Abstract
This study hypothesizes that, bromelain (BL) acts as radiosensitizer of tumor cells and that it protects normal cells from radiation effects. In vitro and in vivo studies have been carried out to prove that assumption. In vitro MTT cell proliferation assay has shown that the irradiated Ehrlich ascites carcinoma (EAC) cell line could be sensitized by BL pretreatment. In vivo: animals were randomly divided into 5 groups, Group 1: control (PBS i.p for 10 days), Group 2: Ehrlich solid tumor (EST) bearing mice, Group 3: EST + γ-radiation (fractionated dose, 1 Gy × 5), Group 4: EST + BL (6 mg/kg, i.p), daily for 10 days, Group 5: EST + BL for 10 days followed by γ-irradiation (1 Gy × 5). The size and weight of tumors in gamma-irradiated EST bearing mice treated with BL decreased significantly with a significant amelioration in the histopathological examination. Besides, BL mitigated the effect of γ-irradiation on the liver relative gene expression of poly ADP ribose polymerase-1 (PARP1), nuclear factor kappa activated B cells (NF-κB), and peroxisome proliferator-activated receptor α (PPAR-α), and it restored liver function via amelioration of paraoxonase1 (PON1) activity, reactive oxygen species (ROS) content, lipid peroxidation (LPO) and serum aspartate transaminase (AST), alanine transaminase (ALT), and albumin (ALB). It is concluded that BL can be considered as a radio-sensitizer and radio-protector, suggesting a possible role in reducing radiation exposure dose during radiotherapy.
Keywords: bromelain, tumor, γ-radiation, radio-sensitizer, radio-protector
Introduction
Radiotherapy has been used for a long time in treating cancer.1 However, from the clinical perspective, radiotherapy provides inadequate success due to the radioresistance of many tumors as well as the high risk of recurrence, and effects on normal cells may occur.2,3 Radioresistance occurs as the microenvironment of solid tumors is hypoxic compared with normal tissue.4 In addition, some tumors have either an intrinsic resistance to ionizing radiation or can attain this property through accumulation of genetic mutations causing an increased survival and proliferation.5 Thus, strategies to improve radiation therapy could include increasing resistance of normal tissues to radiation and/or increasing sensitivity of the tumor cells.6
Radiosensitizing agents increase the sensitivity of tumor cells via enhancing the generation of reactive oxygen species (ROS), increasing lipid peroxidation, depletion of glutathione which leads to DNA damage, inhibition of DNA repair, inhibition of DNA synthesis, induction of cell cycle arrest, induction of apoptosis, and inhibition of proliferation.7 Numerous nutritive cancer chemopreventive compounds having antioxidant properties have been recognized to potentiate radiation therapy-induced cytotoxic effects on cancer cells, inversely decreasing its toxicity on normal adjacent tissues.8,9 In this regard, much research has aimed to develop numerous antioxidant drugs of both natural and synthetic origin, tested in both in vitro and in vivo models and also human clinical trials to overcome injuries caused by IR exposure and to induce killing of cancer cells at the same time. Previous studies have reported that phytochemical soy isoflavones, genistein, daidzein, and glycitein, which exhibit anticarcinogenic properties through their antioxidant activities, could be used as potent radiosensitizers to enhance the efficacy of radiotherapy-mediated suppression of the growth and metastatic ability of cancers.10,11 Along parallel lines, resveratrol and piperine, which possess antitumor activity, have been shown to augment ionizing radiation (IR)-induced apoptosis and loss of mitochondrial membrane potential in murine colon carcinoma and melanoma cells via enhancing IR-induced ROS generation.12 Moreover, pentoxifylline (PTX), a methylxanthine that possesses antioxidant properties, is known for improving tumor tissue oxygenation in murine hypoxic tumors and inhibiting post radiation induced normal tissue injury in mice.13,14 Consequently, searching for a natural product possessing anticancer activity that increases radiosensitivity of tumor cells and radioresistance of normal cells may lead to a potential future drug in cancer therapy.
Among the natural products, bromelain (BL) extract attracts interest due to its anticancer, antioxidant as well as anti-inflammatory effects.15-17 BL, an extract from pineapple stem (Ananas comosus), belongs to a group of protein digesting enzymes. It is a mixture of different thiol endopeptidases and other components like phosphatase, glucosidase, peroxidase, cellulase, escharase, calcium, and several protease inhibitors.18,19 The anticancer activity of BL has been examined in various types of gastrointestinal and breast cancers cell lines. In in vivo models BL has shown antimetastatic effect and reduction in local tumor growth.20-23 It is also used for reducing the severity of such radiation therapy side effects as mucositis, skin reactions, and dysphagia in patients.24 Hence, this study was aimed to evaluate the radiosensitizing and radioprotective effect of BL using in vivo and in vitro approaches.
Materials and Method
In Vitro Studies
MTT cell proliferation assay
The growth and viability of Ehrlich ascites carcinoma (EAC) cell line were tested in vitro by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to Freimoser et al and Buch et al,25,26 to verify the antitumor and radiosensitizing effect of BL. Two plates were designed for this study. The first one contained EAC cells (maintained by serial sub-culturing at the National Cancer Institute, Egypt) incubated for 1 hour before irradiation (IRR) (6 Gy), alone and with different concentrations of bromelain (BL) in phosphate buffer saline (PBS). The second one contained EAC cells (serving as a control) and EAC with different concentrations of BL. Each test was seeded in triplicate into a plate at concentration of 2.5 × 106 cells/well containing RPMI media with 10% FBS, NaHCO3, 100 U/mL penicillin and 100 µg/mL streptomycin and each plate was incubated for 24 hours at 37°C in 5% CO2 and humidity atmosphere. Then, 300 μL MTT reagent (Bio Basic Inc., Canada) was added over the cells in each well and the plate was incubated in the dark for 2 to 4 hours until a purple precipitate was seen and the absorbance was measured at 560 nm. The amount of color produced was directly proportional to the number of viable cells. Viable cell % = (A samples − A blank)/(A control − A blank) × 100. The inhibitory concentration 50% (IC50) is the dose of a drug which reduces the viability to 50%, and was calculated using non-linear regression analysis.
Free radical scavenging assay
The antioxidant activity of bromelain was evaluated by 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical assay and its scavenging power was compared with some antioxidants: naringin (polyphenolic antioxidant), garlic oil and glutathione (sulfur containing antioxidants). About 500 µL of samples (0.25-5 mg/mL, dissolved in dist. water) was added to 500 µL of a solution of DPPH (0.002 g/100 mL, dissolved in 95% v/v methanol). After 30 minutes incubation at room temperature in the dark, the absorbance was read at 517 nm against a blank (500 µL dist. water + 500 µL DPPH/methanol solution). The experiments were done in triplicate according to the method of Braca et al.27 Glutathione (0.007-1 mg/mL) was used as a standard antioxidant. The scavenging percentage of DPPH was calculated according to the following equation:, where B was the absorbance of the blank and A was the absorbance of samples or standard. EC50 is defined as concentration of sample that causes 50% DPPH loss, there values were calculated using non-linear regression analysis.
In Vivo Studies
Radiation processing
Whole body γ-irradiation of mice was carried out using Gamma cell-40 (137Cesium, manufactured by the Atomic Energy of Canada Limited, Ontario, Canada), installed in the National Center for Radiation Research and Technology (NCRRT), Cairo, Egypt. The dose rate was 0.675 Gy/min during the experimental period. Daily correction for humidity, barometric pressure, and temperature were made.
Animals
Adult female Swiss albino mice weighing 25 to 30 g obtained from the breeding unit of NCRRT, Cairo, Egypt. All animal procedures were performed in accordance with the Committee of Scientific Ethics at Faculty of Pharmacy, Al-Azhar University, Egypt, following the guidelines for animal use. The animals were housed in colony cages (10 mice/cage) under proper environmental conditions, that is, 12 hours dark/light cycle, good ventilation condition and temperature, 40% to 55% humidity at the NCRRT animal house, fed with standard diet pellets and provided with water ad libitum. Animals were left 1 week for acclimatization on the lab environment before starting the experiment.
