Abstract
Background:
Lung neuroendocrine carcinoma (NEC) is characterized by aggressive clinical behavior and lack of treatment advances. We evaluate the prognostic and the predictive roles of systemic inflammatory biomarkers in patient circulating blood: neutrophil–lymphocyte ratio (NLR), lactate dehydrogenase (LDH), advanced lung cancer inflammation index (ALI), and the Lung Immune Prognostic Index (LIPI) score.
Methods:
A total of 120 patients with small-cell lung cancer (SCLC) (n = 110) and large cell neuroendocrine carcinoma (LCNEC) (n = 10) were enrolled. Overall survival (OS) was evaluated by Kaplan–Meier estimator and univariate and multivariate Cox proportional hazard analyses were performed to determine prognostic factors associated with OS while χ2 test was used for categorical data.
Results:
NLR cutoff value was 1.93. NLR was measured before and after first-line chemotherapy; 25 (21%) patients had higher NLR (delta NLR >1), whereas NLR was lower in 37 (31%). At the univariate analysis, median OS was 12 months: OS for SCLC and LCNEC were 11 months and 14 months, respectively. OS had a prognostic positive value in patients with pre-treatment NLR <1.93 (p = 0.0002), LDH <600 U/L (p = 0,03) and ALI ⩾34 (p = 0,0065). At the multivariate analysis, Eastern Cooperative Oncology Group performance status, LDH levels and response after first-line chemotherapy were independently associated with OS. Median OS for good, intermediate, and poor LIPI was 15 months, 11 months, and 9 months, respectively(p = 0.091). Patients with higher NLR (>1.93) had an increased probability of tumor progression (p = 0.045, χ2 test).
Conclusion:
This study demonstrated that systemic inflammatory biomarkers could facilitate the understanding of survival differences in the clinical management of lung NEC patients, underlying the need for prospective biomarker-driven studies in the immune checkpoint inhibitors setting.
Keywords: blood-based biomarker, neuroendocrine tumor, predictive biomarker, prognostic biomarker, small-cell lung cancer (SCLC), systemic inflammatory response
Background
Lung cancer is the leading cause of cancer-related death.1 In particular, small cell lung cancer (SCLC) represents 15% of all cases while large cell neuroendocrine carcinoma (LCNEC) accounts for 5%. Lung neuroendocrine carcinomas (NECs) represent poorly differentiated or high-grade lung neuroendocrine neoplasms (NENs), comprising SCLC and LCNEC in accordance with the 2015 World Health Organization (WHO) lung NEN classification.2 Typical and atypical carcinoids are two further categories of this classification, representing well differentiated or low-/intermediate-grade forms. SCLC and LCNEC are NECs characterized by poor survival rates. Furthermore, the American Joint Committee on Cancer Tumor-Node-Metastasis (TNM) classification and staging system has proved to be predictive of overall survival (OS) and a number of clinical indicators such as smoking habit, gender, age and performance status seemed to be associated with prognosis.3 Systemic inflammation plays an important role in tumor promotion and progression. Therefore, it is not surprising that different markers of systemic inflammation have been related to poor outcome in multiple solid neoplasms, including NEC.4 Recently, a variety of novel parameters have emerged as independent prognostic factors with an interesting role in clinical practice such as advanced lung cancer inflammation index (ALI), neutrophil–lymphocyte ratio (NLR), and serum lactate dehydrogenase (LDH). The prognostic significance of ALI, which is calculated by multiplying body mass index (BMI; kg/m2) by the serum albumin/NLR, has been recently described in a study that showed how lower ALI value (<19.5) was associated with poor prognosis in SCLC.5 Zhang et al. in a recent work described the prognostic role of serum LDH, describing elevated values during cell malignant transformation, angiogenesis and hypoxia.6 High levels of LDH were associated with worse prognosis, although LDH levels alone cannot have an independent prognostic significance if not associated with other strong clinical indicators such as the extent of disease, age, NLR or platelets count.6 The prognostic significance of NLR has been evaluated in several studies. Specifically, it has been demonstrated that high preoperative peripheral blood NLR was associated with poor postoperative outcomes.7 The advent of immune checkpoint inhibitors (ICIs) in the SCLC scenario raises the need for prognostic indicators to guide treatment strategies. For this reason, our study aimed to evaluate the prognostic significance of systemic inflammatory biomarkers in patients affected by lung NEC, focusing on NLR, LDH, and ALI. In addition, we tried to stratify our population into three prognostic groups using an innovative immune prognostic score in advanced lung NECs, the Lung Immune Prognostic Index (LIPI).
Methods
Patients
We conducted a retrospective analysis using peripheral blood samples of consecutive patients (aged ⩾18 years) with cytological or histological diagnosis of SCLC and LCNEC (stage IIIB–C/IV (according to version 8 of the International Association for the Study of Lung Cancer TNM Staging System). Clinical characteristics, hematologic tests and outcome data were retrospectively collected and included into an anonymized database. We excluded patients with diagnosis of well-differentiated neuroendocrine tumor, and patients who did not undergo blood sample collection at diagnosis or who have not received chemotherapy treatment for their disease. Patients with a diagnosis of cancer in the last 5 years before diagnosis were excluded. We also excluded patients who were diagnosed with active systemic inflammatory or chronic disease (Crohn’s disease, ulcerative colitis, irritable bowel syndrome, thyroiditis, type 1 and 2 diabetes mellitus, other autoimmune diseases). The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines on Good Clinical Practice. The trial protocol was previously approved by the Independent Ethic Committee at University of Palermo (approval number: 0003/2020).
