Abstract
Nodding syndrome is an uncommon epileptic disorder of childhood onset, which appears to occur exclusively in clusters in sub-Saharan Africa. It was first reported in the 1960s, in what is now southern Tanzania, then in Liberia, and later in South Sudan and northern Uganda, with both epidemic and endemic patterns described. The cause remains unknown. Here we describe the background and development of descriptions of the disorder, review its clinical features and summarize current theories and studies concerning its cause, outlining the principal remaining research questions relating to this highly unusual disease.
Keywords: Nodding syndrome, sub-Saharan Africa
Nodding syndrome is an uncommon, acquired, childhood-onset neurological disorder comprising epilepsy usually combined with cognitive and occasionally other systemic problems. It appears to occur in clusters exclusively in sub-Saharan Africa, in both epidemic and endemic patterns. Here we review its history and clinical features and current hypothesis concerning its cause.
Graphical Abstract
Graphical Abstract.
Introduction
Head nodding as a problematic neurological phenomenon first appeared in the medical literature in the mid-nineteenth century: over the next few decades two separate paediatric contexts became clear—first, head nodding combined with nystagmus and torticollis as part of what became known as the ‘spasmus nutans’ complex (‘nutans’ approximates to the Latin for ‘nodding’); and second, as an epileptic phenomenon in conjunction with ‘salaam’ attacks or West Syndrome in infants (Hadden, 1890; Gibson, 1909; Fukuyama, 2001). Neurologically, ‘nodding’ remained largely confined to these disorders until some 50 years ago when nodding and epilepsy in childhood were again associated—though constrained to rather specific circumstances and geography—in the context of a novel and discrete disorder.
Nodding syndrome (NS) is a childhood-onset epileptic disorder occurring in clusters in sub-Saharan Africa. Louise Jilek-Aall, who worked among the Wapogoro people of Mahenge, Tanzania, in the early 1960s, provided the first description—‘eight patients began two or three months before the major attacks with “nodding head” (to describe this the narrator always let his head fall on his chest)’ (Jilek-Aall, 1965) (Fig. 1). Attacks could be triggered by presentation with food. Later, impaired cognitive and physical development was common, and death not infrequently occurred from burns or drowning. Several instances of multiple affected siblings occurred; Jilek-Aall described considerable therapeutic success with phenobarbitone (Luminal).
Figure 1.

Louise Mathilde Aall-Jilek. Louise Mathilde Aall-Jilek in the Ulanga region, Tanzania, ca 1960. ‘A large number of villages and missions are accessible only on foot or by means of such canoes for many months of the year’ (Jilek-Aall, 1965).
A later study from the Gbawein and Wroughbarh clan region of Liberia described 123 patients with seizures, including 77 with ‘childhood-onset complex partial seizures.....mainly characterized by dorsoventral movements of the head’, often with ‘later secondary generalization’ (van der Waals et al., 1983). The authors stressed the similarities of the disorder—termed ‘see-ee’ in Bassa—‘both in respect to the clinical classification and epidemiology to the seizures described by Jilek-Aall’, and (without lasting success) named the disorder ‘epilepsia nutans’ (nodding epilepsy).
Fuller descriptions followed a decade later. Cases in Western Equatoria, South Sudan, reported to the World Health Organization in 1997, were retrospectively described in 2012 (Tumwine et al., 2012), when the case burden was estimated (by the South Sudanese Ministry of Health) at 5–7000 (WHO, 2012). Finally (so far), cases were reported in Uganda: in the west in 1994 (peer-reviewed description only appearing much later; Kaiser et al., 2015), and then in the Kitgum and Pader Districts of northern Uganda from 2007 onwards (Sejvar et al., 2013) (Fig. 2).
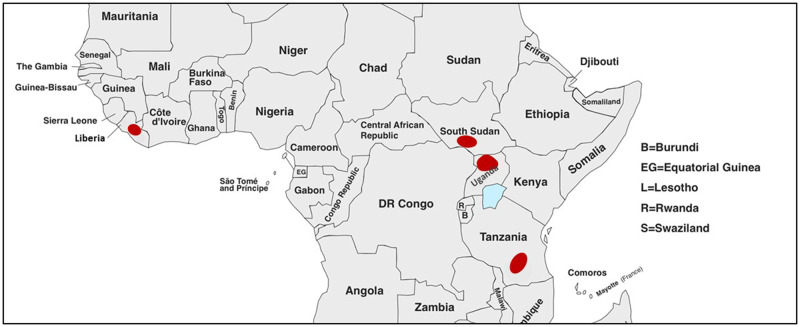
Figure 2.
Geographical distribution of nodding syndrome in sub-Saharan Africa. The earliest reports described cases in Ulanga, Tanzania, and Grand Bassa County, Liberia, later followed by clusters in Western Equatoria, South Sudan, and in the Acoli region of northern Uganda.
