Abstract
The purpose of this article is to describe dependence and withdrawal phenomena related to CNS drugs discontinuation and to clarify issues related to the evaluation of clinical drug withdrawal and rebound as they relate to safety in new drug development. The article presents current understanding and definitions of drug dependence and withdrawal which are also relevant and important features of addiction, though not the same. Addiction, called substance use disorder in DSM-5, affects an individual’s brain and behaviour, represents uncontrollable drug abuse and inability to stop taking a drug regardless of the harm it causes. Characteristic withdrawal syndromes following abrupt discontinuation of CNS-active drugs from numerous drug classes are described. These include drugs both scheduled and non-scheduled in the Controlled Substances Act, which categorizes drugs in five schedules based on their relative abuse potentials and dependence liabilities and for regulatory purposes. Schedules 1 and 2 contain drugs identified as those with the highest abuse potential and strictest regulations. Less recognized aspects of drug withdrawal, such as rebound and protracted withdrawal syndromes for several drug classes are also addressed. Part I presents relevant definitions and describes clinical withdrawal and dependence phenomena. Part II reviews known withdrawal syndromes for the different drug classes, Part III describes rebound and Part IV describes protracted withdrawal syndromes. To our knowledge, this is the first compilation of withdrawal syndromes for CNS drugs. Part V provides details of evaluation of dependence and withdrawal in the clinical trials for CNS drugs, which includes general design recommendations, and several tools, such as withdrawal questionnaires and multiple scales that are helpful in the systematic evaluation of withdrawal. The limitations of different aspects of this method of dependence and withdrawal evaluation are also discussed.
Keywords: dependence, withdrawal syndromes, rebound, human dependence evaluation
This manuscript introduces the concepts of dependence, withdrawal and rebound for CNS-active drugs and provides a comprehensive review of multiple currently known withdrawal syndromes for scheduled and non-scheduled drugs. The manuscript also describes details of evaluation of dependence and withdrawal in clinical trials for drugs that are being developed.
Graphical Abstract
Graphical Abstract.
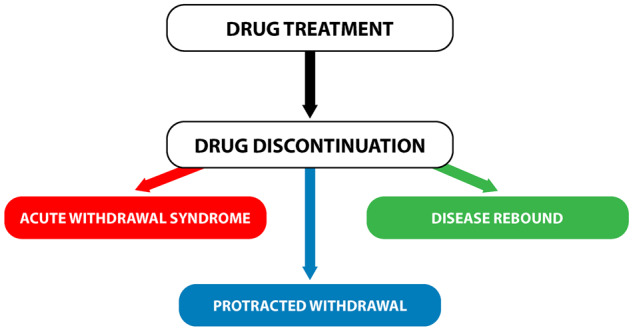
Part I: Introduction, definitions and general considerations of drug dependence, withdrawal and rebound
Introduction
In the United States, the significance of drug dependence, especially as it relates to addiction, came to the foreground and captured public attention only in recent decades, following epidemics of drug abuse, dependence and addiction, sweeping the country, resulting in staggering numbers of deaths. Most cases are related not only to opioid drugs, but also to benzodiazepines (BZ), stimulants and street drugs, including heroin, highly potent fentanyl and its analogues, cathinone-like hallucinogens and dissociative substances, and stimulants similar in action to cocaine and methamphetamine (Dowell et al., 2017; Scholl et al., 2018; Colon-Berezin et al., 2019; Kariisa et al., 2019).
Serious and potentially life-threatening adverse events related to drug withdrawal and dependence may result also from other drug classes generally not associated with abuse and not scheduled under the Controlled Substances Act. Withdrawal of antidepressants and other CNS drugs such as BZ and stimulants may lead to dangerous, even life-threatening, withdrawal symptoms and lead to significant public health concerns, such as suicidality (Risse et al., 1990; Valuck et al., 2009; APA, 2013; Fava et al., 2015).
A widespread misunderstanding regarding dependence and withdrawal is that both need to be related to drug abuse and addiction. Also, in general, the occurrence of dependence and withdrawal may be underestimated, and their significance underappreciated, particularly for drugs not known for abuse potential and which are not scheduled substances in the Controlled Substances Act (Newman et al., 2009; Rabinak and Nirenberg, 2010; Reidenberg, 2011; Hudak et al., 2012; Chouinard and Chouinard, 2015; Fava et al., 2015).
Thus, this article has two main goals, one is to provide brief condensed summaries of withdrawal syndromes for the majority of the CNS-active drug groups which are of interest to prescribers, patients and also clinical scientists, this addresses a gap of knowledge usually not readily available in the drug labels.
The second goal is to emphasize the importance of evaluating dependence and withdrawal for all CNS-active drugs and to provide standardized advice how to assess symptoms of drug withdrawal. The information obtained will be used to provide safety data for warnings on drug labels and to inform patients and physicians about possible dependence, withdrawal and rebound related to the drug—and their consequences.
Definitions of dependence and withdrawal
The phrase ‘drug dependence and withdrawal’ most often and traditionally relates to substance abuse. However, at other times it can relate to a broader concept of ‘dependence and withdrawal’, which potentially may encompass all drug classes, because it may be viewed as a function of an organism’s adaptation to the presence of a drug in the body. In this study, we will discuss both approaches.
In the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; APA, 2013) definitions of drug dependence and withdrawal related to substance use disorder are described in the ‘Substance-Related and Addictive Disorders’ section, and are further defined for drugs and substances known to be related to substance use disorders. These substances include opioids, sedatives, hypnotics, anxiolytics, stimulants, cannabis, caffeine, alcohol and tobacco. Of these substances, cannabis is listed in Schedule 1 of the Controlled Substances Act, many opioids and stimulants are listed in Schedule 2; sedatives, hypnotics and anxiolytics are listed in Schedule 3 or 4, whereas caffeine, alcohol and tobacco are not listed in the Controlled Substances Act (United States Congress, 1970).
However, for drugs, in particular CNS active, which are being developed and evaluated by FDA, we will be using another definition, more appropriate for this function, namely, part (i) of the dependence definition cited by the American Society of Addiction Medicine (ASAM). The recent ASAM definitions of dependence, addiction, tolerance and withdrawal gained support by the American Academy of Pain Medicine and the American Pain Society in 2001, and have since then been updated (Ries et al., 2014).
Physical dependence—used in three different ways: (i) physical dependence is a state of adaptation that is manifested by a drug class-specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, reducing blood level of the drug and/or administration of an antagonist; (ii) psychological dependence is a subjective sense of need for a specific psychoactive substance, either for its positive effects or to avoid negative effects associated with its abstinence; and (iii) one category of psychoactive substance use disorder in previous editions of the Diagnostic and Statistical Manual of Mental Disorders, but not in the DSM-5, published in 2013.
Addiction is characterized by inability to consistently abstain, impairment in behavioural control, craving, diminished recognition of significant problems with one’s behaviours and interpersonal relationships, and a dysfunctional emotional response. Like other chronic diseases, addiction often involves cycles of relapse and remission. Without treatment or engagement in recovery activities, addiction is progressive and can result in disability or premature death.
Tolerance—a state of adaptation in which exposure to a drug over time results in diminution of one or more of the drug’s physiologic effects.
Withdrawal syndrome—the onset of a predictable constellation of signs and symptoms following the abrupt discontinuation of, or rapid reduction in, the dose of a psychoactive substance.
These definitions are important for distinguishing between dependence and addiction or substance use disorder.
To further clarify the concept of dependence it can be said that an individual can be addicted to the drug of abuse, or merely develop dependence to the drug of abuse without being addicted. For drugs not associated with abuse potential, an individual may still develop dependence; but again, this would not be classified as an addiction.
The phenomena of dependence are related to withdrawal, tolerance and rebound. Ashton (1991) described the relation of withdrawal, rebound and tolerance using the example of BZ. The excerpt below is somewhat lengthy for the citation, but it describes withdrawal phenomena, their sequence and physiological background so well that the authors decided to provide it here in full.
Any chronically used drug gradually engenders a series of homeostatic responses which tend to restore normal function despite the presence of the drug. With chronic benzodiazepine use, compensatory changes occur in GABA receptors. Such changes consist of decreased sensitivity of these receptors to GABA, probably as a result of alterations in affinity state and decreased density (Cowen and Nutt, 1982; Nutt and Malizia, 2001).
In addition, there are changes in the secondary systems controlled by GABA, so that the output of excitatory neurotransmitters tends to be restored, and/or the sensitivity of their receptors increases. The whole complex of primary and secondary changes eventually results in benzodiazepine tolerance.
The development of pharmacodynamic tolerance sets the scene for the withdrawal syndrome. Cessation of the drug exposes all the adaptations which have accrued to counteract its presence, releasing a rebound of unopposed activity involving many neurotransmitters and their receptors and many brain systems. Clinically this state is manifested as the withdrawal syndrome, consisting of effects that are largely the opposite of these originally induced by the drug.
Evaluation of dependence and withdrawal is an integral part of FDA’s evaluation of new drugs.
The development of dependence is a general feature of drug effects on the human body which frequently cannot be predicted a priori. Thus, it is important to evaluate thoroughly new drugs for development of dependence, withdrawal and rebound symptoms, as well for development of abuse potential (FDA Abuse Guidance 2017; FDA and Center for Drug Evaluation and Research, 2017).
The evaluation of human dependence and withdrawal is important for regulatory reasons, as it relates to 21U.S.C. 811 and 812 (United States Code, Title 21—FOOD AND DRUGS; U.S. Government, 2006): a drug that is a controlled substance, needs to be monitored for abuse and misuse and new data that relate to abuse and dependence liability resulting from abuse needs to be assessed.
The assessment of dependence and withdrawal is also necessary as a potential warning for physicians and patients and should be provided in the drug label to inform if the drug can be abruptly withdrawn at the end of treatment or must be slowly tapered to avoid potentially dangerous even life-threatening adverse events after abrupt withdrawal. It is also important to inform subjects abusing the drug about health consequences of the development of dependence and of drug withdrawal.
Drug dependence and withdrawal
We present here withdrawal-related phenomena which can be identified for many different drug groups; this list is based largely on a description of clinical aspects of withdrawal emerging after discontinuation of selective serotonin reuptake inhibitors (SSRIs; Chouinard and Chouinard, 2015). These withdrawal phenomena include:
New symptoms (acute withdrawal symptoms): Newly emerging signs and symptoms which occur almost immediately after abrupt drug discontinuation or sometimes even after the dose decrease. These symptoms are related to the disruption of neuroregulatory changes (neuroadaptation) established during drug administration.
Rebound: Recurrence of symptoms of the treated disorder in patients, more severe than before the treatment.
Protracted withdrawal syndrome: Usually appears long past the timeframe of acute withdrawal symptoms, may last for weeks and months, and sometimes may present as a newly emerging disorder.
Relapse. Return of signs and symptoms of the disease after a remission due to natural causes or termination of treatment; it occurs as a phenomenon in the natural history of the disorder. Relapse is not an aspect of dependence; however, it is mentioned here because it occurs during the timeframe of acute withdrawal and needs to be differentiated from the withdrawal.
Symptoms of delayed drug toxicity: May be over-imposed on acute withdrawal symptoms and sometimes are difficult to differentiate from them.
Factors influencing formation of drug dependence
Several studies were performed to evaluate the influence of various factors on the development of dependence. A general consensus is that: (i) timing and rate of exposure(s); (ii) dose; and (iii) drug potency, critically impact the formation of dependence, where with a longer timeframe of use, higher dose and higher drug potency, the likelihood of dependence formation increases (MacKinnon and Parker, 1982; Owen and Tyrer, 1983; Roy-Byrne et al., 1989; Rickels et al., 1990; Busto and Sellers, 1991; Noyes et al., 1991; Chouinard, 2004; Kan et al., 2004).
The neurotransmitter system affected by the drug is also an important variable. Opiate, GABA-ergic and dopaminergic systems tend to be more frequently linked to the development of dependence. However, the impact of other factors, such as gender and age, is less known.
The length of exposure necessary for development of dependence
Probably the first studies examining the relationship between time of drug exposure, dose and formation of dependence and withdrawal were performed in psychiatric patients by Hollister et al. (1961, 1963) using the BZ, chlordiazepoxide and diazepam. Since then, it has been widely recognized that formation of physical dependence resulting in a clinically significant withdrawal syndrome can occur within 6 weeks of BZ administration, especially if sufficiently high doses (two to three times the therapeutic dose) are used. MacKinnon and Parker (1982) reviewed the literature on this topic and found that withdrawal may occur even after only 4–8 weeks of drug exposure; however, in these cases frequently excessively high doses were used.
Kales et al. (1987) observed that after only 7 days of administration of alprazolam (1 mg) insomnia patients develop tolerance and drug lost ∼40% of efficacy, additionally abrupt withdrawal caused rebound insomnia.
Roy-Byrne et al. (1989) compared the withdrawal effects of alprazolam and diazepam in patients with panic disorder after 6 weeks of drug administration. After an initial taper, abrupt discontinuation of drug treatment resulted in relapse and rebound in the patients taking alprazolam and diazepam, with higher prevalence of these symptoms for alprazolam.
Dependence even after therapeutic exposure to opioids such as oxycodone develops even more rapidly, within 2–3 weeks as described by Wakim (2012), Pasero and McCaffery (2010) and Dowell et al. (2016) in Oxycontin label September 2018 and Roxybond label April 2017.
These studies would suggest that dependence formation after administration of opioids can occur within 2–3 weeks using therapeutic doses and for BZ can occur within 6 weeks using therapeutic doses of BZ and in 4 weeks with supratherapeutic doses.
Sex and age differences—a possible factor in severity of withdrawal
Animal withdrawal studies, sex and age impact
Non-clinical withdrawal studies in rodents showed that there are sex and age differences in the severity of symptoms and duration of withdrawal as described in Box 1.
Box 1.
Animal withdrawal studies, sex and age impact
Opioids
Cicero et al. (2002) found that there were sex-related differences in the severity of an opioid withdrawal syndrome after cessation of chronic morphine administration. After stopping the chronic morphine injections or morphine pellet implantation, male rats experienced more severe spontaneous withdrawal syndrome than female rats, consisting of wet-dog shakes, diarrhoea and prominent body weight loss; also, the duration of the withdrawal syndrome was longer in male rats.
In a more recent study, Bobzean et al. (2019) also observed that male morphine-dependent rats expressed more severe symptoms (wet-dog shakes, writhing, global withdrawal score) during the early phases of spontaneous withdrawal compared with females.
Benzodiazepines
Pesce et al. (1994) reported sex differences in withdrawal symptoms of intact and castrated mice that were first treated with subcutaneously implanted pellet of diazepam and then underwent precipitated withdrawal with flumazenil injection. Both sexes experienced seizures and jerks; however, seizures were more pronounced in females. Castration significantly increased the incidence of seizures in male mice.