Study design and treatment
Patients were referred to two Italian cancer centers: University of Chieti and University of Palermo, from January 2002 to January 2018. All patients included in our analysis were newly diagnosed with advanced disease and received platinum-based chemotherapy as first-line regimen. The first-line chemotherapy sensitivity was categorized as chemotherapy-free interval (CFI) >90 days.8 The NLR was calculated as the ratio between the absolute count of neutrophils (ANC) and the absolute count of lymphocytes. Since no validated cut-off value has been reported, NLR was calculated according to the Youden index of the receiver operating characteristic (ROC) curve. The value of ALI was computed as BMI × serum albumin/NLR. The ALI, platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR) and LDH cut-offs were obtained using the median value. The derived (d) NLR was calculated as [ANC/(white blood cell concentration (WBC) − ANC)]. The LIPI score was developed on the basis of dNLR greater than 3 and LDH greater than 600 U/L, stratifying our lung NEC population into three prognostic groups: good, intermediate and poor.9 We categorized advanced lung NEC in two different groups, limited-stage SCLC or extensive stage SCLC, according to the modified version of the Veterans Administration Lung Cancer Study Group (VALSG) staging system that is usually applied to SCLC.10 The comorbidity assessment at baseline was performed using the Charlson comorbidity index (CCI), using the median value as cut-off. Radiological evaluation of efficacy was performed by computed tomography scan every 3 months, thereafter until disease progression and treatment responses were computed according to the response evaluation criteria in solid tumors (RECIST) version 1.1.
Statistical analysis
The ALI, PLR, LMR and LDH raw values were calculated from blood tests obtained from each patient at the same time points. Their median values were computed as the 50th percentile. Categorical variables were compared using χ2 test or Fisher’s exact test. The best response after first line chemotherapy was categorized as complete response (CR), partial response (PR), stable disease (SD), and progression disease, according to RECIST version 1.1. OS was defined as time from diagnosis or primary intervention to last follow-up or death from any cause. The survival curves were estimated using the Kaplan–Meier method, providing median and p values, with the use of the log-rank test for comparisons. Univariate and multivariate analysis for the most significant variables were performed using a logistic regression model. All analyses were considered as statistically significant with a p value ⩽0.05. Statistical analyses were performed using the SPSS Statistics software (IBM SPSS Statistics for Windows, version 22.0. IBM Corp., Armonk, NY, USA).
Results
We screened and included in our study a total of 120 patients. Of them, 110 (92%) were SCLC and 10 (8%) LCNEC. Patients’ baseline characteristics are shown in Table 1. In our population, males were prevalent (n = 101, 84%) and the median age was 65 years (range 39–85). Further, the majority of patients presented with an eastern cooperative oncology group performance status (ECOG PS) score of 0–1 (78%) rather than 2 (6%). Mean BMI was 26 kg/m2 and 53% of the patients were overweight or obese at diagnosis, according to WHO BMI classification. Data on cigarette smoking was available in only 48% of our cohort with most of them being current smokers (96%). As the onset of metastasis is very common in lung NEC disease, the CCI score is usually >6. In fact, in line with previous reports in lung cancer, the mean CCI score was 8 in our study.11,12 Most patients had advanced disease according to the VALSG staging system (58%). Among them, 44% of cases had multi-organ metastatic disease and most common sites of metastasis were liver, bone, and distant lymph nodes. In particular, brain metastases were present in 19 cases (16%). The pre-treatment serum neuron-specific enolase (NSE) level was measured in 63 patients (53%), with an average value of 69 ng/mL. The appropriate cutoff value for the NLR identified by ROC analyses was 1.93, with a sensitivity and specificity for predicting survival of 83% and 46%, respectively. The NLR value was measured before and after first-line chemotherapy and an increased NLR (delta NLR >1) was found in 25 (21%) patients whereas a reduced NLR was found in 37 (31%). All patients had undergone platinum-based chemotherapy. Interestingly, 65% had shown a CR, PR, or SD as disease control after first-line treatment, and 29% had a first-line chemotherapy sensitive tumor according to the pre-specified CFI. Median OS was 12 months. In particular, OS for SCLC and LCNEC was 11 months and 14 months, respectively.
Table 1.
