Abstract
We report a fatal case of COVID-19 in a 51-year-old African American woman with multiple sclerosis on natalizumab. She had multiple risk factors for severe COVID-19 disease including race, obesity, hypertension, and elevated inflammatory markers, but the contribution of natalizumab to her poor outcome remains unknown. We consider whether altered dynamics of peripheral immune cells in the context of natalizumab treatment could worsen the cytokine storm syndrome associated with severe COVID-19. We discuss extended interval dosing as a risk-reduction strategy for multiple sclerosis patients on natalizumab, and the use of interleukin-6 inhibitors in such patients who contract COVID-19.
Keywords: Multiple sclerosis, natalizumab, COVID-19, novel coronavirus disease, cytokine storm syndrome, IL-6 inhibitor
Background
The novel coronavirus disease (COVID-19) emerged as an infectious entity in December 2019 and by 11 March 2020 was declared a global pandemic by the World Health Organization. A majority of infected patients experience mild to moderate upper respiratory symptoms but up to 20% have severe pulmonary disease.1 The risk for severe COVID-19 infection among individuals with multiple sclerosis (MS), many of whom are treated with immunomodulatory therapy, remains unknown.
Case report
We report a fatal case of COVID-19 in a 51-year-old African American woman with MS on natalizumab. Her past medical history includes obesity (body mass index 53 kg/m2), hypertension and recurrent urinary tract infections. She had no known COVID-19 exposure; however, her son and home health attendant both had cough without fever. She typically did not leave her residence except for medical appointments.
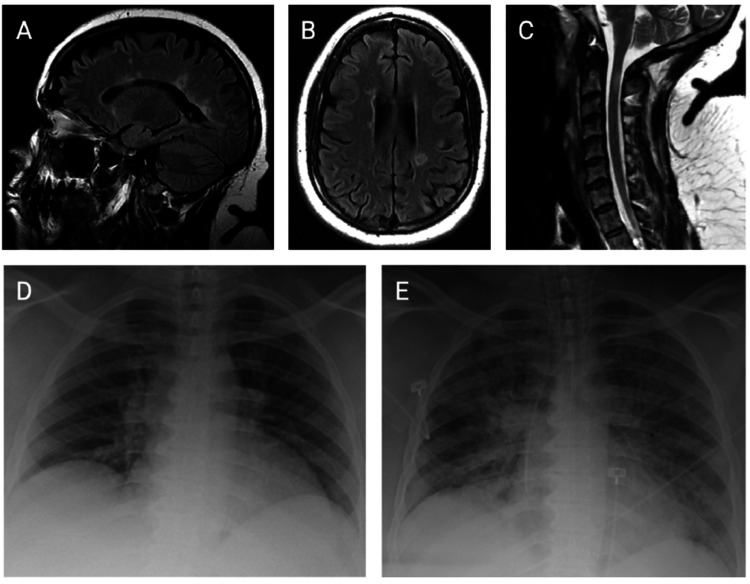
Her MS symptoms began in 2006 with bilateral hand numbness postpartum. In 2010 she developed bilateral leg numbness, weakness, and urinary incontinence. Relapsing–remitting MS was diagnosed in 2012, supported by magnetic resonance imaging (MRI) (Figure 1(a)–(c)). She was treated with interferon beta then natalizumab following a relapse in 2013. She had since received monthly infusions without disease activity. At her last follow-up visit in January 2020, she was at her baseline of Medical Research Council (MRC) grade 4 strength throughout the left leg and grade 3 throughout the right. Her Expanded Disability Status Scale was 6.5 and timed 25-foot walk was 18.5 seconds with a rolling walker. Her most recent natalizumab infusion was administered on 12 March 2020.
Figure 1.
Radiographic studies. Baseline neuroimaging: sagittal (a) and axial (b) views of brain magnetic resonance imaging (MRI), sagittal view of cervical spine MRI (c). Chest radiographs on admission (d) and post-intubation on hospital day (HD) 11 (e).
