Abstract
Levodopa is the first-line treatment for Parkinson’s disease, although the precise mechanisms mediating its efficacy remain elusive. We aimed to elucidate treatment effects of levodopa on brain activity during the execution of fine movements and to compare them with deep brain stimulation of the subthalamic nuclei. We studied 32 patients with Parkinson’s disease using functional MRI during the execution of finger-tapping task, alternating epochs of movement and rest. The task was performed after withdrawal and administration of a single levodopa dose. A subgroup of patients (n = 18) repeated the experiment after electrode implantation with stimulator on and off. Investigating levodopa treatment, we found a significant interaction between both factors of treatment state (off, on) and experimental task (finger tapping, rest) in bilateral putamen, but not in other motor regions. Specifically, during the off state of levodopa medication, activity in the putamen at rest was higher than during tapping. This represents an aberrant activity pattern probably indicating the derangement of basal ganglia network activity due to the lack of dopaminergic input. Levodopa medication reverted this pattern, so that putaminal activity during finger tapping was higher than during rest, as previously described in healthy controls. Within-group comparison with deep brain stimulation underlines the specificity of our findings with levodopa treatment. Indeed, a significant interaction was observed between treatment approach (levodopa, deep brain stimulation) and treatment state (off, on) in bilateral putamen. Our functional MRI study compared for the first time the differential effects of levodopa treatment and deep brain stimulation on brain motor activity. We showed modulatory effects of levodopa on brain activity of the putamen during finger movement execution, which were not observed with deep brain stimulation.
Keywords: Parkinson’s disease, deep brain stimulation, functional magnetic resonance imaging, levodopa, dopaminergic treatment
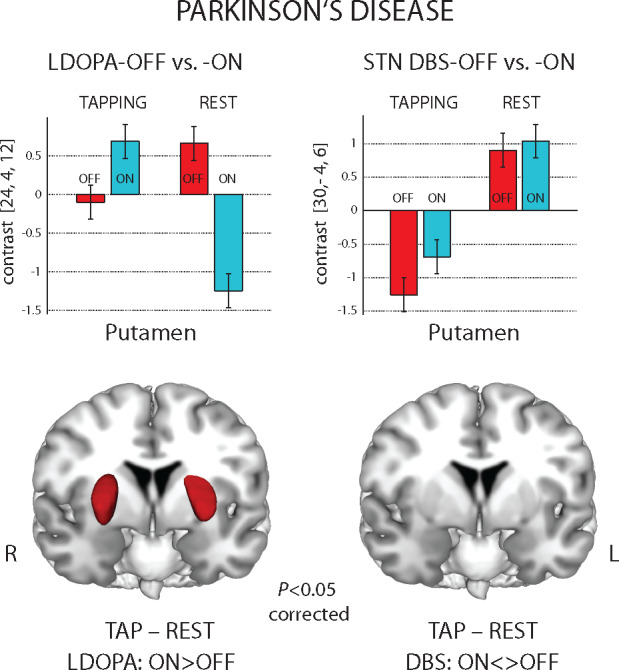
The study shows fundamentally different effects of symptomatic treatment in Parkinson's disease on activity of motor network during motion and rest. The decreased motion-related activity in the putamen after medication withdrawal was reversed by levodopa but not with subthalamic deep brain stimulation.
Graphical Abstract
Graphical Abstract.
Introduction
Parkinson’s disease is a frequent neurodegenerative disease characterized by progressive and relentless loss of motor functions (Fahn, 2006; Poewe et al., 2017). Cardinal motor symptoms with diagnostic validity for Parkinson’s disease are bradykinesia combined with rigidity and/or tremor at rest (Postuma et al., 2015). These symptoms are often accompanied by the loss of postural reflexes, forward bended posture and freezing and also by non-motor manifestations, such as sleep disturbances, constipation, mild cognitive impairment and hallucinations (Fahn et al., 2011). The symptomatology is burdensome for patients and limits everyday activities. No therapeutic option is currently available to stop or revert the neurodegenerative process, but symptomatic treatments are effective and widely used. The most common pharmacological treatments for Parkinson’s disease target the dopaminergic system, exploiting either levodopa (LDOPA), a precursor of dopamine in the brain, or dopamine agonists (Connolly and Lang, 2014). Indeed, a marked loss of dopaminergic nigro-striatal neurons is the most evident brain abnormality in Parkinson’s disease, and the pharmacological restoration of dopamine transmission leads to considerable symptom attenuation (Poewe et al., 2017). Therefore, LDOPA and dopamine agonists represent the foundation of Parkinson’s disease therapy for decades and are the first-choice interventions in early disease stages. Although LDOPA treatment was already introduced in 1961, its precise mechanisms of action in the brain were largely unknown (Hornykiewicz, 2010). Several studies tried to clarify how LDOPA modulates brain functions. In the last two decades, functional MRI (fMRI) studies have investigated the brain activity in patients during the execution of simple movements while unmedicated [levodopa, medication off state (LDOPA-OFF)] and/or after taking the medication [levodopa, medication on state (LDOPA-ON)] (Haslinger et al., 2001; Rowe et al., 2008; Kraft et al., 2009; Maillet et al., 2012; Herz et al., 2014; Michely et al., 2015). Herz et al. (2014) performed a meta-analysis showing that, during movements, Parkinson’s disease patients in the LDOPA-OFF state compared with controls presented a reduced activity in the posterior putamen and a mixed pattern of increased and decreased functional activations in cortical regions, encompassing supplementary and primary motor areas and inferior and superior parietal lobes. Of note, dopaminergic medication attenuated the deficits in the posterior putamen and reduced the hyperactivation of the primary motor cortex. However, studies included in the meta-analysis presented heterogeneous movement paradigms and experimental protocols, thus limiting the generalizability of the findings. Previous results (Holiga et al., 2012; Holiga et al., 2013) indicated that LDOPA increases putamen activation during finger tapping and underlined the importance to control for motor performance and the severity of clinical symptoms for improved sensitivity of fMRI. To date, a clear and extensive evaluation of the effects of LDOPA on brain functions during movement execution is still lacking.
Although LDOPA leads to excellent clinical improvements during the first years of treatment, it is also associated with the emergence of severe side effects, affecting both motor and cognitive/behavioural domains and its efficacy degrades after years of treatment (Fahn, 1989; Obeso et al., 2000). Deep brain stimulation (DBS) was introduced as an additional treatment option for Parkinson’s disease, especially when the pharmacological therapy is less beneficial and its side effects are intolerable (Moro and Lang, 2006; Bronstein et al., 2011). DBS is based on the electrical stimulation of deep brain nuclei with high frequencies, typically focused on either the globus pallidus internus or the subthalamic nucleus (STN) in Parkinson’s disease (Benabid et al., 2009; Bronstein et al., 2011). Indeed, Parkinson’s disease induces brain abnormalities in the firing patterns of the basal ganglia, in particular generating hyperactivity of the globus pallidus internus and the STN that in turn lead to the suppression of thalamo-cortical activity (Galvan et al., 2015). The specific mechanisms of DBS are not completely understood and likely comprehend a variety of mechanisms related to the stimulation (e.g. desynchronization of aberrant oscillations, inhibition of abnormal firing) and not only to the lesion effect (Benabid et al., 2009). It is thus expected that the therapeutic effects of LDOPA and DBS should be mediated by different mechanisms. Previous studies investigated differential effects of DBS and LDOPA on movement performance (Rocchi et al., 2002; Vingerhoets et al., 2002; Timmermann et al., 2008; Bäumer et al., 2009) but did not focus on the modulation of brain activations during movement execution.
In this study, we aim to characterize the modulatory effect of LDOPA on brain activations during movement execution. To this aim, we collected fMRI data from 32 patients with Parkinson’s disease in both LDOPA-OFF and LDOPA-ON states employing a sequential finger-tapping task. Sequential finger–thumb opposition is a useful diagnostic tool to assess motor impairment in Parkinson’s disease, and specifically bradykinesia. It is included in the unified Parkinson’s Disease Rating Scale (UPDRS)-III and has been shown to be more sensitive compared with gross movements (e.g. forearm pronation–supination) because it requires fine motor control (Agostino et al., 1998; Agostino et al., 2003). We hypothesized that LDOPA modulates basal ganglia activity during fine movement execution and expected that fMRI investigations shed light on treatment-induced modulation of the interplay between basal ganglia and cortical regions. The basal ganglia have been shown to influence cortical activity via two mechanisms: (i) facilitation of motor activity via the thalamo-cortical projections and (ii) inhibition of competing motor patterns from unwanted movements (Mink, 1996; Rubchinsky et al., 2003). To further test the specificity of our results, a subgroup of patients, who underwent DBS surgery, performed the same experimental protocol for LDOPA-OFF and LDOPA-ON, and also after DBS implantation with the electrodes switched on [deep brain stimulation, on state (DBS-ON)] or off [deep brain stimulation, off state (DBS-OFF)]. Comparing the treatment effects between LDOPA and DBS provides a rare perspective into treatment-related brain activity changes.