Tumor transplantation
The EAC cell line was supplied by serial sub-culturing at the National Cancer Institute, Cairo University, Egypt. It was implanted in each donor (female Swiss albino mice) by i.p injection of 2.5 × 106 cells/22 g b. wt, and allowed to multiply.28 The Ehrlich solid tumor (EST) was obtained by the intramuscular inoculation of 0.2 mL of 1 × 106 viable EAC in the right lower limb of each mouse.29 Mice with a palpable solid tumor diameter 10 mm3 that was maintained within 7 to 10 days after inoculation were used in the study.
Animal grouping
Animals were randomly divided into 5 groups (10 mice each), Group 1: control not bearing tumor (received PBS i.p for 10 days), Group 2: Ehrlich solid tumor (EST) bearing mice (received PBS i.p for 10 days), Group 3: EST + γ-irradiation (1 Gy × 5) fractionated doses starting 2 days after tumor appearance (10 mm3) and lasting for 5 days, Group 4: EST bearing mice receiving freshly prepared BL dissolved in PBS (6 mg/kg, i.p), daily for 10 days according to pilot study, starting once EST becomes 10 mm3. BL was purchased from Merck KGaA Co. (Darmstadt, Germany), Group 5: EST bearing mice received BL (as in group 4) 2 hours before γ-irradiation (as in group 3). Mice were anesthetized 3 days after last irradiation dose using urethane 1.2 mg/kg.30 Blood samples were collected through cardiac puncture and divided into 2 parts (EDTA coated and plain vials). At that time, they were euthanized by cervical dislocation. Liver and tumor tissues were dissected out, rinsed with ice-cold saline, dried on a filter paper, and weighed, then homogenized in ice-cold PBS (pH 7.4) and stored at −80°C until used for subsequent biochemical analysis.
Estimation of total body, tumor, and liver weights
Animals in each group were checked daily for any adverse clinical symptoms and deaths. After 7 to 10 days post inoculation with EAC, body weights were recorded, so body weight change could be estimated. Tumor and liver weights were measured during sample collection, and then the tumor inhibitory ratio (%) was calculated by the following formula: Inhibition ratio (%) = A−B/A × 100, where A is the tumor weight average of the control, and B is that of the treated group. Also relative liver weight was calculated as liver weight/total body weight × 100.
Histopathological examination
Three tumors of each group were collected and fixed in 10% neutral buffered formalin. The specimens were dehydrated in ascending grades of ethyl alcohol, cleared in xylene, and embedded in paraffin wax. Four micron thick paraffin sections were mounted on clean slides, stained with Ehrlich’s hematoxylin-eosin (H&E),31 and examined using an Olympus microscope (BX41, Hamburg, Germany). Histopathological evaluation was done by assessment of necrosis and calculation of tumor area percentage using image analysis software (Image J, 1.46a, NIH, USA) through the following equation: % of tumor area = (area of tumor/total area of the field) × 100.
Molecular analyses
The mRNA levels of Poly (ADP-Ribose) Polymerase 1 (PARP1), nuclear factor kappa B (NF-κB), and Peroxisome proliferator-activated receptors (PPAR)-α genes; and of the housekeeping gene β-Actin were measured by real time polymerase chain reaction (RT-PCR). Total RNA was isolated from liver tissues using Qiagen tissue extraction kit (Qiagen, USA) in accordance with the manufacturer’s instructions. The extracted RNA (0.5-2 μg) was used for cDNA conversion using high capacity cDNA reverse transcription kit (Fermentas, USA) and 12.5 μL reaction volume SYBR chemistry in Applied Biosystems 2720 Thermal Cycler, USA to amplify PCR under the following conditions: 90°C for denaturation, then 50°C to 60°C for annealing using primers mentioned in Table 1, and 72°C for elongation.
Table 1.
Sequences of Primers for Real-Time Quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| PARP1 NM007415.2 | 5′-CCATCGACGTCAACTACGA-3′ | 5′-GTGCGTGGTAGCATGAGTGT-3′ |
| NF-κB NC000069.6 | 5′-CAATGGCTACACAGGACCA-3′ | 5′-CACTGTCACCTGGAACCAGA-3′ |
| PPAR-α NC000081.6 | 5′-ACTCCACCTGCAGAGCAACCA-3′ | 5′-TAGATCTCCTGCAGTAGCGGG-3′ |
| β-Actin NC000071.6 | 5′-GCGTGGGGACAGCCGCATCTT-3′ | 5′-ATCGGCAGAAGGGGCGGAGA-3′ |
Results were expressed using the comparative ∆∆CT method for relative mRNA quantification of target genes, normalized to an endogenous reference β-Actin and a relevant control, equal to 2−∆∆CT. ∆∆CT is the difference between the mean ∆CT(sample) and mean ∆CT(control), where ∆CT(sample) is the difference between the mean CT(sample) and the mean CT(β-Actin) and ∆CT(control) is the difference between the mean CT(control) and the mean CT(β-Actin).
Estimation of lipid peroxidation (LPO), reactive oxygen species (ROS), and paraoxonase (PON1) in liver homogenate
Liver lipid peroxidation was estimated by measurement of malondialdehyde (MDA) formation using the thiobarbituric acid method of Yoshioka et al.32 A modified technique of Vrablic et al,33 was used to measure the generation of ROS by the intracellular conversion of nitro blue tetrazolium (NBT) to formazan by the action of superoxide anion. Paraoxonase activity was estimated by using fluorometric assay (EnzChek® Kit, Invitrogen, UK) for the organophosphatase activity of paraoxonase, based on the hydrolysis of a fluorogenic organophosphate analog.34
Hematological and biochemical analyses
Whole blood was immediately analyzed for complete blood count with platelet count using the fully automated analyzer (ABX 45 Cobas Micros, Roche, Germany). Estimation of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (ALB) assays follow the recommendations of the International Federation of Clinical Chemistry (IFCC), but were optimized for performance and stability using the Roche/Hitachi Cobas c 311system.
Statistical analysis
The statistical analysis was performed using one-way analysis of variance (ANOVA) and the groups were compared by Tukey-Kramer test. Viability percentage at different concentrations and body weight change analyzed by two-way ANOVA followed by Bonferroni’s posttest. Graphs were sketched using Graph Pad Prism (ISI® software, USA) version (5) software. Data were presented as mean ± standard error (SE) and P values <.05 considered significant.
Results
In Vitro Studies
Effect of bromelain and gamma-irradiation (BL/IRR) on tumor cell growth and viability
The radiosensitizing effect of BL on EAC cells was determined by performing MTT assay. EAC cells exposed to 6 Gy γ-radiation showed high cell viability percentage reflecting a radioresistance of EAC cell line, while BL treatment showed in vitro cytotoxic activity with IC50 value of 2.77 mg/mL. However the maximum cytotoxic effect appeared when the EAC cells were subjected to BL then γ-radiation (6 Gy) compared to control or irradiated group with IC50 = 1.42 mg/mL (Table 2).
Table 2.
Cytotoxic Activity of BL/IRR Against EAC Cell Line.
| Bromelain concentration (mg/mL) | Viability % | |
|---|---|---|
| Non irradiated EAC | Irradiated EAC | |
| 9 | 48a,b | 10a,b |
| 6 | 59a,b | 15.8a,b |
| 3 | 60a,b | 18a,b |
| 1.5 | 69a | 27a,b |
| 0.75 | 78.7a | 52a,b |
| EAC (BL = 0 mg/mL) | 100 | 84.2 |
| IC50 (mg/mL) | 2.77 ± 0.13 | 1.42 ± 0.06 |
Each value indicates the mean of 3 records. Statistical analysis carried out by two-way ANOVA followed by Bonferroni posttests, a: significant versus control Ehrlich ascites carcinoma (EAC) group, where b: significant versus irradiated EAC group at P < .05. IC50 ± SE values were calculated by using non-linear regression analysis.