A more definitive description of NS, confirming its primarily epileptic nature, appeared in 2008, based on Tanzanian cases (Winkler et al., 2008). Finally, at a World Health Organization-convened consensus meeting in Kampala in 2012 (and follow-up meeting in 2015 in Gulu, northern Uganda; WHO, 2012; Spencer et al., 2016a), the term ‘nodding syndrome’ was agreed, and ‘harmonized case definitions were recommended to ensure consistent case identification for surveillance and treatment and comparable research results’ (Table 1).
Table 1.
Proposed case definitions for nodding syndrome (WHO, 2012)
|
Suspected case: (Used at the community level, primarily by marginally trained health teams when asking the mother/caretaker.)
|
|
Probable case: suspect case of head nodding with:
|
|
Confirmed case: is a probable case
|
ReproducedwithpermissionfromtheWHO.
Interestingly, a re-analysis of case records from Jilek-Aall’s original cohort (Jilek-Aall, 1965) in the light of the 2012 World Health Organization meeting concluded that this was indeed NS and noted that Jilek-Aall’s first recorded patient developed nodding symptoms in 1934 (dying in 1963) (Spencer et al., 2013a).
Curiously, while the original Tanzania and Liberia cases appeared endemic (Jilek-Aall, 1965; van der Waals et al., 1983), the South Sudan (Centers for Disease and Prevention, 2012; Tumwine et al., 2012) and northern Uganda (Sejvar et al., 2013) reports described epidemics. Currently, NS appears to be found in southern Tanzania and in northern Uganda and the adjacent southern and central states of South Sudan. In northern Uganda, no new cases are occurring; new cases are reported by non-governmental organizations (though not yet in the peer-reviewed literature) in South Sudan; whether this is true of Tanzania is uncertain.
The current scale of the problem is also uncertain, with no detailed epidemiological prevalence studies. A careful 2018 Uganda Ministry of Health survey documented 2143 cases in northern Uganda (Aceng, 2018). A household survey-based study in South Sudanese villages and communities known to host the disease reported a prevalence of NS among 5–18 year-olds of 5–8%, considered comparable to northern Uganda (Tumwine et al., 2012).
Clinical features, course and diagnosis
NS begins between the ages of 5 and 16 (Jilek-Aall’s 33 cases included a single individual outside this range, with onset at 22 years; Spencer et al., 2013a). The disorder is characterized by repetitive head nodding, 5–20 nods per minute, resulting from momentary but repeated loss of neck extensor tone. Occasionally, an associated loss of tone in the upper trunk and shoulders occurs. Jilek-Aall described a 7-year-old boy whose ‘head constantly dropped to his chest, so that he almost lost his balance and stumbled’, and a ‘nine-year-old boy had to seat himself for almost half an hour every morning with nodding head…. Afterwards he ran about and played quite normally’ (Jilek-Aall, 1965). Remarkably, being presented with food commonly provoked nodding (Jilek-Aall, 1965; Winkler et al., 2008; Idro et al., 2013b; Sejvar et al., 2013). Cold weather could also trigger attacks (Winkler et al., 2008; Idro et al., 2013b; Sejvar et al., 2013). In some cases, nodding was associated with impaired awareness; other affected individuals continued eating and interacting between nods during episodes (Sejvar et al., 2013).
In most cases, after a period of months to a year or more, focal onset to bilateral tonic-clonic fits follow. Focal impaired awareness seizures, tonic-clonic and atypical absence seizures are all reported. Approximately 40% have some form of aura (Spencer et al., 2013a). Photosensitivity may be seen (Jilek-Aall, 1965; van der Waals et al., 1983), bilateral tonic-clonic seizures triggered by the flickering of open fires or sunlight on water helping to explain the widely reported occurrence of burns and of death by drowning (Jilek-Aall, 1965; van der Waals et al., 1983; Tumwine et al., 2012; Sejvar et al., 2013; Kaiser et al., 2015). Status epilepticus represents a third cause of death, accounting for almost 25% of deaths in Jilek-Aall’s Tanzanian cases (Spencer et al., 2013a).
However, NS is not solely an epileptic disorder (Jilek-Aall, 1965; van der Waals et al., 1983). Failure of cognitive development and physical growth retardation are common (Idro et al., 2013b; Sejvar et al., 2013)—though the extent to which poor nutrition and school dropout contribute is unclear. Whether the frequently reported psychological and psychiatric manifestations, including mood changes, sleep disturbances, major depression, aggressive outbursts and episodes of wandering (Spencer et al., 2016a) are distinct, non-epileptic features, reactive secondary consequences, or at least partially explained by seizure events, is similarly uncertain. Reports of catatonia strongly suggest primary psychiatric disorder (Kakooza-Mwesige et al., 2015). Notwithstanding Jilek-Aall’s report of ‘gait disturbance and clumsy movements’ (which she attributed to vitamin deficiencies), and mention of pyramidal, extrapyramidal and cerebellar signs in Liberian NS cases (van der Waals et al., 1983; Spencer et al., 2013a), neurological signs later came to be considered very uncommon (Idro et al., 2013b; Sejvar et al., 2013; Idro et al., 2018).