Materials and methods
LDOPA cohort
Functional MRI was performed in 32 patients with Parkinson’s disease (Hoehn–Yahr Stages II–III, 26 males, age 56.1 ± 7.7 years, mean ± standard deviation; disease duration 12.2 ± 2.5 years, LDOPA treatment duration 9.0 ± 3.0 years). The selection of relatively young patients was based on the rationale that this group was planned to undergo the DBS procedure. Clinical assessment and MRI were performed in two sessions, without dopaminergic medication and after acute LDOPA challenge: LDOPA-OFF and LDOPA-ON. Four days before all measurements, dopamine agonists were substituted by equivalent doses of LDOPA (Tomlinson et al., 2010). Other anti-Parkinson’s disease medications (selegiline, amantadine, anticholinergics) were suspended. After an overnight withdrawal of LDOPA (at least 12 h), clinical and first fMRI data were obtained in the LDOPA-OFF session. Clinical and second fMRI assessment with medication was performed in the LDOPA-ON session ∼1 h after the administration of 250/50 mg of LDOPA/carbidopa after the patient’s clinical improvement. Parkinson’s disease symptoms were assessed with the UPDRS motor score (Part III) in both sessions. All patients gave informed written consent. All procedures conformed to the Declaration of Helsinki. The study protocol had been approved by the Ethics Committee of the General University Hospital in Prague.
LDOPA-DBS cohort
For a subgroup of 18 patients (Hoehn–Yahr Stages II–III, 15 males, age 54.6 ± 7.1 years, disease duration 12.2 ± 2.7 years, LDOPA treatment duration 9.5 ± 3.1 years), implantation of the DBS system was performed separately in two surgeries following previously described procedures (Jech et al., 2001). Within 15.5 ± 12.5 days after the LDOPA-OFF and LDOPA-ON sessions, the first DBS surgery was carried out in awake state, during which the patient with attached Leksell stereotactic frame and motor microdriver underwent electrophysiology mapping of the subthalamic area with five parallel microelectrodes. Then, the intraoperative stimulation by macroelectrode was performed in a region with a neuronal signal typical for STN to confirm clinical benefit and to monitor potential adverse effects of DBS. The macroelectrode was eventually replaced by the permanent electrode (type 3389; Medtronic, Minneapolis, MN, USA) connected to external leads.
Within 1–3 days after the first surgery, DBS-OFF and DBS-ON with clinical assessment and fMRI were scheduled when the electrodes were externalized and connected to an external stimulator working in bipolar mode (Dual Screen 3628; Medtronic). Note that clinical assessment in the DBS-ON session used bilateral STN DBS, while fMRI was performed using unilateral STN DBS contralateral to finger tapping. The DBS parameters were kept below threshold for dyskinesias and above the threshold for rigidity and akinesa in all patients (dyskinesias were not observed with STN DBS during MRI). Since the therapeutic effect of STN DBS might last even after switching off the neurostimulator, the DBS-OFF and DBS-ON conditions were randomized across the group to avoid order effects. Implantation of the internal pulse generator in the subclavial region was done under general anaesthesia 1 day after fMRI.
MRI data acquisition
Functional MRI data were obtained in two sessions (LDOPA-OFF and LDOPA-ON) for the LDOPA cohort and in four sessions (LDOPA-OFF, LDOPA-ON, DBS-OFF and DBS-ON) for the LDOPA-DBS subgroup. In each session, the patients performed a simple tapping task for each hand separately while lying supine with both hands in a resting position, resulting in four and eight data sets for the LDOPA and the LDOPA-DBS cohorts, respectively. The finger tapping experiment consisted of 25 consecutive movement and rest epochs [TAP (finger tapping, experimental condition) and REST (resting, experimental condition)], each lasting 10 s, resulting in a total session duration of 500 s. During rest epochs, a visual ‘rest signal’ (centred static red fixation cross on a black background) was presented on a projection screen, whereas during movement epochs, 10 pacing ‘movement cues’ (yellow square behind the fixation cross displayed for 100 ms) were presented with a frequency of 1 Hz. While viewing the ‘rest signal’, patients were instructed to remain motionless. They had to perform a unilateral index finger–thumb opposition whenever the ‘movement signal’ appeared.
All data were acquired with a 1.5-T Siemens Symphony scanner (Siemens, Erlangen, Germany) using a gradient-echo echo-planar imaging sequence (repetition time = 1000 ms, echo time = 54 ms, nominal in-plane resolution 3 mm × 3 mm, 3 mm slice thickness, 1 mm inter-slice gap). Ten oblique slices were acquired, oriented along the central sulcus and covering the rolandic cortex, basal ganglia and thalamus in a region between the anterior border of the caudate nuclei and the posterior border of the red nuclei. For image registration and further morphological analysis, axial T1-weighted magnetization prepared rapid acquisition gradient-echo (repetition time = 2140 ms, echo time = 3.93 ms, nominal in-plane resolution 0.46 mm × 0.46 mm, 1.65 mm slice thickness) and T2-weighted turbo spin echo (repetition time = 5520 ms, echo time = 86 ms, nominal in-plane resolution 0.45 mm × 0.45 mm, 4 mm slice thickness) images were acquired.
Data pre-processing and first-level analysis
Functional MRI data were processed using SPM12 (Wellcome Trust Centre for Neuroimaging, UCL, London, UK) and MATLAB (The MathWorks Inc., Natick, MA, USA). Standard pre-processing included realignment, normalization to the Montreal Neurological Institute space based on the unified segmentation approach (Ashburner and Friston, 2005) and spatial filtering using a Gaussian kernel with 10-mm full width at half maximum. The quality of pre-processing was carefully assessed by visual inspection to exclude misalignment and segmentation faults.
Each data set was further processed by least-squares parameter estimation using the general linear model with serially correlated observations (first-level analysis) (Friston et al., 2002a, b). A high-pass filter was used for baseline correction with a cut-off frequency of 1/96 Hz. The design matrix was generated with the onsets of all 25 TAP and 25 REST blocks. Using the standard model in SPM12, the stimulus function (generated from the onsets) was convolved with a canonical hemodynamic response function (HRF) (Friston et al., 1998) including its first derivative resulting in two columns for each condition. Finally, parameter maps (beta images) were estimated for both conditions and a contrast image was generated by subtracting the beta images (TAP–REST).
To investigate dynamic changes during finger tapping and rest in relationship to medication, another first-level analysis was performed using a design matrix generated with a finite impulse response (FIR) model for an entire 20-s cycle of TAP and REST. Thus, instead of 4 basis functions in the model described above (2 conditions and 2 temporal derivatives), the FIR model was implemented with 20 basis functions (i.e. 1 basis function for each functional volume of the cycle). Parameter estimation was performed for each individual data set resulting in 20 parameter maps. Note that this FIR model did not include any assumption about the shape of the HRF.
Statistical analysis (second-level analysis)
For all types of analyses described below, significant differences were obtained with P < 0.05 using family-wise error correction at the voxel level as well as a minimum cluster size of 25 voxels. This combination of voxel-level family-wise error correction plus a minimum cluster size substantially reduces the appearance of false-positive clusters (see voxel inference displayed in Figure 1 in Eklund et al., 2016, right column). However, to reduce false-negative findings, we additionally show all results using an uncorrected voxel threshold of P < 0.001 in combination with family-wise error correction at the cluster level at P < 0.05 in the Supplementary material. Note that this ‘cluster-defining threshold’ (CDT) procedure is prone to produce false-positive findings and should be used carefully (see statistical parametric mapping results Figure 1 in Eklund et al., 2016, middle column, using a CDT of P < 0.001).
Four types of second-level analyses were performed for the LDOPA cohort:
(1.1) The first analysis was performed with all TAP–REST contrast images using a flexible factorial design with factors LEFT/RIGHT (left hand finger tapping/right hand finger tapping) and OFF/ON (LDOPA state) for 32 patients with Parkinson’s disease (i.e. 32 × 2 × 2 = 128 contrast images). The model was implemented with both factors as main effects for investigating both OFF/ON and LEFT/RIGHT differences in a paired fashion. After parameter estimation, contrast images and t-statistics were computed for OFF/ON and LEFT/RIGHT differences.
(1.2) Two additional group analyses were performed using the individual beta images to find OFF/ON and LEFT/RIGHT differences for the TAP and the REST condition separately (identical design as above with 128 beta images for both TAP and REST). Contrast images and t-statistics were computed for OFF/ON and LEFT/RIGHT differences separately for the TAP and the REST condition.
(1.3) To investigate interactions between TAP/REST and OFF/ON differences, a model was generated with factors TAP/REST, LEFT/RIGHT and OFF/ON. The design matrix was created implementing an interaction of factors TAP/REST and OFF/ON (factor LEFT/RIGHT as main effect; 32 × 2 × 2 × 2 = 256 beta images). Two contrasts for testing both directions of interaction between factors TAP/REST and OFF/ON were computed and processed with t-statistics. An F-contrast was computed to investigate the amount of variance explained by both factors TAP/REST and OFF/ON within the model.