Sloan et al. (2000) observed the sex differences in the number in seizures, respiratory rate and vocalization in rats which first had implanted diazepam and then underwent withdrawal precipitated with either with PK11195, a peripheral BZ antagonist, and/or flumazenil. Flumazenil induced higher withdrawal scores in females, also female rats vocalized significantly more than male rats; however, flumazenil tended to produce more tachypnoea in male than in female rats.
Cannabis
Marusich et al. (2014) studied impact of gonadal hormones on Δ9-tetrahydrocannabinol (THC) dependence in gonadectomized rats. Rimonabant, selective cannabinoid 1 receptor blocker, precipitated withdrawal in rats with increased forepaw tremors, licking, and increased startle amplitude. However, testosterone treatment of gonadectomized males decreased withdrawal-induced licking, whereas oestradiol and progesterone treatment of gonadectomized females increased withdrawal-induced chewing, and progesterone increased withdrawal-induced sniffing.
Harte-Hargrove and Dow-Edwards (2012) who studied the sex differences in adolescent THC-dependent rats after the spontaneous THC withdrawal showed increased anxiety-like behaviour in females and decreased anxiety-like behaviour in males early in withdrawal, also female rats showed greater locomotor depression than males.
Nicotine
Torres et al. (2013) reported that during nicotine withdrawal, adult female rats displayed higher levels of anxiety-like behaviour than adult males. However, adolescents displayed less nicotine withdrawal than adults, but adolescent males displayed some increase in anxiety-like behaviour.
Hamilton et al. (2010) observed more severe withdrawal behaviours in adolescent male Long-Evans than females. However, these sex differences were not seen in the adolescent Sprague Dawley male and female rats, both sexes had significant withdrawal behaviours.
Sex and age differences in humans during drugs withdrawal
There are limited human data available regarding difference in severity of withdrawal symptoms by sex; some studies discuss this topic for different pharmacological groups (Agrawal et al., 2008; Copersino et al., 2010; Chen et al., 2014; Chartier et al., 2015; Herrmann et al., 2015; Cuttler et al., 2016; Rungnirundorn et al., 2017).
Opioids
Back et al. (2011), studied demographic characteristics and substance use parameters among opioid-dependent men and women participating in a multisite effectiveness trial.
Withdrawal symptoms in this population were assessed using the Opiate Withdrawal Symptoms and Craving (COWS), Adjective Rating Scale for Withdrawal (ARSW) and Visual Analogue Scale (VAS). The results showed that gender difference examined with COWS was small (effect size = 0.12), also no statistically significant gender-related differences in withdrawal symptoms were revealed with the ARSW or VAS. Subjective craving as measured with VAS was significantly higher in women than men.
However, further research studies with humans, males and females, are needed to investigate sex and gender variables that influence the various conditions affected by opioids, including treating pain, occurrence of adverse events and development of addiction (Koons et al., 2018).
Cannabis
Agrawal et al. (2008) examined symptoms of cannabis withdrawal in subjects who reported its use during the past 12 months to the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). It was noted that nausea was more frequently reported by women (3.2% versus 1.7%) and goose bumps and pupil dilation more frequently reported by men (2.2% versus 4.6%) at P = 0.02.
Copersino et al. (2010), in a study that evaluated sociodemographic characteristics of the cannabis withdrawal experience, reported that women were more likely than men to report physical withdrawal symptoms. Also, during cannabis withdrawal women reported more symptoms of upset stomach but significantly fewer instances of craving or increased sex drive compared with men.
Herrmann et al. (2015) reported on sex differences during cannabis withdrawal which were assessed with the Marijuana Withdrawal Checklist (MWC), then converted into a composite Withdrawal Discomfort Scale (WDS) score. Women had significantly higher composite WDS scores than men as well as higher scores than men on six individual symptoms in two domains: mood symptoms that included irritability, restlessness, increased anger and violent outbursts; and gastrointestinal symptoms, that included nausea and stomach pain.
Cuttler et al. (2016) based on the online survey that assessed the cannabis users’ practices and experiences found that during the withdrawal period men more often than women reported insomnia and vivid dreams, whereas women more often reported nausea and anxiety. Also, significantly more women (44.9%) than men (36.7%) did not experience withdrawal symptoms.
Stimulants
Chartier et al. (2015) examined sex-specific factors associated with severity of stimulant withdrawal in a population of abusing or dependent subjects undergoing treatment. The results of the study that used Stimulant Selective Severity Assessment (SSSA), showed that women reported greater severity of stimulant withdrawal, namely 13.78 (SD 11.9) than did men 10.44 (SD 11.1). This difference was seen in anxiety-related withdrawal symptoms (anxiety, tension, attention and irritability) which were more frequent in women.
Rungnirundorn et al. (2017) evaluated withdrawal symptoms in both genders of methamphetamine users. Females were more likely than males to be methamphetamine dependent (79% versus 60%), and to experience withdrawal (65.3% versus 48.9%). Also, some withdrawal symptoms were more frequent in women versus men, including hypersomnia (77.2% versus 64.8%), fatigue (77.5% versus 70.3%).
Ketamine
Chen et al. (2014) analysed sex differences in subjective withdrawal symptoms associated with ketamine use. Results of the study showed that female ketamine users, compared with male users, reported meaningful difference in withdrawal symptoms, such as anxiety (23.4% versus 17.4%), dysphoria (24.1% versus 15.9%) and cravings (23% versus 17.9%).
Nicotine
Foulkner et al. (2018) examined in young adult smokers impact of varied yields of nicotine on sex differences in craving, withdrawal and affect. Women reported greater psychological withdrawal, greater sedation and trend towards greater craving than men during abstinence.
Age as a factor in dependence formation and severity of withdrawal syndrome
There are some data available describing impact of age on dependence formation, mainly in relation to opioids and BZ. It was noted that in children undergoing treatment with opioids, physical dependence may develop in as little as 2–3 days of continuous opioid therapy (Fisher et al., 2013). Also, it seems that signs of opioid withdrawal are more severe in older children than in younger children and infants; thus, adolescents and children more than 7 years old are particularly at risk for increased severity of withdrawal (Anand and Ingraham, 1996).
Regarding BZ withdrawal in children, in addition to classic BZ withdrawal symptoms, children may show visual hallucinations, agitation, facial grimacing, aerophagia, myoclonus, ataxia, choreoathetoid movements and generalized seizures (Anand and Ingraham, 1996). Dependence and resulting withdrawal syndrome may develop in only 7–17 days, although doses of midazolam used in the cases described were high (Sury et al., 1989).
Also, older adults (aged >50 years) seem to develop somewhat different withdrawal symptoms than do younger people after BZ discontinuation. These include emergence of confusion and disorientation, sometimes with hallucinations in older people instead of anxiety, insomnia and perceptual changes noted in younger people (Kruse, 1990; Simoni-Wastila and Yang, 2006). Also, it was observed that catatonia after BZ withdrawal occurs mainly in older adults (Rosebush and Mazurek, 1996; Lebin and Cerimele, 2017).
Five Half-life times rule—issues and misunderstandings
Some investigators consider that withdrawal symptoms last only for a maximum of five half-lives of the drug, which is obviously incorrect, although it is not clear where this statement originated as we did not find the source. For example, heroin half-life is 2-6 min, but duration of the withdrawal syndrome is approximately 4–10 days (see Fig. 1), although this delay may be explained by the fact that the main active molecule in the brain after heroin administration is not heroin itself but rather morphine and 6-acetylmorphine, which have longer half-lives and thus intoxication and withdrawal is longer lasting (Inturrisi et al., 1983; Rook et al., 2006). For amphetamines, half-life is ∼9–11 h, whereas withdrawal symptoms only start 2–4 days after drug discontinuation (New South Wales Government, 2008) and last at least 2–4 weeks. Generally, acute withdrawal symptoms for drugs with very short lifetimes (e.g. heroin) last about a week; for drugs with a medium half-life of ∼10–20 h (e.g. short-acting BZ), the acute withdrawal syndrome may last 2–4 weeks; whereas for drugs with longer half-lives (e.g. long-half-life BZ) the withdrawal phase may last 2–8 weeks (Fig. 2). However, this generalization applies only to acute withdrawal syndrome; symptoms of protracted withdrawal may last for weeks and months.
Figure 1.
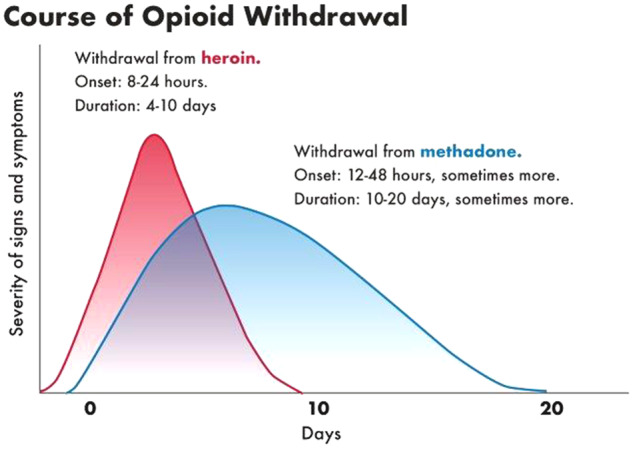
Course of opioid withdrawal. The figure shows different time course for opioids heroine (red curve) and methadone (blue curve). Although heroin half-life is 2–6 min, the withdrawal symptoms start usually at 6–24 h after the last dose, reach a peak at 24–48 h, and resolve after 4–10 days, although this delay as already mentioned, maybe explained by the fact that the main active molecule in the brain after heroin administration is not heroin itself but rather morphine and 6-acetylmorphine, which have much longer half-lives and thus longer-lasting intoxication and withdrawal; for methadone, with a half-life of 15–55 h, the withdrawal symptoms start usually at 36–48 h after the last dose, the withdrawal may be prolonged, and last 3–6 weeks. Adapted from Drug and Alcohol Withdrawal Clinical Practice Guidelines—NSW (New South Wales Government, 2008).
Figure 2.
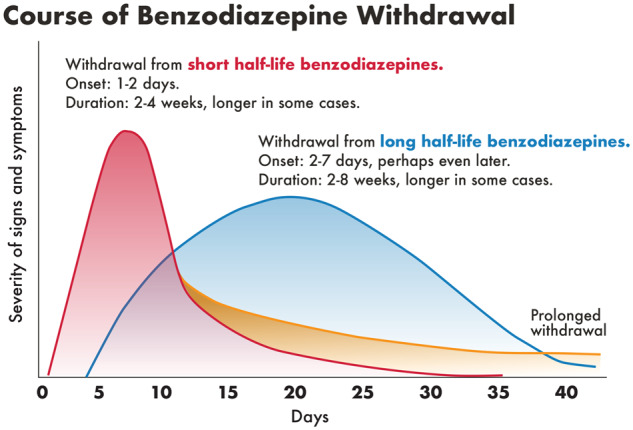
Course of BZ withdrawal. The figure shows different time course for short (red curve) and long half-life BZ (blue curve) which is related to the drugs half-lives. It shows also a formation of protracted withdrawal (yellow curve) for short half-life BZ. Adapted from Drug and Alcohol Withdrawal Clinical Practice Guidelines—NSW (New South Wales Government, 2008).
Current theories on the neurobiological basis of dependence and withdrawal
Opponent process theory of drug withdrawal
Because few drug groups discussed in this manuscript namely opioids, stimulants and BZ are also involved in addictive disorders alternative concepts of dependence and withdrawal are presented (Koob and Le Moal, 2008a, b).
Addiction is depicted as a compulsive disorder with excessive drug intake and loss of control over intake, with decreases in reward neurotransmission and activation of brain stress systems. Addiction is a disorder that involves elements of both impulsivity and compulsivity in which impulsivity predominates at the early stages of addiction (preoccupation and anticipation) whereas compulsivity dominates at the final stages of withdrawal and negative affect.
The key brain structures involved in these processes are ventral striatum with neurotransmitters dopamine and opioid peptides and extended amygdala with corticotropin-releasing factor, norepinephrine and dynorphin. In fact, it is proposed that all main drugs of abuse act through the central nucleus of the amygdala producing increases in reward thresholds, anxiety-like responses and extracellular levels of corticotropin-releasing factor. Specifically, during development of dependence and withdrawal brain antireward systems are recruited and include corticotropin-releasing factor, norepinephrine and dynorphin which produce aversive states such as dysphoria and stress. The components of acute drug withdrawal include decrease of activity of the mesocorticolimbic dopaminergic system and decreased activity in the nucleus accumbens/amygdala mediated by opioid peptides, GABA and glutamate.
Summary points
Dependence and withdrawal are results of neuroadaptation that occur during drug treatment.
Dependence and withdrawal are not equated with drug addiction although may coexist.
The shortest known time to acquire dependence occurs with opioid drugs where 2–3 weeks or longer exposure at the therapeutic dose may produce dependence and upon drug discontinuation or exposure to antagonist (naloxone) also an acute withdrawal syndrome.
Part II: Specific acute withdrawal syndromes acute withdrawal syndrome—general features
Ries et al. (2014) report the American Society of Addiction Medicine (ASAM) definition of withdrawal as ‘The onset of a predictable constellation of signs and symptoms following the abrupt discontinuation of, or rapid reduction in, the dose of a psychoactive substance’.
Chouinard and Chouinard (2015) describe in a very comprehensive manner general features of the emergence of new, acute withdrawal symptoms after drug discontinuation.
New withdrawal symptoms include the symptoms that first appeared during the drug discontinuation or dose decrease and are not related to symptoms of the patient’s original illness.
Different classes of CNS drugs have some common withdrawal symptoms, as well as some specific to the drug class withdrawal symptoms.
New withdrawal symptoms which are common to many CNS drugs include: nausea, anxiety, headaches, decreased concentration, irritability, agitation, tremor, sleep disturbances, dysphoria and depression.
- Some new withdrawal symptoms may be characteristic of a specific CNS drug class:
- • Lacrimation, rhinorrhoea and sneezing for opioids
- • Electric-shock sensations, confusion and myoclonus for SSRIs.
The new withdrawal symptoms are usually transient and reversible.
Major complications of abrupt withdrawal may occur, such as seizures and psychoses in cases of BZ withdrawal (Preskorn and Denner, 1977; Petursson, 1994), seizures in cases of sertraline (Zoloft label) and zolpidem withdrawal (Aragona, 2000), and suicidality involving antidepressants (Yerevanian et al., 2004; Valuck et al., 2009) and MAO inhibitors such as phenelzine (Dilsaver, 1994).
The onset of withdrawal symptoms depends on the duration of action; usually, short-acting drugs have an early peak of onset.
Drugs with higher potency and short/intermediate half-life have greater risks for formation of drug dependence and withdrawal symptoms.
There are well-known withdrawal syndromes in different classes of scheduled and unscheduled drugs. These are described in detail below, along with symptoms and time course of withdrawal syndrome.