The natural history of NS also remains unclear. Some authors describe a prodromal phase, with dizziness and lethargy but no explicit mention of seizures (Idro et al., 2018). Most, but not all sufferers progress from nodding attacks to other seizure types. Some of this latter group develop additional cognitive, psychological and/or physical problems—with the caveat mentioned above, namely that these difficulties may be intrinsic to the disorder or represent secondary consequences of repeated seizures and/or social and educational isolation (Idro et al., 2018). Also uncertain is whether such individuals, who appear to have progressed through recognizable if overlapping stages (Idro et al., 2018), then enter a chronic but stable phase, or whether they continue inexorably to progress to a severely disabled and then fatal outcome. Early anecdotal reports of NS described a rapidly progressive course from the onset of nodding, with increasing neurological and cognitive disabilities, then progressive deterioration of consciousness and death (Lacey, 2003; Wasswa, 2012; Idro et al., 2018). But one of the few studies with follow-up (12 Ugandan patients over 8 months) reported no general neurological deterioration, some worsening in seizures (frequency or type) in six patients, no improvement in any and no deaths (Sejvar et al., 2013). No studies report any instance of spontaneous recovery.
Clinical electrophysiology, imaging and spinal fluid in NS
EEGs in 10 patients were normal in four, non-specifically abnormal (including diffuse slowing) in four, and showed more clearly epileptic interictal activity in two, with intermittent generalized slowing and sharp wave activity (Winkler et al., 2008).
Later studies differed, reporting interictal changes in the great majority of cases (Tumwine et al., 2012; Idro et al., 2013b; Sejvar et al., 2013). In one, epileptiform activity comprised frequent runs of generalized, 2.5–3.0 Hz spike-and-slow-wave activity, multifocal spikes, and polyspike-and-wave activity, without clinical accompaniment (Sejvar et al., 2013). Others, however, found no consistent frequency to spikes or sharp waves (Idro et al., 2013b).
Nodding attacks, captured during video-EEG in two children, were ‘associated with generalized electrodecrement along with drop-out of cervical paraspinal electromyography, followed by generalized sharply contoured rhythmic theta activity, findings consistent with atonic seizures’ (Sejvar et al., 2013). None of the studies sought EEG-photosensitivity.
The clinical and electrophysiological picture is therefore complex: multiple seizure types occur. ‘Simple’ nodding spells represent atonic attacks, often occurring as a form of reflex epilepsy. Later, tonic-clonic, myoclonic and atypical absence seizures, with both focal and generalized onset, are seen, with abnormal interictal EEG including slow spikewave and polyspike-and-wave activity (Idro et al., 2013b; Sejvar et al., 2013).
MRI reports, including 48 NS children in total, describe no disease-specific changes and no aetiological clues. Some scans were normal; many showed cortical and/or cerebellar atrophy (Winkler et al., 2008; Idro et al., 2013b; Sejvar et al., 2013; Winkler et al., 2013). Some report unilateral (either side) or bilateral hippocampal sclerosis or atrophy; some describe sub-cortical gliotic lesions (‘dot-like’ hyperintensities) on T2-weighted and fluid-attenuated inversion recovery (FLAIR) scans in any of the four lobes (Winkler et al., 2008; Winkler et al., 2013). Others reported no such cortical lesions, and normal hippocampi (Idro et al., 2013b; Sejvar et al., 2013).
CSF examination has been similarly uninformative: most report normal cell counts and protein. Oligoclonal bands were not sought in either of the principal studies (Winkler et al., 2008; Sejvar et al., 2013). (One study published only in abstract form, studying three siblings, reported isolated oligoclonal bands in one; Soldatos et al., 2015.) Other studies report performing spinal fluid tests, but included no results in their accounts (Tumwine et al., 2012; Foltz et al., 2013). The difficulties of conducting such analyses given the often very remote location of patients (Fig. 3) are substantial.
Figure 3.
Nodding syndrome in Uganda. A community affected by Nodding Syndrome in Pader District, northern Uganda (left). The great majority live in remote rural settings, presenting practical challenges in particular to neurological investigations (right).
Treatment response
In these settings, most anti-epileptic drugs are unavailable, unaffordable or both. Phenytoin, carbamazepine and sodium valproate are accessible; phenobarbital is also used. There have been no randomized controlled treatment trials, but retrospective assessments of therapeutic responsivity are reported.