Characteristics of study population.
| Variable | Category | All cases No. (%) |
HR | 95% CI Univariate |
p-value | HR | 95% CI Multivariate |
p-value |
|---|---|---|---|---|---|---|---|---|
| Histotype | SCLC | 110 (92) | 1 (ref.) | |||||
| LCNEC | 10 (8) | 1.14 | 0.54–2.38 | 0.7 | ||||
| Gender | Male | 101 (84) | 1 | |||||
| Female | 19 (16) | 0.88 | 0.52–1.47 | 0.62 | ||||
| Age, years | < 65 | 58 (48) | 1 | |||||
| ⩾65 | 62 (52) | 1.9 | 1.21–2.8 | 0.0019 | ||||
| BMI |
Normal weight
Overweight or obese |
51 (43) 64 (53) |
1 0.87 |
0.56–1.35 | 0.50 | |||
| ECOG PS | 0 | 44 (37) | 1 | 1.1–15.8 | 1 | 1 | 1 | 4 |
| 1 | 49 (41) | 2.5 | 0.66–9.66 | 0.01 | 3.57 | 1.05–12.5 | 0.04 | |
| 2 | 7 (6) | 4.16 | 1.1–16.6 | 0.0004 | 4.34 | 1.20–16.6 | 0.0025 | |
| Charlson score |
<8
⩾8 |
42 (35) 59 (49) |
1 2.2 |
1.40–3.40 | 0.0003 | |||
| VALGS stage at diagnosis |
LD
ED |
31 (26) 70 (58) |
1 1.6 |
1.02–2.54 | 0.04 | |||
| Multi-organ metastases, only metastatic | No | 39 (56) | 1 | |||||
| Yes | 31 (44) | 1.72 | 0.99–3.0 | 0.03 | ||||
| Brain metastases |
No
Yes |
81 (67) 19 (16) |
1 1.17 |
0.64–2.11 | 0.57 | |||
| NLR | <1.93 | 23 (19) | 1 | |||||
| ⩾1.93 | 90 (75) | 2.6 | 1.68–4.05 | 0.0002 | ||||
| ΔNLR | ⩽1 | 37 (31) | 1 | |||||
| >1 | 25 (21) | 1.18 | 0.66–2.13 | 0.50 | ||||
| LMR | <2.82 | 54 (45) | 1 | |||||
| ⩾2.82 | 55 (46) | 0.79 | 0.5–1.22 | 0.26 | ||||
| PLR |
<146
⩾146 |
57 (47) 56 (47) |
1 1.22 |
0.79–1.88 | 0.34 | |||
| LDH, U/L | <600 | 41 (34) | 1 | 1 | ||||
| ⩾600 | 38 (32) | 1.69 | 1.01–2.81 | 0.03 | 6.6 | 1.31–33.3 | 0.022 | |
| Albumin, g/dL | <3.9 | 33 (28) | 1 | |||||
| ⩾3.9 | 46 (38) | 0.86 | 0.51–1.46 | 0.57 | ||||
| Serum sodium, mmol/L | <130 | 5 (4) | 1 | |||||
| ⩾130 | 94 (79) | 1.82 | 0.48–6.87 | 0.22 | ||||
| CRP, mg/dL | <1 | 14 (12) | 1 | |||||
| ⩾1 | 12 (10) | 1.84 | 0.74–4.6 | 0.14 | ||||
| NSE, ng/mL | <20 | 19 (16) | 1 | |||||
| ⩾20 | 44 (37) | 1.14 | 0.60–2.16 | 0.69 | ||||
| ALI, BMI*ALB/NLR | <34 | 38 (32) | 1 | |||||
| ⩾34 | 37 (31) | 0.49 | 0.28–0.86 | 0.0065 | ||||
| 6-month disease control, CR+PR+SD | No | 31 (34) | 1 | 1 | ||||
| Yes | 58 (65) | 0.25 | 0.11–0.60 | <0.0001 | 0.15 | 0.04–0.55 | 0.004 | |
| First line sensitivity | No | 50 (42) | 1 | |||||
| Yes | 35 (29) | 0.39 | 0.24–0.63 | <0.0001 |
Univariate and multivariate analysis; SCLC; LCNEC; body mass index was categorized as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (⩾25.0 kg/m2). First line sensitivity was categorized as chemotherapy free-interval >90 days.
ALB, albumin; ALI, advanced lung cancer inflammation index; BMI, body mass index; CI, confidence interval; CR, complete response; CRP, C-reactive protein; ΔNLR: pre-treatment to post-treatment NLR changes; ED, extensive disease; HR, hazard ratio; LCNEC; large cell neuroendocrine carcinoma; LD, limited disease; LDH, lactate dehydrogenase; LMR, lymphocyte-to-monocyte ratio; NLR, neutrophil-to-lymphocyte ratio; NSE, serum neuron specific enolase; PLR, platelet-to-lymphocyte ratio; PR, partial response; ECOG PS, Eastern Cooperative Oncology Group performance status; ref., reference; SCLC, small cell lung cancer; SD, stable disease; VALGS, Veterans Administration Lung Study Group.
As regards the univariate analysis, a positive prognostic value for OS was found in patients with pre-treatment NLR <1.93 (log-rank test p = 0.0002), LDH <600 U/L (p = 0.03) and ALI ⩾34 (p = 0.0065) (Figures 1–3 and Table 1). Moreover, other clinical meaningful parameters such as age at diagnosis <65 years (p = 0.0019), lower ECOG PS (1 versus 0 p = 0.02; 2 versus 0 p = 0.0004), CCI <8 (p = 0.0003), limited disease (p = 0,04), one site of distant metastasis (p = 0.03), disease control after first-line chemotherapy (CR+PR+SD; p ⩽ 0.0001) and first line sensitivity (p ⩽ 0.0001) were significantly associated with a better survival. At the multivariate analysis, ECOG PS, LDH levels and response after first-line chemotherapy maintained their significance for OS and should be considered as independent prognostic factors in our series (Table 1).