(1.4) In addition to the above analyses based on the canonical HRF, another second-level analysis was based on the beta images obtained with the FIR model. Here, we generated a model with the factors LEFT/RIGHT, OFF/ON and TIME (time factor, represented by the 20 basis functions for a full TAP-REST cycle). Note that instead of two levels of each factor in the other analyses, the factor TIME included 20 levels. The design matrix was created implementing an interaction of factors TIME and OFF/ON (factor LEFT/RIGHT as main effect; 32 × 20 × 2 × 2 = 2560 beta images). Two contrasts for testing both directions of interaction between the factors TIME and OFF/ON were computed and further processed with t-statistics. An F-contrast was computed to investigate the amount of variance explained by both factors.
Five types of second-level analyses were additionally performed for the LDOPA-DBS cohort:
(2.1) The first analysis aimed at detecting activity differences between OFF and ON states of both treatment approaches using the TAP–REST contrast images. The OFF–ON comparison for LDOPA treatment was identical as in analysis (1.1) in the LDOPA cohort. The same OFF–ON analysis was also performed for the DBS-OFF and DBS-ON sessions (each 18 × 2 × 2 = 72 contrast images). The model was implemented with both factors OFF/ON and LEFT/RIGHT to investigate differences in a paired fashion. After parameter estimation, contrast images and t-statistics were computed for OFF/ON and LEFT/RIGHT differences.
(2.2) After investigating OFF–ON differences for LDOPA and DBS separately, a three-factorial model was generated with the factors LDOPA/DBS, OFF/ON and LEFT/RIGHT (interaction between factors LDOPA/DBS and OFF/ON, factor LEFT/RIGHT implemented as main effect; 18 × 2 × 2 × 2 = 144 TAP–REST contrast images). Two contrasts for testing both interaction directions were computed and further processed with t-statistics. An F-contrast was computed to investigate the amount of variance explained by both factors LDOPA/DBS and OFF/ON.
(2.3) The third analysis aimed at detecting activity differences upon the treatment change using all TAP–REST contrast images in both ON states LDOPA-ON and DBS-ON (two-factorial model with factors LDOPA/DBS and LEFT/RIGHT; 18 × 2 × 2 = 72 contrast images). Subsequently, the same analysis was repeated to compare the TAP–REST contrast images from the LDOPA-OFF and DBS-OFF sessions. Both models were implemented with factors LDOPA/DBS and LEFT/RIGHT as main effects, and contrast images and t-statistics were computed for LDOPA/DBS and LEFT/RIGHT differences. Note that the difference between both ON–ON and OFF–OFF effects can be expressed by the same interaction analysis as in (2.2).
(2.4) Further analyses included the beta images to investigate both conditions TAP and REST separately. To study OFF–ON differences for LDOPA and DBS, beta images of the TAP and the REST condition were used in a flexible factorial model implementing an interaction between factors OFF/ON and TAP/REST (factor LEFT/RIGHT as main effect; 18 × 2 × 2 × 2 = 144 beta images). In addition to their interaction, we studied the main effect of both factors OFF/ON and TAP/REST and computed the F-contrasts to look at contrast estimates.
(2.5) Finally, an analysis was performed including beta images (TAP and REST) from all sessions (LDOPA-OFF, LDOPA-ON, DBS-OFF, DBS-ON). A flexible factorial model was created using factors LDOPA/DBS, OFF/ON and TAP/REST as an interaction and LEFT/RIGHT as main effect (18 × 2 × 2 × 2 × 2 = 288 beta images). Potential interaction between factors OFF/ON and TAP/REST were first tested separately for LDOPA treatment and DBS. Thereafter, a statistical analysis was performed including all three factors. In addition, we computed an F-contrast containing all columns of the design matrix associated with the three factors. This contrast was used to plot contrast estimates within regions of interest.
Visualization
Figures showing orthogonal brain slices were generated using the Mango software v4.1 (Research Imaging Institute, UTHSCSA) with the ‘Build Surface’ option and the ‘Cut Plane’ feature. Finally, statistical parametric maps were imported using the ‘Add Overlay’ function. The bar plots for the contrast estimates were directly obtained from SPM12 and plotted with MATLAB.
Data availability
Datasets analysed during the current study are available on reasonable request. All data will be anonymized. Functional MRI data will be available in a pre-processed fashion in the neuroimaging informatics technology initiative (NIfTI) format without any personal meta-data.
Results
LDOPA cohort
After the overnight withdrawal of dopaminergic treatment, the patients showed moderate Parkinson’s disease symptoms in the LDOPA-OFF session with a UPDRS-III score of 33.0 ± 8.5. One hour after the single dose of 250/50 mg of LDOPA/carbidopa, all 32 patients improved in the LDOPA-ON session showing fewer Parkinson’s disease symptoms resulting in a decreased UPDRS-III score (11.2 ± 5.3). A paired t-test showed a significant decrease with P < 10−17. The analysis of the fMRI data revealed significant results in the motor system, particularly in the primary left and right motor cortex and in the left and right putamen. Note that all results described below were obtained with P < 0.05 using family-wise error correction at the voxel level to prevent false-positive findings (Eklund et al., 2016). However, all results were re-checked using the more liberal CDT approach to reduce false-negative findings (see tables in the Supplementary material). All reported non-significant results remained non-significant with the CDT approach.
(1.1) The pairwise comparison of the TAP–REST contrast images between the LDOPA-OFF and LDOPA-ON states (i.e. the OFF/ON factor in the general linear model) revealed a significant increase in the left and right putamen (see O in Table 1; Supplementary Table 1; Fig. 1). We did not find any significant OFF/ON decrease in the TAP–REST contrast. As a verification of the experimental design and plausibility of the data analysis, we also performed a pairwise comparison of the TAP–REST contrast images between finger tapping with the left and the right hand (i.e. the LEFT/RIGHT factor in the general linear model). As expected, we obtained significant LEFT–RIGHT and RIGHT–LEFT differences in the contralateral primary motor cortex, i.e. in the right and left primary motor cortex, respectively.
(1.2) The second analysis aimed at investigating LDOPA-OFF/ON differences for the TAP and the REST condition separately using the beta images. For the TAP condition, we obtained a significant increase in brain activity with LDOPA medication in the left putamen (see A in Table 1 and A in Fig. 2; see also Supplementary Table 1). (In the right putamen, we observed an activity increase with an uncorrected threshold of P < 0.001, see values in Table 1.) Using the beta images from the REST condition, we obtained an inverse pattern showing significant activity decrease with LDOPA medication in both left and right putamen (see B in Table 1 and B in Fig. 2). We also looked at inverse contrasts for brain activity decrease with TAP and for brain activity increase with REST; however, we did not find any significant results. For both analyses with TAP and REST, we also looked at the LEFT/RIGHT factor that was included in both models looking at LEFT–RIGHT and RIGHT–LEFT differences. With TAP, we obtained similar results as described under (1.1); however, when using the REST beta images, we obtained an inverse pattern showing LEFT–RIGHT and RIGHT–LEFT differences in the ipsilateral primary motor cortex, i.e. in the left and right primary motor cortex, respectively.
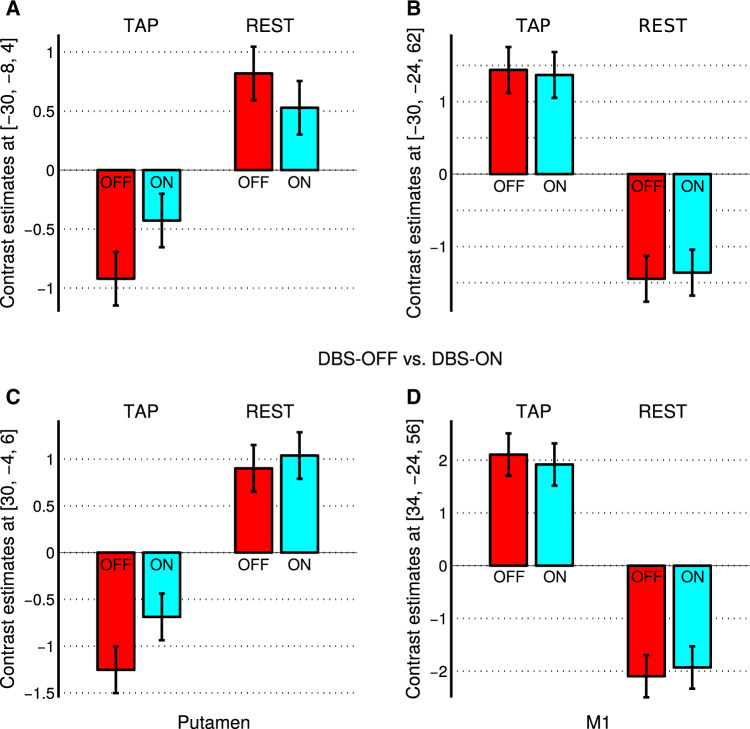
(1.3) The third analysis aimed at investigating the differential results on LDOPA-induced brain activity change with the TAP and the REST condition. Using a full model including all beta files, we obtained a significant interaction between the factors TAP/REST and OFF/ON showing a differential pattern of putamen activity change with LDOPA treatment in the TAP (increase) and in the REST (decrease) condition (see A–B in Table 1 and A–B in Fig. 2; see also Supplementary Table 1). The inverse interaction contrast did not show significance, i.e. we did not observe any decrease in putamen activity during TAP or any increase in putamen activity during REST with LDOPA treatment. Finally, an F-contrast including all experimental conditions related to the interaction between the factors TAP/REST and OFF/ON yielded a differential pattern of brain activity change in the left and right putamen with dopaminergic treatment during finger tapping and rest. Figure 3 shows the contrast estimates using the local maxima of the TAP/REST–OFF/ON interaction. The putamen activity decrease in the OFF versus ON state during REST appeared much more prominent than the increase during TAP, which is in line with our other analyses, particularly with analysis (1.1) using the TAP–REST contrast images. To understand the TAP–REST increase in the ON state in Fig. 1, REST bars shown in Fig. 3 would need to be flipped because of the subtraction of REST in the contrast TAP–REST.