Effect of bromelain and some natural compounds as free radical scavengers
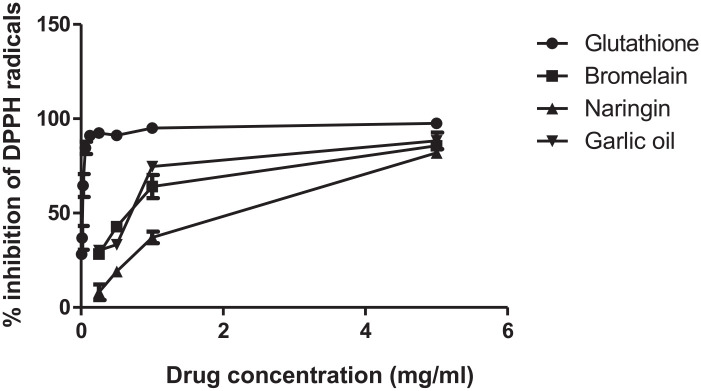
The inhibitory percentage of each compound is shown in Figure 1. The EC50 value (concentration of sample that causes 50% DPPH activity loss) is a reliable way for estimation of the radical scavenging activity. The EC50 value of glutathione (referenced antioxidant) is 0.03 mg/mL, while bromelain and garlic oil EC50 are almost equal (0.81 and 0.82 mg/mL, respectively), however the naringin (phenolic antioxidant) is the least potent one (EC50 = 1.303 mg/mL) in this comparison.
Figure 1.
DPPH (1, 1-diphenyl-2-picrylhydrazyl) reduction curve for glutathione, bromelain, naringin, and garlic oil, each value represents mean ± SE, all experiments were replicated 3 times.
In Vivo studies
Effect of bromelain and gamma-irradiation (BL/IRR) on tumor weight and volume
Table 3 shows a significant decrease in tumor weight in groups treated with BL and/or γ-irradiation as compared to the EST non-treated group. The more drastic decrease in the tumor weight ratio observed in the combined therapy group (BL + IRR) compared with the EST-irradiated group as well as EST group indicates that combination therapy is more significantly effective than single agent therapy. The photograph of EST xenografts at the time of sacrifice shows the synergistic effect of BL and IRR on tumor volume (Figure 2).
Table 3.
Tumor Weight and Inhibition Ratio of Ehrlich Solid Tumor (EST)-Bearing Mice Treated With Gamma-Irradiation (IRR) (1 Gy × 5) and/or Bromelain (BL) (6 mg/kg).
| Groups | Tumor weight (g) | Tumor inhibitory ratio (%) |
|---|---|---|
| EST | 2.1 ± 0.18 | — |
| EST + IRR | 1.25 ± 0.04a | 42.18 ± 2.4a |
| EST + BL | 0.9 ± 0.05a | 57.83 ± 1.4a,b |
| EST + BL + IRR | 0.72 ± 0.05a,b | 65.71 ± 2.04a,b |
Abbreviations: EST, Ehrlich solid tumor; EST + IRR, Ehrlich solid tumor + irradiation; EST + BL, Ehrlich solid tumor + bromelain; EST + BL + IRR, Ehrlich solid tumor + bromelain + irradiation.
The values shown are mean ± SE of data, a: significant versus EST group, where b: significant versus EST-irradiated group at P < .05.
Figure 2.
A photograph of Ehrlich solid tumor (EST) xenografts at the time of scarification showing the effect of bromelain and gamma-irradation (BL/IRR) on tumor volume. E, Ehrlich solid tumor; E + IR, Ehrlich solid tumor + irradiation; E + Br, Ehrlich solid tumor + bromelain; E + IR + Br, Ehrlich solid tumor + bromelain + irradiation.
Effect of bromelain and gamma-irradiation (BL/IRR) on tumor histopathological features of EST bearing mice
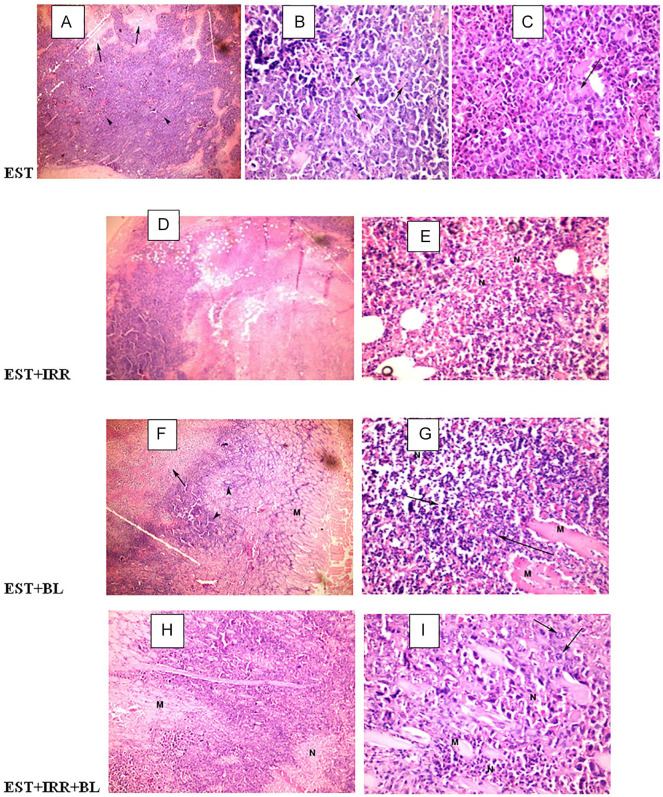
Histopathological examination of solid tumor sections revealed typical malignant features including sheets of malignant cells infiltrating adjacent muscular tissue. The malignant cells show pleomorphism, hyperchromatism and mitotic activity, while the necrotic cells appear as nonviable homogenous structureless material with degenerated or karyorrhectic nuclei. Untreated EST bearing group shows a deeply stained tumor cells (arrow head) and areas of necrosis (arrow) (Figure 3A, 100×); also, it displays intact cancer cells (arrow) and giant cells (arrow) (Figure 3B and C, 400×). The EST-irradiated group shows muscle fibers invaded by deeply stained tumor cells (arrow head) and large areas of necrosis (arrow) (Figure 3D, 100×), also displays a notable necrosis of cancer cells (N) (Figure 3E, 400×). The BL treated group shows a wide area of necrosis (arrow and N), few groups of cancer cells (arrow head) and muscle fiber (M) (Figures 3F, 100× and 4G, 400×). However, combined treatment (BL + IRR) displays muscle fiber (M), significant regression of tumor or wide areas of necrotic cancer cells (N), and few groups of intact cancer cells (arrow). (Figure 3H, 100× and 3I, 400×).
Figure 3.
Photo micrograph of Ehrlich solid tumor (EST) xenografts in different animal groups, EST sections show the degree of tumorogenesis, necrosis (N) regression of tumor by appearance of muscle fibers (M), 100× and 400×. A, B, C: EST (Ehrlich solid tumor); D, E: EST + IRR (Ehrlich solid tumor + irradiation); F, G: EST + BL (Ehrlich solid tumor + bromelain); H, I: EST + BL + IRR (Ehrlich solid tumor + bromelain + irradiation).
The tumor area percentage per total tissue area could determine the degree of proliferation. As seen in Figure 4, there is a great regression of tumor area in the group treated with BL alone (14.83%) or BL and IRR (11.28%) compared with untreated EST (65%) or EST-irradiated group (31.1%), indicating that combination therapy significantly more effective than single agent therapy.
Figure 4.
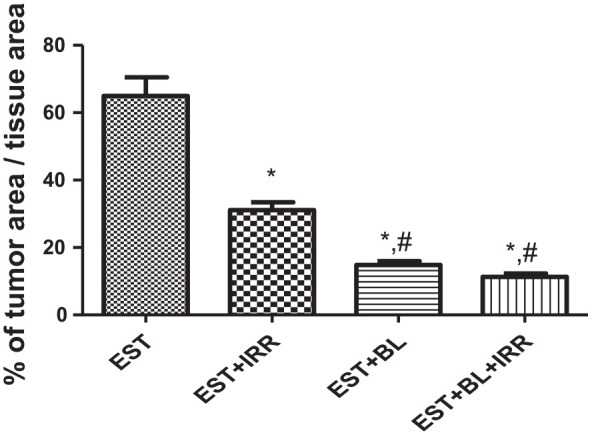
Percentage of tumor area/total tissue area of Ehrlich solid tumor (EST) bearing mice treated with gamma-irradiation (IRR) (1 Gy × 5) and/or BL (6 mg/kg). The values shown in the plotted area are mean of 15 records from 3 animals ± SE, *: significant versus EST group, where #: significant versus EST-irradiated group at P < .05.
