Abstract
Nonbacterial thrombotic endocarditis, a form of noninfectious thrombotic endocarditis, is mainly characterized by deposition of sterile platelet thrombi on heart valves. Usually, it is observed in advanced malignancy. Herein, we report a case of a previously healthy male with recent unprovoked deep vein thrombosis presented with acute ischemic stroke. The echocardiogram revealed aortic and mitral valve masses. Eventually, he was discovered to have advanced cholangiocarcinoma. The present case, apart from being the youngest reported case, is among the few reported cases which manifest the association between cholangiocarcinoma and nonbacterial thrombotic endocarditis.
Keywords: Thrombotic endocarditis, endocarditis, thrombophilia, cholangiocarcinoma
Introduction
The pathogenesis of thrombophilia, associated with cancer and its treatment, is highly complex and multifactorial.1 Indeed, advanced malignancy is associated with the formation of noninfectious thrombotic lesions on the heart valves, a rare condition which is termed as nonbacterial thrombotic endocarditis (NBTE). Moreover, in affected patients, it can lead to substantial morbidity.2 The NBTE is potentially overlooked and often confused with classic infective endocarditis. Consequently, if it is left unchecked, it can cause valvular dysfunction, heart failure, and systemic embolization.3
Primarily, the NBTE is usually found to be allied up with hypercoagulable states of advanced malignancy mainly adenocarcinomas.4 In one study, 15% of cancer patients suffer a thromboembolic event during their clinical course, whereas 50% of cancer patients exhibit signs of venous thromboembolism on postmortem examination.5 The present case, apart from being the youngest reported case, is among the few reported cases which manifest the association between cholangiocarcinoma and NBTE.
Case report
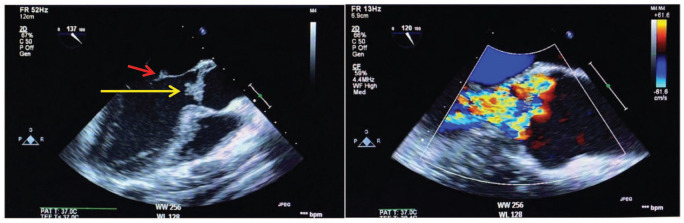
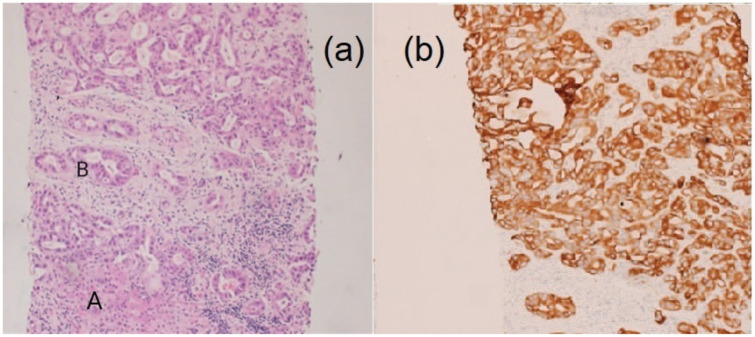
A 36-year-old male football player with no significant past medical history recently diagnosed with unprovoked deep venous thrombosis of the lower limb, presented with acute right-sided hemiplegia, hemi-sensory loss, facial upper motor neuron lesion and expressive aphasia. Prior to the presentation, the patient had a history of undocumented fever, but no reported history of chest pain, shortness of breath, weight loss, night sweating, drug abuse, alcohol consumption or family history of similar presentation. With the aid of the above information, the computed tomography (CT) of the brain showed multiple bilateral occipital and frontoparietal areas of hypodensities more on the left cerebral hemisphere. The transthoracic echocardiogram revealed left ventricular ejection fraction of 60%, the presence of masses attached to mitral and aortic valves needed to be evaluated further by tranesophageal echocardiogram (TEE). The TEE showed multiple highly mobile, irregular shape masses attached to right and left coronary cusps of the aortic valve, largest mass measuring 20 × 18 mm2 with eccentric severe aortic regurgitation, no associated abscess or fistula, similar echogenicity mass attached to anterior mitral valve measures 7 × 4 mm2, and posterior mitral valve mass measures 5 × 3 mm2, and there was mild mitral regurgitation and no intracardiac shunt (Figure 1(a) and (b)). Magnetic resonance imaging (MRI) of the brain (Figure 2(a) and (b)) revealed multifocal diffusion restrictions involving bilateral high frontal and left thalamus, bilateral occipital lobes, and bilateral cerebellar hemispheres, with no significant mass effect. Forty-eight hours of follow-up CT of the brain confirmed the presence of hemorrhagic transformation. Clinically, the patient showed improvement in the expressive aphasia and the right-sided hemiplegia during the first week of admission. The patient was clinically stable throughout his hospitalization course. The follow-up investigations of all the tests performed on the patient leads to the following conclusions: (1) all blood cultures were negative; (2) Brucella titer and serology for Q fever were negative; (3) markers for tumors came back positive (CA-125 102 U/mL, CA19-9 5247 U/mL, CEA 13.6 U/mL); (4) thrombophilic evaluation, including anti-nuclear antibody (ANA), anti-neutrophil cytoplasmic antibody (ANCA), antiphospholipid antibodies (cardiolipin IgG and IgM), Factor-V Leiden mutation, homocysteine, Protein-S, Protein-C and Antithrombin-III levels, was unremarkable; and (5) CT scan of chest, abdomen and pelvis unveiled the presence of hypovascular hepatic mass having dimensions of 7.8 × 5.7 × 6.4 cm3, which invaded the right portal vein as well as the right hepatic duct with multiple porta hepatis and retroperitoneal lymphadenopathies, thereby leading to an alarming stage of cholangiocarcinoma (Figure 3). On a similar note, the peripheral right lung showed lesion with central cavitation probably infarction or metastasis, in addition to the presence of bilateral hypodense renal cervical lesions. The patient underwent ultrasound-guided liver biopsy showed well-differentiated adeno-carcinoma, consistent with the diagnosis of cholangiocarcinoma (Figure 4(a) and (b)).
Figure 1.
(a) Tranesophageal echocardiogram (TEE). Large mass attached to the aortic valve (yellow arrow). Small mass attached to the anterior mitral leaflet (red arrow) and (b) TEE color Doppler shows severe aortic regurgitation.
Figure 2.
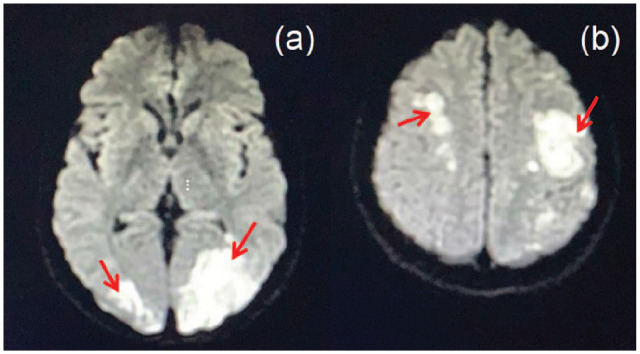
(a) Magnetic resonance imaging (MRI) of brain: (a) bilateral occipital acute ischemic stroke and (b) bilateral parietal acute ischemic stroke.
Figure 3.
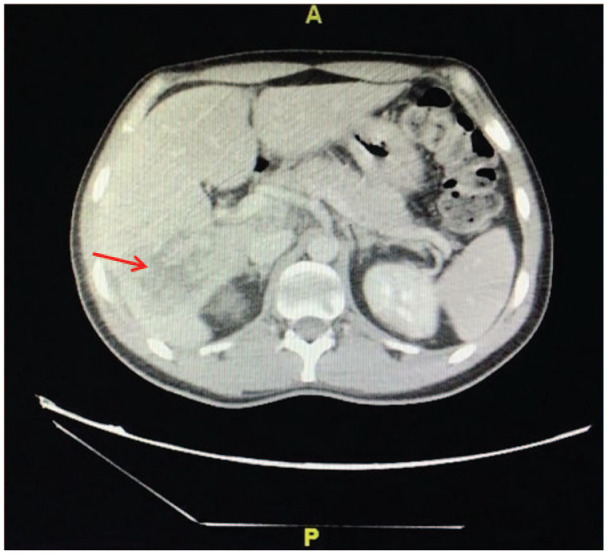
Computed tomography (CT) of the abdomen showing the hypovascular liver mass.