(1.4) In the fourth analysis, employing the FIR model instead of an HRF, we obtained a significant interaction between TIME and OFF/ON in the left and right putamen. Contrast estimates for each basis function allowed investigating the temporal dynamics of the fMRI signal in the OFF and in the ON state of LDOPA medication without prior assumptions about form and shape of the HRF. Looking at contrast estimates for the left and right putamen, we obtained different response patterns in the two different medication states. Most interestingly, we obtained an HRF-shaped response with the FIR model that supports the usefulness of our HRF models. In addition to the interaction analysis between the factors TIME and OFF/ON, the main effect of the LEFT/RIGHT factor yielded a significant LEFT–RIGHT and RIGHT–LEFT differences in the contralateral primary motor cortex, i.e. in the right and the left primary motor cortex, respectively. Note that we did not find an LDOPA-related differential response in the motor cortex. Here, we obtained the same response pattern of brain activity in both medication states showing an increase with finger tapping.
Table 1.
List of significant clusters of brain activity change with LDOPA treatment for the LDOPA cohort including 32 patients with Parkinson’s diseasea
| Cluster level |
Voxel level |
x | y | z | ||||
|---|---|---|---|---|---|---|---|---|
| pFWE | kE | pFWE | T | Z | ||||
| O: TAP–REST: ON > OFF | <0.001 | 590 | <0.001 | 7.60 | 6.69 | −26 | −2 | 10 |
| <0.001 | 543 | <0.001 | 6.86 | 6.16 | 24 | 6 | 10 | |
| A: TAP: ON > OFF | 0.016 | 25 | 0.016 | 4.42 | 4.20 | −24 | −2 | 10 |
| 0.172 | 3.60 | 3.48 | 24 | 4 | 12 | |||
| B: REST: ON < OFF | <0.001 | 644 | <0.001 | 7.58 | 6.68 | −26 | −2 | 12 |
| <0.001 | 476 | <0.001 | 6.84 | 6.14 | 24 | 2 | 12 | |
| A–B | 0.003 | 160 | <0.001 | 5.73 | 5.52 | −24 | 0 | 12 |
| 0.003 | 170 | <0.001 | 5.30 | 5.14 | 24 | 4 | 12 | |
aThe upper rows of the table show significant clusters of a pairwise comparison of the TAP–REST contrast images between the LDOPA-ON and LDOPA-OFF states using a flexible factorial design (O, see also clusters in Fig. 1). Middle rows show LDOPA-OFF–ON differences for the TAP and the REST condition separately (A and B, respectively). During the TAP condition, we obtained a significant brain activity increase with LDOPA treatment in the left putamen (A, below significance for the right putamen, see also A in Fig. 2). During the REST condition, we found a reversed pattern of major brain activity decrease with LDOPA treatment (B, see also B in Fig. 2). The lower rows of the table show clusters of a significant interaction between both factors OFF/ON and TAP/REST within a flexible factorial design (A–B, see also A–B in Fig. 2). Height threshold P < 0.05 FWE corrected at the voxel level.
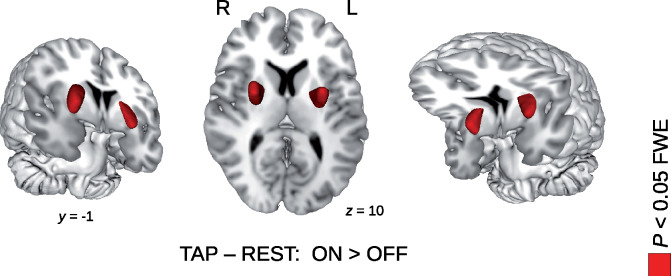
Figure 1.
Brain activity increase with LDOPA treatment in the LDOPA cohort of 32 patients with Parkinson’s disease. Using an experiment of consecutive blocks of finger tapping (TAP) and rest (REST), contrast images of TAP–REST were created for each participant. With a pairwise comparison of these contrast images (between the ON and OFF sessions with and without LDOPA treatment, respectively), a significant increase in the TAP–REST contrast was obtained after LDOPA treatment in the left and right putamen (P < 0.05 FWE corrected at the voxel level, see Table 1 for details).
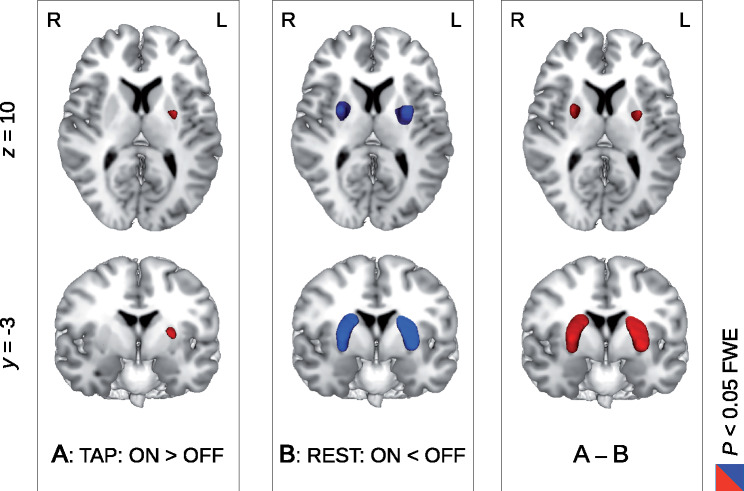
Figure 2.
Differential pattern of brain activity change with LDOPA treatment during finger tapping and rest within the LDOPA cohort of 32 patients with Parkinson’s disease. Using an experimental design with consecutive blocks of finger tapping and rest in both treatment states with (ON) and without (OFF) LDOPA medication, we observed a differential pattern of brain activity change in the putamen. During phases of finger tapping (TAP), an increased brain activity was obtained with LDOPA medication (left column, A, colour coded in red). In contrast, during resting periods (REST), putamen activity was decreased with LDOPA (middle column, B, colour coded in blue). A significant interaction between both factors of experimental condition (TAP/REST) and LDOPA treatment (OFF/ON) was observed in the left and right putamen (right column, A–B). All results were obtained with P < 0.05 with FWE correction at the voxel level (see Table 1 for details).
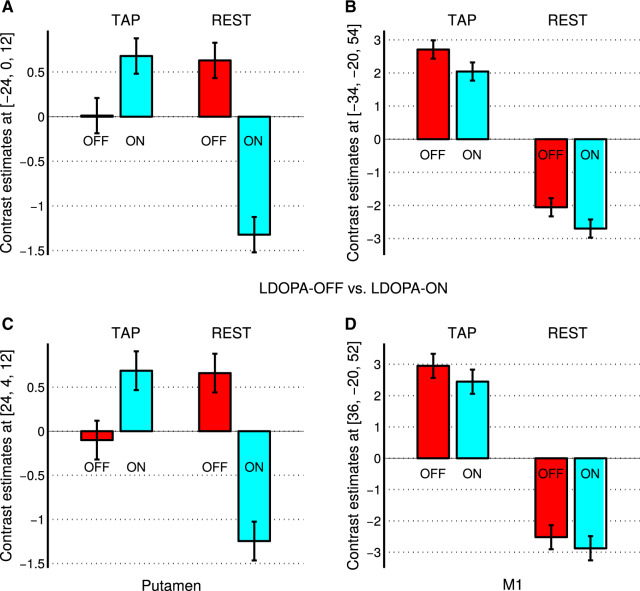
Figure 3.
Contrast estimates of a factorial model containing both experimental conditions of finger tapping and rest in both treatment states without and with LDOPA medication for the LDOPA cohort of 32 patients with Parkinson’s disease. Contrast estimates of the putamen showed a differential pattern of brain activity change after LDOPA treatment (ON versus OFF) during finger tapping (TAP) and rest (REST). In particular, during REST periods, we found a significant activity decrease (see A and C on the left, see also B in Fig. 2). In contrast to the differential pattern of brain activity in the left and right putamen, we did not observe any brain activity differences between the OFF and ON states in the left or right motor cortex M1, neither in the TAP nor in the REST condition (see B and D on the right).