Effect of bromelain and gamma-irradiation (BL/IRR) on body weight change and relative liver weight
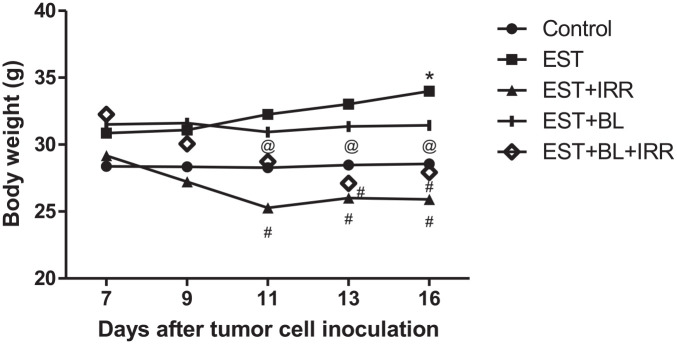
Regarding the day by day documented recording of body weight (b.wt) illustrated in Figure 5, there is almost no change in b.wt of BL treated group, while it increases significantly in the untreated EST group; conversely EST-irradiated groups with or without BL treatment show a significant decrease in b.wt when compared with the control group (Table 4).
Figure 5.
Effect of bromelain and gamma-irradiation (BL/IRR) on body weight during experiment period, each value represents the mean of 6 records ± SE, *: significant versus control group, where #: significant versus EST group and @: significant versus EST-irradiated group at P < .05. EST, Ehrlich solid tumor; EST + IRR, Ehrlich solid tumor + irradiation; EST + BL, Ehrlich solid tumor + bromelain; EST + BL + IRR, Ehrlich solid tumor + bromelain + irradiation.
Table 4.
Change in Body Weight and Relative Liver Weight of Control Mice and Ehrlich Solid Tumor (EST) Bearing Mice Treated With Gamma-Irradiation (IRR) (1 Gy × 5) and/or Bromelain (BL) (6 mg/kg).
| Groups | Body weight change (%) | Relative liver weight (%) |
|---|---|---|
| Control | 0.48 ± 0.04 | 5.61 ± 0.27 |
| EST | 10.23 ± 1.01a | 7.79 ± 0.26a |
| EST + IRR | −12.54 ± 2.17a,b | 7.19 ± 0.22a |
| EST + BL | −0.23 ± 2.01b | 7.09 ± 0.26a |
| EST + BL + IRR | −16.04 ± 3.41a,b | 6.92 ± 0.26a,b |
Abbreviations: EST, Ehrlich solid tumor; EST + IRR, Ehrlich solid tumor + irradiation; EST + BL, Ehrlich solid tumor + bromelain; EST + BL + IRR, Ehrlich solid tumor + bromelain + irradiation.
Each value represents the mean ± SE, a: significant versus control group, where b: significant versus EST group at P < .05. Body weight changes percent are related to the initial weight of animals.
Relative liver weight was compared after normalization to 100 mg body weight. Untreated EST-bearing group shows a significant increase in liver weight by 39% (hepatomegaly), while non-significant changes were observed in BL treated groups compared to the normal group (Table 4).
Effect of bromelain and gamma-irradiation (BL/IRR) on the poly (ADP-Ribose) polymerase 1 (PARP1), nuclear factor kappa B (NF-κB), and peroxisome proliferator-activated receptors (PPAR)-α relative gene expression of Ehrlich solid tumor (EST) bearing mice
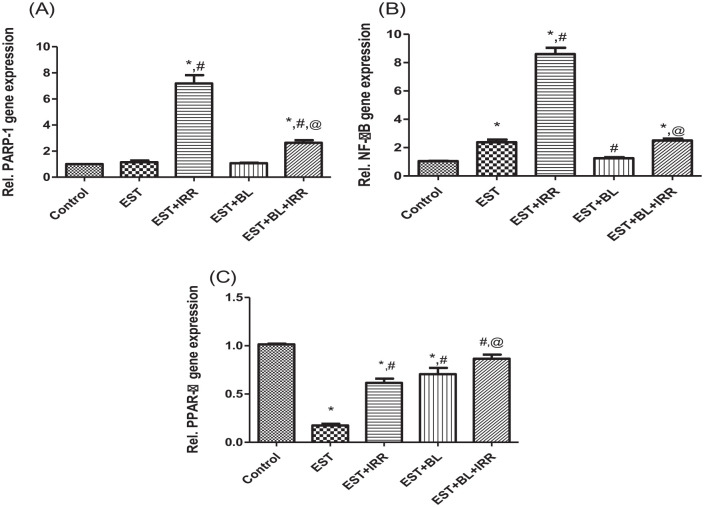
To test the possibility that BL reduces radiation damage to the liver, mRNA gene expression of PARP1, NF-κB, and PPAR-α was measured in the liver homogenates of EST bearing mice and compared to control PBS treated mice. The results, illustrated in Figure 6, show that IRR causes significant increases in PARP1 and NF-κB expression compared to the control group, however combined treatment (BL + IRR) shows a significant increase in PARP1 and NF-κB expression compared to control group and a significant attenuation compared to EST-irradiated group.
Figure 6.
Effect of bromelain and gamma-irradiation (BL/IRR) on relative gene expression of liver (A) PARP1, (B) NF-κB, and (C) PPAR-α, each value represents the mean of 6 records ± SE, *: significant versus control group, where #: significant versus EST group and @: significant versus EST-irradiated group at P < .05. EST, Ehrlich solid tumor; EST + IRR, Ehrlich solid tumor + irradiation; EST + BL, Ehrlich solid tumor + bromelain; EST + BL + IRR, Ehrlich solid tumor + bromelain + irradiation.
Moreover, all EST bearing groups show significant decreases in hepatic PPAR-α relative gene expression compared to the control group, except the combined therapy group (BL + IRR) shows non-significant change. Additionally, BL + IRR group significantly upregulated PPAR-α expression compared with EST and EST-irradiated groups; indicating that BL might have a hepato- as well as radio-protective effect (Figure 6).
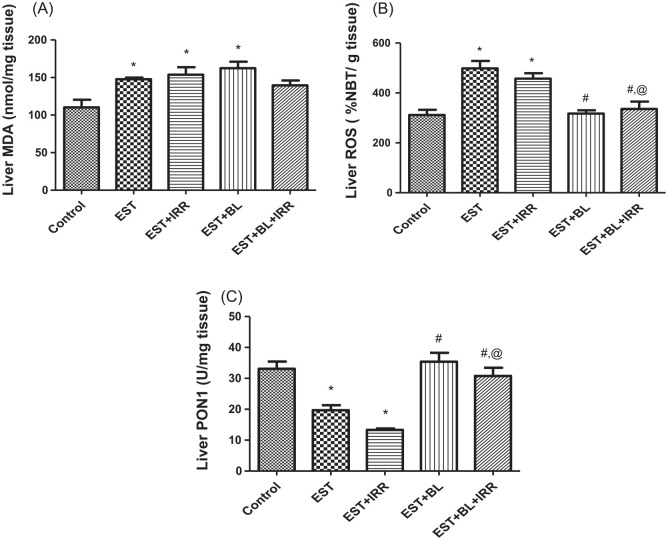
Effect of bromelain and gamma-irradiation (BL/IRR) on the hepatic lipid peroxidation (LPO) level, reactive oxygen species (ROS) content, and paraoxonase-1 (PON1) activity of Ehrlich solid tumor (EST) bearing mice
LPO in liver tissues significantly increased in all EST bearing groups compared to the control group, except the combined treated group (IRR + BL) succeeded in returning MDA (LPO measured as MDA (malondialdehyde)) level to the normal level. However, liver ROS significantly increased only in untreated and γ-irradiated EST bearing groups when compared to the control group, while a significant decrease in liver ROS showed in EST-irradiated mice treated with BL in comparison with both EST untreated and EST-irradiated groups. PON1 activity in liver homogenate was significantly decreased in EST untreated and EST-irradiated groups when compared with the control group. BL treated groups revealed significant increases in PON1 when compared with both EST untreated and EST-irradiated groups; showing that BL might have a hepato- and radio-protective effect (Figure 7).