Withdrawal syndromes for scheduled drug classes apply to:
Opioids
Benzodiazepines
Z-Drugs (Zolpidem, Zaleplon and Eszopiclone)
Barbiturates
Stimulants (amphetamine, cocaine and methamphetamine)
Testosterone and androgenic anabolic steroids
Ketamine
Cannabis
Withdrawal syndromes for unscheduled drug classes apply to:
Antidepressants: SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs)
Tricyclic antidepressants
Antipsychotics
Anti-Parkinsonian drugs
Monoamine oxidase inhibitors
Gabapentin
Nicotine/tobacco
Corticosteroids
Statins
Aspirin
Acute withdrawal syndromes—scheduled drugs—examples
Opioid withdrawal syndrome
The first descriptions of opioid withdrawal are those by Light and Torrence (1929) and Himmelsbach (1939). The symptoms include yawning, rhinorrhoea, piloerection, perspiration, lacrimation, mydriasis, hand tremors, hot and cold flashes, restlessness, muscle twitches, abdominal cramps, anxiety and chills, myalgia, irritability, backache, joint pain, weakness, insomnia, nausea, anorexia, vomiting, diarrhoea, or increased blood pressure, respiratory rate or heart rate (Light and Torrence, 1929; Himmelsbach, 1939; Handelsman et al., 1987), morphine label (morphine sulphate extended-release tablets, morphine sulfate Contin, label 2018; Table 1). Symptoms of withdrawal may start as early as 6–24 h after the last dose (heroin), peak at 24–48 h, and resolve after 5–10 days.
Table 1.
Summary table for the most common withdrawal syndromes
| Adverse events | Opioids | BZ | Stimulants | SSRIs-SNRIs | Cannabis | Nicotine | Anti-psychotics | Androgenic anabolic steroids and testosterone |
|---|---|---|---|---|---|---|---|---|
| Acute withdrawal syndrome | ||||||||
| Anxiety | + | + | + | + | + | + | + | + |
| Depression/depressed mood | + | + | + | + | + | + | ||
| Irritability | + | + | + | + | + | + | ||
| Agitation | + | + | + | + | ||||
| Insomnia | + | + | + | + | + | + | + | + |
| Somnolence/lethargy | + | + | + | + | ||||
| Psychotic behaviours | + | + | ||||||
| Hallucinations | + | + | + | |||||
| Suicidality (rare) | + | + | + | + | ||||
| Tremors | + | + | + | + | ||||
| Restlessness | + | + | + | + | + | + | + | |
| Seizures | + Neonates | + | ||||||
| Nausea/vomiting | + | + | + | + | ||||
| Diarrhoea | + | + | + | |||||
| Constipation | + | |||||||
| Appetite | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | |
| Weight | ↓ | ↓ | ↑ | |||||
| Heart rate | ↑ | ↑ | ↓ | ↑ | ↓ | |||
| Blood pressure | ↑ | ↓Postural | ||||||
| Sweating | ↑ | ↑ | ↑ | ↑ | ↑ | |||
| Drug group specific AEs | Piloerection/mydriasis/yawning/rhinorrhea | Depersonalization/derealization/delirium | Vivid, unpleasant dreams/paranoid ideation | Brain zaps/ derealization/depersonalization/myoclonus | Disturbing dreams/abdominal pain | Rhinorrhea/rare NMS | Decreased libido/hypogonadotropic hypogonadism | |
Acute withdrawal syndrome.
|
|
|
|
|
|
|
|
|
Rebound
|
|
|
|
|
||||
Protracted withdrawal
|
|
|
|
|
|
|
Rebound insomnia is specific to short and intermediate half-life BZ compounds.
, symptom/syndrome present; ↑, symptom increased; ↓, symptom decreased; BZ = benzodiazepines; INF = information not found; NMS = neuroleptic malignant syndrome; SSRIs = selective serotonin reuptake inhibitors; SNRIs = selective serotonin-norepinephrine reuptake inhibitors.
For long-acting opioid (methadone) withdrawal starts after 36–48 h after the last dose, and may last 3–6 weeks (New South Wales Government, 2008).
The withdrawal symptoms of oxycodone products include restlessness, lacrimation, rhinorrhoea, yawning, perspiration, chills, myalgia and mydriasis, also other symptoms may develop such as irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhoea, increased blood pressure, respiratory rate and heart rate (Oxycontin label Sep 2018, Roxybond label April 2017).
Opioid withdrawal symptoms have been also observed in children undergoing treatment with opioids. In these cases, physical dependence developed as early as 2–3 days following continuous opioid therapy; the most commonly seen symptoms of withdrawal in children included:
Neurological adverse events such as anxiety, agitation, grimacing, insomnia, increased muscle tone, abnormal tremors and choreoathetoid movements.
Gastrointestinal symptoms included vomiting, diarrhoea and poor appetite.
Autonomic signs included tachypnoea, tachycardia, fever, sweating and hypertension (Ista et al., 2008; Anand et al., 2010; Fisher et al., 2013).
Neonatal abstinence syndrome (NAS) is unusual in that the withdrawal symptoms are related to the maternal opioid intake but then manifest in the newborn. The clinical presentation of NAS includes signs of neurological excitability such as tremors, hyperirritability, increased wakefulness, excessive high-pitched crying, increased muscle tone, hyperactive deep tendon reflexes, exaggerated Moro reflex, seizures, frequent yawning and sneezing, and also symptoms of gastrointestinal dysfunction such as poor feeding, unco-ordinated and constant sucking, hyperphagia, vomiting, diarrhoea and dehydration. Onset varies with the opioid pharmacology, in case of maternal use of heroin NAS may start 24 h of birth, whereas in case of withdrawal from methadone NAS usually starts around 24–72 h of age (Hudak et al., 2012; Kocherlakota, 2014).
Although generally not life-threating, in some cases opioid withdrawal may lead to serious adverse events such as Takotsubo cardiomyopathy ‘broken heart syndrome’ in cases of methadone and extended-release long-acting oxycodone withdrawal (Rivera et al., 2006; Lemesle et al., 2010; Saiful et al., 2011; Spadotto et al., 2013), or seizures due to neonatal abstinence syndrome (Hudak et al., 2012; Kocherlakota, 2014).
The time course of the severity of acute withdrawal symptoms for opioid drugs depends usually on the half-life of the drug, although it can be quite variable. Figure 1 shows onset, peak, duration and severity of acute withdrawal symptoms for two frequently used and abused opioids, heroin and methadone (New South Wales Government, 2008).
It is important to note that opioid withdrawal also includes a protracted phase of withdrawal which occurs after the acute withdrawal phase and is further discussed in the ‘Protracted Withdrawal Syndrome’ section.
As mentioned in the ‘Definitions of dependence and withdrawal’ section, there is a difference between withdrawal syndrome and addiction.
Opioid addiction is a complex disorder and its development can vary. An individual’s risk in developing addiction likely relates to a combination of health, social, economic, lifestyle and genetic factors. A history of substance abuse, psychiatric disorders, mistreatment, poverty, and access to prescription or illegal opioids may also contribute to a person’s risk of opioid addiction (Webster et al., 2011).
Benzodiazepine withdrawal syndrome
BZ withdrawal symptoms may vary in intensity. Mild withdrawal may include anxiety, apprehension, fearfulness, insomnia, irritability, agitation, restlessness, dizziness, headache, anorexia, weight loss, difficulty in concentration, muscle stiffness and pain, hyperosmia, metallic taste, perceptual changes, sweating and intolerance to light and sound. More severe symptoms include nausea, vomiting, vertigo, cramps, weakness, tremor, tachycardia, postural hypotension, hyperthermia, panic attacks, depression, psychotic reactions, depersonalization, de-realization, delirium, delusions and hallucinations (MacKinnon and Parker, 1982; Petursson, 1994; New South Wales Government, 2008). The most serious adverse events which may occur are psychosis, delirium, suicidality, seizures and in older people catatonia (Preskorn and Denner, 1977; Owen and Tyrer, 1983; Risse et al., 1990; Lader, 1994; Petursson, 1994; Rosebush and Mazurek, 1996; Lebin and Cerimele, 2017; Reeves and Kamal, 2019; Table 1).
The timing of withdrawal symptoms depends on several factors; the main one is BZ half-life. Long-acting BZs include diazepam, chlordiazepoxide, flurazepam and clorazepate; shorter-acting BZs include oxazepam, lorazepam and triazolam. For the long-acting BZs, there is a lag period of 3–7 days for onset of withdrawal symptoms following the drug discontinuation; whereas the short-acting BZs symptoms of withdrawal may occur within 24 h. The correlation between drug half-life and onset of symptoms and length of the withdrawal is presented in Fig. 2, below (New South Wales Government, 2008). Figure 2 also shows formation of protracted withdrawal which starts at the end of the acute withdrawal phase.
Use of BZ during the later stages of pregnancy can result in withdrawal symptoms in the neonate such as apnoea, bradycardia, hypertonia, irritability, hypothermia, hyperactivity, tachypnoea, restlessness, tremors, hyperreflexia, inconsolable crying, cyanosis, diarrhoea, vomiting and feeding difficulties (Iqbal et al., 2002; Hudak et al., 2012).
Z-drugs withdrawal syndrome (zolpidem and zopiclone)
Dependence following the use of zolpidem has been known since the 1990s as occurring mainly in people abusing it. There are also a considerable number of zolpidem dependence case reports in the scientific literature (Aragona, 2000; Tripodianakis et al., 2003; Cubala and Landowski, 2007; Victorri-Vigneau et al., 2007; Victorri-Vigneau et al., 2014; Chattopadhyay et al., 2016).
The withdrawal syndrome after abrupt withdrawal of zolpidem was already noted during the clinical studies of Ambien (zolpidem tartrate). Adverse events reported within 48 h of drug discontinuation included fatigue, nausea, flushing, lightheadedness, uncontrolled crying, emesis, stomach cramps, panic attack, nervousness, abdominal discomfort, hyperventilation, shallow breathing, angry feelings, cramping muscles and restlessness (Watsky, 1996; Sethi and Khandelwal, 2005) and labels for zolpidem (e.g. Ambien, Edluar 2019). Withdrawal seizures after zolpidem discontinuation were reported mainly in cases of use of supratherapeutic doses (Aragona, 2000; Tripodianakis et al., 2003; Sethi and Khandelwal, 2005; Cubala and Landowski, 2007).
Also, withdrawal symptoms after discontinuation of zopiclone were reported, and included cravings, rebound insomnia, anxiety or panic attacks, weakness, palpitations, tachycardia, tremor and withdrawal seizures. These symptoms were mainly seen in subjects using supratherapeutic doses; rebound insomnia and rebound anxiety were also noted (Cimolai, 2007).
Barbiturate withdrawal syndrome
Abrupt discontinuation of a barbiturate after prolonged use or abuse can result in a withdrawal syndrome which is sometimes life-threatening. Withdrawal symptoms include weakness, tremor, muscle twitches, agitation, anxiety, anorexia, nausea, vomiting, weight loss, disorientation in time and place, agitation, tremulousness, insomnia, delusions, visual and auditory hallucinations, postural hypotension and high fever. The most serious adverse events of withdrawal are delirium, generalized convulsions and cardiovascular collapse that may lead to death; however, severe withdrawal occurs mainly after dependence on short- or intermediate-acting barbiturates such as pentobarbital, secobarbital, amobarbital or butalbital (Fraser et al., 1953; Essig, 1968; Gault, 1976; Gussow and Carlson, 2013).
Also, neonatal withdrawal syndrome associated with barbiturates has been identified.
Infants born to mothers physically dependent on barbiturates may develop dependence and subsequently experience withdrawal. The withdrawal may manifest within the first few days of life and symptoms include excessive crying, hyperphagia, vomiting, diarrhoea, tremors, irritability, hyperacusis, vasomotor instability, sweating, restlessness, sleeplessness, increased tone and sometimes seizures (Desmond et al., 1972; Hudak et al., 2012; Gresham and LoVecchio, 2015) and label Butisol (2019).
Stimulant withdrawal syndrome
Withdrawal from stimulants such as amphetamine (including dexamphetamine and methamphetamine), MDMA (ecstasy) and cocaine usually occurs in three phases (crash, withdrawal and extinction phase), Fig. 3. The crash phase, starts as stimulants wear off, can last for several days and includes fatigue, flat affect, increased sleep and reduced cravings; severe depressive symptoms may occur. The withdrawal phase typically starts 2–4 days after the last amphetamine use and 1–2 days after the last cocaine use. Withdrawal phase symptoms may include strong cravings, fluctuating mood and energy levels, psychomotor retardation or agitation, irritability, restlessness, anxiety, fatigue, lack of energy, anhedonia, vivid and unpleasant dreams, insomnia or hypersomnia, general aches and pains, headaches, muscle tension, increased appetite, poor concentration and attention, disturbances of thought (paranoid ideation, strange beliefs) and hallucinations; bradycardia may be present and is a reliable measure of stimulant withdrawal (Kosten and O’Connor, 2003; New South Wales Government, 2008; APA, 2013; Table 1). The withdrawal symptoms gradually decrease over 2–4 weeks for amphetamine and 1–2 weeks for cocaine, although some symptoms persist during the extinction phase/protracted withdrawal.
Figure 3.
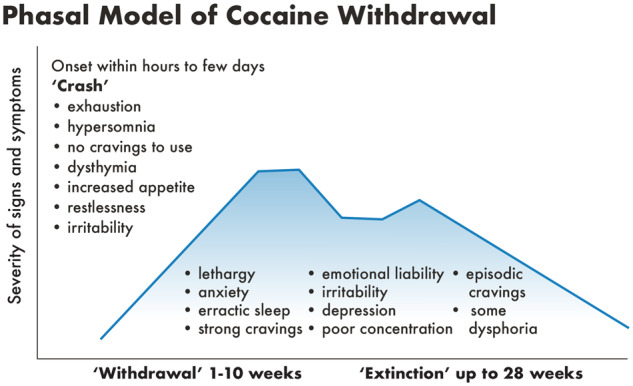
Phasal model of cocaine withdrawal. The figure shows three phases of cocaine withdrawal: crash, withdrawal and extinction, which have somewhat different symptoms profile. Adapted from Baker et al. (2004).
Withdrawal from stimulant drugs is generally not medically dangerous; however, depressive symptoms with suicidal ideation or behaviour may occur and are generally the most serious problems seen during the stimulant withdrawal (APA, 2013).