LDOPA-DBS cohort
The subgroup of 18 patients with additional DBS showed a similar improvement in Parkinson’s disease symptoms with both treatment approaches. After withdrawal of dopaminergic treatment, patients showed Parkinson’s disease symptoms in the LDOPA-OFF session with a UPDRS-III score of 31.7 ± 8.8. One hour after the single dose of 250/50 mg of LDOPA/carbidopa, all patients improved in the LDOPA-ON session resulting in a decreased UPDRS-III score (9.3 ± 4.4; P < 10−9). After implanting the electrodes, patients showed Parkinson’s disease symptoms in the DBS-OFF session with a UPDRS-III score of 23.0 ± 6.0. The observed Parkinson’s disease symptoms in DBS-OFF were significantly reduced compared with LDOPA-OFF (the so-called microlesion effect, P < 0.0003). Finally, we obtained a significant UPDRS-III decrease in the DBS-ON session (10.0 ± 4.6) compared to both LDOPA-OFF (P < 10−7) and DBS-OFF (P < 10−9).
Although LDOPA-ON and DBS-ON led to similar improvement in UPDRS-III scores, the analysis of the fMRI data revealed different patterns of brain activity change for LDOPA treatment and DBS. We observed a differential pattern of putamen activity with LDOPA, however, not with DBS. Moreover, we found a significant difference between the LDOPA- and DBS-related putamen response patterns that reflect different mechanisms of both treatment approaches.
(2.1) The comparison between LDOPA-ON and LDOPA-OFF states yielded increased TAP–REST contrast under treatment in the left and right putamen (see A in Table 2 and A in Fig. 4; see also Supplementary Table 2). Thus, the main finding from the entire LDOPA cohort (see O in Table 1 and Fig. 1) was replicated with the LDOPA-DBS subgroup. The comparison between the DBS-ON and DBS-OFF states showed no significant differences, neither positive nor negative, even when using the CDT approach (see B in Table 2 and B in Fig. 4; see Supplementary Table 2).
(2.2) To disentangle the LDOPA effects from the DBS effects obtained in the analyses described in (2.1) above, we investigated the interaction between the factors LDOPA/DBS and OFF/ON including all eight TAP–REST contrast images for each subject within a single model. Here, we found a significant interaction between the factors LDOPA/DBS and OFF/ON in both left and right putamen (see A–B in Table 2 and A–B in Fig. 4). No further interaction was observed in other brain regions. The inverse interaction contrast (activity decrease with LDOPA treatment) did not yield significant results.
(2.3) Although differences between the UPDRS-III scores in the LDOPA-ON and DBS-ON sessions were insignificant (P = 0.58), the fMRI data revealed significant brain activity differences between both ON states. Using the TAP–REST contrast images, we obtained a significant decrease in the TAP–REST contrast with the treatment switch (from LDOPA-ON to DBS-ON) in the left and right putamen (see C in Table 2 and C in Fig. 4; see also Supplementary Table 2). The opposite contrast of an increase in the TAP–REST difference with the treatment switch did not reveal significant results. Comparing the DBS-OFF and LDOPA-OFF sessions, we found a significant improvement in Parkinson’s disease symptoms, presumably due to microlesion effects. Such decreased UPDRS-III values were accompanied by a decrease in the TAP–REST contrast. Using the same flexible factorial design but including both OFF states instead of the ON states, we observed significant activity decrease in various subcortical regions in the vicinity of the anterior thalamus and the internal globus pallidus (see D in Table 2, and D in Fig. 4). The inverse contrast of an increase in the TAP–REST difference upon switching from LDOPA-OFF to DBS-OFF did not reveal significant results. Using the same analysis as in (2.2), we found a significant interaction between the factors LDOPA/DBS and OFF/ON in both left and right putamen (see C–D in Table 2 and C–D in Fig. 4), which allows to separate treatment effects from microlesion effects. The interaction was observed in the left and right putamen but not in other subcortical regions that is in line with both ON–ON and OFF–OFF analyses.
(2.4) Investigating only DBS using the DBS-ON and the DBS-OFF session with the beta images from both the TAP and the REST condition, we were looking for a potential interaction between the factors OFF/ON and TAP/REST. However, in contrast to our findings with the LDOPA cohort, we did not find a significant interaction between both factors (both directions). Moreover, we did not find significant OFF–ON differences, neither for the TAP nor for the REST condition (Fig. 5). However, independent of the OFF or ON state of DBS, we found a reversed pattern of putamen and motor cortex activity. As expected, activity increased with tapping in the primary motor cortex but decreased in left and right putamen (Fig. 5). Note that a similar decrease in the putamen was observed for LDOPA-OFF (see Fig. 3, left column, bars in red colour) but not for LDOPA-ON.
(2.5) The final analysis was performed using all beta images of the TAP and the REST condition for all sessions LDOPA-OFF, LDOPA-ON, DBS-OFF and DBS-ON. The comparison between LDOPA-OFF and LDOPA-ON revealed a significant interaction between the factors OFF/ON and TAP/REST that is a replication of the result of analysis (1.3) for the subgroup of 18 patients with Parkinson’s disease of the LDOPA-DBS cohort. There was no significant interaction between the factors DBS-OFF/DBS-ON and TAP/REST; however, similar to analysis (2.4), we found significant brain activity decrease with finger tapping in the left and right putamen. The analysis including all three factors LDOPA/DBS, OFF/ON and TAP/REST did not reveal a significant interaction; however, using a merged contrast containing the interaction between LDOPA-OFF/LDOPA-ON and TAP/REST, and REST > TAP for both DBS-OFF and DBS-ON, we obtained significant clusters in the left and right putamen (Table 3; see also Supplementary Table 3).
Table 2.
List of significant clusters of brain activity change with LDOPA treatment and DBS using the TAP–REST contrast images of the LDOPA–DBS cohort of 18 patients with Parkinson’s diseasea
| Cluster level |
Voxel level |
x | y | z | ||||
|---|---|---|---|---|---|---|---|---|
| pFWE | kE | pFWE | T | Z | ||||
| A: LDOPA: ON > OFF | <0.001 | 627 | <0.001 | 8.54 | 6.72 | −28 | −2 | 8 |
| <0.001 | 584 | <0.001 | 7.23 | 5.99 | 26 | 0 | 8 | |
| B: DBS: ON <> OFF | n.s. | n.s. | ||||||
| A–B | 0.001 | 183 | <0.001 | 6.30 | 5.85 | −28 | 0 | 8 |
| 0.001 | 270 | <0.001 | 5.33 | 5.05 | 26 | 4 | 6 | |
| C: ON: LDOPA > DBS | 0.004 | 154 | <0.001 | 5.70 | 5.00 | −26 | 2 | 4 |
| 0.012 | 65 | 0.001 | 5.21 | 4.65 | 22 | 0 | 0 | |
| <0.001 | 522 | <0.001 | 5.77 | 5.05 | 10 | −2 | 20 | |
| D: OFF: LDOPA > DBS | <0.001 | 597 | <0.001 | 7.30 | 6.03 | 10 | −4 | 8 |
| C–D | 0.001 | 183 | <0.001 | 6.30 | 5.85 | −28 | 0 | 8 |
| 0.001 | 270 | <0.001 | 5.33 | 5.05 | 26 | 4 | 6 | |
aThe upper part of the table (A, B) shows clusters of a pairwise comparison of the TAP–REST contrast images between the ON and OFF states of LDOPA treatment (A) and DBS (B) in the subgroup of 18 patients with Parkinson’s disease of the LDOPA–DBS cohort. Here, we obtained a significant brain activity increase with LDOPA treatment in the putamen (A, see A in Fig. 4, see also Fig. 1 and Table 1 for the full LDOPA cohort). In contrast to the LDOPA case, we did not find any significant differences between the TAP–REST contrast images of the DBS-OFF and DBS-ON states (B, see also B in Fig. 4) even when using a more liberal cluster-defining threshold approach (see Supplementary Table 2). We obtained a significant interaction between both factors LDOPA/DBS and OFF/ON within a flexible factorial design (A–B, see also A–B in Fig. 4). The lower part of the table (C, D) shows a direct comparison of the TAP–REST contrast images between both treatment approaches, LDOPA and DBS, in ON and OFF states separately (ON–ON and OFF–OFF). Comparing both ON states of LDOPA and DBS, we obtained significant brain activity differences in the putamen (C, see also C in Fig. 4). The middle part of the table shows the pairwise comparison of the TAP–REST contrast images between the LDOPA-OFF and DBS-OFF states (the so-called microlesion effect) showing a subcortical region in the vicinity of the anterior thalamus and the internal globus pallidus (D, see also D in Fig. 4). To investigate a pure effect of LDOPA–DBS treatment change, the microlesion effect (OFF–OFF) must be subtracted from the treatment change (ON–ON) (C–D), which can be performed by the same interaction analysis between the factors LDOPA/DBS and OFF/ON already shown in the upper part of the table (A–B). Height threshold P < 0.05 FWE corrected at the voxel level.
n.s. = not significant.