Figure 1.
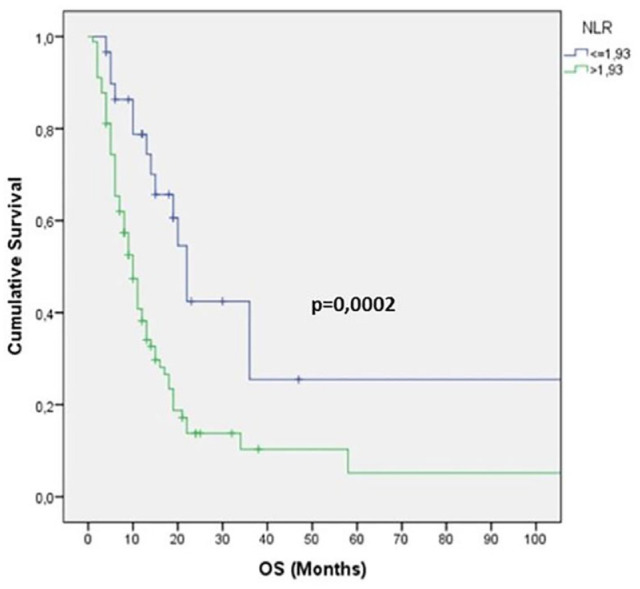
Kaplan–Meier survival curve for overall survival (OS) according to neutrophil–lymphocyte ratio (NLR) values.
Figure 2.
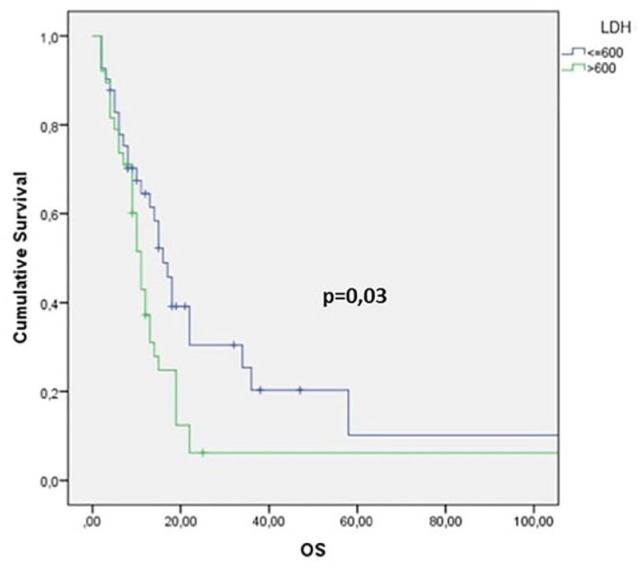
Kaplan–Meier survival curve for overall survival (OS) according to lactate dehydrogenase (LDH) values.
Figure 3.
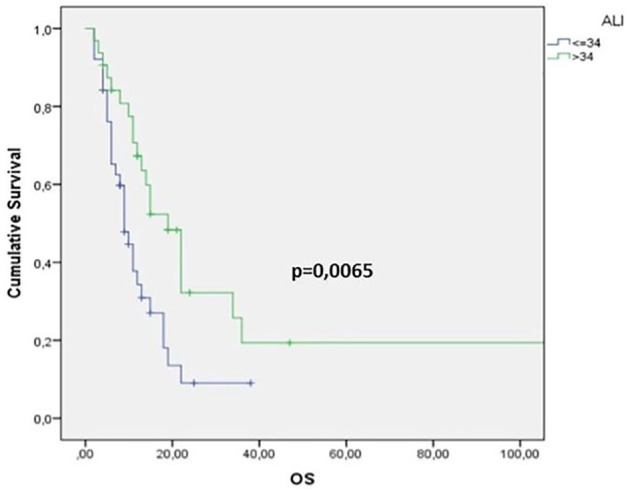
Kaplan–Meier survival curve for overall survival (OS) according to lung cancer inflammation index (ALI) values.
Forty-one (34%) patients without baseline LDH or dNLR were excluded from the LIPI analysis. Among the 78 evaluable patients, 34 (43%) had good LIPI score, 35 (45%) had intermediate LIPI score, and nine (11%) had poor LIPI score. About LIPI and OS, our analysis showed OS values of 15 months versus 11 months versus 9 months according to good, intermediate, and poor LIPI groups, respectively, showing a trend toward statistical significance (p = 0.091) (Figure 4). A significantly increased probability of tumor progression was observed in those patients with higher NLR before treatment, while patients with NLR <1.93 showed a lower probability of tumor progression (p = 0.045, χ2 test) (Figure 5).
Figure 4.