Ketamine withdrawal syndrome
Ketamine is known for the formation of severe psychological dependence and significant, rapidly developing tolerance (Kamaya and Krishna, 1987; Hurt and Ritchie, 1994; Jansen and Darracot-Cankovic, 2001; Pal et al., 2002) without a prominent physical withdrawal syndrome. However, the presence of physical withdrawal symptoms after ketamine discontinuation has also been described, and includes craving, dysphoria, shaking, sweating, palpitations, tiredness, low appetite, low mood, chills, autonomic arousal, lacrimation, restlessness, anxiety, nightmares, paranoia, delusions and hallucinations (Lim, 2003; Critchlow, 2006; Chen et al., 2014). Addiction websites (American Addiction Centers and Addiction Center) additionally list the following adverse events occurring in ketamine abusing subjects during the withdrawal period: agitation, confusion, psychosis, including delusion and hallucination; loss of motor skills, rage, nausea, decreased respiratory and cardiac functions, insomnia, cognitive impairment, loss of appetite, tremors, restlessness, depression, nightmares and chills.
Timelines of ketamine withdrawal include: (i) acute withdrawal symptoms that typically begin within 24 h of discontinuing ketamine use and last approximately 3 days; symptoms include shaking, fatigue, insomnia, rage, depression, hallucinations, delusions, tremors, nausea and rapid breathing; (ii) withdrawal symptoms that may persist for 2 weeks, but begin to decrease; and (iii) after approximately 2 weeks, most withdrawal symptoms usually stabilize.
Testosterone and anabolic-androgenic steroids withdrawal syndrome
The withdrawal syndrome after a chronic intake of supratherapeutic doses of testosterone and androgenic anabolic steroids can last for weeks or months and is characterized by depressed mood, major depression, suicidality, fatigue, craving, headache, muscle and joint pain, anorexia, anxiety, restlessness, irritability, insomnia or hypersomnia, decreased libido, hypogonadotropic hypogonadism and suppression of the hypothalamic–pituitary–testicular (HPT) axis (Thiblin et al., 1999; Medras and Tworowska, 2001; Brower, 2002, 2009; Kanayama et al., 2009) and Testosterone label, 2017 (Table 1).
Cannabis withdrawal syndrome
Withdrawal symptoms of cannabis include irritability, anger, aggression, decreased appetite, weight loss, nervousness, anxiety, restlessness, sleep difficulties including insomnia and strange or disturbing dreams, abdominal pain, shakiness, tremors, fever and headache. Less common symptoms include chills, depressed mood and sweating (Budney et al., 2004; Budney and Hughes, 2006; New South Wales Government, 2008; Gorelick et al., 2012; APA, 2013; Bonnet and Preuss, 2017; Schlienz et al., 2017; Table 1).
Withdrawal symptoms usually start within the first 1–4 days of abstinence, peak within the first week, and then resolve within 2–3 weeks of drug discontinuation (Schlienz et al., 2017). Strange dreams and sleep difficulties can persist longer (Budney and Hughes, 2006; Herrmann et al., 2015). Also, there is temporal variation in the occurrence of specific symptoms, with the early onset of insomnia and shakiness, followed by irritability and anxiety (day 4–5) and anger and aggression peaking after 2 weeks of abstinence (Fig. 4) setting. The timing of the severity of withdrawal symptoms to some degree reflects the changes at the cannabinoid 1 (CB1) receptors level in the brain where regular cannabis intake causes a desensitization and down-regulation of CB1 receptors, and during the first 2 days of withdrawal this begins to reverse and the receptors begin to return to normal functioning within 4 weeks of abstinence (Bonnet and Preuss, 2017).
Figure 4.
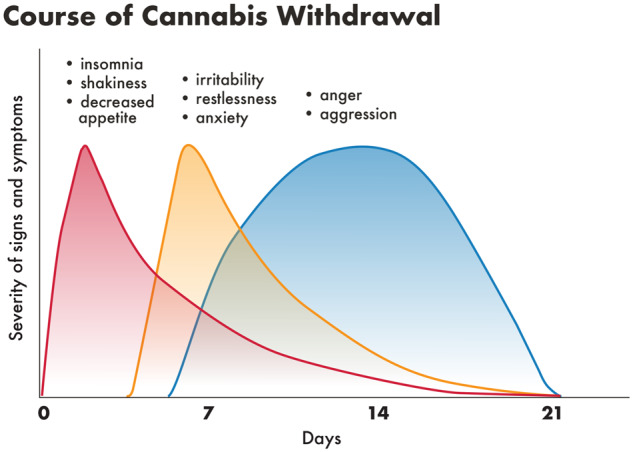
Course of cannabis withdrawal. The figure shows different phases of occurrence of cannabis withdrawal symptoms, with the early phase (red curve) of insomnia, shakiness, decreased appetite symptoms, middle phase (yellow curve) with irritability, anxiety, restlessness symptoms and late somewhat prolonged phase (blue curve) of anger and aggression. Adapted from Drug and Alcohol Withdrawal Clinical Practice Guidelines – NSW (New South Wales Government, 2008).
Acute withdrawal syndromes—non-scheduled drugs—examples
Antidepressant SSRIs and SNRIs withdrawal syndrome
Some more frequently used SSRIs include: fluoxetine, fluvoxamine, paroxetine, citalopram and sertraline. Some more frequently used SNRIs include: venlafaxine, desvenlafaxine and duloxetine.
The SSRIs and SNRIs withdrawal syndrome is characterized by the following symptoms, general: headaches, flu-like symptoms, tachycardia; gastrointestinal: nausea, vomiting, diarrhoea, anorexia; sleep-related: vivid dreams, nightmares, insomnia or hypersomnia; neuropsychiatric: anxiety, depression, suicidality, hypomania, agitation, dysphoria, aggression, hallucinations, de-realization and depersonalization, decreased concentration, dizziness, ataxia, electric-shock sensations (‘brain-zaps’), confusion and myoclonus (Leiter et al., 1995; Rosenbaum et al., 1998; Haddad, 2001; Yerevanian et al., 2004; Chouinard and Chouinard, 2015; Fava et al., 2015) and labels e.g. Paxil, 2014; Prozac, 2017; Zoloft, 2017, Table 1. SSRIs and SNRIs withdrawal symptoms have their peak of onset at 36–96 h or later, depending on the drug’s half-life, and may last up to 6 weeks, also depending on drug half-life (Chouinard and Chouinard, 2015). The individual SSRIs have somewhat different profiles of adverse events, and it seems that severity might be related to half-life (Rosenbaum et al., 1998; Fava et al., 2015). Also, it was observed that withdrawal syndrome was more common in young patients than in the elderly (Himei and Okamura, 2006; Fava et al., 2015). Frequently, acute withdrawal syndrome may be followed by disease rebound (see Rebound section) and protracted withdrawal syndrome (see Protracted Withdrawal Syndrome section).
Of note, there is a tendency in the scientific literature to call SSRIs and SNRIs withdrawal syndrome a ‘discontinuation syndrome’, which in our opinion is both scientifically incorrect and misleading, as it may suggest an absence of the withdrawal syndrome. A comment by Chouinard and Chouinard (2015) explains this issue well: ‘The terminology discontinuation refers to the medical prescribing act or a patient’s self-discontinuation of medication. Furthermore, the term discontinuation syndrome is misleading since withdrawal may occur without discontinuation, for example, in between two doses of rapid-onset and short-acting drugs (e.g. clock watching syndrome) and with a decrease in medication’. Fava et al. (2015) adds, ‘The term “discontinuation syndrome” minimizes the vulnerabilities induced by SSRI and should be replaced by “withdrawal syndrome”’. However, discussion on the topic of this nomenclature continues in the scientific literature (Schatzberg et al., 1997; Shelton, 2006; Nielsen et al., 2012; Chouinard and Chouinard, 2015; Fava et al., 2015).
In addition, neonatal withdrawal syndrome associated with SSRIs has been identified. It includes respiratory distress, cyanosis, seizures, feeding difficulty, vomiting, hypoglycaemia, hypotonia or hypertonia, hyperreflexia, tremor, jitteriness, irritability, pulmonary hypertension; and with paroxetine use, necrotizing enterocolitis (Stiskal et al., 2001; Sanz et al., 2005; Koren and Boucher, 2009; Hudak et al., 2012) and SSRI labels e.g. Paxil, 2014; Prozac, 2017; Zoloft, 2017.
Tricyclic antidepressant withdrawal syndrome
Some more frequently used tricyclic antidepressants include: imipramine, clomipramine, amitriptyline, amoxapine, desipramine, doxepin and nortriptyline. Tricyclic antidepressant withdrawal symptoms can be grouped into four discrete syndromes: (i) gastrointestinal and general somatic distress with occasional anxiety and agitation, also nausea, vomiting, diarrhoea, abdominal pain and anorexia; (ii) sleep disturbances, such as initial and middle insomnia, vivid dreams and nightmares; (iii) parkinsonism including bradykinesia, cogwheel rigidity, tremor or akathisia; and (iv) paradoxical mania. Sometimes withdrawal syndrome presents as a ‘flu-like’ syndrome; which in addition to gastrointestinal symptoms, also includes diaphoresis, dizziness, chills, malaise, fatigue, myalgia and headache; also suicidality and delirium may occur (Dilsaver, 1990; Ceccherini-Nelli et al., 1993; Garner et al., 1993; Dilsaver, 1994; Haddad, 2001; Yerevanian et al., 2004) and label Anafranil, 2014.
In paediatric populations, the most frequent symptoms are irritability, decreased appetite, drowsiness, fatigue, insomnia; also nausea, vomiting and abdominal cramps, which, if severe, may lead to dehydration (Garner et al., 1993).
There are also neonatal withdrawal symptoms that are attributed to maternal use of tricyclic antidepressants; these adverse events include irritability, respiratory difficulty, hypothermia, poor feeding, tremors, jitteriness, myoclonus and hyperactive Moro reflex. Serious withdrawal reactions may be present, such as tachycardia, cyanosis and convulsions (Cowe et al., 1982; Bloem et al., 1999; Haddad, 2001; Hudak et al., 2012; ter Horst et al., 2012) and label Anafranil (2014).
Antipsychotic drug withdrawal syndrome
Antipsychotic drugs belong to multiple pharmacological groups: phenothiazines, thioxanthenes, butyrophenones and atypical antipsychotics. For these drugs, the common withdrawal symptoms are nausea, vomiting, diarrhoea, anorexia, influenza-like syndrome, rhinorrhoea, diaphoresis, myalgia, paraesthesia, anxiety, agitation, restlessness, insomnia, vertigo and tremor (Dilsaver, 1994; Borison, 1996; Shiovitz et al., 1996) and labels e.g. Clozaril February 2017, Table 1. Symptoms may appear 36–96 h or later after drug discontinuation, dose reduction or switch, depending on the drug’s duration of action and last up to 6 weeks (Chouinard et al., 2017). Of note is that during the withdrawal period, not uncommon is also psychosis rebound which results in re-emergence or worsening of psychosis (see Rebound section) or supersensitivity psychosis and occurrence of withdrawal-emergent tardive dyskinesia (Crane, 1968; Chouinard and Jones, 1980; Borison et al., 1988; Schultz et al., 1995; Margolese et al., 2005; Karas et al., 2016; Chouinard et al., 2017).
A rare, but potentially life-threatening reaction with 10% mortality rate (Strawn et al., 2007), to antipsychotic withdrawal is a neuroleptic malignant syndrome (NMS), characterized by hyperthermia, muscular rigidity, rhabdomyolysis, autonomic instability, mental status changes, diaphoresis, incontinence and elevated creatine phosphokinase (Corrigan and Coulter, 1988; Kasckow et al., 1990; Spivak et al., 1990; Amore and Zazzeri, 1995).
Anti-psychotics taken during the third trimester of pregnancy may cause neonatal withdrawal symptoms which may include agitation, tremor, somnolence, hypertonia, hypotonia, respiratory distress and feeding disorder (e.g. label Risperdal 2019; Haldol 2019, Clozaril 2017).
Levodopa-withdrawal malignant syndrome (withdrawal-emergent hyperpyrexia and confusion)
This is an uncommon, but potentially fatal complication (Sechi et al., 1984) which follows reduction or cessation of anti-parkinsonian medications, including levodopa, dopamine agonists, and even amantadine (Friedman et al., 1985; Rosse and Ciolino, 1985; Rainer et al., 1991; Mizuno et al., 2003; Serrano-Duenas, 2003; Brantley et al., 2009; Newman et al., 2009) and labels e.g. Apokyn 2004, Parlodel 2012, Sinemet 2017, Requip 2017. Typically, symptoms develop between 18 h and 7 days following anti-parkinsonian drug discontinuation. Clinical features include high fever, marked rigidity, consciousness disturbances ranging from drowsiness to coma, autonomic dysfunction such as tachycardia, perspiration, non-paralytic ileus, blood pressure fluctuation, rhabdomyolysis and elevation of serum creatinine phosphokinase. Symptoms are similar to those of NMS.
Amantadine withdrawal may precipitate NMS also in patients with other than Parkinson’s disease, namely with influenza A encephalopathy (Ito et al., 2001).
Dopamine agonist withdrawal syndrome in Parkinson’s patients
The syndrome was described by Rabinak and Niremberg (2010) in Parkinson’s disease patients who were withdrawn from long-term treatment with dopamine agonists due to the development of impulse control disorders. The syndrome presents as a constellation of neuropsychiatric and autonomic symptoms: depression, anxiety, agoraphobia, fatigue, dysphoria, irritability, agitation, pain, sleep disturbances, diaphoresis, orthostatic hypotension and drug cravings.
Nicotine/tobacco withdrawal syndrome
The nicotine withdrawal syndrome includes subsets of somatic components, cognitive, and affective components; the somatic signs include bradycardia, constipation, increased appetite and weight gain; affective symptoms include depressed mood and anhedonia, anxiety, irritability, frustration, dysphoria, emotional lability, anger, other symptoms are restlessness, insomnia, fatigue, somnolence, difficulty concentrating, sweating, confusion and craving. Tobacco/nicotine withdrawal usually begins within 24–72 h of stopping or decreasing tobacco use, peaks at 2–3 days after abstinence, and lasts 2–4 weeks (Hughes, 2007; New South Wales Government, 2008; APA, 2013; Jackson et al., 2015) and label Nicotrol 2010, Table 1.
Treatment and prevention of acute withdrawal syndrome
For the drugs with known acute withdrawal syndromes, the main treatments and prevention measures are: (i) taper at the end of treatment; (ii) after abrupt drug discontinuation immediate substitute with the drug with similar mechanism of action or reinstitute the discontinued drug, if possible, and taper much slower; (iii) treat symptomatically the dangerous or unpleasant symptoms such as seizures, psychosis, depression, headaches, vomiting, diarrhoea (Ashton, 1994; Kosten and O’Connor, 2003; O'Brien C, 2005; New South Wales Government, 2008; Chouinard and Chouinard, 2015; Chouinard et al., 2017).
The above presented list of acute withdrawal syndromes is by no means complete; it only represents better known syndromes. However, it is important to emphasize that the list of new withdrawal syndromes is growing along with new drug approvals and the identification of new withdrawal syndromes for older drugs that are frequently recognized only during the post-marketing period.