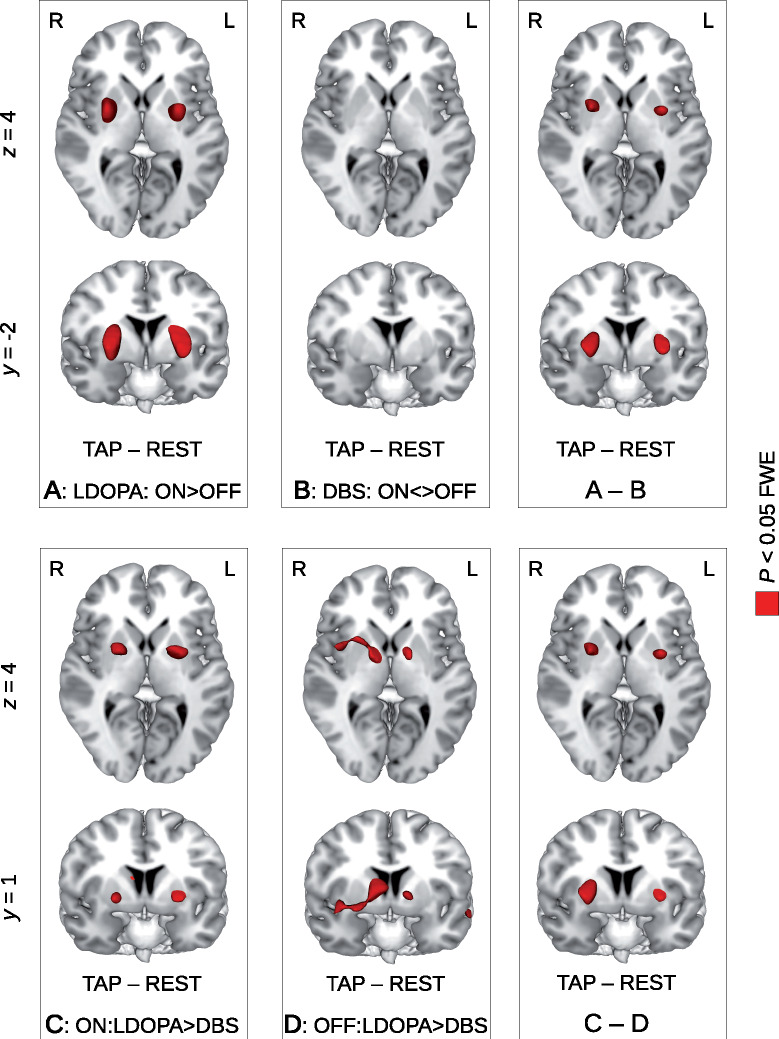
Figure 4.
Differential pattern of brain activity change with finger tapping during LDOPA treatment and deep brain stimulation (DBS) in the LDOPA-DBS cohort of 18 patients with Parkinson’s disease. Using the subcohort of patients who underwent DBS, the pairwise ON–OFF comparison revealed a brain activity increase with LDOPA treatment with finger tapping in the left and the right putamen (top row, A, colour coded in red, P < 0.05 FWE corrected at the voxel level, see also Fig. 1 for the full cohort). In contrast, we did not observe any significant brain activity change when comparing the ON and OFF states of DBS even when using the more liberal CDT approach (top row, B, see also Table 2 and Supplementary Table 2). The interaction model using a flexible factorial design with both factors LDOPA/DBS and OFF/ON revealed a significant result in the left and right putamen showing a significant difference between the ON–OFF differences of LDOPA and DBS (top row, A and B). The pairwise comparison between both ON states of LDOPA treatment and DBS revealed a significant brain activity decrease with finger tapping when changing the treatment from LDOPA to DBS (bottom row, C). Comparing both OFF states between LDOPA and DBS (the so-called microlesion effect), we did not find any significant brain activity differences in the left and right putamen but in the vicinity of the anterior thalamus and the internal globus pallidus (bottom row, D). Note that the interaction C–D is exactly the same as A–B shown in the top row.
Figure 5.
Contrast estimates of a factorial model containing both experimental conditions of finger tapping and rest in both treatment states without and with DBS within the subgroup of 18 patients with Parkinson’s disease. In contrast to a differential pattern of brain activity change with LDOPA treatment (see Fig. 3), we did not find any significant brain activity differences with DBS (ON versus OFF), neither for finger tapping (TAP) nor for the rest (REST) condition. Independent of the ON or OFF state of DBS, we found a reversed pattern of brain activity in the putamen (see A and C on the left) and in the primary motor cortex M1 (see B and D on the right).
Table 3.
List of significant clusters obtained by a three-factorial model containing (i) both experimental conditions of finger tapping and rest (TAP/REST), (ii) both treatment approaches with LDOPA and DBS (LDOPA/DBS), and (iii) both treatment states (OFF/ON), for the LDOPA–DBS cohort of 18 patients with Parkinson’s diseasea
| Cluster level |
Voxel level |
x | y | z | |||
|---|---|---|---|---|---|---|---|
| pFWE | kE | pFWE | T | Z | |||
| 0.001 | 195 | <0.001 | 5.52 | 5.36 | −28 | −2 | 8 |
| <0.001 | 461 | <0.001 | 6.54 | 6.29 | 28 | 0 | 8 |
aThe table shows the result of a three-factorial model containing the factors ‘condition’ (TAP/REST), ‘treatment approach’ (LDOPA/DBS), and ‘treatment state’ (OFF/ON) using a merged contrast containing the interaction between LDOPA-OFF/LDOPA-ON and TAP/REST, and REST > TAP for both DBS-OFF and DBS-ON. Two significant clusters were found in the left and right putamen. Height threshold P < 0.05 FWE corrected at the voxel level.
Discussion
Using fMRI, we investigated brain connectivity alterations in patients with Parkinson’s disease related to acute treatment effects. Overall, we showed that, during finger tapping, LDOPA specifically modulates activity in the basal ganglia but not in the motor cortex. We validated the robustness of our findings in two independent analyses and assessed their specificity for LDOPA through the comparison against DBS. To the best of our knowledge, our fMRI study is the first to present the comparison between therapeutic effects of LDOPA and DBS on brain motor activity.
Brain activity changes with LDOPA during finger tapping
Concerning the LDOPA effects, the motor cortex in both LDOPA-ON and LDOPA-OFF conditions showed an activity pattern related to finger tapping consistent with the previous literature (Witt et al., 2008; Gountouna et al., 2010) and expectations from basic brain physiology (Kandel et al., 2000). The REST condition was characterized by a low cortical activity, while tapping execution was associated with significant activity increases in the contralateral primary motor cortex and, in particular, in the hand areas. On the contrary, during the LDOPA-OFF state, the basal ganglia, and more specifically the bilateral putamen, showed an elevated activity during REST condition and a lower activity during the TAP execution. The LDOPA-ON state reverted this activity pattern in the putamen, so that REST and TAP were associated, respectively, with lower and higher activities, thus resembling the pattern observed in cortical motor regions. Of note, we found a significant interaction in the putamen between LDOPA medication (OFF/ON) and task (TAP/REST). We propose that the increased activity in the putamen during REST in the LDOPA-OFF state might have a pathological meaning, reflecting the derangement of the basal ganglia network in Parkinson’s disease as shown with electrophysiological recordings (Galvan and Wichmann, 2008; Galvan et al., 2015). For example, Singh et al. (2016) reported with in vivo electrophysiology increased firing rates in striatal projections neurons (both from putamen and, to a lower extent, caudate nucleus) in patients with Parkinson’s disease. Nevertheless, a conclusive statement concerning the aberrant nature of the increased putaminal activity in LDOPA-OFF would require the comparison with a group of healthy controls, which was not available for the present study. Here, the previous literature on finger tapping in healthy subjects might provide valuable information to clarify this point. Several studies, recently summarized in a quantitative meta-analysis, reported the brain functional correlates of finger tapping (Witt et al., 2008). Gountouna et al. (2010) assessed their consistency across centres and the robustness against confounds such as scanner variability. Specifically, the TAP–REST comparison has been mostly associated with activations focused in the primary motor, premotor and supplementary motor areas, and also in the basal ganglia, thalamus and cerebellum. This means that the activation in the putamen in normal controls is higher in the TAP condition as compared with the REST condition. We observed this same pattern (TAP > REST) in patients with Parkinson’s disease for the LDOPA-ON state, but not for the LDOPA-OFF state, thus supporting the idea that the LDOPA-OFF state represents a difference from a normal healthy cohort. Moreover, patients with Parkinson’s disease in our study showed reduced activations in the putamen during the TAP condition that was reversed by the LDOPA intake. A similar activity pattern in the putamen has been previously reported by Holiga et al. (2012; 2013) comparing LDOPA-OFF and LDOPA-ON in a subset of our cohort, and also in a meta-analysis of fMRI studies including 283 patients with Parkinson’s disease compared with healthy controls (Herz et al., 2014). The meta-analysis showed consistent reductions in the activity of the posterior putamen comparing LDOPA-OFF patients with Parkinson’s disease and controls during the execution of movement. Interestingly, consistent with our observations, this deficit was attenuated by LDOPA medication. This finding supports the idea that neural activity in striatal regions is impaired due to the lack of nigro-striatal dopaminergic input in Parkinson’s disease. Finally, at difference with our findings, previous investigations reported that the basal ganglia hypoactivity also associates with presumably compensatory hyperactivity in cortical and cerebellar regions in Parkinson’s disease (Thobois et al., 2000; Yu et al., 2007). However, Haslinger et al. (2001) reported that only cerebellar hyperactivity has a compensatory meaning, while motor cortex hyperactivity relates to specific motor symptoms (i.e. upper limb rigidity).