In the first, Tanzanian cases, a striking response was reported to only small doses of Luminal (phenobarbital)—‘more than half….could be kept free of fits’ with phenobarbital (Jilek-Aall, 1965). NS in Liberia appeared less responsive (van der Waals et al., 1983).
Subsequent reports confirmed a seizure reduction of 50% (from 22.1 ± 21.7 seizures per month to 10.9 ± 11.5), using (mostly) either phenobarbital or phenytoin (Winkler et al., 2008). There was a similar >50% reduction in seizure frequency in 22 Ugandan children with sodium valproate monotherapy (Idro et al., 2013b). In the largest study so far (484 northern Ugandan patients), a >70% seizure frequency reduction occurred with valproate monotherapy (Idro et al., 2014). The Ugandan Ministry of Health clinical management response included a formal set of treatment guidelines (Idro et al., 2013a). Sadly, a very recent later follow-up found low levels of implementation of and adherence to these guidelines, mostly because of financial constraints (Abbo et al., 2019).
Perhaps unsurprisingly, the general benefits of targeted dietary supplementation in NS children are clear (Gazda and Kitara, 2018). Furthermore, management of behavioural and emotional difficulties, nutritional therapy and physical rehabilitation significantly improved levels of independence in basic self-care, school enrolment and behavioural and emotional symptomatology (Idro et al., 2014).
Possible aetiological mechanisms
Many hypothetical causes of NS have been advanced, some rather speculative, others with more persuasive supporting evidence, but none proven. Prions, climate change (Donnelly, 2012), antibody-mediated channel blockade, monkey meat consumption (Lacey, 2003), toxic residue from munitions (Lacey, 2003), mitochondrial dysfunction, toxic traditional medicines, forms of autism (Spencer et al., 2016a), neuropsychiatric trauma precipitated by war exposure (Musisi et al., 2013) and measles have all been invoked.
Jilek-Aall herself postulated unspecified nutritional deficiency. The Wapogoro tribe were conspicuous even within the local geography and amongst neighbouring tribes for their ‘extreme poverty….always on the verge of starvation…with famine every year.’ Wapogoro nodding patients were all ‘chronic sufferers from malnutrition, avitaminosis and anaemia…with hook and round worms, chronic malaria, bilharzia and amoebiasis in almost every stool’ (Jilek-Aall, 1965). Two studies have reported B6 hypovitaminosis (Donnelly, 2012; Obol et al., 2016), though NS would be most unlike the known manifestations of B6 deficiency. Others reported no reduction in vitamin B6 levels in NS cases (Kyu et al., 2017)—and no association with a history of malnutrition (Kyu et al., 2017).
Alternative diet-associated possibilities have included food-related toxins. A northern Uganda CDC case-control study associated NS with consumption of (unspecified) ‘crushed roots’ used in traditional medicine (Foltz et al., 2013). No other dietary components, including red sorghum grain, a major dietary component previously anecdotally implicated, were associated. A later case-control study in the same area differed, finding no link to cassava. Rather, an association with the consumption of World Food Programme emergency food was reported, particularly maize that had become mouldy (Spencer et al., 2016b). A link with (mouldy) food aid and Internally Displaced Person (IDP) camps would be consistent with the two epidemic-like occurrences of NS in Northern Uganda and South Sudan, but plainly could not explain the more endemic NS in Tanzania and Liberia. But, as Spencer has pointed out, ‘the NS-susceptible Pogoro people of Tanzania show parallels with the NS-susceptible [South Sudan population] given their probable common…dependence on [foods] subject to spoilage and fungal contamination’ (Spencer et al., 2016b).
However, a subsequent comprehensive exploration of mycotoxins in a range of grains found no evidence to associate NS with consumption of mycotoxins in maize or any other contaminated food. The levels of aflatoxin and ochratoxin in maize were no different to those in any of the grain types tested; and there was no correlation between the total concentration of any of the various types of mycotoxin with the presence of children with NS in households (Echodu et al., 2018)—though to exclude this possibility more definitively, testing immediately at the onset of the disease would be required. Mycotoxins are widespread in core food products throughout Africa, including maize, spices and groundnuts (Darwish et al., 2014), rather starkly contrasting with the highly (oligo-)focal distribution of NS, also militating against their involvement.
Also related to IDP camps—and more specifically, conflict—is an alternative neurotoxin hypothesis, namely that munitions-related chemical or biological agents are implicated. An elegant epidemiological study explored the relationship between the emergence of NS in northern Uganda and conflict. Case incidence showed clear and striking peaks 5–6 years after peaks in wartime conflicts and deaths. NS peaks similarly lagged 5–7 years after the movement of families into internally displaced person camps (Landis et al., 2014). A case-control study of Ugandan children suggested that (self-reported) exposure to munitions was associated with NS (Foltz et al., 2013).