The reason for listing the above non-scheduled drugs, which are used by innumerable numbers of patients, is to point out that these drugs not only can produce dependence, withdrawal and rebound; but in some cases, these adverse events may be life-threatening, and the labels do not necessarily reflect this fact and provide necessary warnings for patients and physicians.
We provide the additional listing of acute withdrawal syndromes in the Supplementary material 2 for less frequently used drugs and/or few not CNS-active drugs although frequently used including monoamine oxidase inhibitors, gabapentin, corticosteroids, statins and aspirin.
In Table 1 are listed the most frequent and relevant acute withdrawal syndromes, rebound and protracted withdrawal syndromes.
Summary points
Majority of CNS-active drugs, scheduled and not scheduled, produce acute withdrawal syndromes.
Onset and duration of withdrawal depends on drugs’ duration of action and half-life.
The characteristic of withdrawal syndrome mainly depends on neurotransmitter system affected, and generally presents opposite symptoms to that seen during drug administration.
Part III. Rebound phenomena—definition and examples
Another aspect of withdrawal, rebound effect, also called rebound phenomenon, occurs in a similar timeframe to that of acute withdrawal. Rebound phenomenon is a rapid return of the patient’s original symptoms at a greater intensity than before the treatment. Rebound may cause in a small subset of susceptible individuals serious and fatal adverse events after abrupt drug discontinuation, such as severe psychosis after neuroleptics (Chouinard et al., 2017), severe worsening of multiple sclerosis after immunomodulatory drugs (Gonzalez-Suarez et al., 2017; Larochelle et al., 2017) or suicidality after antidepressants (Valuck et al., 2009). However, unlike protracted withdrawal syndromes rebound symptoms are transient and reversible and return after days or weeks to the baseline.
Lupolover et al. (1982) reviewed several CNS-active and non-CNS-active drugs and provided the following conclusion. ‘The survey of the medical literature with regard to the “rebound phenomenon” after withdrawal of drugs shows that it can appear after all classes of drugs, irrespective of their chemical formulation or pharmacological action. Cardiovascular drugs, diuretics, hormones, anticoagulants, antacids and neuropsychiatric drugs have been said to produce “rebound phenomena” on withdrawal’.
Emergence of rebound is variable for different drugs and may be seen in only 36–96 h for SSRIs and oral antipsychotics (Chouinard and Chouinard, 2015; Chouinard et al., 2017) and in 1–5 days for some BZ such as in patients with anxiety experiencing rebound after abrupt discontinuation of bromazepam and diazepam (Fontaine et al., 1984) and Fig. 6 and in the cases of patients with insomnia after abrupt withdrawal of triazolam (Kales et al., 1987) or lorazepam (Scharf et al., 1982).
Figure 6.
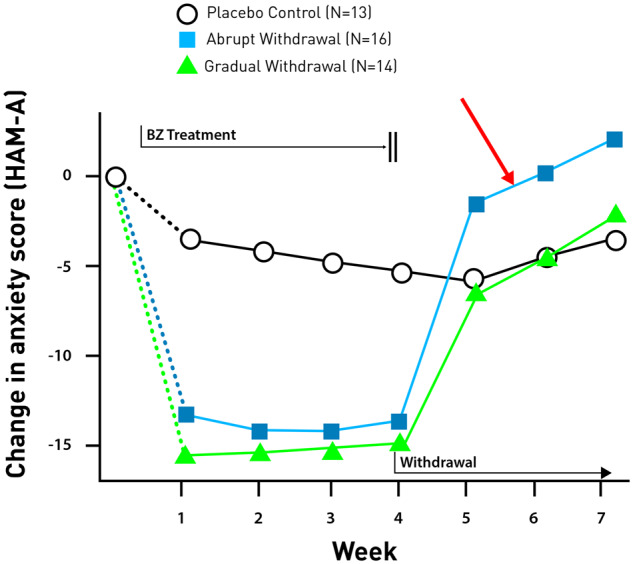
Rebound anxiety in patients treated with diazepam or bromazepam who were abruptly withdrawn. The figure shows anxiety scores (HAM-A) for three arms in the study: patients who were treated with BZ but then either discontinued drug abruptly (blue line) or discontinued drug gradually (green line), the study included also a placebo arm (black line). After abrupt drug discontinuation the symptoms of anxiety not only return to pre-treatment state, but there is also a rebound of anxiety (red arrow), that is a worsening of the symptoms above disease baseline. However, the gradual drug discontinuation does not produce the rebound and is similar to placebo arm. Adapted from Fontaine et al. (1984).
The mechanisms that underlie the occurrence of the rebound effect are not yet fully elucidated; also, for the different CNS drugs the brain mechanism implicated in rebound may differ.
Generally, rebound is explained as a consequence of down-regulation and desensitization of the receptors targeted by the therapeutic drug, where increased severity of disease symptoms is related to the lag in time for receptors to be synthesized after the decrease in receptor sites during treatment (Fontaine et al., 1984; Bhanji et al., 2006). An alternative explanation is that there is a lag in production and replacement of endogenous neurotransmitters after their production was suppressed during treatment with the drug (Kales et al., 1983b).
The treatment options for the rebound are the same as for acute withdrawal syndrome, but there is one more option, namely, the rebound symptoms also often improve rapidly after reintroduction of the discontinued drug, if it is not medically contraindicated (Chouinard and Chouinard, 2015; Chouinard et al., 2017), then the drug should be tapered very slowly.
Benzodiazepine rebound
One of the most common examples is anxiety and insomnia rebound following abrupt discontinuation of treatment with BZ. Symptoms usually appear within 1–5 days of drug discontinuation, depending on the half-life of the individual drug, and may last at least 3 days for insomnia rebound (Kales et al., 1983a, 1987; Bixler et al., 1985), and at least 2 weeks for anxiety rebound (Fontaine et al., 1984), but frequently the rebound was not evaluated for all its full duration. Patients experienced rebound insomnia after treatment with several short- and intermediate-acting BZ used for insomnia, including alprazolam, lorazepam, nitrazepam, triazolam, midazolam, flunitrazepam and lormetazepam (Kales et al., 1978, 1983a, 1987; Bixler et al., 1985; Lader and Lawson, 1987; Roehrs et al., 1992). Figure 5 shows rebound insomnia after abrupt withdrawal of treatment with triazolam (Kales et al., 1983b). However, rebound insomnia was not reported following use of long-acting BZ such as quazepam and flurazepam (Kales et al., 1982; Bixler et al., 1985).
Figure 5.
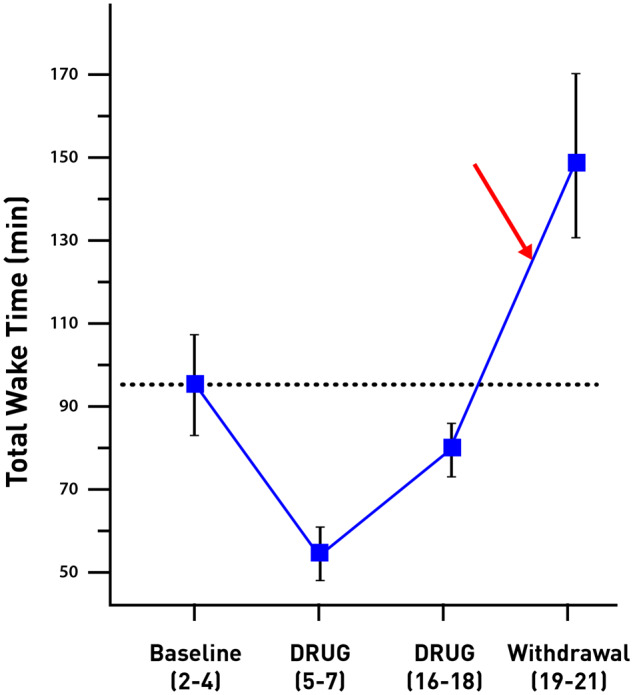
Rebound insomnia in patients treated with triazolam after abrupt drug withdrawal. The figure shows that after abrupt drug discontinuation the symptoms of insomnia (measured as total wake time) not only promptly return but there is a rebound of insomnia, that is worsening of the symptoms far above disease baseline from pre-treatment period (red arrow), in fact, this worsening is considerably greater than the maximal degree of improvement of sleep during the drug treatment. Adapted from Kales et al. (1983a).
Rebound anxiety or panic attacks resulted from abrupt withdrawal of BZ such as alprazolam, lorazepam, diazepam, clorazepate and bromazepam (Scharf et al., 1982; Fontaine et al., 1984; Pecknold et al., 1988; Rickels et al., 1988; Roy-Byrne et al., 1989; Chouinard, 2004). Anxiety patients more frequently experience rebound with short- and intermediate-actin BZ than long-acting (Chouinard, 2004).
Rebound insomnia was also reported for a non-BZ hypnotic drug, zolpidem (Voshaar et al., 2004).
Following is an example of rebound anxiety after withdrawal of BZ in patients with generalized anxiety disorder treated successfully with diazepam or bromazepam (Fontaine et al., 1984) and Fig. 6. The patients were treated for 4 weeks with BZ, then one group was abruptly withdrawn, and the second group gradually withdrawn. The scores of patients withdrawn abruptly increased 10% or more on both the Hamilton Rating Scale for Anxiety and the Self Rating Symptom Scale. In patients with rebound anxiety, previous anxiety symptoms returned and were more severe than before treatment, beginning in some cases within 24 h of drug withdrawal.
Also, Z-drugs such as zopiclone were reported to cause rebound insomnia (Soldatos et al., 1999; Tsutsui and Zolipidem Study Group, 2001; Cimolai, 2007).
Stimulant rebound
Stimulants such as methylphenidate and amphetamine may cause rebound in children with attention-deficit/hyperactivity disorder, which can include serious and consistent deterioration of behaviour manifested in sadness, crying, emotional lability, irritability, social withdrawal, restlessness, distractibility, belligerence, excitability, talkativeness, euphoria and insomnia, emerging after the last stimulant dose has worn off (Rapoport et al., 1978; Porrino et al., 1983; Smucker and Hedayat, 2001; Carlson and Kelly, 2003; Connor, 2015). In fact, up to one-third of children taking methylphenidate for attention-deficit/hyperactivity disorder experience a rebound as interdose recurrence of symptoms in the late afternoon when the medication wears off and symptoms of attention-deficit/hyperactivity disorder, such as irritability and non-compliance, return (Riccio et al., 2001); however, the total duration is not known as the next dose of drug is given; in more severe cases the medication is discontinued.
Selective serotonin reuptake inhibitor’s rebound
Rebound involves the return of symptoms of the treated disorder, but at greater intensity. These include: anxiety, panic, agitation, insomnia, depression, obsessions and compulsions (Bhanji et al., 2006; Chouinard and Chouinard, 2015). Generally, peak of onset is 36–96 h which depends mainly on drug duration of action, whereas the duration can be up to 6 weeks, which depends on drug elimination half-life (Chouinard and Chouinard, 2015).
Antipsychotic rebound
Rebound psychosis, was noted not only for many classic antipsychotics, such as haloperidol; but also, atypical antipsychotics such as quetiapine and clozapine. Rebound psychosis may include positive symptoms from pre-treatment level, however, at greater severity: illusions, hallucinations, delusions, catatonia, Schneider passivity experiences (Borison et al., 1988; Kahne, 1989; Margolese et al., 2002; Chouinard et al., 2017). The peak of onset is 36–96 h after drug discontinuation or dose reduction and may last up to 6 weeks depending on drug elimination (Chouinard et al., 2017).
Immunomodulatory drug rebound
Recently, a new rebound syndrome was identified for immunomodulatory drugs indicated for treatment of multiple sclerosis, namely Gilenya (fingolimod) and natalizumab. The abrupt withdrawal of these drugs produces severe, sometimes life-threatening or fatal rebound in multiple sclerosis patients, accompanied by drastic increases in new lesions or enhancing lesions seen on MRI (Sorensen et al., 2014; Faissner et al., 2015; Hatcher et al., 2016; Czlonkowska et al., 2017; Gunduz et al., 2017; Larochelle et al., 2017).
Summary points
Rebound reflects drug’s ability to affect organism’s neuroadaptation expressed as tolerance and dependence which are exposed during drug discontinuation; thus, rebound is more severe for drugs which produce tolerance and dependence within a short time and to a higher degree.
Rebound coincides in time with acute withdrawal syndrome but is differentiated from it by the fact that represents the symptom of the underlying disorder but of the greater severity than before the treatment, whereas acute withdrawal represents a new symptom.
Rebound is of relatively short duration and self-terminating, although at times may be severe and life-threatening.
Part IV. Protracted withdrawal syndrome—definition and examples
Protracted withdrawal also called post-acute withdrawal syndrome (PAWS), persistent post-withdrawal syndrome, and prolonged withdrawal syndrome, describes a set of persistent impairments that occur after withdrawal from some drug classes, such as BZ, opioids, antipsychotics and antidepressants. Symptoms of protracted withdrawal frequently appear later, well past the expected timeframe for acute withdrawal, last longer, and sometimes are irreversible. Because many names are used to describe protracted withdrawal symptoms, which makes identification confusing at times, we present a list of all known to date names of this syndrome, compiled by the Substance Abuse and Mental Health Services Administration (SAMHSA, 2010).
Chronic withdrawal
Extended withdrawal
Late withdrawal
Long-term withdrawal
Persistent post-use symptoms
Post-acute withdrawal syndrome
Post-use syndrome
Protracted abstinence
Sobriety-based symptoms
Subacute withdrawal
The most characteristic feature of this disorder is its duration, sometimes persisting for weeks and months after cessation of the drug; additionally, new disorders may emerge.
Protracted withdrawal syndrome is not only characteristic of drugs of abuse but also was described for non-scheduled drug classes, such as SSRIs and antipsychotics. In this study, we present some known examples of post-acute withdrawal syndromes.
Scheduled drugs
Opioid protracted withdrawal syndrome
Protracted withdrawal symptoms related to opioid withdrawal include: opioid cravings, memory problems, inability to think clearly, fatigue, anxiety, depression, dysphoria, irritability, sleep disturbances, insomnia, palpitations, restlessness, periodic diarrhoea and hypomania (Kolb, 1928; Satel et al., 1993; SAMHSA, 2010). Some symptoms, such as fatigue, insomnia and restlessness can last for weeks or months following withdrawal from opioids, in some cases, 6–9 months.
Some opioid drugs may produce physiological protracted withdrawal symptoms. During abrupt morphine withdrawal, Martin and Jasinski (1969) observed after the acute withdrawal phase occurrence of a protracted or secondary phase which usually began to emerge between the sixth and ninth weeks after drug withdrawal and persisted through the weeks 26–30. This phase was characterized by decreased blood pressure, heart rate and body temperature, as well as miosis and hyposensitivity of the respiratory centre to CO2.