As aforementioned, we report here the interaction between finger-tapping task and LDOPA medication in both the LDOPA cohort and the LDOPA-DBS cohort, thus providing an internal validation of our results. However, for external validation, our results need to be replicated with different cohorts of patients with Parkinson’s disease. Note that validation is of particular importance as recent studies pointed at poor reproducibility as one of the major pitfalls of neuroimaging studies (Pernet and Poline, 2015). We further suggest using a particularly stringent correction for multiple comparisons (P < 0.05 family-wise error at the voxel level), thus minimizing the likelihood of false positives (Nichols and Hayasaka, 2003; Eklund et al., 2016).
Differences between LDOPA and DBS
The comparison between LDOPA and DBS revealed that the modulation of basal ganglia activity during finger tapping is specific for LDOPA, and it is not a general feature of treatment effects in Parkinson’s disease. Indeed, we found an interaction between LDOPA/DBS and OFF/ON factors in the bilateral putamen. This finding is in line with previous knowledge concerning the therapeutic activity of LDOPA and DBS. The former aims at restoring the impaired dopaminergic transmission in the nigro-striatal system (Connolly and Lang, 2014; LeWitt and Fahn, 2016), while the latter specifically interferes with the electrical signalling of the hyperactive subthalamic nuclei (Benabid et al., 2009). More specifically, in ON treatment conditions, the activity associated with finger tapping was higher in the bilateral putamen with LDOPA than with DBS (i.e. LDOPA-ON > DBS-ON). Note that the DBS-ON condition comprises both the effect of active DBS itself and the mircolesion effect due to the electrode placement during the surgery that is known to modulate brain network organization and activity (Singh et al., 2012; Holiga et al., 2015). In the OFF condition, instead, we found an increased activity in various brain regions in the LDOPA-OFF state as compared with the DBS-OFF state (i.e. as compared with the microlesion effect alone, LDOPA-OFF > DBS-OFF). This finding might be a result of the microlesion effect that attenuates the basal ganglia hyperactivity during motor execution (Jech et al., 2012).
However, we were not able to show a specific difference between DBS-ON and DBS-OFF during finger tapping, even using the more liberal CDT approach with the correction for multiple comparisons. The relatively small sample size of the LDOPA-DBS cohort might be a factor that limits our ability to capture subtle changes due to DBS. In addition, we recognize at least three more reasons that might explain this negative finding. First, our analysis was focused on the core motor regions, including the motor and premotor cortex, basal ganglia and thalamus, but excluding several other areas whose activity might be modulated by the treatment. Indeed, previous fMRI studies during both motor activity and rest showed that the dopaminergic system has a broad influence on the brain, encompassing both motor and non-motor regions (Postuma and Dagher, 2006; Wu et al., 2012; Ballarini et al., 2018). For example, a previous resting-state fMRI study from our group showed that DBS, as compared with LDOPA, is associated with increases in functional interconnectedness of the motor cortical regions that are in turn more connected to the thalamus and the cerebellum (Mueller et al., 2018). Second, the presence of electrodes in the brain after DBS surgery requires that implantation foci and neighbouring regions are masked out from the analysis. This hinders the detection of treatment-related activity changes in deep brain regions in the proximity of the electrodes. It has been indeed proposed that DBS, through the electrical stimulation of the subthalamic nuclei, influences cortical activity either through reducing activity in the indirect pathway (Bergman et al., 1990) or directly via the hyperdirect pathway (Nambu et al., 2002; Akram et al., 2017) of the basal ganglia. Third, the still present microlesion effect in the DBS-OFF state modifies brain activity related to finger tapping compared to the LDOPA-OFF state. This effect is known to be transitory and might hide from our analysis the ‘true’, long-term effect of DBS-ON (Jech et al., 2012).
Limitations and Strengths
A first limitation of our study is the lack of a healthy control cohort that could provide additional information regarding the specificity of our finding for Parkinson’s disease. However, the implemented study design provided an unprecedented framework to investigate the core question of our research, namely the differential effects of LDOPA and DBS within patients with Parkinson’s disease. Indeed, since patients with Parkinson’s disease did not receive LDOPA during the DBS part of the study, the pre-surgery LDOPA sessions can be used as a baseline for the post-surgery DBS one and vice versa. Of note, the distance between LDOPA and DBS sessions was on average about 2 weeks, thus minimizing the impact of long-term disease-related changes. A second limitation of our study is the fixed order (OFF before ON) of clinical and fMRI assessments in the LDOPA condition. As the study was performed in a clinical setting typical for conventional LDOPA test, this experimental design was necessary to document responsiveness of dominant clinical symptoms to dopaminergic treatment routinely required before DBS implantation procedure. On the other hand, DBS-ON and DBS-OFF conditions were randomized, thus mitigating in the interaction analyses the fault of the fixed LDOPA order.
As an additional cautionary note for the interpretation of our results, one has to consider that fMRI sessions with DBS were run 1–3 days after surgery. Therefore, our findings likely reflect short-term effects of DBS and we cannot exclude that further brain functional changes would come into play in a later—chronic—DBS stage. In addition, due to the randomized order of the DBS conditions DBS-ON and DBS-OFF, we were able to take the microlesion effect into account (Jech et al., 2012). Finally, despite most of the current fMRI research is acquiring MRI data at 3 T or even stronger magnetic field strength, we performed our measurements on a 1.5 T device. This choice was imposed by safety concerns regarding the application of higher magnetic fields in patients with DBS and by recommendations of manufacturers producing implantable DBS devices (Tagliati et al., 2009).
Conclusions
Our investigation provides an in-depth perspective on the effects of LDOPA therapy for Parkinson’s disease on activity in the basal ganglia during finger movement execution. We showed a strong interaction between LDOPA effects and finger tapping in the bilateral putamen, but not in the motor cortex. The LDOPA-OFF state was associated with an abnormal pattern of activity in the putamen, where the activity during REST exceeded that during TAP. The medication (LDOPA-ON) normalized this pattern, so that the activity in the putamen during the TAP phase was larger than in the REST one, as reported for healthy controls (Witt et al., 2008; Gountouna et al., 2010). Moreover, the within-group comparison with DBS treatment highlighted the specificity of our findings for the LDOPA medication. Here, we found a significant interaction between LDOPA/DBS and OFF/ON in the bilateral putamen, showing that LDOPA medication, but not DBS, has a modulatory effect on basal ganglia activity.
Funding
This study was supported by the Czech Science Foundation (grant 16-13323S), the Czech Ministry of Health (AZV NV19-04-00233), the Charles University in Prague (project PROGRES Q27), the Parkinson’s Disease Foundation (project PDF-IRG-1307) and the Michael J. Fox Foundation (project MJFF-11362).
Competing interests
R. Jech received honoraria and consultancies from Medtronic Czechia, Cardion, Ipsen and Abbvie. D. Urgošík received consultancies from Medtronic Czechia and Cardion.