Figure 4.
(a) The photomicrographs show a normal liver tissue (A) exhibiting a tumor formed of cells arranged in glands and tubules, the tumor cells display hyperchromatic pleomorphic nuclei (B) with increased mitotic activity, the tumor is surrounded by desmoplastic stroma and (b) the tumor showed positive immunohistochemical reaction for CytoKeratin7 (CK7). The above-mentioned features are in keeping with cholangiocarcinoma.
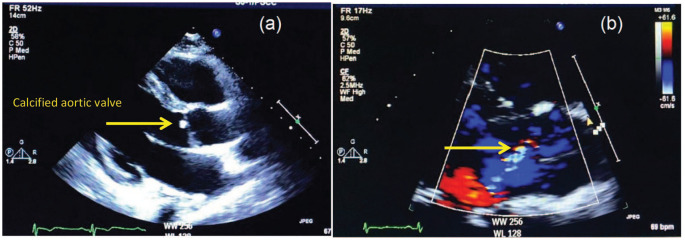
The follow-up transthoracic echocardiogram after 10 days of admission showed the presence of a small calcified aortic valve mass, which measures 6 × 4 mm2, mild aortic and mitral regurgitation and no evidence of mitral valve mass (Figure 5(a) and (b)). At this stage, upon his and his family request, the patient was transferred to an oncology center in the United States, where he was recommended for palliative treatment. Unfortunately, 6 months later, the patient died.
Figure 5.
(a) The follow-up transthoracic echocardiogram showing small calcified aortic mass and no mass on the mitral valve and (b) color Doppler showing mild aortic regurgitation.
Discussion
The former marantic endocarditis, now known as NBTE, is a rare condition mainly associated with the formation of sterile vegetations on heart valve leaflets, which in turn can cause valvular dysfunction, heart failure, and systemic embolization.3 Although the pathogenesis of NBTE is not fully clear, the main theories of factors implicated in the initiation of NBTE include malignancy especially adenocarcinoma,4 autoimmune disease, hypercoagulable state and hypoxia, which can cause endothelial damage and subsequent exposure of the subendothelial connective tissue to the circulating platelets.6 NBTE secondary to malignancy is a part of clinical spectrum of Trousseau’s syndrome, which describes the association of malignancies and thromboembolic disorders that extends from uncomplicated superficial thrombophlebitis to life-threatening thromboembolism such as NBTE.7 The first case of Trousseau’s syndrome secondary to cholangiocarcinoma was reported, by Ching.8 Since then very few cases, with a total of only 11 cases including an autopsy case, had been reported.9–13 Among the few previously reported cases, only three cases had confirmed NBTE with echocardiography finding of valve mass or vegetations, the present case is the youngest case.9,13,14 Cholangiocarcinoma associated with NBTE has poor prognosis and most of the reported cases were advance at diagnosis or were diagnosed on autopsy.9 The definitive diagnosis can be made pathologically by the demonstration of platelet thrombi on autopsy or surgical specimens. However, the routine acquisition of valvular tissue is not practical such that clinicians are reliant upon a constellation of clinical, echocardiographic, and absence of microbiologic findings for the diagnosis of NBTE. The NBTE should be suspected in patients with acute stroke or coronary ischemia with underlying cancer, systemic lupus erythematosus or antiphospholipid syndrome. It should also be suspected in patients with acute stroke or multiple widely distributed emboli of unknown etiology as well as in those with presumed infective endocarditis who are unresponsive to, or progressing poorly on, antibiotic therapy.15
Management of NBTE depends on treating the underlying reason and anticoagulation for systemic embolization risk.2,3,16 In the present case, large cerebral ischemic stroke with hemorrhagic transformation was a contraindication for anticoagulation. However, generally patients with thromboemboli or NBTE-related malignancy should continue on anticoagulation if there is no contraindication.2,3,16 Unfractionated heparin appears to be most effective.2,3,16 Although experience is more limited, low molecular weight heparin has also been useful.2,3,13,16 Vitamin K antagonists such as warfarin are not suggested in malignancy-associated NBTE, due to the risk of recurrent thromboembolic events.2,3,16 The novel oral anticoagulants (NOACs) are currently considered an attractive option but remains unknown whether it will be effective. Valvular surgery represents a valuable therapeutic alternative in patients with large mobile vegetations, valvular dysfunction or recurrent embolic events despite anticoagulation.17 In our case, heart valve surgery was deferred based on the clinical neurological improvement, presence of cerebral hemorrhagic transformation and advance cholangiocarcinoma at diagnosis.