Others, however, failed to reproduce this association in South Sudan NS cases (Centers for Disease and Prevention, 2012; Tumwine et al., 2012; Spencer et al., 2013b). The absence of NS in other theatres of war in Africa (or elsewhere), and the occurrence of NS as an endemic disease in non-warfare situations, both also strongly weigh against this possibility. In particular, the description of NS with an onset in 1934 (Spencer et al., 2013a), long pre-dating the introduction to rural sub-Saharan African of agricultural or weaponized artificial chemicals, helps exclude man-made toxins as a potential cause.
Infectious—or infection-related—causes for NS have also been implicated, principally onchocerciasis (river blindness). Neither original report from Tanzania or Liberia mention river blindness, but both emphasized the extremely common co-incidence of various infections in nodding-affected children (Jilek-Aall, 1965; van der Waals et al., 1983), and the specific areas studied both have a high onchocerciasis prevalence (Spencer et al., 2013a).
Despite casual observations of a link, however, the first detailed study suggested an association was at best ‘debatable’ (Winkler et al., 2008)—and spinal fluid polymerase chain reaction (PCR) and other CSF tests for Onchocerca volvulus, were negative in all patients. Other, later case-control studies reported a positive link with onchocercal infection (Tumwine et al., 2012; Foltz et al., 2013)—though the consistently negative CSF PCR tests for onchocercal DNA in NS patients have been replicated (Winkler et al., 2013).
Others assert that O. volvulus is only a ‘bystander’ in NS (Spencer et al., 2016b; Spencer et al., 2017), swayed by the absence of any evidence of onchocerciasis in a significant proportion of patients with NS, the absence of any evidence of CNS infiltration in any NS patients, the widespread global distribution of onchocerciasis, contrasting with the highly focal and limited incidence of NS and then finally the lack of age restriction in onchocercal infection contrasting with the consistent age-related onset of NS.
An alternative onchocerciasis-based hypothesis proposes that, rather than direct infection, the organism triggers auto-immune epilepsy by generating cross-reactive antibodies. Recently, leiomodin-1 antibodies were reported in both the sera and CSF of some 53% of NS patients, compared to 31% unaffected controls from the same village (Johnson et al., 2017). Leiomodin-1 was expressed in human neurons in vitro - and, at least in the rodent, is also expressed in CNS neuronal populations in vivo (Takebayashi et al., 2009); anti-leiomodin-1 antibodies were neurotoxic in vitro; and NS patient-derived leiomodin-1 antibodies cross-reacted with O. volvulus antigens (Johnson et al., 2017).
This hypothesis is arguably supported indirectly by the suggestion that onchocerciasis causes river blindness by comparable mechanisms—not directly infecting the eye, but eliciting a secondary inflammatory response to some of its antigens (McKechnie et al., 2002). The problem, however, remains that river blindness is seen globally, wherever ochocerciasis occurs, in stark contrast to NS. In addition, almost half of NS patients have no leiomodin antibodies, while a third of normal controls do have antibodies. Leiomodin is also strongly expressed in muscle, which appears unaffected in NS.
Concerning the related if less exotic immunological possibility of ion channel-mediated autoimmunity, no evidence of circulating anti-N-methyl-d-aspartate (NMDA) or voltage gated potassium channel-complex (VGKC) antibodies were found in NS (Dietmann et al., 2014).
Finally, autoimmune suggestions receive no support from the limited number of published autopsy studies, which report none of the inflammatory changes seen in ion channel antibody-mediated encephalitis (Winkler et al., 2013; Idro et al., 2016; Hotterbeekx et al., 2019).
Measles virus has also been implicated, with NS suggested as a post-measles complication in malnourished children, based partly on the anecdotal (but likely incorrect) hypothesis that the clinical features and course of NS—an inevitable progression to death—were comparable to sub-acute sclerosing panencephalitis (Spencer et al., 2016b). A case-control study in northern Uganda reported a significant association of NS with prior measles infection (Spencer et al., 2016b). However, other NS studies, in south Sudan and in the same area of northern Uganda, found no measles relationship (Tumwine et al., 2012; Foltz et al., 2013). Autopsy studies also showed no evidence of viral or other infection (Winkler et al., 2013; Hotterbeekx et al., 2019).
Finally on the infectious front, one South Sudan study reported a link with Mansonella perstans infection (a midge-borne filarial nematode; Tumwine et al., 2012). Confirmation has not been forthcoming, and the incidence of Mansonella perstans-related disease in numerous areas where NS is not seen, together with the absence of evidence of parasite infection in autopsy studies (Pollanen et al., 2018; Hotterbeekx et al., 2019) challenge this suggestion.