Benzodiazepine protracted withdrawal syndrome
Protracted withdrawal symptoms include: anxiety, depression, psychotic reactions, memory impairment, motor symptoms (muscle jerking, blepharospasm), paraesthesia, formication, tinnitus and irritable bowel syndrome, and there is a characteristic fluctuation of symptoms that may wax and wane (Smith and Wesson, 1983; Ashton, 1991; SAMHSA, 2010). Generally, the symptoms of protracted withdrawal start ∼4–6 weeks after drug discontinuation and may last 6–12 months, but some symptoms, such as anxiety, may persist for up to 2 years (Ashton, 1991).
Stimulants protracted withdrawal
Protracted withdrawal sometimes called the extinction phase is characterized by symptoms such as fluctuations in mood and energy levels, irritability, restlessness, anxiety, agitation, fatigue, lacking energy and anhedonia, cravings and disturbed sleep and this phase may last for weeks to months (New South Wales Government, 2008).
Non-scheduled drugs
SSRIs and SNRIs protracted withdrawal syndrome
Protracted withdrawal symptoms include: tardive insomnia, major depression, bipolar disorder, panic disorder, agitation, anxiety disorders and panic attacks, emotional lability, mood swings, irritability, aggression, impaired concentration, impaired memory and pathological gambling. These disorders can last for several months to years when the previous drug treatment is not restarted (Bhanji et al., 2006; Belaise et al., 2012, 2014; Chouinard and Chouinard, 2015).
Antipsychotic protracted withdrawal syndrome
Main protracted withdrawal symptoms for antipsychotics include tardive dyskinesia and supersensitivity psychosis (Crane, 1968; Chouinard and Jones, 1980; Margolese et al., 2005; Chouinard and Chouinard, 2008; Oda et al., 2015; Chouinard et al., 2017; Nakata et al., 2017). The peak of onset is 24 h to 6 weeks after drug discontinuation or dose reduction or switch and protracted withdrawal may last several months (Chouinard et al., 2017).
Summary points
Protracted withdrawal is a relatively less known aspect of dependence and withdrawal; however, its duration and severity affect the ability of subjects to terminate therapeutic drugs and drugs of abuse and addiction.
Protracted withdrawal may represent very slowly reversible or permanent pathological changes in the neurocircuitry.
Part V: Evaluation of drug dependence in clinical studies—regulatory considerations
Human dependence study—design and considerations
Evaluation of dependence, withdrawal and rebound for new drugs being developed is of great importance, as it provides information related to potential drug scheduling and safe use. The evaluation provides critically important safety information for patients and physicians, especially in cases when the drug must be abruptly withdrawn due to serious, life-threatening adverse events.
It is probably important to note here that for regulatory purposes an animal dependence study is not an alternative to a human dependence evaluation. It is only a first step, one which is important for gathering information about potentially serious adverse events of withdrawal, such as convulsions.
The recommended design of a clinical dependence study contains consideration of the following components: population, design, statistical considerations, dose, pharmacodynamic and pharmacokinetic assessments, evaluation of rebound, adverse events collection and analysis. All listed technical details and considerations are available as the Supplementary material 1.
Discussion
Major issues in the evaluation of dependence and withdrawal involve the definitions and nomenclature currently used. Both can possibly contribute to misunderstanding and confusion. This particularly affects different authors’ naming conventions for acute withdrawal syndrome, and even more so, for protracted withdrawal syndrome.
In general, the authors consider the nomenclature of withdrawal syndrome and the time course of withdrawal phenomena, presented by Chouinard and Chouinard (2015) to be clear and comprehensive. This nomenclature and the presentation of the different aspects of withdrawal phenomena could be adapted to describe withdrawal from other drugs and drug classes.
It is important to note that some drugs and drug classes were not mentioned in this short review. This does not necessarily mean that these drugs do not produce a withdrawal syndrome, it may simply mean that withdrawal has never been evaluated and described.
Conclusions
As discussed above, the evaluation of dependence and withdrawal is critically important for CNS-active drugs, which are likely to be scheduled. However, the withdrawal evaluation is also important for non-scheduled CNS-active drugs, mainly for safety reasons. Reidenberg (2011) summarizing issues related to drug dependence and withdrawal, stated: ‘Drug discontinuation effects can occur and are usually neglected in pharmacology and medicine until adverse clinical events force them to be noticed’. It is vital that dependence, withdrawal and rebound be recognized and understood by healthcare practitioners, because these effects may constitute major safety issues. The data on acute withdrawal symptoms and rebound obtained from the study and evaluation of dependence will provide physicians and patients with information on drug tapering and constitute a warning about possible effects related to abrupt drug discontinuation (Kosten and O’Connor, 2003).
Except for a few drug groups comprising highly addictive drugs, such as opioids, BZ and stimulants; and non-scheduled drugs such as SSRIs, there is relatively little knowledge of different aspects of the withdrawal phenomena for most currently used drugs. But even for these drug classes mentioned above, product labels often do not always reflect all current information available in the scientific literature and fail to provide adequate warnings for patients and physicians.
The evaluation of dependence and withdrawal for drugs in paediatric population is very important and should be also addressed in the future. The current available data are more than sparse and only available for some opioid and BZ drugs and some stimulants. Also, the sex differences in the withdrawal symptomatology should be further explored as the data for many scheduled and unscheduled drug is not available.
This is the authors’ hope that future drug development will incorporate many of the methods described in this review as the knowledge of the withdrawal symptoms is critical for patients’ safety. Also, equally important is incorporating knowledge on withdrawal and dependence in the drug labels.
Supplementary Material
Acknowledgements
The authors thank Joanne M. Berger, FDA Lead Librarian, and Vivianna P. Cowl, FDA Project Manager, for proofreading the manuscript, FDA, CDER-Designz visual information specialists Geninne John-Crosland and Joshua Budich for adapting and creating the figures and FDA ORISE Fellow Sasha Farley, BS, for editorial work.
Disclaimer
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies. Contact FDA to obtain the most recent and updated recommendations regarding evaluation of drug dependence, withdrawal and rebound. The information provided here is not intended to replace the pertinent FDA Guidance for Industry, which is updated continuously.
Competing interests
Dr. A.L. is employed by the FDA and has no competing interests to disclose. Dr. M.K. provides consulting services to companies on drugs that affect the central nervous system. Dr. M.K. is Principal of Controlled Substance Scientific Solutions, LLC and Member of the Scientific Advisory Board, Analgesic Solutions LLC. He has been a consultant for INC Research, IQVIA, Cote Orphan LLC, and Pinney Associates.
Glossary
- ARSW
Adjective Rating Scale for Withdrawal
- ASAM
American Society of Addiction Medicine
- BZ
benzodiazepines
- CB1
cannabinoid 1 receptors
- COWS
Clinical Opiate Withdrawal Scale
- DSM-5
diagnostic and statistical manual of mental disorders, fifth edition
- FDA
Food and Drug Administration
- INF
information not found
- MAOIs
monoamine oxidase inhibitors
- NMS
neuroleptic malignant syndrome
- NAS
neonatal abstinence syndrome
- SSRIs
selective serotonin reuptake inhibitors
- SNRIs
serotonin–norepinephrine reuptake inhibitors
- THC
Δ9-tetrahydrocannabinol
- VAS
Visual Analogue Scale
References
- Agrawal A, Pergadia ML, Lynskey MT.. Is there evidence for symptoms of cannabis withdrawal in the national epidemiologic survey of alcohol and related conditions? Am J Addict 2008; 17: 199–208. [DOI] [PubMed] [Google Scholar]
- Amore M, Zazzeri N.. Neuroleptic malignant syndrome after neuroleptic discontinuation. Prog Neuropsychopharmacol Biol Psychiatry 1995; 19: 1323–34. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Ingraham J.. Pediatric. Tolerance, dependence, and strategies for compassionate withdrawal of analgesics and anxiolytics in the pediatric ICU. Crit Care Nurse 1996; 16: 87–93. [PubMed] [Google Scholar]
- Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J.. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics 2010; 125: e1208–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders, 5th Edition: DSM-5. 5th edn.Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Aragona M. Abuse, dependence, and epileptic seizures after zolpidem withdrawal: review and case report. Clin Neuropharmacol 2000; 23: 281–3. [DOI] [PubMed] [Google Scholar]
- Ashton H. Protracted withdrawal syndromes from benzodiazepines. J Subst Abuse Treat 1991; 8: 19–28. [DOI] [PubMed] [Google Scholar]
- Ashton H. The treatment of benzodiazepine dependence. Addiction 1994; 89: 1535–41. [DOI] [PubMed] [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, et al. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse 2011; 37: 313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A, Lee NK, Jenner L, editors. Models of intervention and care for psychostimulant users In: Psychostimulant withdrawal and detoxification. 2nd edn, Chap. 7, National Drug Strategy Monograph Series No. 51. Canberra: Australian Government Department of Health and Ageing, p. 102–19. [Google Scholar]
- Belaise C, Gatti A, Chouinard VA, Chouinard G.. Patient online report of selective serotonin reuptake inhibitor-induced persistent postwithdrawal anxiety and mood disorders. Psychother Psychosom 2012; 81: 386–8. [DOI] [PubMed] [Google Scholar]
- Belaise C, Gatti A, Chouinard VA, Chouinard G.. Persistent postwithdrawal disorders induced by paroxetine, a selective serotonin reuptake inhibitor, and treated with specific cognitive behavioral therapy. Psychother Psychosom 2014; 83: 247–8. [DOI] [PubMed] [Google Scholar]
- Bhanji NH, Chouinard G, Kolivakis T, Margolese HC.. Persistent tardive rebound panic disorder, rebound anxiety and insomnia following paroxetine withdrawal: a review of rebound-withdrawal phenomena. Can J Clin Pharmacol 2006; 13: e69–74. [PubMed] [Google Scholar]
- Bixler EO, Kales JD, Kales A, Jacoby JA, Soldatos CR.. Rebound insomnia and elimination half-life: assessment of individual subject response. J Clin Pharmacol 1985; 25: 115–24. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Lammers GJ, Roofthooft DW, De Beaufort AJ, Brouwer OF.. Clomipramine withdrawal in newborns. Arch Dis Child Fetal Neonatal Ed 1999; 81: F77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean SAM, Kokane SS, Butler BD, Perrotti LI.. Sex differences in the expression of morphine withdrawal symptoms and associated activity in the tail of the ventral tegmental area. Neurosci Lett 2019; 705: 124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet U, Preuss UW.. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil 2017; 8: 9–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borison RL. Changing antipsychotic medication: guidelines on the transition to treatment with risperidone. The Consensus Study Group on Risperidone Dosing. Clin Ther 1996; 18: 592–607; discussion 591. [DOI] [PubMed] [Google Scholar]
- Borison RL, Diamond BI, Sinha D, Gupta RP, Ajiboye PA.. Clozapine withdrawal rebound psychosis. Psychopharmacol Bull 1988; 24: 260–3. [PubMed] [Google Scholar]
- Brantley E, Cohn J, Babu K.. Case files of the program in medical toxicology at brown university: amantadine withdrawal and the neuroleptic malignant syndrome. J Med Toxicol 2009; 5: 92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatry Rep 2002; 4: 377–87. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Anabolic steroid abuse and dependence in clinical practice. Phys Sportsmed 2009; 37: 131–40. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR.. The cannabis withdrawal syndrome. Curr Opin Psychiatry 2006; 19: 233–8. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R.. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 2004; 161: 1967–77. [DOI] [PubMed] [Google Scholar]
- Busto UE, Sellers EM.. Anxiolytics and sedative/hypnotics dependence. Br J Addict 1991; 86: 1647–52. [DOI] [PubMed] [Google Scholar]
- Carlson GA, Kelly KL.. Stimulant rebound: how common is it and what does it mean? J Child Adolesc Psychopharmacol 2003; 13: 137–42. [DOI] [PubMed] [Google Scholar]
- Ceccherini-Nelli A, Bardellini L, Cur A, Guazzelli M, Maggini C, Dilsaver SC.. Antidepressant withdrawal: prospective findings. Am J Psychiatry 1993; 150: 165. [DOI] [PubMed] [Google Scholar]
- Chartier KG, Sanchez K, Killeen TK, Burrow A, Carmody T, Greer TL.. Men and women from the STRIDE clinical trial: an assessment of stimulant abstinence symptom severity at residential treatment entry. Am J Addict 2015; 24: 336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay AC, Shukla L, Kandasamy A, Benegal V.. High-dose zolpidem dependence—psychostimulant effects? A case report and literature review. Ind Psychiatry J 2016; 25: 222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Huang MC, Lin SK.. Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst Abuse Treat Prev Policy 2014; 9: 39.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard G. Issues in the clinical use of benzodiazepines: potency, withdrawal, and rebound. J Clin Psychiatry 2004; 65 (Suppl 5): 7–12. [PubMed] [Google Scholar]
- Chouinard G, Chouinard VA.. Atypical antipsychotics: CATIE study, drug-induced movement disorder and resulting iatrogenic psychiatric-like symptoms, supersensitivity rebound psychosis and withdrawal discontinuation syndromes. Psychother Psychosom 2008; 77: 69–77. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Chouinard VA.. New classification of selective serotonin reuptake inhibitor withdrawal. Psychother Psychosom 2015; 84: 63–71. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Jones BD.. Neuroleptic-induced supersensitivity psychosis: clinical and pharmacologic characteristics. Am J Psychiatry 1980; 137: 16–21. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Samaha AN, Chouinard VA, Peretti CS, Kanahara N, Takase M, et al. Antipsychotic-induced dopamine supersensitivity psychosis: pharmacology, criteria, and therapy. Psychother Psychosom 2017; 86: 189–219. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER.. Gender-linked differences in the expression of physical dependence in the rat. Pharmacol Biochem Behav 2002; 72: 691–7. [DOI] [PubMed] [Google Scholar]