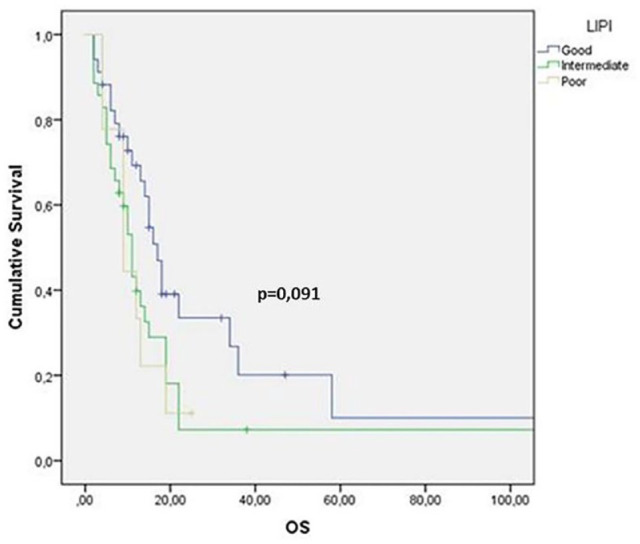
Kaplan–Meier survival curve for overall survival (OS) according to Lung Immune Prognostic Index (LIPI) values.
Figure 5.
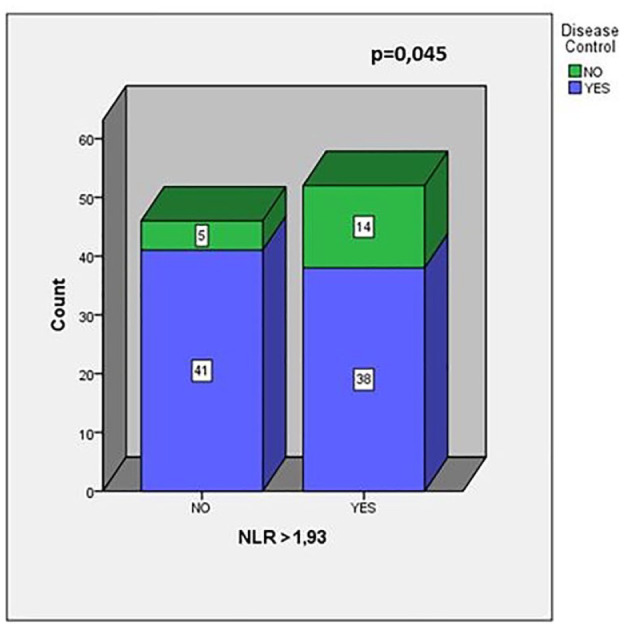
Number of patients who reach disease control stratified by neutrophil–lymphocyte ratio (NLR). In the χ2 test patients were divided into two groups (NLR ⩽1.93 and NLR >1.93). Patients in the NLR >1.93 group showed a significantly higher progressive disease rate than patients in the NLR ⩽1.93 group.
Discussion
Lung NECs are considered as rare tumors characterized by lack of treatment advances over the last 30 years. Over recent decades, ICIs revolutionized cancer care of several aggressive solid tumors, including non-small cell lung cancer, melanoma and Merkel-cell carcinoma. The strong association with smoking habit and high mutational rate suggested a potential immunogenicity of lung NEC and provided the rationale for adding ICIs to chemotherapy to enhance antitumor immunity and improve outcomes.13
The role of ICIs is currently under investigation in LCNEC, whereas recent studies demonstrated encouraging data from SCLC patients.
Due to the rarity of lung NECs, identification of independent prognostic and predictive factors is an important unmet clinical need. Gender, extent of disease, ECOG PS, hemoglobin, LDH, NSE and chemotherapy disease control are well-recognized prognostic factors for SCLC and LCNEC.14–20
Despite that emerging evidences have confirmed an important role of inflammatory response in tumor progression, there is no robust evidence regarding systemic inflammatory biomarkers in this setting.
Moreover, since different trials showed that improvements from ICIs are limited to a small subset of patients,21 further identification of predictive biomarkers is crucial in order to improve a better selection of patients.
Besides the well-known inflammatory biomarkers, including LDH and hypoalbuminemia, novel parameters have been investigated, such as NLR, ALI and LIPI score, all of which are associated with survival in cancer.
As novel biomarkers, NLR and dNLR measure the inflammation/immunity ratio in various solid tumors. The dNLR differs from NLR because it includes monocytes and other granulocyte subpopulations that could contribute to the process.9 Elevated NLR values are related to neutrophilia and lymphocytopenia.
Neutrophils seem to dominate the lung cancer landscape, as showed by Kargl et al., being responsible for the ICI resistance.22 The tumor microenvironment, rich in neutrophils, can be artificially manipulated and stimulated in order to develop a pro-inflammatory status related to tumor progression.23 This “negative inflammation”, responsible for ICI resistance, may be reflected by the aforementioned inflammatory biomarkers (NLR, LIPI).