Autopsy studies are often invaluable in elucidating disease origins, and a significant advance appeared possible from the first major study in NS, which proposed that the disorder was a novel tauopathy (Pollanen et al., 2018). Neuropathologic findings in five northern Ugandan NS cases revealed tau-positive neuronal neurofibrillary tangles, pre-tangles, neuropil threads, and ‘dot-like lesions’ in the cerebral cortex, subcortical nuclei and brainstem. The authors also reported neurofibrillary tangles and threads in the mesencephalopontine tegmental nuclei, substantia nigra and locus coeruleus (Pollanen et al., 2018).
However, as the authors themselves cautiously pointed out, both chronic trauma and repeated seizures can precipitate tau deposition (McKee et al., 2009; Tai et al., 2016)—indeed they drew attention to partial similarities between the patchy cortical involvement in NS and that of chronic traumatic encephalopathy. (Seizure control in NS patients being notoriously poor, repeated head trauma is almost invariable.) The stable, non-progressive course in many NS individuals also militates against a primary tauopathy. Other authors have pointed out the absence of control brain studies (local chronic, non-NS epilepsy, for example), while unpublished NS autopsy studies showing no tau deposits are described (Spinney, 2018).
A more recent autopsy study described cerebellar atrophy and Purkinje cell loss—but no generalized tauopathy. Rather, changes implying ‘past ventriculitis’ were reported in four of the five affected brains. Clusters of CD68-positive macrophages were observed in all cases (Hotterbeekx et al., 2019).
Other unpublished observations include findings of ‘polarizable crystal-like materials of different sizes, mainly in the brainstem, but also in the white matter’. These elicited no local inflammatory reaction, and their nature was unknown (Idro et al., 2016). But others found no such deposits and suggested they were ‘Buscaino bodies,’ post-mortem artefacts (Hotterbeekx et al., 2018). Indeed, the original description indicated that the bodies dissolved when brains were stored in 70% alcohol (Idro et al., 2016)—and promptly harvesting and transporting brains from remote areas in an equatorial climate presents major logistical difficulties.
Discussion and outstanding questions
Much about NS remains unclear. That we do not know the cause of the disorder is perhaps least surprising—the number of acquired neurological diseases whose aetiology is well understood is not large. The idiosyncrasies of NS—its highly focal (or oligo-focal) localization in four non-contiguous parts of Africa, its apparent occurrence in both epidemic and endemic forms, that the former appear time-restricted (no new cases in northern Uganda since 2012, for example), its curious age sensitivity (onset exclusively between the 5 and 16 years), to name but four—are tantalizing, but so far have not, despite significant efforts, yielded aetiological answers.
Many questions also remain about its fundamental clinical nature. We are not at all certain of its natural history. Nodding is an early feature, but the proportion who develop other forms of seizure, or other neurological features, and the clinical course itself remain to be clarified. Early reports of rapid progression leading inexorably to death have not been substantiated. While some individuals die from (presumably) seizure-related drowning or burns, and some from status epilepticus (Spencer et al., 2013a), whether there are other causes of mortality in NS is unknown. We are not even certain whether the syndrome varies between locations—comparative studies are lacking. The imaging findings are non-specific, with small but significant differences between studies. Electroencephalographic changes are well-reported, though without exploration of photosensitivity, despite clear suggestions of bright or flashing light-induced seizures from the earliest clinical NS descriptions. Many aspects of the neuropathology remain uncertain. The extremely remote location of the great majority of sufferers helps explain some of these areas of uncertainty.
Still, it is important to remember that the disorder was only accepted as a distinct epileptic entity a decade ago (Winkler et al., 2008), and much has been learned since then. There are now many known knowns, with a clear list of known unknowns. Much more will doubtless emerge in the next 10 years, highly likely including a sprinkling of unknown unknowns. Given the frequency with which an understanding of rare disease provides major insights into common disorders, perhaps particularly neurological, this area may be both interesting and profitable to observe.
Funding
We are grateful to the Burden Neurological Institute for financial support for this study.
Competing interests
The authors report no competing interests.
Glossary
- NS =
nodding syndrome
References
- Abbo C, Mwaka AD, Opar BT, Idro R.. Qualitative evaluation of the outcomes of care and treatment for children and adolescents with nodding syndrome and other epilepsies in Uganda. Infect Dis Poverty 2019; 8: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceng J. Statement on Nodding Syndrome in Northern Uganda. Uganda Ministry of Health 2018. https://www.health.go.ug/download/file/fid/1800 (December 2019, date last accessed).