- Cimolai N. Zopiclone: is it a pharmacologic agent for abuse? Can Fam Physician 2007; 53: 2124–9. [PMC free article] [PubMed] [Google Scholar]
- Colon-Berezin C, Nolan ML, Blachman-Forshay J, Paone D.. Overdose deaths involving fentanyl and fentanyl analogs—New York City, 2000–2017. MMWR Morb Mortal Wkly Rep 2019; 68: 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor DF. Stimulant and nonstimulant medications for childhood ADHD In: Barkley RA, editor. Attention-deficit hyperactivity disorder, a handbook for diagnosis and treatment. 4th edn.The Guilford Press; 2015. p. 666–85. [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, et al. Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. Am J Drug Alcohol Abuse 2010; 36: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan FM, Coulter F.. Neuroleptic malignant syndrome, amitriptyline, and thioridazine. Biol Psychiatry 1988; 23: 320–1. [DOI] [PubMed] [Google Scholar]
- Cowe L, Lloyd DJ, Dawling S.. Neonatal convulsions caused by withdrawal from maternal clomipramine. Br Med J (Clin Res Ed) 1982; 284: 1837–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen PJ, Nutt DJ.. Abstinence symptoms after withdrawal of tranquillising drugs: is there a common neurochemical mechanism? Lancet 1982; 2: 360–2. [DOI] [PubMed] [Google Scholar]
- Crane GE. Tardive dyskinesia in schizophrenic patients treated with psychotropic drugs. Agressologie 1968; 9: 209–18. [PubMed] [Google Scholar]
- Critchlow DG. A case of ketamine dependence with discontinuation symptoms. Addiction 2006; 101: 1212–3. [DOI] [PubMed] [Google Scholar]
- Cubala WJ, Landowski J.. Seizure following sudden zolpidem withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 539–40. [DOI] [PubMed] [Google Scholar]
- Cuttler C, Mischley LK, Sexton M.. Sex differences in cannabis use and effects: a cross-sectional survey of cannabis users. Cannabis Cannabinoid Res 2016; 1: 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czlonkowska A, Smolinski L, Litwin T.. Severe disease exacerbations in patients with multiple sclerosis after discontinuing fingolimod. Neurol Neurochir Pol 2017; 51: 156–62. [DOI] [PubMed] [Google Scholar]
- Desmond MM, Schwanecke RP, Wilson GS, Yasunaga S, Burgdorff I.. Maternal barbiturate utilization and neonatal withdrawal symptomatology. J Pediatr 1972; 80: 190–7. [DOI] [PubMed] [Google Scholar]
- Dilsaver SC. Heterocyclic antidepressant, monoamine oxidase inhibitor and neuroleptic withdrawal phenomena. Prog Neuropsychopharmacol Biol Psychiatry 1990; 14: 137–61. [DOI] [PubMed] [Google Scholar]
- Dilsaver SC. Withdrawal phenomena associated with antidepressant and antipsychotic agents. Drug Saf 1994; 10: 103–14. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA 2016; 315: 1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Noonan RK, Houry D.. Underlying factors in drug overdose deaths. JAMA 2017; 318: 2295–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essig CF. XIV. Addiction to barbiturate and nonbarbiturate sedative drugs. Res Publ Assoc Res Nerv Ment Dis 1968; 46: 188–98. [PubMed] [Google Scholar]
- Faissner S, Hoepner R, Lukas C, Chan A, Gold R, Ellrichmann G.. Tumefactive multiple sclerosis lesions in two patients after cessation of fingolimod treatment. Ther Adv Neurol Disord 2015; 8: 233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkner P, Petersen N, Ghahremani DG, Cox CM, Tyndale RF, Hellemann GS, et al. Sex differences in tobacco withdrawal and responses to smoking reduced-nicotine cigarettes in young smokers. Psychopharmacology (Berl). 2018; 235: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava GA, Gatti A, Belaise C, Guidi J, Offidani E.. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychother Psychosom 2015; 84: 72–81. [DOI] [PubMed] [Google Scholar]
- Fisher D, Grap MJ, Younger JB, Ameringer S, Elswick RK.. Opioid withdrawal signs and symptoms in children: frequency and determinants. Heart Lung 2013; 42: 407–13. [DOI] [PubMed] [Google Scholar]
- Fontaine R, Chouinard G, Annable L.. Rebound anxiety in anxious patients after abrupt withdrawal of benzodiazepine treatment. Am J Psychiatry 1984; 141: 848–52. [DOI] [PubMed] [Google Scholar]
- Fraser HF, Shaver MR, Maxwell ES, Isbell H.. Death due to withdrawal of barbiturates. Ann Intern Med 1953; 38: 1319–25. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Feinberg SS, Feldman RG.. A neuroleptic malignantlike syndrome due to levodopa therapy withdrawal. JAMA 1985; 254: 2792–5. [PubMed] [Google Scholar]
- Garner EM, Kelly MW, Thompson DF.. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother 1993; 27: 1068–72. [DOI] [PubMed] [Google Scholar]
- Gault FP. A review of recent literature on barbiturate addiction and withdrawal. Bol Estud Med Biol 1976; 29: 75–83. [PubMed] [Google Scholar]
- Gonzalez-Suarez I, Rodriguez de Antonio L, Orviz A, Moreno-Garcia S, Valle-Arcos MD, Matias-Guiu JA, et al. Catastrophic outcome of patients with a rebound after Natalizumab treatment discontinuation. Brain Behav 2017; 7: e00671.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Levin KH, Copersino ML, Heishman SJ, Liu F, Boggs DL, et al. Diagnostic criteria for cannabis withdrawal syndrome. Drug Alcohol Depend 2012; 123: 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham C, LoVecchio F.. Barbiturates In: Tintinalli JE, editor. Tintinalli’s emergency medicine: a comprehensive study guide. 8th edn.Chap. 182, Mc Graw-Hill Education; 2015, 1–11. [Google Scholar]
- Gunduz T, Kurtuncu M, Eraksoy M.. Severe rebound after withdrawal of fingolimod treatment in patients with multiple sclerosis. Mult Scler Relat Disord 2017; 11: 1–3. [DOI] [PubMed] [Google Scholar]
- Gussow L, Carlson A.. Sedative hypnotics In: Marx JA, Hockberger R, Walls RM, Biros MH, editors. Rosen’s emergency medicine: Concepts and clinical practice. 8th edn Chap. 165, Philadelphia, PA: Elsevier Saunders; 2013. p. 2076–83. [Google Scholar]
- Haddad PM. Antidepressant discontinuation syndromes. Drug Saf 2001; 24: 183–97. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Perry ME, Berger SS, Grunberg NE.. Behavioral effects of nicotine withdrawal differ by genetic strain in male and female adolescent rats. Nicotine Tob Res 2010; 12: 1236–45. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD.. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse 1987; 13: 293–308. [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Dow-Edwards DL.. Withdrawal from THC during adolescence: sex differences in locomotor activity and anxiety. Behav Brain Res 2012; 231: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS.. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol 2016; 73: 790–4. [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Weerts EM, Vandrey R.. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol 2015; 23: 415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himei A, Okamura T.. Discontinuation syndrome associated with paroxetine in depressed patients: a retrospective analysis of factors involved in the occurrence of the syndrome. CNS Drugs 2006; 20: 665–72. [DOI] [PubMed] [Google Scholar]
- Himmelsbach CK. Studies of certain addiction characteristics of (a) dihydromorphine (“Paramorphan”); (b) dihydrodesoxymorphineD (“Desomorphine”); (c) dihydrodesoxycodeine (“Desocodeine”) and (d) methyldihydromorphinone (“Metopon”). Pharmacol Exp Ther 1939; 67: 239–2. [Google Scholar]
- Hollister LE, Bennett JL, Kimbell I Jr, Savage C, Overall JE.. Diazepam in newly admitted schizophrenics. Dis Nerv Syst 1963; 24: 746–50. [PubMed] [Google Scholar]
- Hollister LE, Motzenbecker FP, Degan RO.. Withdrawal reactions from chlordiazepoxide (“Librium”). Psychopharmacologia 1961; 2: 63–8. [DOI] [PubMed] [Google Scholar]
- Hudak ML, Tan RC; Committee on Drugs, Committee on Fetus and Newborn, American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics 2012; 129: e540–60. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 2007; 9: 315–27. [DOI] [PubMed] [Google Scholar]
- Hurt PH, Ritchie EC.. A case of ketamine dependence. Am J Psychiatry 1994; 151: 779.. [DOI] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ.. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci 1983; 33 (Suppl 1): 773–6. [DOI] [PubMed] [Google Scholar]
- Iqbal MM, Sobhan T, Ryals T.. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr Serv 2002; 53: 39–49. [DOI] [PubMed] [Google Scholar]
- Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M.. Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: a first evaluation. Crit Care Med 2008; 36: 2427–32. [DOI] [PubMed] [Google Scholar]
- Ito T, Shibata K, Watanabe A, Akabane J.. Neuroleptic malignant syndrome following withdrawal of amantadine in a patient with influenza A encephalopathy. Eur J Pediatr 2001; 160: 401. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Muldoon PP, De Biasi M, Damaj MI.. New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology 2015; 96: 223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen KL, Darracot-Cankovic R.. The nonmedical use of ketamine, part two: a review of problem use and dependence. J Psychoactive Drugs 2001; 33: 151–8. [DOI] [PubMed] [Google Scholar]
- Kahne GJ. Rebound psychoses following the discontinuation of a high potency neuroleptic. Can J Psychiatry 1989; 34: 227–9. [DOI] [PubMed] [Google Scholar]
- Kales A, Bixler EO, Soldatos CR, Vela-Bueno A, Jacoby J, Kales JD.. Quazepam and flurazepam: long-term use and extended withdrawal. Clin Pharmacol Ther 1982; 32: 781–8. [DOI] [PubMed] [Google Scholar]
- Kales A, Bixler EO, Vela-Bueno A, Soldatos CR, Manfredi RL.. Alprazolam: effects on sleep and withdrawal phenomena. J Clin Pharmacol 1987; 27: 508–15. [DOI] [PubMed] [Google Scholar]
- Kales A, Scharf MB, Kales JD.. Rebound insomnia: a new clinical syndrome. Science 1978; 201: 1039–41. [DOI] [PubMed] [Google Scholar]
- Kales A, Soldatos CR, Bixler EO, Goff PJ, Vela-Bueno A.. Midazolam: dose-response studies of effectiveness and rebound insomnia. Pharmacology 1983a; 26: 138–49. [DOI] [PubMed] [Google Scholar]
- Kales A, Soldatos CR, Bixler EO, Kales JD.. Rebound insomnia and rebound anxiety: a review. Pharmacology 1983b; 26: 121–37. [DOI] [PubMed] [Google Scholar]
- Kamaya H, Krishna PR.. Ketamine addiction. Anesthesiology 1987; 67: 861–2. [DOI] [PubMed] [Google Scholar]
- Kan CC, Hilberink SR, Breteler MH.. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry 2004; 45: 88–94. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG Jr.. Anabolic-androgenic steroid dependence: an emerging disorder. Addiction 2009; 104: 1966–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas H, Guduk M, Saatcioglu O.. Withdrawal-emergent dyskinesia and supersensitivity psychosis due to olanzapine use. Arch Neuropsychiatr 2016; 53: 178–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariisa M, Scholl L, Wilson N, Seth P, Hoots B.. Drug overdose deaths involving cocaine and psychostimulants with abuse potential—United States, 2003–2017. MMWR Morb Mortal Wkly Rep 2019; 68: 388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasckow J, Dolor R, Lipper S.. Delayed onset of neuroleptic malignant syndrome after discontinuation of thioridazine. J Clin Psychopharmacol 1990; 10: 146. [DOI] [PubMed] [Google Scholar]
- Kocherlakota P. Neonatal abstinence syndrome. Pediatrics 2014; 134: e547–61. [DOI] [PubMed] [Google Scholar]
- Kolb L. Clinical contribution to drug addiction: the struggle for cure and the conscious reasons for relapse. Edinb Med J 1928; 35: 740–1. [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M.. Addiction and the brain antireward system. Annu Rev Psychol 2008a; 59: 29–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M.. Neurobiological mechanisms for opponent motivational processes in addiction. Phil Trans R Soc B 2008b; 363: 3113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koons AL, Rayl Greenberg M, Cannon RD, Beauchamp GA.. Women and the experience of pain and opioid use disorder: a literature-based commentary. Clin Ther 2018; 40: 190–6. [DOI] [PubMed] [Google Scholar]
- Koren G, Boucher N.. Adverse effects in neonates exposed to SSRIs and SNRI in late gestation–Motherisk Update 2008. Can J Clin Pharmacol 2009; 16: e66–7. [PubMed] [Google Scholar]
- Kosten TR, O’Connor PG.. Management of drug and alcohol withdrawal. N Engl J Med 2003; 348: 1786–95. [DOI] [PubMed] [Google Scholar]
- Kruse WH. Problems and pitfalls in the use of benzodiazepines in the elderly. Drug Saf 1990; 5: 328–44. [DOI] [PubMed] [Google Scholar]
- Lader M. Anxiety or depression during withdrawal of hypnotic treatments. J Psychosom Res 1994; 38 (Suppl 1): 113–23; discussion 118–23. [DOI] [PubMed] [Google Scholar]
- Lader M, Lawson C.. Sleep studies and rebound insomnia: methodological problems, laboratory findings, and clinical implications. Clin Neuropharmacol 1987; 10: 291–312. [DOI] [PubMed] [Google Scholar]
- Larochelle C, Metz I, Lécuyer M-A, Terouz S, Roger M, Arbour N, et al. Immunological and pathological characterization of fatal rebound MS activity following natalizumab withdrawal. Mult Scler 2017; 23: 72–81. [DOI] [PubMed] [Google Scholar]
- Lebin LG, Cerimele JM.. Recurrent benzodiazepine withdrawal catatonia in an older adult. Am J Psychiatry 2017; 174: 1001–2. [DOI] [PubMed] [Google Scholar]
- Leiter FL, Nierenberg AA, Sanders KM, Stern TA.. Discontinuation reactions following sertraline. Biol Psychiatry 1995; 38: 694–5. [DOI] [PubMed] [Google Scholar]
- Lemesle F, Lemesle F, Nicola W, Pierre Jonville-Bera A.. First case of stress cardiomyopathy as a result of methadone withdrawal secondary to drug-drug interaction. Am J Emerg Med 2010; 28: 387.e5–6. [DOI] [PubMed] [Google Scholar]
- Light AB, Torrence EG.. Opium addiction. Arch Intern Med (Chic) 1929; 44: 1–16. [Google Scholar]
- Lim DK. Ketamine associated psychedelic effects and dependence. Singapore Med J 2003; 44: 31–4. [PubMed] [Google Scholar]
- Lupolover R, Dazzi H, Ward J.. Rebound phenomena: results of a 10 years’ (1970–1980) literature review. Int Pharmacopsychiatry 1982; 17: 194–237. [DOI] [PubMed] [Google Scholar]
- MacKinnon GL, Parker WA.. Benzodiazepine withdrawal syndrome: a literature review and evaluation. Am J Drug Alcohol Abuse 1982; 9: 19–33. [DOI] [PubMed] [Google Scholar]