Figure 7.
Effect of bromelain and gamma-irradiation (BL/IRR) on (A) lipid peroxidation (LPO; MDA level), (B) reactive oxygen species (ROS) content, and (C) paraoxonase-1 (PON1) activity in the liver homogenate. Each value represents the mean of 6 records ± SE, *: significant versus control group, where #: significant versus EST group and @: significant versus EST-irradiated group at P < .05. EST, Ehrlich solid tumor; EST + IRR, Ehrlich solid tumor + irradiation; EST + BL, Ehrlich solid tumor + bromelain; EST + BL + IRR, Ehrlich solid tumor + bromelain + irradiation.
Effect of bromelain and gamma-irradiation (BL/IRR) on hematological measurements
WBCs and PLTs were significantly elevated while HGB and HCT significantly decreased in the untreated EST-bearing mice in comparison with control mice. However, γ-irradiation resulted in a significant decrease in WBCs, RBCs, PLT, HGB, and HCT% compared with the control mice. Treatment of the EST-bearing mice with BL shows a significant amelioration in WBCs, PLT, and HCT compared to EST untreated mice. Combined treatment (BL + IRR) shows an enhancement in WBCs, PLT, and HCT compared to EST untreated and gamma-irradiated EST bearing mice (Table 5).
Table 5.
White Blood Cell (WBCs), Red Blood Cell (RBCs) and Platelet (PLT) Counts, Hemoglobin (HGB) Concentration, and Hematocrit (HCT%) of Normal and Ehrlich Solid Tumor (EST) Bearing Mice Treated With Gamma-Irradiation (IRR, 1 Gy × 5) and/or Bromelain (BL, 6 mg/kg).
| Groups | WBCs ×103/UL |
RBCs ×106/UL |
PLT ×103/UL |
HGB (g/dL) |
HCT (%) |
|---|---|---|---|---|---|
| Control | 2.7 ± 0.09 | 8.8 ± 0.26 | 837 ± 42.7 | 14.1 ± 0.24 | 44.98 ± 0.7 |
| EST | 10.9 ± 0.4a | 7.5 ± 0.5 | 1184 ± 70.2a | 9.6 ± 0.5a | 26.38 ± 0.7a |
| EST + IRR | 0.8 ± 0.05a,b | 6.1 ± 0.2a | 105 ± 4.9a,b | 10.53 ± 0.5a | 26.5 ± 1.1a |
| EST + BL | 5.1 ± 0.6a,b | 8.6 ± 0.7 | 670 ± 53.9b | 10.52 ± 0.4a | 33.9 ± 1a,b |
| EST + BL + IRR | 2.1 ± 0.19b,c | 6.3 ± 0.08a | 317 ± 20a,b,c | 9.9 ± 0.1a | 33.5 ± 0.2a,b,c |
Abbreviations: EST, Ehrlich solid tumor; EST + IRR, Ehrlich solid tumor + irradiation; EST + BL, Ehrlich solid tumor + bromelain; EST + BL + IRR, Ehrlich solid tumor + bromelain + irradiation.
Each value represents the mean of 6 records ± SE, a: significant versus control group, where b: significant versus EST group and c: significant versus EST-irradiated group at P < .05.
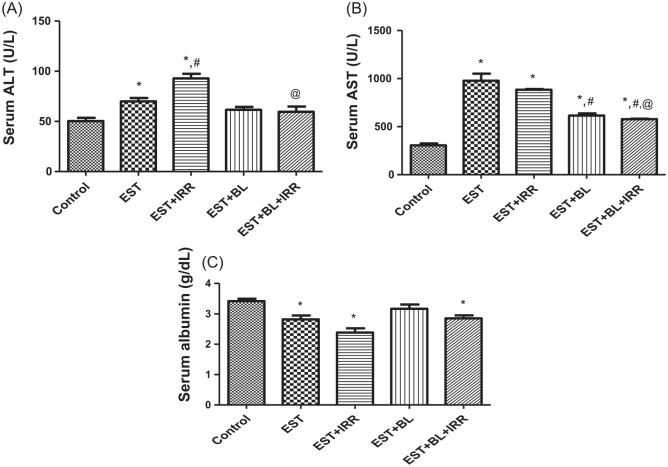
Effect of bromelain and gamma-irradiation (BL/IRR) on the serum alanine transaminase (ALT), aspartate transaminase (AST), and albumin (ALB)
To investigate the cytoprotective effects of BL against irradiation, the levels of serum ALT, AST, and ALB were measured (Figure 8). It was found that ALT and AST significantly increased, and conversely ALB significantly decreased in EST untreated and EST-irradiated groups compared with the control group. However, EST-bearing mice treated with BL alone show nearly the same result of ALT and ALB as control values. EST-bearing mice treated with BL in combination with irradiation initiated a significant decrease in AST and ALT as compared with EST-irradiated group, which may reflect a potential hepatic radio-protective effect of BL.
Figure 8.
Effect of bromelain and gamma-irradiation (BL/IRR) on serum (A) alanine transaminase (ALT), (B) aspartate transaminase (AST), and (C) albumin (ALB). Each value represents the mean of 6 records ± SE, *: significant versus control group, where #: significant versus EST group and @: significant versus EST-irradiated group at P < .05. EST, Ehrlich solid tumor; EST + IRR, Ehrlich solid tumor + irradiation; EST + BL, Ehrlich solid tumor + bromelain; EST + BL + IRR, Ehrlich solid tumor + bromelain + irradiation.
Discussion
Resistance of tumor cells to chemo-radiotherapy as well as the damaging effects to nearby normal tissues remains a major obstacle to successful cancer management. Therefore, the current study has been conducted to estimate the effect of bromelain (BL) as a tumor radiosensetizer and to show to what extent it can protect normal tissue from radiation hazards.
Radiosensitizers are compounds that when combined with radiation therapy, achieve greater cytotoxicity; they can be determined in vitro by the MTT assay.26,35 The present study has found that the radioresistant EAC cells could be sensitized when incubated with BL before irradiation. It was known previously that in vitro treatment with BL on mouse tumor cell lines resulted in inhibition of cell growth and invasion capacities.36,37 The anticancer property of BL has been mainly attributed to the protease component through digestion and diffusion in tumor cells.38 It may also be due to the BL enhancement of p53 expression, as well as another activator of apoptosis (eg, Bax).39 In addition, it decreases the activity of cell survival regulators such as Akt and Erk; it also deactivates Akt-dependent pro-apoptotic regulator FOXO3A, thus promoting apoptotic cell death in tumors.40
It is well known that during cancer and radiotherapy, excessive energy is used from the host,41 ultimately contributing to mechanisms that promote loss of weight as shown in the present study, which also showed that BL could return body weight to a normal level by decreasing tumor weight and volume. Currently, the combined therapy (BL + IRR) has been shown to be more effective than single agent therapy in reducing tumor volume and weight, indicating that BL could possess a radiosensitizing effect. In addition, the combined therapy has revealed a drastic decrease in tumor area percentage, wide areas of necrotic cancer cells and presence of muscle fiber in the histopathological examination, compared with the control EST and EST-irradiated groups. This seems to be in agreement with other findings of the role of BL in reducing metastasis and local tumor growth.23,42 In chemically induced mouse skin papillomas, topical application of BL reduced tumor formation, tumor volume, and caused apoptotic cell death.39 BL is a hydrolytic enzymatic complex which shows an efficient digestion and diffusion in tumor cells through attacking the glycosidic linkages and hence denatures glycoproteins. Thus it protects against tumor growth.37 Another study has demonstrated the use of controlled proteolytic activity on tumor as a successful strategy to increase therapeutic efficacy.43
The aim of the radiotherapy protocols is to achieve the maximum curative effect on tumor cells with minimal damaging effect on normal cells. Hence, antioxidants and other nutrients which do not interfere with therapeutic modalities for cancer may enhance the killing property, decrease side effects, and protect normal tissue.44
For estimation of the antioxidant ability of BL, DPPH assay was conducted in vitro and the free radical inhibitory action of BL was compared with some antioxidant compounds. It was found that BL has a powerful free radical scavenging power. BL belongs to thiol proteases in which the catalytic nucleophile is sulfhydryl groups of cysteine residues, which in turn accounts for its antioxidant activity.45
The involvement of ROS, MDA and PON1 are important mechanisms that play a vital role during radiation toxicity. The use of antioxidants is an important preventive to decrease the toxic and pathological effects associated with oxidative stress caused by radiation.46 The attained results show a hepatic impairment on the third day from exposure to γ-radiation (elevation of LPO and ROS levels, and inhibition of PON1 activity) compared to normal mice. However, treatment with BL revealed an amelioration in hepatic damage caused by irradiation. These results were in accordance with Liu et al,47 who described the effect of radiation induced ROS generation which in turn might attack cell membrane phospholipids and circulating lipids and, thus, increases production of MDA.48 LPO acts as a sensitive biomarker for oxidative stress that occurs as part of the pathogenesis of irradiation.49 BL has sulfhydryl groups, consequently accounting for its antioxidant activity,45 thus it could act as ROS scavenger.