- Centers for Disease and Prevention. Nodding syndrome—South Sudan, 2011. MMWR Morb Mortal Wkly Rep 2012; 61: 52–4. [PubMed] [Google Scholar]
- Darwish WS, Ikenaka Y, Nakayama SM, Ishizuka M.. An overview on mycotoxin contamination of foods in Africa. J Vet Med Sci 2014; 76: 789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietmann A, Wallner B, Konig R, Friedrich K, Pfausler B, Deisenhammer F, et al. Nodding syndrome in Tanzania may not be associated with circulating anti-NMDA-and anti-VGKC receptor antibodies or decreased pyridoxal phosphate serum levels-a pilot study. Afr Health Sci 2014; 14: 434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly J. CDC planning trial for mysterious nodding syndrome. Lancet (London, England) 2012; 379: 299. [DOI] [PubMed] [Google Scholar]
- Echodu R, Edema H, Malinga GM, Hendy A, Colebunders R, Moriku Kaducu J, et al. Is nodding syndrome in northern Uganda linked to consumption of mycotoxin contaminated food grains? BMC Res Notes 2018; 11: 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz JL, Makumbi I, Sejvar JJ, Malimbo M, Ndyomugyenyi R, Atai-Omoruto AD, et al. An epidemiologic investigation of potential risk factors for nodding syndrome in Kitgum District, Uganda. PLoS One 2013; 8: e66419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama Y. History of clinical identification of West syndrome–in quest after the classic. Brain Dev 2001; 23: 779–87. [DOI] [PubMed] [Google Scholar]
- Gazda S, Kitara DL.. Treatment and rehabilitation outcomes of children affected with nodding syndrome in Northern Uganda: a descriptive case series. Pan Afr Med J 2018; 29: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C. Two cases of head-nodding in infants. Br Med J 1909; 2: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden WB. On head-nodding and head-jerking in children, commonly associated with nystagmus. Lancet 1890; 135: 1293–5. [Google Scholar]
- Hotterbeekx A, Lammens M, Idro R, Akun PR, Lukande R, Akena G, et al. Neuroinflammation and not tauopathy is a predominant pathological signature of nodding syndrome. J Neuropathol Exp Neurol 2019; 78: 1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotterbeekx A, Onzivua S, Menon S, Colebunders R.. Histological examination of post-mortem brains of children with nodding syndrome. Ann Transl Med 2018; 6: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idro R, Musubire KA, Byamah Mutamba B, Namusoke H, Muron J, Abbo C, et al. Proposed guidelines for the management of nodding syndrome. Afr Health Sci 2013. a; 13: 219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idro R, Namusoke H, Abbo C, Mutamba BB, Kakooza-Mwesige A, Opoka RO, et al. Patients with nodding syndrome in Uganda improve with symptomatic treatment: a cross-sectional study. BMJ Open 2014; 4: e006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idro R, Ogwang R, Kayongo E, Gumisiriza N, Lanyero A, Kakooza-Mwesige A, et al. The natural history of nodding syndrome. Epileptic Disord 2018; 20: 508–16. [DOI] [PubMed] [Google Scholar]
- Idro R, Opar B, Wamala J, Abbo C, Onzivua S, Mwaka DA, et al. Is nodding syndrome an Onchocerca volvulus-induced neuroinflammatory disorder? Uganda’s story of research in understanding the disease. Int J Infect Dis 2016; 45: 112–7. [DOI] [PubMed] [Google Scholar]
- Idro R, Opoka RO, Aanyu HT, Kakooza-Mwesige A, Piloya-Were T, Namusoke H, et al. Nodding syndrome in Ugandan children–clinical features, brain imaging and complications: a case series. BMJ Open 2013. b; 3: e002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek-Aall LM. Epilepsy in the Wapogoro tribe in Tanganyika. Acta Psychiat Scand 1965; 41: 57–86. [Google Scholar]
- Johnson TP, Tyagi R, Lee PR, Lee MH, Johnson KR, Kowalak J, et al. Nodding syndrome may be an autoimmune reaction to the parasitic worm Onchocerca volvulus. Sci Transl Med 2017; 9. pii: eaaf6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C, Rubaale T, Tukesiga E, Kipp W, Asaba G.. Nodding syndrome, western Uganda, 1994. Am J Trop Med Hyg 2015; 93: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakooza-Mwesige A, Dhossche DM, Idro R, Akena D, Nalugya J, Opar BT.. Catatonia in Ugandan children with nodding syndrome and effects of treatment with lorazepam: a pilot study. BMC Res Notes 2015; 8: 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyu HH, Mumford JE, Stanaway JD, Barber RM, Hancock JR, Vos T, et al. Mortality from tetanus between 1990 and 2015: findings from the global burden of disease study 2015. BMC Public Health 2017; 17: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M. Nodding disease: mystery of southern Sudan. Lancet Neurol 2003; 2: 714. [DOI] [PubMed] [Google Scholar]