On 22 March 2020 the patient developed fever, cough, and worsening paraparesis, which led to hospitalization that evening. Initial vital signs were a temperature of 39.3°C, heart rate of 112 beats per minute, blood pressure of 161/89, respiratory rate of 33 breaths per minute, and oxygen saturation of 98% on ambient air. Physical exam revealed clear lungs with comfortable breathing, normal heart sounds, and MRC grade 3 paraparesis. A complete blood count with differential demonstrated an elevated white blood cell count, elevated neutrophil percentage, and reduced lymphocyte percentage (Table 1). A complete metabolic panel was within normal limits except for a decreased albumin level of 3.6 g/dL. Inflammatory markers were not elevated except for C-reactive protein (CRP) and lactate dehydrogenase (LDH) (Table 1). Chest radiograph showed low lung volumes (Figure 1(d)). Blood and urine cultures were negative; however, SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) from nasopharyngeal swab returned positive. She received 5 days of hydroxychloroquine and azithromycin for COVID-19. Despite scheduled acetaminophen, the patient continued to have fevers up to 39.4°C. By hospital day (HD) 4, she required 2–4 L of supplemental oxygen. Her antibiotics were broadened with vancomycin and piperacillin-tazobactam. In light of the persistent fevers and oxygen requirement, there was consideration of experimental therapy with sarilumab, an antibody directed against the interleukin-6 (IL-6) receptor, but she was excluded from the clinical trial because of MS history and recent natalizumab infusion. On HD 10 her oxygen requirement acutely worsened to 15 L, and repeat inflammatory markers were elevated (Table 1). Chest radiograph showed diffuse airspace opacities (Figure 1(e)). She developed severe acute respiratory distress syndrome (ARDS) and vasodilatory shock on HD 11. Despite ventilatory support and high-dose vasopressors, she remained hypotensive with evidence of multi-organ failure. She expired on HD 12.
Table 1.
Laboratory trends.
| Date | 14 October 2013 Pre-NTZ |
14 January 2020 Baseline |
22 March 2020 ED |
23 March 2020 HD 1 |
31 March 2020 HD 9 |
2 April 2020 HD 11 |
|---|---|---|---|---|---|---|
| CBC with differential | ||||||
| White blood count (×103 cells/uL) |
4.5 | 8.71 | 16.12 | 8.27 | 5.44 | 10.54 |
| Neutrophil (%) | 50 | 54.5 | 78.2 | 59.9 | 54 | 75 |
| Absolute neutrophil count (cells/uL) | 2280 | 4750 | 12610 | 4950 | 2940 | 7910 |
| Lymphocyte (%) | 40 | 36.4 | 14.0 | 25.3 | 42 | 21 |
| Absolute lymphocyte count (cells/uL) | 1800 | 3170 | 2250 | 2090 | 2500 | 2420 |
| Monocyte (%) | 9 | 6.5 | 5.6 | 13.2 | ||
| Absolute monocyte count (cells/uL) | 400 | 570 | 900 | 1090 | ||
| Hemoglobin (g/dL) | 10.7 | 7.9 | 7.1 | 6.3 | 7.2 | 7.4 |
| Platelet count (×103 cells/uL) |
383 | 337 | 457 | 344 | 224 | 384 |
| Inflammatory markers | ||||||
| C-reactive protein (mg/L) | 11.77 | 131.32 | 174.34 | |||
| Erythrocyte sedimentation rate (mm/h) | 78 | |||||
| Procalcitonin (ng/mL) | 0.07 | 0.89 | ||||
| Interleukin-6 (pg/mL) | 12.3 | 105.0 | ||||
| Lactate dehydrogenase (U/L) | 333 | 709 | ||||
| Ferritin (ng/mL) | 10.8 | 709.9 | ||||
| D-dimer (ug/mL) | 0.7 | 6.95 | 4.25 |
CBC: complete blood count; ED: emergency department; HD: hospital day; NTZ: natalizumab.
Discussion
This patient had multiple risk factors associated with severe COVID-19 including race, hypertension, obesity, high fever (≥39°C), and elevated inflammatory markers (IL-6, CRP, LDH, ferritin, D-dimer).1–4 Unequivocally, her unfortunate outcome was driven by those factors. Whether natalizumab could have contributed to the poor outcome is unknown, but the theoretical risk remains a concern amidst the current COVID-19 pandemic.
