [PubMed] [Google Scholar]
- 11. Rajasekaran SA. Therapeutic potential of curcumin in gastrointestinal diseases. World J Gastrointest Pathophysiol. 2011;15:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sasaki H, Sunagawa Y, Takahashi K, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34:660-665. [DOI] [PubMed] [Google Scholar]
- 13. Nakagawa Y, Mukai S, Yamada S, et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19:933-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akai M, Doi T, Fujino K, Iwaya T, Kurosawa H, Nasu T. An outcome measure for Japanese people with knee osteoarthritis. J Rheumatol. 2005;32:1524-1532. [PubMed] [Google Scholar]
- 15. Kato M, Nishikawa S, Ikehata A, et al. Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol Nutr Food Res. 2017 Mar;61. doi: 10.1002/mnfr.201600471. [DOI] [PubMed] [Google Scholar]
- 16. Okuda M, Omokawa S, Okahashi K, Akahane M, Tanaka Y. Validity and reliability of the Japanese Orthopaedic Association score for osteoarthritic knee. J Orthop Sci. 2012;17:750-756. [DOI] [PubMed] [Google Scholar]
- 17. Sterzi S, Giordani L, Morrone M, et al. The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: a randomized, double-blind, placebo-controlled study. Eur J Phys Rehabil Med. 2016;52:321-330. [PubMed] [Google Scholar]
- 18. Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A, Sahebkar A. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytother Res. 2014;28:1625-1631. [DOI] [PubMed] [Google Scholar]
- 19. Haroyan A, Mukuchyan V, Mkrtchyan N, et al. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: a comparative, randomized, double-blinded, placebo-controlled study. BMC Complement Altern Med. 2018;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Appelboom T, Maes N, Albert A. A new Curcuma extract (Flexofytol®) in osteoarthritis: results from a Belgian real-life experience. Open Rheumatol J. 2014;8:77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belcaro G, Dugall M, Luzzi R, et al. Meriva®+glucosamine versus Condroitin + glucosamine in patients with knee osteoarthritis: an observational study. Eur Rev Med Pharmacol Sci. 2014;18:3959-3963. [PubMed] [Google Scholar]
- 23. Panahi Y, Alishiri GH, Parvin S, Sahebkar A. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: results of a randomized controlled trial. J Diet Suppl. 2016;13:209-220. [DOI] [PubMed] [Google Scholar]
- 24. Henrotin Y, Gharbi M, Dierckxsens Y, et al. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement Altern Med. 2014;14:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belcaro G, Cesarone MR, Dugall M, et al. Product-evaluation registry of Meriva®, a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010;52:55-62. [PubMed] [Google Scholar]
- 26. Zhang Z, Leong DJ, Xu L, et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res Ther. 2016;18:128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 27. Zhang G, Cao J, Yang E, et al. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Bioscience Rep. 2018;28:BSR20171691. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Park S, Lee LR, Seo JH, Kang S. Curcumin and tetrahydrocurcumin both prevent osteoarthritis symptoms and decrease the expression of pro-inflammatory cytokines in estrogen-deficient rats. Genes Nutrition. 2016;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clutterbuck AL, Allaway D, Harris P, Mobasheri A. Curcumin reduces prostaglandin E2, matrix metalloproteinase-3 and proteoglycan release in the secretome of interleukin 1β-treated articular cartilage. F1000Res. 2013;2:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J, Ma J, Gu JH, et al. Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1β mediated by curcumin via inhibition of NF-kB signaling in rat chondrocytes. Mol Med Rep. 2017;16:1837-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao Z, Dou C, Dong S. Curcumin inhibits chondrocyte hypertrophy of mesenchymal stem cells through IHH and Notch signaling pathways. Chem Pharm Bull (Tokyo). 2017;65:762-767. [DOI] [PubMed] [Google Scholar]
- 32. Daily JW, Yang M, Park S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J Med Food. 2016;19:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanai M, Otsuka Y, Otsuka K, et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol. 2013;71:1521-1530. [DOI] [PubMed] [Google Scholar]
- 34. Chin KY. The spice for joint inflammation: anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des Devel Ther. 2016;10:3029-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]