- Landis JL, Palmer VS, Spencer PS.. Nodding syndrome in Kitgum District, Uganda: association with conflict and internal displacement. BMJ Open 2014; 4: e006195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKechnie NM, Gurr W, Yamada H, Copland D, Braun G.. Antigenic mimicry: Onchocerca volvulus antigen-specific T cells and ocular inflammation. Invest Ophthalmol Vis Sci 2002; 43: 411–8. [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009; 68: 709–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musisi S, Akena D, Nakimuli-Mpungu E, Abbo C, Okello J.. Neuropsychiatric perspectives on nodding syndrome in northern Uganda: a case series study and a review of the literature. Afr Health Sci 2013; 13: 205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obol JH, Arony DA, Wanyama R, Moi KL, Bodo B, Odong PW, et al. Reduced plasma concentrations of vitamin B6 and increased plasma concentrations of the neurotoxin 3-hydroxykynurenine are associated with nodding syndrome: a case control study in Gulu and Amuru districts, Northern Uganda. Pan Afr Med J 2016; 24: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollanen MS, Onzivua S, Robertson J, McKeever PM, Olawa F, Kitara DL, et al. Nodding syndrome in Uganda is a tauopathy. Acta Neuropathol 2018; 136: 691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ, Kakooza AM, Foltz JL, Makumbi I, Atai-Omoruto AD, Malimbo M, et al. Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: an observational case series. Lancet Neurol 2013; 12: 166–74. [DOI] [PubMed] [Google Scholar]
- Soldatos A, Nutman T, Groden C, Wahl C, Inati S, Buckler G, et al. Evaluation and immunomodulatory treatment at the NIH of children with nodding syndrome from Northern Uganda (S37.005). Neurology 2015; 84: S37.005. [Google Scholar]
- Spencer PS, Kitara DL, Gazda SK, Winkler AS.. Nodding syndrome: 2015 International Conference Report and Gulu Accord. eNeurologicalSci 2016. a; 3: 80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PS, Mazumder R, Palmer VS, Lasarev MR, Stadnik RC, King P, et al. Environmental, dietary and case-control study of Nodding Syndrome in Uganda: a post-measles brain disorder triggered by malnutrition? J Neurol Sci 2016. b; 369: 191–203. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Palmer VS, Jilek-Aall L.. Nodding syndrome: origins and natural history of a longstanding epileptic disorder in sub-Saharan Africa. Afr Health Sci 2013. a; 13: 176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PS, Schmutzhard E, Winkler AS.. Nodding syndrome in the spotlight—placing recent findings in perspective. Trends Parasitol 2017; 33: 490–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer PS, Vandemaele K, Richer M, Palmer VS, Chungong S, Anker M, et al. Nodding syndrome in Mundri county, South Sudan: environmental, nutritional and infectious factors. Afr Health Sci 2013. b; 13: 183–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinney L. Link to Alzheimer’s seen in nodding syndrome. Science 2018; 362: 1341. [DOI] [PubMed] [Google Scholar]
- Tai XY, Koepp M, Duncan JS, Fox N, Thompson P, Baxendale S, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain 2016; 139: 2441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Yamamoto N, Umino A, Nishikawa T.. Developmentally regulated and thalamus-selective induction of leiomodin2 gene by a schizophrenomimetic, phencyclidine, in the rat. Int J Neuropsychopharmacol 2009; 12: 1111–26. [DOI] [PubMed] [Google Scholar]
- Tumwine JK, Vandemaele K, Chungong S, Richer M, Anker M, Ayana Y, et al. Clinical and epidemiologic characteristics of nodding syndrome in Mundri County, southern Sudan. Afr Health Sci 2012; 12: 242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waals FW, Goudsmit J, Gajdusek S. ee-ee: clinical characteristics of highly prevalent seizure disorders in the Gbawein and Wroughbarh Clan Region of Grand Bassa County, Liberia. Neuroepidemiology 1983; 2: 35–44. [Google Scholar]
- WHO. International scientific meeting on Nodding Syndrome. World Health Organization: Kampala; 2012. [Google Scholar]
- Wasswa H. Ugandan authorities deal with a mysterious ailment that leaves people nodding continuously. BMJ 2012; 344: e349. [DOI] [PubMed] [Google Scholar]
- Winkler AS, Friedrich K, Konig R, Meindl M, Helbok R, Unterberger I, et al. The head nodding syndrome–clinical classification and possible causes. Epilepsia 2008; 49: 2008–15. [DOI] [PubMed] [Google Scholar]
- Winkler AS, Friedrich K, Velicheti S, Dharsee J, Konig R, Nassri A, et al. MRI findings in people with epilepsy and nodding syndrome in an area endemic for onchocerciasis: an observational study. Afr Health Sci 2013; 13: 529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]