Supplementary Material
Glossary
- CDT
cluster-defining threshold
- DBS
deep brain stimulation
- DBS-OFF
deep brain stimulation, off state
- DBS-ON
deep brain stimulation, on state
- FIR
finite impulse response
- fMRI
functional MRI
- FWE
family-wise error
- HRF
hemodynamic response function
- LEFT
left hand finger tapping
- LDOPA
levodopa
- LDOPA-OFF
levodopa, medication off state
- LDOPA-ON
levodopa, medication on state
- OFF
treatment off state
- ON
treatment on state
- REST
resting, experimental condition
- RIGHT
right hand finger tapping
- STN
subthalamic nucleus
- TAP
finger tapping, experimental condition
- TIME
time factor
- UPDRS
Parkinson’s Disease Rating Scale
References
- Agostino R, Berardelli A, Currà A, Accornero N, Manfredi M.. Clinical impairment of sequential finger movements in Parkinson’s disease. Mov Disord 1998; 13: 418–21. [DOI] [PubMed] [Google Scholar]
- Agostino R, Currà A, Giovannelli M, Modugno N, Manfredi M, Berardelli A.. Impairment of individual finger movements in Parkinson’s disease. Mov Disord 2003; 18: 560–5. [DOI] [PubMed] [Google Scholar]
- Akram H, Sotiropoulos SN, Jbabdi S, Georgiev D, Mahlknecht P, Hyam J, et al. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson’s disease. Neuroimage 2017; 158: 332–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ.. Unified segmentation. Neuroimage 2005; 26: 839–51. [DOI] [PubMed] [Google Scholar]
- Ballarini T, Růžička F, Bezdicek O, Růžička E, Roth J, Villringer A, et al. Unraveling connectivity changes due to dopaminergic therapy in chronically treated Parkinson’s disease patients. Sci Rep 2018; 8: 14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumer T, Hidding U, Hamel W, Buhmann C, Moll CK, Gerloff C, et al. Effects of DBS, premotor rTMS, and levodopa on motor function and silent period in advanced Parkinson’s disease. Mov Disord 2009; 24: 672–6. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P.. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol 2009; 8: 67–81. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR.. Reversal of experimental Parkinsonism by lesions of the subthalamic nucleus. Science 1990; 249: 1436–8. [DOI] [PubMed] [Google Scholar]
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 2011; 68: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly BS, Lang AE.. Pharmacological treatment of Parkinson disease: a review. JAMA 2014; 311: 1670–83. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H.. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016; 113: 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. Adverse effects of levodopa in Parkinson’s disease. In: Calne DB, editor. Drugs for the treatment of Parkinson’s disease. Handbook of experimental pharmacology, vol 88. New York: Springer-Verlag Berlin Heidelberg; 1989. p. 385–409. [Google Scholar]
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci 2006; 991: 1–14. [DOI] [PubMed] [Google Scholar]
- Fahn S, Jankovic J, Hallett M.. Principles and practice of movement disorders E-book: expert consult. Elsevier Health Sciences; 2011. https://www.elsevier.com/books/principles-and-practice-of-movement-disorders/9781437723694. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg M, Turner R.. Event-related fMRI: characterizing differential responses. Neuroimage 1998; 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J.. Classical and Bayesian inference in neuroimaging: applications. Neuroimage 2002a; 16: 484–512. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J.. Classical and Bayesian inference in neuroimaging: theory. Neuroimage 2002b; 16: 465–83. [DOI] [PubMed] [Google Scholar]
- Galvan A, Devergnas A, Wichmann T.. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front Neuroanat 2015; 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Wichmann T.. Pathophysiology of parkinsonism. Clin Neurophysiol 2008; 119: 1459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gountouna V-E, Job DE, McIntosh AM, Moorhead TWJ, Lymer GKL, Whalley HC, et al. Functional Magnetic Resonance Imaging (fMRI) reproducibility and variance components across visits and scanning sites with a finger tapping task. Neuroimage 2010; 49: 552–60. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kämpfe N, Boecker H, Rummeny E, Schwaiger M, et al. Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 2001; 124: 558–70. [DOI] [PubMed] [Google Scholar]
- Herz DM, Eickhoff SB, Løkkegaard A, Siebner HR.. Functional neuroimaging of motor control in parkinson’s disease: A meta‐analysis. Hum Brain Mapp 2014; 35: 3227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holiga Š, Möller HE, Sieger T, Schroeter ML, Jech R, Mueller K.. Accounting for movement increases sensitivity in detecting brain activity in Parkinson’s disease. PLoS One 2012; 7: e36271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holiga Š, Mueller K, Möller HE, Sieger T, Schroeter ML, Vymazal J, et al. Motor matters: tackling heterogeneity of Parkinson’s disease in functional MRI studies. PLoS One 2013; 8: e56133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holiga Š, Mueller K, Möller HE, Urgošík D, Růžička E, Schroeter ML, et al. Resting-state functional magnetic resonance imaging of the subthalamic microlesion and stimulation effects in Parkinson’s disease: indications of a principal role of the brainstem. Neuroimage Clin 2015; 9: 264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. A brief history of levodopa. J Neurol 2010; 257: 249–52. [DOI] [PubMed] [Google Scholar]
- Jech R, Mueller K, Urgošík D, Sieger T, Holiga Š, Růžička F, et al. The subthalamic microlesion story in Parkinson’s disease: electrode insertion-related motor improvement with relative cortico-subcortical hypoactivation in fMRI. PLoS One 2012; 7: e49056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jech R, Urgošík D, Tintěra J, Nebuželský A, Krásenský J, Liščák R, et al. Functional magnetic resonance imaging during deep brain stimulation: a pilot study in four patients with Parkinson’s disease. Mov Disord 2001; 16: 1126–32. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, Biochemistry D, Jessell MBT, Siegelbaum S, et al. Principles of Neural Science. New York: McGraw-Hill; 2000.
- Kraft E, Loichinger W, Diepers M, Lule D, Schwarz J, Ludolph AC, et al. Levodopa-induced striatal activation in Parkinson’s disease: a functional MRI study. Parkinsonism Relat Disord 2009; 15: 558–63. [DOI] [PubMed] [Google Scholar]
- LeWitt PA, Fahn S.. Levodopa therapy for Parkinson disease: a look backward and forward. Neurology 2016; 86(14 Suppl 1): S3–12. [DOI] [PubMed] [Google Scholar]
- Maillet A, Krainik A, Debû B, Troprès I, Lagrange C, Thobois S, et al. Levodopa effects on hand and speech movements in patients with Parkinson’s disease: a FMRI study. PLoS One 2012; 7: e46541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michely J, Volz LJ, Barbe MT, Hoffstaedter F, Viswanathan S, Timmermann L, et al. Dopaminergic modulation of motor network dynamics in Parkinson’s disease. Brain 2015; 138: 664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 1996; 50: 381–425. [DOI] [PubMed] [Google Scholar]
- Moro E, Lang AE.. Criteria for deep-brain stimulation in Parkinson’s disease: review and analysis. Exp Rev Neurother 2006; 6: 1695–705. [DOI] [PubMed] [Google Scholar]
- Mueller K, Jech R, Růžička F, Holiga Š, Ballarini T, Bezdicek O, et al. Brain connectivity changes when comparing effects of subthalamic deep brain stimulation with levodopa treatment in Parkinson’s disease. Neuroimage Clin 2018; 19: 1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M.. Functional significance of the cortico–subthalamo–pallidal ‘hyperdirect’pathway. Neurosci Res 2002; 43: 111–7. [DOI] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S.. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res 2003; 12: 419–46. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Olanow CW, Nutt JG.. Levodopa motor complications in Parkinson’s disease. Trends Neurosci; 2000; 23: S2–S7. [DOI] [PubMed] [Google Scholar]
- Pernet C, Poline J-B.. Improving functional magnetic resonance imaging reproducibility. Gigascience 2015; 4: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers 2017; 3: 17013. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015; 30: 1591–601. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A.. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 2006; 16: 1508–21. [DOI] [PubMed] [Google Scholar]
- Rocchi L, Chiari L, Horak F.. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2002; 73: 267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Hughes L, Ghosh B, Eckstein D, Williams-Gray C, Fallon S, et al. Parkinson’s disease and dopaminergic therapy—differential effects on movement, reward and cognition. Brain 2008; 131: 2094–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubchinsky LL, Kopell N, Sigvardt KA.. Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus–pallidal circuits. Proc Natl Acad Sci USA 2003; 100: 14427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kammermeier S, Mehrkens JH, Bötzel K.. Movement kinematic after deep brain stimulation associated microlesions. J Neurol Neurosurg Psychiatry 2012; 83: 1022–6. [DOI] [PubMed] [Google Scholar]
- Singh A, Mewes K, Gross RE, DeLong MR, Obeso JA, Papa SM.. Human striatal recordings reveal abnormal discharge of projection neurons in Parkinson’s disease. Proc Natl Acad Sci USA 2016; 113: 9629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliati M, Jankovic J, Pagan F, Susatia F, Isaias IU, Okun MS, et al. Safety of MRI in patients with implanted deep brain stimulation devices. Neuroimage 2009; 47 (Suppl 2): T53–7. [DOI] [PubMed] [Google Scholar]
- Thobois S, Dominey P, Decety J, Pollak P, Gregoire MC, Broussolle E.. Overactivation of primary motor cortex is asymmetrical in hemiparkinsonian patients. Neuroreport 2000; 11: 785–9. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Braun M, Groiss S, Wojtecki L, Ostrowski S, Krause H, et al. Differential effects of levodopa and subthalamic nucleus deep brain stimulation on bradykinesia in Parkinson’s disease. Mov Disord 2008; 23: 218–27. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE.. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 2010; 25: 2649–53. [DOI] [PubMed] [Google Scholar]
- Vingerhoets FJ, Villemure J-G, Temperli P, Pollo C, Pralong E, Ghika J.. Subthalamic DBS replaces levodopa in Parkinson’s disease: two-year follow-up. Neurology 2002; 58: 396–401. [DOI] [PubMed] [Google Scholar]
- Witt ST, Laird AR, Meyerand ME.. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage 2008; 42: 343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang J, Wang C, Hallett M, Zang Y, Wu X, et al. Basal ganglia circuits changes in Parkinson’s disease patients. Neurosci Lett 2012; 524: 55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE.. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 2007; 35: 222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets analysed during the current study are available on reasonable request. All data will be anonymized. Functional MRI data will be available in a pre-processed fashion in the neuroimaging informatics technology initiative (NIfTI) format without any personal meta-data.