The significance of these systemic inflammatory markers could be explained by the role of neutrophils in promoting inflammation and consequently an adequate environment for tumor growth.24 Neutrophils promote remodeling of the extracellular matrix and tumor progression by activating a large variety of inflammatory markers such as vascular endothelial growth factor and anti-apoptotic factors such as nuclear factor kappa-light-chain-enhancer of activated B cells.25 On the contrary, lymphocyte count reflects the immune system activation and the consequent inhibiting effect on tumor proliferation and migration.24 It is well known that inflammation reduces albumin levels irrespective of the patient’s nutritional status,26 and low albumin has also been recognized as a negative prognostic factor in several diseases, especially solid tumors.27 In addition, the relationship between immune system and energetic metabolism, in both obese and cachectic patients, and its impact on tumor progression are active fields of investigation.28 Accordingly, previous studies investigated ALI score, based on albumin level and BMI together with NLR, to validate the degree of systemic inflammation at diagnosis.5,29 As mentioned previously, LIPI score, a combined score including dNLR and LDH, has proved to be useful for stratifying those patients treated with ICIs in prognostic groups,30 raising the hypothesis that it might be useful as a predictive tool. Our aim was to evaluate the role of combined routine blood parameters, such as systemic inflammatory biomarkers, in order to investigate the effect of inflammatory status on lung NEC outcomes. Results of this article confirmed recent reports about the prognostic role of NLR and other factors such as LIPI also in patients who underwent conventional chemotherapy.31,32 After the introduction of ICIs in SCLC, it should be interesting to evaluate the role of steroids (above all ⩾10 mg of prednisone or equivalent) because of its role as neutrophilia and lymphopenia factor and its relationship with LIPI and prognosis worsening, as suggested by studies on non-small cell lung cancer.33
LDH is an important and widely studied inflammatory biomarker, which acquired a prognostic and predictive role in melanoma patients treated with Program Death 1 inhibitors. LDH has also been studied in SCLC with its role being still controversial, even in the definition of cut-off values. Nonetheless, a recent meta-analysis showed that a high LDH value was associated with poor prognosis.30
Furthermore, our results showed a favorable survival in patients with pre-treatment NLR <1.93, LDH <600 U/L and ALI ⩾34. Moreover, analysis of other clinical parameters showed that age at diagnosis <65 years, lower ECOG PS, CCI <8, limited disease, low tumor burden, disease control after first-line chemotherapy and first line sensitivity were significantly associated with a better survival. Only ECOG PS, LDH levels and response after first-line chemotherapy maintained their significance for OS at the multivariate analysis. Stratification through LIPI score allowed us to identify three prognostic groups, categorized as good, intermediate and poor, with a trend toward statistical significance.
Finally, our results showed that patients with NLR >1.93 reflect a higher probability of tumor progression, while patients with NLR values lower than 1.93 seemed to have a decreased probability of disease progression (Figure 5). The association between high level of NLR and poor prognosis is supported by strong evidences suggesting that systemic inflammatory response influences tumor progression.
Conclusion
With our retrospective analysis, we provided evidence that levels of NLR, LDH and ALI evaluated at diagnosis showed a significant prognostic role in lung NEC, while LIPI stratified patients into three prognostic groups: good, intermediate and poor. Limitations include the retrospective design and the low number of centers involved, which could affect the study for selection bias. However, to reduce the risk of bias, we have recruited consecutive patients. The prognostic role of LIPI did not achieve the statistical significance probably because of the lack of power in our sample size and data on LDH. Multivariate analysis contributes to adjust our results for clinical characteristics. Therefore, a future multicenter study is needed for a prospective validation of our results. To the best of our knowledge, this is the first attempt to evaluate the prognostic role of LIPI in advanced lung NEC. Our results are consistent with previous studies focused on lung NEC, as summarized in Table 2,6,7,9,29,34–36 and could facilitate the understanding of survival differences in advanced lung NEC patients in the clinical setting, suggesting a role of systemic inflammatory biomarkers in the management of advanced lung NEC patients.
Table 2.
Summary of published data for routine inflammatory biomarkers.
| Reference | Tumor | No. of patients | Study | Marker | Outcomes | Significance |
|---|---|---|---|---|---|---|
| Eun Young Kim et al.19 | SCLC | 186 | Retrospective | ALI | Low ALI Good prognosis |
Univariate
HR 2.10 95% CI 1.50 ± 2.94 p value <0.001 Multivariate HR 1.67 95% CI 1.17 ± 2.37 p value 0.004 |
| Masayuki Okui et al.7 | LCNEC | 1890 | Retrospective | NLR | High NLR Poor prognosis |
Multivariate
HR 8.559 95% CI 1.260–58.139 p value 0.028 |
| Laura Mezquita et al.8 | NSCLC | 466 | Retrospective | LIPI | High LIPI Poor prognosis |
3 months [95% CI, 1 month to not reached (NR)] versus 10 months (95% CI, 8 months to NR) versus 34 months (95% CI, 17 months to NR) for the poor, intermediate, and good LIPI groups, respectively (p < 0.001) |
| Xiuxiu Zhang et al.6 | SCLC | 4785 | Meta-analysis | LDH | High LDH Poor prognosis |
HR 1.45 95% CI 1.27–1.66 |
| Xuan Hong et al.21 | SCLC | 919 | Retrospective | NLR | High NLR Poor prognosis |
HR 0.908 95% CI 0.721–1.144 p value 0.413 |
| Min Deng et al.22 | SCLC | 320 | Retrospective | NLR, LDH | High NLR Poor prognosis High LDH Poor prognosis |
NLR
HR 1.35 95% CI 1.02–1.79 p value 0.039 LDH HR 1.46 95% CI 1.10–1.96 p value 0.010 |
| Dan Liu et al.24 | SCLC | 139 | Retrospective | NLR | High NLR Poor prognosis |
Univariate analysis
NLR >4.55 versus ⩽4.55 HR 3.309 95% CI 2.088–5.244 p value 0.000 Multivariate analysis HR 2.093 95% CI 1.079–4.063 p value 0.029 |
| Xin Wang et al.23 | SCLC | 153 | Retrospective | NLR | High NLR Poor prognosis |
HR 1.724 95% CI 1.116–2.663 p value 0.014 |
ALI, advanced lung cancer inflammation index; CI, confidence interval; HR, hazard ratio; LCNEC, large cell neuroendocrine carcinoma; LDH, lactate dehydrogenase; LIPI, lung inflammation prognostic index; NLR, neutrophil-to-lymphocyte ratio; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer.