Conclusion
The present case is among the few reported cases which manifests an association between cholangiocarcinoma and NBTE. The NBTE is often not readily diagnosed and should be considered in patients with valvular vegetations, absence of microbiologic findings, significant valvular dysfunction, multiple embolic events or venous thrombosis.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article. A general consent form to publish patient information was obtained from the legal representative(s).
ORCID iD: Abdullah Alkhushail  https://orcid.org/0000-0001-6677-7945
https://orcid.org/0000-0001-6677-7945
References
- 1. Chu G, Versteeg HH, Verschoor AJ, et al. Atrial fibrillation and cancer—An unexplored field in cardiovascular oncology. Blood Rev 2019; 35: 59–67. [DOI] [PubMed] [Google Scholar]
- 2. el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007; 12(5): 518–523. [DOI] [PubMed] [Google Scholar]
- 3. Liu J, Frishman WH. Nonbacterial thrombotic endocarditis: pathogenesis, diagnosis, and management. Cardiol Rev 2016; 24(5): 244–247. [DOI] [PubMed] [Google Scholar]
- 4. Mazokopakis EE, Syros PK, Starakis IK. Nonbacterial thrombotic endocarditis (marantic endocarditis) in cancer patients. Cardiovasc Hematol Disord Drug Targets 2010; 10(2): 84–86. [DOI] [PubMed] [Google Scholar]
- 5. Deitcher SR. Cancer and thrombosis: mechanisms and treatment. J Thromb Thrombolysis 2003; 16(1–2): 21–31. [DOI] [PubMed] [Google Scholar]
- 6. Asopa S, Patel A, Khan OA, et al. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg 2007; 32(5): 696–701. [DOI] [PubMed] [Google Scholar]
- 7. Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau’s syndrome revisited. Blood 1983; 62(1): 14–31. [PubMed] [Google Scholar]
- 8. Ching CK. Trousseau’s syndrome in a patient with cholangiocarcinoma. Am J Gastroenterol 1991; 86(7): 928–929. [PubMed] [Google Scholar]
- 9. Yuri T, Kato K, Hirohara Y, et al. Trousseau’s syndrome caused by intrahepatic cholangiocarcinoma: an autopsy case report and literature review. Case Rep Oncol 2014; 7(2): 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sasaki R, Ohya Y, Hayashida S, et al. A case of Trousseau’s syndrome due to intrahepatic cholangiocarcinoma with an extremely high level of CA19-9. Surg Case Rep 2020; 6(1): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blum MF, Ma VY, Betbadal AM, et al. Trousseau’s syndrome in cholangiocarcinoma: the risk of making the diagnosis. Clin Med Res 2016; 14(1): 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn T, Rudrapatna VA, Hesse S, et al. Arterial thromboses heralding cholangiocarcinoma. Am J Med 2017; 130(8): e341–e342. [DOI] [PubMed] [Google Scholar]
- 13. Nadkarni N, Lee YJ, Hoefen R, et al. Cholangiocarcinoma manifesting as non-bacterial thrombotic endocarditis in a young patient. Am J Med 2020; S0002–9343: 30033–30034. [DOI] [PubMed] [Google Scholar]
- 14. Yih JS, Chao Y, Chen WY, et al. Nonbacterial thrombotic endocarditis and cerebral embolism in a patient with cholangiocarcinoma. Zhonghua Yi Xue Za Zhi (Taipei) 1993; 51(3): 238–240. [PubMed] [Google Scholar]
- 15. Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985; 64(1): 16–35. [DOI] [PubMed] [Google Scholar]
- 16. Dardiotis E, Aloizou AM, Markoula S, et al. Cancer-associated stroke: pathophysiology, detection and management (Review). Int J Oncol 2019; 54(3): 779–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rabinstein AA, Giovanelli C, Romano JG, et al. Surgical treatment of nonbacterial thrombotic endocarditis presenting with stroke. J Neurol 2005; 252(3): 352–355. [DOI] [PubMed] [Google Scholar]