Severe COVID-19 infection is associated with lymphopenia, lung infiltration by monocytes/macrophages, and markers of systemic inflammation.1–4 Elevations of IL-6, IL-2R, tumor necrosis factor (TNF) alpha, CRP, LDH, ferritin, and D-dimer result from cytokine storm syndrome where uncontrolled immune cell activation leads to excess cytokine release, ARDS, hypercoagulation, and multi-organ dysfunction.4,5 Unlike many MS treatments, natalizumab does not suppress the peripheral immune system. Rather, this monoclonal antibody against the α4β1-integrin prevents leukocyte transmigration across the blood-brain barrier and often causes relative increases in peripheral lymphocyte, monocyte, and eosinophil counts within the normal range,6,7 as observed in our patient (Table 1). Natalizumab treatment also increases cytokine-producing T cells in peripheral blood, particularly those expressing TNF, interferon-γ, IL-6, and IL-17.8
In considering the relationship between this patient’s natalizumab treatment and her COVID-19 infection, the frequent interaction with healthcare facilities necessitated by a monthly infusion could have increased her risk of contracting COVID-19. Extended interval natalizumab dosing is under investigation for minimizing opportunistic infection risk while maintaining efficacy. Observational data from extended interval dosing (every 6 weeks) suggest preservation of the MS therapeutic effect relative to standard dosing.9 Additionally, the effect of natalizumab on peripheral immune cells begins to reverse between 4 and 8 weeks from the last dose.7 During the COVID-19 pandemic, temporarily implementing extended interval dosing on a case-by-case basis could both reduce exposure to high-risk healthcare facilities and modulate the peripheral immune cell subpopulations that facilitate the cytokine storm.5,7
In COVID-19-infected patients, strategies for treating cytokine storm include glucocorticoids, intravenous immunoglobulin, and monoclonal antibodies against the IL-6 pathway, sarilumab and tocilizumab.4,5 Our patient was not considered a candidate for sarilumab or tocilizumab because of perceived baseline immunosuppression. The inhibition of the IL-6 pathway could be a relevant strategy in MS patients with severe COVID-19 infection, and in such cases MS specialists are important advocates to include in the inpatient discussions on immunomodulatory management.
Conclusion
Individual case reports are useful for generating hypotheses and reflecting on practice patterns. Ongoing MS case series and registries will provide the statistically robust findings needed to guide immunomodulatory care of MS patients in the context of COVID-19.
Contributor Information
Genna Braverman, New York Presbyterian Hospital, United States of America; Department of Medicine, Columbia University Irving Medical Center, United States of America.
Yun Kyoung Ryu, New York Presbyterian Hospital, United States of America; Department of Medicine, Columbia University Irving Medical Center, United States of America.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kathryn Rimmer, Genna Braverman, Dina Podolsky, Lauren Sutherland, Christopher Migliore, Yun Kyoung Ryu, and Libby Levine have no disclosures. Rebecca Farber served as a consultant for VielaBio. She received a pilot grant and a fellowship training grant from the National MS Society. Kiran Thakur received grant NIH 1K23NS105935-01. Seth Levin received a fellowship training grant through Genentech and honoraria for advisory work with Biogen. Wendy Vargas served as a paid consultant for Biogen Idec, Alexion Pharmaceuticals, EMD Serono, Genentech, and Octapharma. She is the recipient of the grant NIH K23HD098312. Philip L De Jager served on the scientific advisory board for Neuroimmunology Newco, Roche, Biogen, and Celgene, has a sponsored research agreement with Biogen and Roche, and has fellowship funding through Genentech. Claire S Riley served as a consultant or advisor for Biogen Idec, EMD Serono, Genentech, TG Therapeutics, and Third Rock Ventures.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Kathryn Rimmer https://orcid.org/0000-0001-7900-5215
Christopher Migliore https://orcid.org/0000-0001-7477-0022
Seth Levin https://orcid.org/0000-0001-9222-1094
Philip L De Jager https://orcid.org/0000-0002-8057-2505
References
- 1.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020; 395(10,239): 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online ahead of print, 2020 Mar 13]. JAMA Intern Med 2020;e200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China (published correction appears in Lancet, 30 January 2020). Lancet 2020; 395(10,223): 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The perspectives of clinical immunologists from China. Clin Immunol 2020; 214: 108,393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: Immunopathology in COVID-19 (published online ahead of print, 15 April 2020). Arthritis Rheumatol 2020; 10.1002/art.41285. [DOI] [PMC free article] [PubMed]
- 6.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2003; 348(1): 15–23. [DOI] [PubMed] [Google Scholar]
- 7.Plavina T, Muralidharan KK, Kuesters G, et al. Reversibility of the effects of natalizumab on peripheral immune cell dynamics in MS patients. Neurol 2017; 89(15): 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kivisäkk P, Healy BC, Viglietta V, et al. Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurol 2009; 72(22): 1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerico M, De Mercanti SF, Signori A, et al. Extending the interval of natalizumab dosing: Is efficacy preserved? Neurotherapeutics 2020; 17(1): 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]