Acknowledgments
The authors thank the patients and the study staff of both medical centers for their contributions to research. All authors thank Dr. Chiara Drago for her English revision.
Footnotes
Author contribution: Conception and design: MP, AG, AAG, VB, AR. Acquisition of data: AG, AAG, MP, CN, VG, MDT, AG, LI, NB, MC, AB, VB and AR. Analysis and interpretation of data: AG, MP and AAG. Drafting of the manuscript: AG, AAG, MP, SR, VB and AR. Critical revision of the manuscript for important intellectual content: CN, VG, MDT, AG, LI, NB, MC, AB, VB and AR. Statistical analysis: AG. Supervision: VB, CN.
Availability of data and materials: Data is available upon reasonable request.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: The trial protocol was previously approved by the Independent Ethic Committee at University of Palermo (approval number: 0003/2020).
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Antonio Russo  https://orcid.org/0000-0002-4370-2008
https://orcid.org/0000-0002-4370-2008
Contributor Information
Antonio Galvano, Department of Surgical, Oncological and Stomatological Sciences, Medical Oncology Unit, University of Palermo, Palermo, Italy.
Marta Peri, Department of Surgical, Oncological and Stomatological Sciences, Medical Oncology Unit, University of Palermo, Palermo, Italy.
Aurelia Ada Guarini, Department of Surgical, Oncological and Stomatological Sciences, Medical Oncology Unit, University of Palermo, Palermo, Italy.
Marta Castiglia, Department of Surgical, Oncological and Stomatological Sciences, Medical Oncology Unit, University of Palermo, Palermo, Italy.
Antonino Grassadonia, Department of Medical, Oral and Biotechnological Sciences University “G. D’Annunzio”, Chieti-Pescara, Italy.
Michele De Tursi, Department of Medical, Oral and Biotechnological Sciences University “G. D’Annunzio”, Chieti-Pescara, Italy.
Luciana Irtelli, Department of Medical, Oral and Biotechnological Sciences University “G. D’Annunzio”, Chieti-Pescara, Italy.
Sergio Rizzo, Department of Surgical, Oncological and Stomatological Sciences, Medical Oncology Unit, University of Palermo, Palermo, Italy.
Alessandro Bertani, Division of Thoracic Surgery and Lung Transplantation, IRCCS ISMETT – UPMC, Palermo, Italy.
Valerio Gristina, Department of Surgical, Oncological and Stomatological Sciences, Medical Oncology Unit, University of Palermo, Palermo, Italy.
Nadia Barraco, Department of Surgical, Oncological and Stomatological Sciences, Medical Oncology Unit, University of Palermo, Palermo, Italy.
Antonio Russo, Department of Surgical, Oncological and Stomatological Sciences, Medical Oncology Unit, University of Palermo, A.O.U.P. “P. Giaccone” University Hospital, Via del Vespro 129, Palermo, 90127, Italy.
Clara Natoli, Department of Medical, Oral and Biotechnological Sciences University “G. D’Annunzio”, Chieti-Pescara, Italy.