Measurement of PON1 post-radiotherapy could be an effective clinical biomarker of hepatic and systemic oxidative stress and may be used as an index of the usefulness of radiotherapy.50 It has been demonstrated to catalyze hydrolysis of lipid hydroperoxides and lactones.51 PON1 protects serum HDL and LDL particles against lipid peroxidation.52 In the present study, the decreased activity of PON1 upon radiation exposure, might be due to its super saturation of lipid hydroperoxides and lactones. Upon treatment with BL, the activity of PON1 was restored near to the normal level. Hence, the PON1 protein has an antioxidant function against oxidative stress-induced cell death through inhibiting phosphorylation of NF-κB (p65 and IκBα) and decreasing ROS production.46,53,54 Also, interleukin 6 (IL-6) has significantly increased both the function and protein level of PON1 in the human hepatocyte cell line. As IL-6 increases IκB kinase activity and IκB phosphorylation, causing inhibition of NF-κB,55 this is in accordance with our results. On the other hand, BL and papain enhanced IL-6 production in an in vitro study due to their protease activity.56 The enhancement of PON1 activity by BL could be new mechanism for its potential radioprotective effect.
There is evidence showing that inhibiting NF-κB has potential as a treatment of cancer and chronic inflammatory diseases.40 The current study has revealed a significant increase in the hepatic PARP1 and NF-κB expression upon exposure to gamma radiation. In contrast, upon the BL treatment significant amelioration in PARP1 and NF-κB expression has been reported compared to EST-irradiated group. NF-κB is a redox-regulated sensor for oxidative stress,57 and is activated by low doses of ROS.58 Ionizing radiations are inducers of inflammation, as ROS produced during irradiation mediate the NF-κB activation which is a family of closely related protein that regulates genes involved in inflammation.59-61 Selective COX-2 inhibitors like BL have been tested for their therapeutic efficacy when combined with chemotherapy.62,63 Nevertheless, BL has not been tested for its anti-inflammatory efficacy when combined with radiation.
PARP1 is a transcriptional modulating enzyme that facilitates inflammatory responses.64 Also, PARP1 is a mandatory for ionizing radiation-induced NF-κB activation through stimulating the DNA-binding of the NF-κB complex.65-67 On the same line, the existing study has revealed that PARP1 expression has been increased in liver tissue with the increase of NF-κB after irradiation, so it is feasible that ionizing radiation-induced NF-κB activation may be partially mediated by PARP1 activation. Pretreatment of EST-irradiated mice with BL in the current study revealed an amelioration in hepatic PARP expression compared to EST-irradiated group reflecting a hepatic protection.
In the current study, γ-radiation induced inhibition of hepatic PPAR-α mRNA level, while treatment the EST-irradiated mice with BL caused amelioration in the PPAR-α expression. These results were in accordance with the analysis of Linard et al.68 It was found that PPAR agonists can inhibit the radiation-induced inflammation by inhibition of the NF-κB signaling pathway.69 An important antagonistic crosstalk could be suggested between PARP1 and PPAR-α. PARP1 is the major nicotinamide adenine dinucleotide (NAD+) consumer in the cell during stress responses.70 PARP inhibitors would enhance the DNA binding activity of PPARs by increasing binding of non-modified PARP1 to PPARs.71 On the other hand, PPARs are negatively regulated by the NF-κB signaling pathways, which provoked its anti-inflammatory action.72
In the current study, irradiation causes an increase in serum ALT and AST and a decrease in albumin levels compared to the control group. However, BL causes a significant amelioration in ALT and AST which are disturbed by IRR, but not albumin compared with the EST-irradiated group. IRR affected cell membrane integrity that caused a release of the enzymes from the liver leading to an increase in the serum ALT and AST.47 It has been shown that proteolytic enzymes like bromelain or papain or both can modulate blood protein, antioxidant, and ROS.23 Recent study has suggested that pineapple juice which contains BL has the potential to be hepatoprotective through reducing ALT and AST and its potential is not significantly different from that of silymarin.73
Whole body gamma irradiation resulted in a significant decrease in WBCs, RBCs, and PLT count, HGB concentration and HCT% on the third day from latest irradiation dose compared with the control mice. However, BL treatment has shown a significant amelioration in WBCs, PLT, and HCT compared with the EST-irradiated group. Ionizing radiation has well documented effects on blood cells and these effects contribute to the hematopoietic syndrome observed in animals and human, following exposure to total body irradiation.74,75 Moreover, it was reported that the increase in the count of PLT (thrombocytosis) and WBCs is associated with many tumor types.76-78 BL was previously described in vitro and in vivo as an inhibitor of blood platelet aggregation.18,79 The mechanism of anti-inflammatory and anti-platelet aggregation action of BL is mediated by increasing serum fibrinolytic activity, reducing plasma fibrinogen, and bradykinin levels. It may also increase PGI2 causing vasodialation which in order decreases PLT aggregation.80-82 Thus BL could ameliorate the disturbances in PLT and WBCs caused by cancer and IRR.
The current results have proposed an insight into the molecular mechanism and the potential clinical application of BL as a radiation protector to selectively protect normal tissues which is a complementary approach to sensitizing tumors to radiation and allowing more cancers to be cured by exposing them to higher doses of radiation
Conclusion
In conclusion, BL may constitute a novel therapeutic modality to sensitize cancer cells and at the same time protect normal cells in the radiotherapy course. Further, studies in this field may be designed to bring out BL as a lead radioprotector in the market for cancer patients and radiation workers.