- Margolese HC, Chouinard G, Beauclair L, Belanger MC.. Therapeutic tolerance and rebound psychosis during quetiapine maintenance monotherapy in patients with schizophrenia and schizoaffective disorder. J Clin Psychopharmacol 2002; 22: 347–52. [DOI] [PubMed] [Google Scholar]
- Margolese HC, Chouinard G, Kolivakis TT, Beauclair L, Miller R.. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatry 2005; 50: 541–7. [DOI] [PubMed] [Google Scholar]
- Martin WR, Jasinski DR.. Physiological parameters of morphine dependence in man–tolerance, early abstinence, protracted abstinence. J Psychiatr Res 1969; 7: 9–17. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL.. Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend 2014; 137: 20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medras M, Tworowska U. [Treatment strategies of withdrawal from long-term use of anabolic-androgenic steroids]. Pol Merkur Lekarski 2001; 11: 535–8. [PubMed] [Google Scholar]
- Mizuno Y, Takubo H, Mizuta E, Kuno S.. Malignant syndrome in Parkinson’s disease: concept and review of the literature. Parkinsonism Relat Disord 2003; 9 (Suppl 1): S3–9. [DOI] [PubMed] [Google Scholar]
- Nakata Y, Kanahara N, Iyo M.. Dopamine supersensitivity psychosis in schizophrenia: concepts and implications in clinical practice. J Psychopharmacol 2017; 31: 1511–8. [DOI] [PubMed] [Google Scholar]
- New South Wales Government. Drug and alcohol withdrawal clinical practice guidelines—NSW In: Ministry of Health NG, AU, editor. Sydney, NSW: NSW Department of Health; 2008. [Google Scholar]
- Newman EJ, Grosset DG, Kennedy PG.. The parkinsonism-hyperpyrexia syndrome. Neurocrit Care 2009; 10: 136–40. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Hansen EH, Gotzsche PC.. What is the difference between dependence and withdrawal reactions? A comparison of benzodiazepines and selective serotonin re-uptake inhibitors. Addiction 2012; 107: 900–8. [DOI] [PubMed] [Google Scholar]
- Noyes R Jr, Garvey MJ, Cook B, Suelzer M.. Controlled discontinuation of benzodiazepine treatment for patients with panic disorder. Am J Psychiatry 1991; 148: 517–23. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL.. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry 2001; 179: 390–6. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Benzodiazepine use, abuse, and dependence. J Clin Psychiatry 2005; 66 (Suppl 2): 28–33. [PubMed] [Google Scholar]
- Oda Y, Kanahara N, Iyo M.. Alterations of dopamine D2 receptors and related receptor-interacting proteins in schizophrenia: the pivotal position of dopamine supersensitivity psychosis in treatment-resistant schizophrenia. Int J Mol Sci 2015; 16: 30144–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen RT, Tyrer P.. Benzodiazepine dependence. A review of the evidence. Drugs 1983; 25: 385–98. [DOI] [PubMed] [Google Scholar]
- Pal HR, Berry N, Kumar R, Ray R.. Ketamine dependence. Anaesth Intensive Care 2002; 30: 382–4. [DOI] [PubMed] [Google Scholar]
- Pasero C, McCaffery M.. Misconception that hamper assessment and treatment of patients who report pain. In: Pain assessment and pharmacologic management Chap. 2. St. Louis, MO: Elsevier Health Sciences; 2010, 20–48. [Google Scholar]
- Pecknold JC, Swinson RP, Kuch K, Lewis CP.. Alprazolam in panic disorder and agoraphobia: results from a multicenter trial. III. Discontinuation effects. Arch Gen Psychiatry 1988; 45: 429–36. [DOI] [PubMed] [Google Scholar]
- Pesce ME, Acevedo X, Pinardi G, Miranda HF.. Gender differences in diazepam withdrawal syndrome in mice. Pharmacol Toxicol 1994; 75: 353–5. [DOI] [PubMed] [Google Scholar]
- Petursson H. The benzodiazepine withdrawal syndrome. Addiction 1994; 89: 1455–9. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Rapoport JL, Behar D, Ismond DR, Bunney WE Jr.. A naturalistic assessment of the motor activity of hyperactive boys. II. Stimulant drug effects. Arch Gen Psychiatry 1983; 40: 688–93. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Denner LJ.. Benzodiazepines and withdrawal psychosis. Report of three cases. JAMA 1977; 237: 36–8. [PubMed] [Google Scholar]
- Rabinak CA, Nirenberg MJ.. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol 2010; 67: 58–63. [DOI] [PubMed] [Google Scholar]
- Rainer C, Scheinost NA, Lefeber EJ.. Neuroleptic malignant syndrome. When levodopa withdrawal is the cause. Postgrad Med 1991; 89: 175–8, 180. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Zahn TP, Weingartner H, Ludlow C, Mikkelsen EJ.. Dextroamphetamine: cognitive and behavioral effects in normal prepubertal boys. Science 1978; 199: 560–3. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Kamal A.. Complicated withdrawal phenomena during benzodiazepine cessation in older adults. J Am Osteopath Assoc 2019; 119: 327–31. [DOI] [PubMed] [Google Scholar]
- Reidenberg MM. Drug discontinuation effects are part of the pharmacology of a drug. J Pharmacol Exp Ther 2011; 339: 324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P.. Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. J Neuropsychiatry Clin Neurosci 2001; 13: 326–35. [DOI] [PubMed] [Google Scholar]
- Rickels K, Schweizer E, Case GW, Garcia-Espana F.. Benzodiazepine dependence, withdrawal severity, and clinical outcome: effects of personality. Psychopharmacol Bull 1988; 24: 415–20. [PubMed] [Google Scholar]
- Rickels K, Schweizer E, Case WG, Greenblatt DJ.. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47: 899–907. [DOI] [PubMed] [Google Scholar]
- Ries RK, Fiellin DA, Miller SC, Saitz R.. The ASAM principles of addiction medicine. Chevy Chase, MD: American Academy of Addiction Medicine Wolters Kluver; 2014. [Google Scholar]
- Risse SC, Whitters A, Burke J, Chen S, Scurfield RM, Raskind MA.. Severe withdrawal symptoms after discontinuation of alprazolam in eight patients with combat-induced posttraumatic stress disorder. J Clin Psychiatry 1990; 51: 206–9. [PubMed] [Google Scholar]
- Rivera JM, Locketz AJ, Fritz KD, Horlocker TT, Lewallen DG, Prasad A, et al. “Broken heart syndrome” after separation (from OxyContin). Mayo Clin Proc 2006; 81: 825–8. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Merlotti L, Zorick F, Roth T.. Rebound insomnia in normals and patients with insomnia after abrupt and tapered discontinuation. Psychopharmacology (Berl) 1992; 108: 67–71. [DOI] [PubMed] [Google Scholar]
- Rook E, Huitema A, Brink W, Ree J, Beijnen J.. Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: review of the literature. Curr Clin Pharmacol 2006; 1: 109–18. [DOI] [PubMed] [Google Scholar]
- Rosebush PI, Mazurek MF.. Catatonia after benzodiazepine withdrawal. J Clin Psychopharmacol 1996; 16: 315–9. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB.. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry 1998; 44: 77–87. [DOI] [PubMed] [Google Scholar]
- Rosse R, Ciolino C.. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985; 142: 270–1. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Dager SR, Cowley DS, Vitaliano P, Dunner DL.. Relapse and rebound following discontinuation of benzodiazepine treatment of panic attacks: alprazolam versus diazepam. Am J Psychiatry 1989; 146: 860–5. [DOI] [PubMed] [Google Scholar]
- Rungnirundorn T, Verachai V, Gelernter J, Malison RT, Kalayasiri R.. Sex differences in methamphetamine use and dependence in a thai treatment center. J Addict Med 2017; 11: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiful FB, Lafferty J, Jun CH, Teli S, Duvvuri S, Khattri S, et al. Takotsubo cardiomyopathy due to iatrogenic methadone withdrawal. Rev Cardiovasc Med 2011; 12: 164–7. [PubMed] [Google Scholar]
- SAMHSA—Substance Abuse and Mental Health Services Administration. Protracted Withdrawal. Substance Abuse Treatment Advisory 2010; 9: 1–8. [Google Scholar]
- Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R.. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 2005; 365: 482–7. [DOI] [PubMed] [Google Scholar]
- Satel SL, Kosten TR, Schuckit MA, Fischman MW.. Should protracted withdrawal from drugs be included in DSM-IV? Am J Psychiatry 1993; 150: 695–704. [DOI] [PubMed] [Google Scholar]
- Scharf MB, Kales A, Bixler EO, Jacoby JA, Schweitzer PK.. Lorazepam-efficacy, side effects, and rebound phenomena. Clin Pharmacol Ther 1982; 31: 175–9. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Haddad P, Kaplan EM, Lejoyeux M, Rosenbaum JF, Young AH, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation Consensus panel. J Clin Psychiatry 1997; 58 (Suppl 7): 5–10. [PubMed] [Google Scholar]
- Schlienz NJ, Budney AJ, Lee DC, Vandrey R.. Cannabis withdrawal: a review of neurobiological mechanisms and sex differences. Curr Addict Rep 2017; 4: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G.. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep 2018; 67: 1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SK, Miller DD, Arndt S, Ziebell S, Gupta S, Andreasen NC.. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry 1995; 38: 713–9. [DOI] [PubMed] [Google Scholar]
- Sechi GP, Tanda F, Mutani R.. Fatal hyperpyrexia after withdrawal of levodopa. Neurology 1984; 34: 249–51. [DOI] [PubMed] [Google Scholar]
- Serrano-Duenas M. Neuroleptic malignant syndrome-like, or–dopaminergic malignant syndrome–due to levodopa therapy withdrawal. Clinical features in 11 patients. Parkinsonism Relat Disord 2003; 9: 175–8. [DOI] [PubMed] [Google Scholar]
- Sethi PK, Khandelwal DC.. Zolpidem at supratherapeutic doses can cause drug abuse, dependence and withdrawal seizure. J Assoc Physicians India 2005; 53: 139–40. [PubMed] [Google Scholar]
- Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry 2006; 67 (Suppl 4): 3–7. [PubMed] [Google Scholar]
- Shiovitz TM, Welke TL, Tigel PD, Anand R, Hartman RD, Sramek JJ, et al. Cholinergic rebound and rapid onset psychosis following abrupt clozapine withdrawal. Schizophr Bull 1996; 22: 591–5. [DOI] [PubMed] [Google Scholar]
- Simoni-Wastila L, Yang HK.. Psychoactive drug abuse in older adults. Am J Geriatr Pharmacother 2006; 4: 380–94. [DOI] [PubMed] [Google Scholar]
- Sloan JW, Wala EP, Jing X, Holtman JR Jr.. The pharmacodynamics of PK 11195 in diazepam-dependent male and female rats. Pharmacol Biochem Behav 2000; 66: 751–64. [DOI] [PubMed] [Google Scholar]
- Smith DE, Wesson DR.. Benzodiazepine dependency syndromes. J Psychoactive Drugs 1983; 15: 85–95. [DOI] [PubMed] [Google Scholar]
- Smucker WD, Hedayat M.. Evaluation and treatment of ADHD. Am Fam Physician 2001; 64: 817–29. [PubMed] [Google Scholar]
- Soldatos CR, Dikeos DG, Whitehead A.. Tolerance and rebound insomnia with rapidly eliminated hypnotics: a meta-analysis of sleep laboratory studies. Int Clin Psychopharmacol 1999; 14: 287–303. [PubMed] [Google Scholar]
- Sorensen PS, Koch-Henriksen N, Petersen T, Ravnborg M, Oturai A, Sellebjerg F.. Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol 2014; 261: 1170–7. [DOI] [PubMed] [Google Scholar]
- Spadotto V, Zorzi A, Elmaghawry M, Meggiolaro M, Pittoni GM.. Heart failure due to ‘stress cardiomyopathy’: a severe manifestation of the opioid withdrawal syndrome. Eur Heart J Acute Cardiovasc Care 2013; 2: 84–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak B, Weizman A, Wolovick L, Hermesh H, Tyano S, Munitz H.. Neuroleptic malignant syndrome during abrupt reduction of neuroleptic treatment. Acta Psychiatr Scand 1990; 81: 168–9. [DOI] [PubMed] [Google Scholar]
- Stiskal JA, Kulin N, Koren G, Ho T, Ito S.. Neonatal paroxetine withdrawal syndrome. Arch Dis Child Fetal Neonatal Ed 2001; 84: F134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Keck PE Jr, Caroff SN.. Neuroleptic malignant syndrome. Am J Psychiatry 2007; 164: 870–6. [DOI] [PubMed] [Google Scholar]
- Sury MR, Billingham I, Russell GN, Hopkins CS, Thornington R, Vivori E.. Acute benzodiazepine withdrawal syndrome after midazolam infusions in children. Crit Care Med 1989; 17: 301–2. [DOI] [PubMed] [Google Scholar]
- ter Horst PG, van der Linde S, Smit JP, den Boon J, van Lingen RA, Jansman FG, et al. Clomipramine concentration and withdrawal symptoms in 10 neonates. Br J Clin Pharmacol 2012; 73: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiblin I, Runeson B, Rajs J.. Anabolic androgenic steroids and suicide. Ann Clin Psychiatry 1999; 11: 223–31. [DOI] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE.. Biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Front Psychiatry 2013; 4: 38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodianakis J, Potagas C, Papageorgiou P, Lazaridou M, Matikas N.. Zolpidem-related epileptic seizures: a case report. Eur Psychiatry 2003; 18: 140–1. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Zolipidem Study Group.. A double-blind comparative study of zolpidem versus zopiclone in the treatment of chronic primary insomnia. J Int Med Res 2001; 29: 163–77. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry - assessment of abuse potential of drugs. Silver Spring, MD 20993-0002; 2017. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessment-abuse-potential-drugs.
- U.S. Government PO. Title 21 - FOOD AND DRUGS, United States Code, Supplement 5 2006.
- United States Congress. Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970 US Government. 1970.
- Valuck RJ, Orton HD, Libby AM.. Antidepressant discontinuation and risk of suicide attempt: a retrospective, nested case-control study. J Clin Psychiatry 2009; 70: 1069–77. [DOI] [PubMed] [Google Scholar]
- Victorri-Vigneau C, Dailly E, Veyrac G, Jolliet P.. Evidence of zolpidem abuse and dependence: results of the French Centre for Evaluation and Information on Pharmacodependence (CEIP) network survey. Br J Clin Pharmacol 2007; 64: 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorri-Vigneau C, Gerardin M, Rousselet M, Guerlais M, Grall-Bronnec M, Jolliet P.. An update on zolpidem abuse and dependence. J Addict Dis 2014; 33: 15–23. [DOI] [PubMed] [Google Scholar]
- Voshaar RC, van Balkom AJ, Zitman FG.. Zolpidem is not superior to temazepam with respect to rebound insomnia: a controlled study. Eur Neuropsychopharmacol 2004; 14: 301–6. [DOI] [PubMed] [Google Scholar]
- Wakim JH. Alleviating symptoms of withdrawal from an opioid. Pain Ther 2012; 1: 4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watsky E. Management of zolpidem withdrawal. J Clin Psychopharmacol 1996; 16: 459.. [DOI] [PubMed] [Google Scholar]
- Webster LR, Cochella S, Dasgupta N, Fakata KL, Fine PG, Fishman SM.. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med 2011; 12 (Suppl 2): S26–35. [DOI] [PubMed] [Google Scholar]
- Yerevanian BI, Koek RJ, Feusner JD, Hwang S, Mintz J.. Antidepressants and suicidal behaviour in unipolar depression. Acta Psychiatr Scand 2004; 110: 452–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