Viviana Bazan, Department of Experimental Biomedicine and Clinical Neurosciences, School of Medicine, University of Palermo, Palermo, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–1260. [DOI] [PubMed] [Google Scholar]
- 3. Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl. 6): vi99–105. [DOI] [PubMed] [Google Scholar]
- 4. Russo A, Russano M, Franchina T, et al. Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and outcomes with Nivolumab in Pretreated Non-Small Cell Lung Cancer (NSCLC): a large retrospective multicenter study. Adv Ther 2020; 37: 1145–1155. [DOI] [PubMed] [Google Scholar]
- 5. He X, Zhou T, Yang Y, et al. Advanced lung cancer inflammation index, a new prognostic score, predicts outcome in patients with small-cell lung cancer. Clin Lung Cancer 2015; 16: e165–e171. [DOI] [PubMed] [Google Scholar]
- 6. Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: a systematic review with meta-analysis. Cancer Biomark 2016; 16: 415–423. [DOI] [PubMed] [Google Scholar]
- 7. Okui M, Yamamichi T, Asakawa A, et al. Prognostic significance of neutrophil-lymphocyte ratios in large cell neuroendocrine carcinoma. Gen Thorac Cardiovasc Surg 2017; 65: 633–639. [DOI] [PubMed] [Google Scholar]
- 8. Cheng S, Evans WK, Stys-Norman D, et al. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2007; 2: 348–354. [DOI] [PubMed] [Google Scholar]
- 9. Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol 2018; 4: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalemkerian GP. Staging and imaging of small cell lung cancer. Cancer Imaging 2012; 11: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao L, Leung LH, Wang J, et al. Association between Charlson comorbidity index score and outcome in patients with stage IIIB-IV non-small cell lung cancer. BMC Pulm Med 2017; 17: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh N, Singh PS, Aggarwal AN, et al. Comorbidity assessment using Charlson comorbidity index and simplified comorbidity score and its association with clinical outcomes during first-line chemotherapy for lung cancer. Clin Lung Cancer 2016; 17: 205–213.e1. [DOI] [PubMed] [Google Scholar]
- 13. Weber MM, Fottner C. Immune checkpoint inhibitors in the treatment of patients with neuroendocrine neoplasia. Oncol Res Treat 2018; 41: 306–312. [DOI] [PubMed] [Google Scholar]
- 14. Bremnes RM, Sundstrom S, Aasebø U, et al. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer 2003; 39: 303–313. [DOI] [PubMed] [Google Scholar]
- 15. Tortorici S, Corrao S, Natoli G, et al. Prevalence and distribution of oral mucosal non-malignant lesions in the western Sicilian population. Minerva Stomatol 2016; 65: 191–206. [PubMed] [Google Scholar]
- 16. Albain KS, Crowley JJ, LeBlanc M, et al. Determinants of improved outcome in small-cell lung cancer: an analysis of the 2,580-patient Southwest Oncology Group data base. J Clin Oncol 1990; 8: 1563–1574. [DOI] [PubMed] [Google Scholar]
- 17. Lim JH, Ryu JS, Kim JH, et al. Gender as an independent prognostic factor in small-cell lung cancer: Inha Lung Cancer Cohort study using propensity score matching. PLoS One 2018; 13: e0208492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peri M, Botteri E, Pisa E, et al. A single-institution retrospective analysis of metachronous and synchronous metastatic bronchial neuroendocrine tumors. J Thorac Dis 2018; 10: 3928–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee CH, Lin C, Wang CY, et al. Premorbid BMI as a prognostic factor in small-cell lung cancer-a single institute experience. Oncotarget 2018; 9: 24642–24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Q, Xu Z, Chen X, et al. Clinicopathological characteristics and prognostic factors of pulmonary large cell neuroendocrine carcinoma: a large population-based analysis. Thorac Cancer 2019; 10: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379: 2220–2229. [DOI] [PubMed] [Google Scholar]
- 22. Kargl J, Busch SE, Yang GH, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun 2017; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016; 16: 431–446. [DOI] [PubMed] [Google Scholar]
- 24. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer 2016; 16: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishida S, Hashimoto I, Seike T, et al. Serum albumin levels correlate with inflammation rather than nutrition supply in burns patients: a retrospective study. J Med Invest 2014; 61: 361–368. [DOI] [PubMed] [Google Scholar]
- 27. Ouyang X, Dang Y, Zhang F, et al. Low serum albumin correlates with poor survival in gastric cancer patients. Clin Lab 2018; 64: 239–245. [DOI] [PubMed] [Google Scholar]
- 28. Brocco D, Di Marino P, Grassandonia A. From cachexia to obesity: the role of host metabolism in cancer immunotherapy. Curr Opin Support Palliat Care 2019; 13: 305–310. [DOI] [PubMed] [Google Scholar]
- 29. Kim EY, Kim N, Kim YS, et al. Prognostic significance of modified Advanced Lung Cancer Inflammation Index (ALI) in patients with small cell lung cancer_ comparison with original ALI. PLoS One 2016; 11: e0164056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyers DE, Stukalin I, Vallerand IA, et al. The lung immune prognostic index discriminates survival outcomes in patients with solid tumors treated with immune checkpoint inhibitors. Cancers (Basel) 2019; 11: 1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sorich MJ, Rowland A, Karapetis CS, et al. Evaluation of the lung immune prognostic index for prediction of survival and response in patients treated with atezolizumab for NSCLC: pooled analysis of clinical trials. J Thorac Oncol 2019; 14: 1440–1446. [DOI] [PubMed] [Google Scholar]
- 32. Kazandjian D, Gong Y, Keegan P, et al. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer. JAMA Oncol 2019; 5: 1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019; 4: e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 2015; 236: 297–304. [DOI] [PubMed] [Google Scholar]
- 35. Deng M, Ma X, Liang X, et al. Are pretreatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio useful in predicting the outcomes of patients with small-cell lung cancer? Oncotarget 2017; 8: 37200–37207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Teng F, Kong L, et al. Pretreatment neutrophil-to-lymphocyte ratio as a survival predictor for small-cell lung cancer. Onco Targets Ther 2016; 9: 5761–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]