Acknowledgments
The authors appreciate Dr. Sahar Kamal Darwish, associate professor and head of Histopathology Department, National Organization of Drug Control and Research, for her support during histological examination.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hanan A. Fahmy  https://orcid.org/0000-0002-9593-9664
https://orcid.org/0000-0002-9593-9664
References
- 1. Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129-1137. [DOI] [PubMed] [Google Scholar]
- 2. Deng WG, Kawashima H, Wu G, et al. Synergistic tumor suppression by coexpression of FUS1 and p53 is associated with down-regulation of murine double minute-2 and activation of the apoptotic protease-activating factor 1-dependent apoptotic pathway in human non-small cell lung cancer cells. Cancer Res. 2007;67:709-717. [DOI] [PubMed] [Google Scholar]
- 3. Sarsenov D, Aktepe F, Ozmen V. Radiation fibrosis syndrome imitating breast cancer recurrence: a case report. J Breast Health. 2017;13:40-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002;7:492-508. [DOI] [PubMed] [Google Scholar]
- 5. Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014;21:260-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yazlovitskaya EM, Uzhachenko R, Voziyan PA, Yarbrough WG, Ivanova AV. A novel radioprotective function for the mitochondrial tumor suppressor protein Fus1. Cell Death Dis. 2013;4:e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arora R, Gupta D, Chawla R, et al. Radioprotection by plant products: present status and future prospects. Phytother Res. 2005;19:1-22. [DOI] [PubMed] [Google Scholar]
- 8. Mut-Salud N, Alvarez PJ, Garrido JM, Carrasco E, Aranega A, Rodriguez-Serrano F. Antioxidant intake and antitumor therapy: toward nutritional recommendations for optimal results. Oxid Med Cell Longev. 2016;2016:6719534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim SH, Khil MS, Ryu S, Kim JH. Enhancement of radiation response on human carcinoma cells in culture by pentoxifylline. Int J Radiat Oncol Biol Phys. 1993;25:61-65. [DOI] [PubMed] [Google Scholar]
- 10. Malik A, Sultana M, Qazi A, et al. Role of natural radiosensitizers and cancer cell radioresistance: an update. Anal Cell Pathol (Amst). 2016;2016:6146595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raffoul JJ, Sarkar FH, Hillman GG. Radiosensitization of prostate cancer by soy isoflavones. Curr Cancer Drug Targets. 2007;7:759-765. [DOI] [PubMed] [Google Scholar]
- 12. Tak JK, Lee JH, Park JW. Resveratrol and piperine enhance radiosensitivity of tumor cells. BMB Rep. 2012;45:242-246. [DOI] [PubMed] [Google Scholar]
- 13. Song CW, Hasegawa T, Kwon HC, Lyons JC, Levitt SH. Increase in tumor oxygenation and radiosensitivity caused by pentoxifylline. Radiat Res. 1992;130:205-210. [PubMed] [Google Scholar]
- 14. Zhang X, Sharma RK, Agarwal A, Falcone T. Effect of pentoxifylline in reducing oxidative stress-induced embryotoxicity. J Assist Reprod Genet. 2005;22:415-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muhammad ZA, Ahmad T. Therapeutic uses of pineapple-extracted bromelain in surgical care—A review. J Pak Med Assoc. 2017;67:121-125. [PubMed] [Google Scholar]
- 16. Rosenberg L, Shoham Y, Krieger Y, et al. Minimally invasive burn care: a review of seven clinical studies of rapid and selective debridement using a bromelain-based debriding enzyme (Nexobrid(R)). Ann Burns Fire Disasters. 2015;28:264-274. [PMC free article] [PubMed] [Google Scholar]
- 17. Cassileth B. Complementary therapies, herbs, and other OTC agents. Bromelain. Oncology (Williston Park). 2011;25:195. [PubMed] [Google Scholar]
- 18. Pavan R, Jain S, Shraddha Kumar A. Properties and therapeutic application of bromelain: a review. Biotechnol Res Int. 2012;2012:976203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Lencastre Novaes LC, Jozala AF, Lopes AM, de Carvalho Santos-Ebinuma V, Mazzola PG, Pessoa Junior A. Stability, purification, and applications of bromelain: a review. Biotechnol Prog. 2016;32:5-13. [DOI] [PubMed] [Google Scholar]
- 20. Pauzi AZ, Yeap SK, Abu N, et al. Combination of cisplatin and bromelain exerts synergistic cytotoxic effects against breast cancer cell line MDA-MB-231 in vitro. Chin Med. 2016;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amini A, Masoumi-Moghaddam S, Ehteda A, Liauw W, Morris DL. Depletion of mucin in mucin-producing human gastrointestinal carcinoma: results from in vitro and in vivo studies with bromelain and N-acetylcysteine. Oncotarget. 2015;6:33329-33344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhatnagar P, Pant AB, Shukla Y, Panda A, Gupta KC. Hyaluronic acid grafted PLGA copolymer nanoparticles enhance the targeted delivery of Bromelain in Ehrlich’s ascites carcinoma. Eur J Pharm Biopharm. 2016;105:176-192. [DOI] [PubMed] [Google Scholar]
- 23. Beuth J, Braun JM. Modulation of murine tumor growth and colonization by bromelaine, an extract of the pineapple plant (Ananas comosum L.). In Vivo. 2005;19:483-485. [PubMed] [Google Scholar]
- 24. Gujral MS, Patnaik PM, Kaul R, et al. Efficacy of hydrolytic enzymes in preventing radiation therapy-induced side effects in patients with head and neck cancers. Cancer Chemother Pharmacol. 2001;47:S23-S28. [DOI] [PubMed] [Google Scholar]
- 25. Freimoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbiol. 1999;65:3727-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buch K, Peters T, Nawroth T, Sanger M, Schmidberger H, Langguth P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT Assay—a comparative study. Radiat Oncol. 2012;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79:379-381. [DOI] [PubMed] [Google Scholar]
- 28. Osman AM, Alqahtani AA, Damanhouri ZA, et al. Dimethylsulfoxide excerbates cisplatin-induced cytotoxicity in Ehrlich ascites carcinoma cells. Cancer Cell Int. 2015;15:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Somasagara RR, Hegde M, Chiruvella KK, Musini A, Choudhary B, Raghavan SC. Extracts of strawberry fruits induce intrinsic pathway of apoptosis in breast cancer cells and inhibits tumor progression in mice. PLoS ONE. 2012;7:e47021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flecknell PA. Anaesthesia of animals for biomedical research. Br J Anaesth. 1993;71:885-894. [DOI] [PubMed] [Google Scholar]
- 31. Bancroft D, Stevens A, Turmer R. Theory and Practice of Histological Techniques. 4th ed. Edinburgh, London, Melbourne: Churchill Living Stone; 1996. [Google Scholar]
- 32. Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135:372-376. [DOI] [PubMed] [Google Scholar]
- 33. Vrablic AS, Albright CD, Craciunescu CN, Salganik RI, Zeisel SH. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl-rac-glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J. 2001;15:1739-1744. [DOI] [PubMed] [Google Scholar]
- 34. Furlong CE, Richter RJ, Seidel SL, Costa LG, Motulsky AG. Spectrophotometric assays for the enzymatic hydrolysis of the active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Anal Biochem. 1989;180:242-247. [DOI] [PubMed] [Google Scholar]
- 35. Fathy MM, Fahmy HM, Saad OA, Elshemey WM. Silica-coated iron oxide nanoparticles as a novel nano-radiosensitizer for electron therapy. Life Sci. 2019;234:116756. [DOI] [PubMed] [Google Scholar]
- 36. Guimaraes-Ferreira CA, Rodrigues EG, Mortara RA, et al. Antitumor effects in vitro and in vivo and mechanisms of protection against melanoma B16F10-Nex2 cells by fastuosain, a cysteine proteinase from Bromelia fastuosa. Neoplasia. 2007;9:723-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pillai K, Ehteda A, Akhter J, Chua TC, Morris DL. Anticancer effect of bromelain with and without cisplatin or 5-FU on malignant peritoneal mesothelioma cells. Anticancer Drugs. 2014;25:150-160. [DOI] [PubMed] [Google Scholar]
- 38. Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Cell Mol Life Sci. 2001;58:1234-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalra N, Bhui K, Roy P, et al. Regulation of p53, nuclear factor kappaB and cyclooxygenase-2 expression by bromelain through targeting mitogen-activated protein kinase pathway in mouse skin. Toxicol Appl Pharmacol. 2008;226:30-37. [DOI] [PubMed] [Google Scholar]
- 40. Chobotova K, Vernallis AB, Majid FA. Bromelain’s activity and potential as an anti-cancer agent: current evidence and perspectives. Cancer Lett. 2010;290:148-156. [DOI] [PubMed] [Google Scholar]
- 41. Seyfried TN, Flores RE, Poff AM, D’Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2014;35:515-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baez R, Lopes MT, Salas CE, Hernandez M. In vivo antitumoral activity of stem pineapple (Ananas comosus) bromelain. Planta Med. 2007;73:1377-1383. [DOI] [PubMed] [Google Scholar]
- 43. Parodi A, Haddix SG, Taghipour N, et al. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8:9874-9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simone CB, 2nd, Simone NL, Simone V, Simone CB. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part 1. Altern Ther Health Med. 2007;13:22-28. [PubMed] [Google Scholar]
- 45. Yon-Kahn J, Herve G. Examples of structure–function relationships in enzymatic systems. In: Molecular and Cellular Enzymology. New York: Springer; 2010:451-542. [Google Scholar]
- 46. Soliman AM, Karam HM, Mekkawy MH, Ghorab MM. Antioxidant activity of novel quinazolinones bearing sulfonamide: potential radiomodulatory effects on liver tissues via NF-kappaB/PON1 pathway. Eur J Med Chem. 2020;197:112333. [DOI] [PubMed] [Google Scholar]
- 47. Liu H, Wang S, Wu Z, et al. Glibenclamide, a diabetic drug, prevents acute radiation induced liver injury of mice via up-regulating intracellular ROS and subsequently activating Akt-NF-kappaB pathway. Oncotarget. 2017;8:40568-40582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soliman AM, Karam HM, Mekkawy MH, Higgins M, Dinkova-Kostova AT, Ghorab MM. Radiomodulatory effect of a non-electrophilic NQO1 inducer identified in a screen of new 6, 8-diiodoquinazolin-4(3H)-ones carrying a sulfonamide moiety. Eur J Med Chem. 2020;200:112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ping X, Junqing J, Junfeng J, Enjin J. Radioprotective effects of troxerutin against gamma irradiation in mice liver. Int J Radiat Biol. 2012;88:607-612. [DOI] [PubMed] [Google Scholar]
- 50. Arenas M, Garcia-Heredia A, Cabre N, et al. Effect of radiotherapy on activity and concentration of serum paraoxonase-1 in breast cancer patients. PLoS ONE. 2017;12:e0188633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zaki ME, El-Bassyouni H, Kamal S, El-Gammal M, Youness E. Association of serum paraoxonase enzyme activity and oxidative stress markers with dyslipidemia in obese adolescents. Indian J Endocrinol Metab. 2014;18:340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martinelli N, Consoli L, Girelli D, Grison E, Corrocher R, Olivieri O. Paraoxonases: ancient substrate hunters and their evolving role in ischemic heart disease. Adv Clin Chem. 2013;59:65-100. [DOI] [PubMed] [Google Scholar]
- 53. Kim MJ, Jeong HJ, Kim DW, et al. PEP-1-PON1 protein regulates inflammatory response in raw 264.7 macrophages and ameliorates inflammation in a TPA-induced animal model. PLoS ONE. 2014;9:e86034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kamble P, Selvarajan K, Litvinov D, et al. Induction of Sirt1 and PGC-1α are important for aspirin ability to induce ApoE and PON1 genes. Biol Med. 2017;9:1. [Google Scholar]
- 55. Cheng CC, Hsueh CM, Chen CY, Chen TH, Hsu SL. Interleukin-6 upregulates paraoxonase 1 gene expression via an AKT/NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2013;437:55-61. [DOI] [PubMed] [Google Scholar]
- 56. Rose B, Herder C, Loffler H, et al. Dose-dependent induction of IL-6 by plant-derived proteases in vitro. Clin Exp Immunol. 2006;143:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999;13:1137-1143. [PubMed] [Google Scholar]
- 58. Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 1992;17:221-237. [DOI] [PubMed] [Google Scholar]
- 59. Di Maggio FM, Minafra L, Forte GI, et al. Portrait of inflammatory response to ionizing radiation treatment. J Inflamm (Lond). 2015;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang C, Jiang H, Wang P, Liu H, Sun X. Transcription factor NF-kappa B represses ANT1 transcription and leads to mitochondrial dysfunctions. Sci Rep. 2017;7:44708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang H, Ahn KS, Alharbi SA, et al. Celastrol alleviates gamma irradiation-induced damage by modulating diverse inflammatory mediators. Int J Mol Sci. 2020;21:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davis TW, Hunter N, Trifan OC, Milas L, Masferrer JL. COX-2 inhibitors as radiosensitizing agents for cancer therapy. Am J Clin Oncol. 2003;26:S58-S61. [DOI] [PubMed] [Google Scholar]
- 63. Bhui K, Prasad S, George J, Shukla Y. Bromelain inhibits COX-2 expression by blocking the activation of MAPK regulated NF-kappa B against skin tumor-initiation triggering mitochondrial death pathway. Cancer Lett. 2009;282:167-176. [DOI] [PubMed] [Google Scholar]
- 64. Bai P, Virag L. Role of poly(ADP-ribose) polymerases in the regulation of inflammatory processes. FEBS Lett. 2012;586:3771-3777. [DOI] [PubMed] [Google Scholar]
- 65. Veuger SJ, Hunter JE, Durkacz BW. Ionizing radiation-induced NF-kappaB activation requires PARP-1 function to confer radioresistance. Oncogene 2009;28:832-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chang WJ, Alvarez-Gonzalez R. The sequence-specific DNA binding of NF-kappa B is reversibly regulated by the automodification reaction of poly (ADP-ribose) polymerase 1. J Biol Chem. 2001;276:47664-47670. [DOI] [PubMed] [Google Scholar]
- 67. Pazzaglia S, Pioli C. Multifaceted role of PARP-1 in DNA repair and inflammation: pathological and therapeutic implications in cancer and non-cancer diseases. Cells. 2019;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Linard C, Gremy O, Benderitter M. Reduction of peroxisome proliferation-activated receptor gamma expression by gamma-irradiation as a mechanism contributing to inflammatory response in rat colon: modulation by the 5-aminosalicylic acid agonist. J Pharmacol Exp Ther. 2008;324:911-920. [DOI] [PubMed] [Google Scholar]
- 69. Xi Y, Zhang Y, Zhu S, Luo Y, Xu P, Huang Z. PPAR-mediated toxicology and applied pharmacology. Cells. 2020;9:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fouquerel E, Sobol RW. ARTD1 (PARP1) activation and NAD(+) in DNA repair and cell death. DNA Repair (Amst). 2014;23:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang D, Yang C, Wang Y, Liao Y, Huang K. PARP-1 suppresses adiponectin expression through poly(ADP-ribosyl)ation of PPAR gamma in cardiac fibroblasts. Cardiovasc Res. 2009;81:98-107. [DOI] [PubMed] [Google Scholar]
- 72. Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453-459. [DOI] [PubMed] [Google Scholar]
- 73. Yantih N, Harahap Y, Sumaryono W, Setiabudy R, Rahayu L. Hepatoprotective activity of pineapple (Ananas comosus) juice on isoniazid-induced rats. J. Biol. Sci. 2017;17:388-393. [Google Scholar]
- 74. Billings PC, Romero-Weaver AL, Kennedy AR. Effect of gender on the radiation sensitivity of murine blood cells. Gravit Space Res. 2014;2:25-31. [PMC free article] [PubMed] [Google Scholar]
- 75. Vasudeva V, Tenkanidiyoor YS, Radhakrishna V, et al. Palliative effects of lutein intervention in gamma-radiation-induced cellular damages in Swiss albino mice. Indian J Pharmacol. 2017;49:26-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carr BI, Guerra V. Thrombocytosis and hepatocellular carcinoma. Dig Dis Sci. 2013;58:1790-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gakis G, Fritsche HM, Hassan F, et al. Prognostic relevance of postoperative platelet count in upper tract urothelial carcinoma after radical nephroureterectomy. Eur J Cancer. 2014;50:2583-2591. [DOI] [PubMed] [Google Scholar]
- 78. Mantas D, Kostakis ID, Machairas N, Markopoulos C. White blood cell and platelet indices as prognostic markers in patients with invasive ductal breast carcinoma. Oncol Lett. 2016;12:1610-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Heinicke RM, van der Wal L, Yokoyama M. Effect of bromelain (Ananase) on human platelet aggregation. Experientia. 1972;28:844-845. [DOI] [PubMed] [Google Scholar]
- 80. Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and anti-inflammatory activity. Asia Pac J Clin Nutr. 2006;15:143-152. [PubMed] [Google Scholar]
- 81. Metzig C, Grabowska E, Eckert K, Rehse K, Maurer HR. Bromelain proteases reduce human platelet aggregation in vitro, adhesion to bovine endothelial cells and thrombus formation in rat vessels in vivo. In Vivo. 1999;13:7-12. [PubMed] [Google Scholar]
- 82. Ley CM, Tsiami A, Ni Q, Robinson N. A review of the use of bromelain in cardiovascular diseases. Zhong Xi Yi Jie He Xue Bao. 2011;9:702-710. [DOI] [PubMed] [Google Scholar]