Abstract
Alzheimer’s disease is associated with chronic response of innate immune system, referred as neuroinflammation. PET radioligands binding to the 18 kDa translocator protein are potential biomarkers of neuroinflammation. Translocator protein PET studies in mild cognitive impairment and Alzheimer’s disease have indicated controversial results, possibly reflecting interindividual variation and heterogeneity of study populations. We controlled for genetic and environmental effects by studying twin pairs discordant for episodic memory performance. Episodic memory impairment is a well-known cognitive hallmark of early Alzheimer’s disease process. Eleven same-sex twin pairs (four monozygotic pairs, six female pairs, age 72–77 years) underwent [11C]N-acetyl-N-(2-methoxybenzyl)-2-phenoxy-5-pyridinamine ([11C]PBR28) PET imaging, structural magnetic resonance imaging and neuropsychological testing in 2014–17. Main PET outcome was the volume-weighted average standardized uptake value of cortical regions vulnerable to Alzheimer’s disease pathology. Ten pairs were discordant for episodic memory performance. In the eight pairs with identical translocator protein genotype, twins with poorer episodic memory had ∼20% higher cortical [11C]PBR28 binding compared with their better-performing co-twins (mean intra-pair difference 0.21 standardized uptake value, 95% confidence interval 0.05–0.37, P = 0.017). The result remained the same when including all discordant pairs and controlling for translocator protein genotype. Increased translocator protein PET signal suggests that increased microglial activation is associated with poorer episodic memory performance. Twins with worse episodic memory performance compared with their co-twins had on average 20% higher uptake of the neuroinflammatory marker translocator protein PET tracer 11[11C]PBR28. The findings support a negative association between neuroinflammation and episodic memory and the use of translocator protein positron emission tomography as a useful indicator of Alzheimer’s disease process.
Keywords: mild cognitive impairment, neuroinflammation, PET imaging, Alzheimer’s disease, twins
Twins with worse episodic memory performance compared with their co-twins had on average 20% higher uptake of the neuroinflammatory marker translocator protein PET tracer 11 [11C]PBR28. The findings support a negative association between neuroinflammation and episodic memory and the use of translocator protein positron emission tomography as a useful indicator of Alzheimer’s disease process.
Graphical Abstract
Graphical Abstract.
Introduction
Increasing evidence indicates that chronic response of innate immune system characterized by reactive glial cells and elevated levels of inflammatory mediators is a key player in the development of Alzheimer’s disease from the early stage (Heppner et al., 2015). Particularly, aberrant function of microglial cells has revealed to be critical in the pathogenesis of Alzheimer’s disease.
The 18-kDa translocator protein (TSPO) is the most commonly used marker of neuroinflammation in clinical PET studies. Previous studies using the second-generation TSPO tracer [11C]N-acetyl-N-(2-methoxybenzyl)-2-phenoxy-5-pyridinamine ([11C]PBR28) have shown conflicting results with regard to increased TSPO PET signal as a marker of early stages of Alzheimer’s disease (Kreisl et al., 2013; Dani et al., 2018; Fan et al., 2018; Schain et al., 2018; Femminella et al., 2019). The high interindividual variance in [11C]PBR28 binding may contribute to the conflicting results (Collste et al., 2016; Tuisku et al., 2019).
We aimed to minimize the effect of genetic and environmental variation by studying the brain uptake of TSPO tracer [11C]PBR28 in monozygotic and dizygotic twin pairs discordant for episodic memory (EM) performance. EM impairment is a well-known cognitive hallmark of early Alzheimer’s disease process. Our aims were to determine if increased uptake of [11C]PBR28 is detected in twins with worse EM performance as compared with their better-performing co-twins and in twins with amnestic mild cognitive impairment (aMCI) as compared with their co-twins with age-normative EM performance. In addition, we investigated within-twin pair differences in continuous measure of TSPO PET binding in relation to within-twin pair differences in continuous EM (including verbal immediate and delayed recall, visual delayed recall and incidental memory) and global cognitive function measures.
Materials and methods
Cognitively discordant same-sex twin pairs born in 1938–44 were recruited from the older Finnish Twin Cohort study using a telephone interview including the telephone assessment for dementia and the modified Telephone Interview for Cognitive Status instruments in 2013–17 (Lindgren et al., 2019). A total of 1110 same-sex twin pairs were invited to participate in the telephone interview. Cognitively discordant twin pairs based either on the interview or on a previous diagnosis of Alzheimer’s disease or memory impairment in one twin sibling were asked to participate in the brain imaging study. Out of 559 pairs in which both co-twins participated, 39 cognitively discordant pairs were excluded from the brain imaging study due to neurological and psychiatric disorders other than Alzheimer’s disease or mild cognitive impairment, including history of major stroke or head trauma, significant medical conditions affecting the ability to undergo the study or contraindications for MRI and PET scanning, and 36 discordant pairs were excluded because one or both co-twins declined permission to contact or declined to participate in the imaging study. After genotyping for the rs6971 (C/T) polymorphism in the TSPO gene and excluding 2 twin pairs with TT genotype (low-affinity binders), 11 twin pairs participated to [11C]PBR28 imaging at the Turku PET Centre, Finland, in 2014–17.
The participants received the mean injection of 492 (SD 21) MBq of [11C]PBR28 with >99.9% radiochemical purity and the mean molar activity of 293 (SD 104) MBq/nmol and underwent a 70-min dynamic PET scan using a High Resolution Research Tomograph (Siemens/CTI, Knoxville, TN, USA). Twins had PET scans within 1 week from each other. The details of [11C]PBR28 synthesis, PET acquisition and preprocessing have been described elsewhere (Tuisku et al., 2019). T1-weighted MRI scans were acquired using a 3-T scanner (Philips Ingenuity TF PET/MR, Philips Medical Systems, Cleveland, OH, USA). After applying an automated region of interest generation with FreeSurfer software (version 6.0.0) and using PET data from 30 to 70 min after injection, a grey matter (GM) composite standardized uptake value [SUV, the ratio of tissue radioactivity concentration (kBq/ml) and administered dose (MBq) divided by body weight (kg)] were calculated as the volume-weighted average SUV across six region of interests (prefrontal, parietal, lateral temporal, precuneus, posterior cingulate and mesial temporal cortex). For 18 twins (7 full pairs) with available metabolite-corrected arterial input function, a GM distribution volume (VT) and a delivery rate constant (K1) were estimated with two-tissue-compartment model by using an in-house created software (http://www.turkupetcentre.net/petanalysis/tpcclib/doc/fitk4.html, accessed 12 March 2020), where metabolite and delay-corrected arterial plasma curve were used as an input function, blood volume fraction was fixed to 5% and the estimation was weighted by using the frame lengths.
The participants were also administered a neuropsychological test battery including multiple cognitive measures. The primary measures of EM that were used in our classification of discordance were the delayed word list recall from the Consortium to Establish a Registry for Alzheimer’s disease Neuropsychological Battery (CERAD-NB) and Logical Memory delayed recall from the Wechsler Memory Scale-Revised. The test performances were transformed into standard deviation (SD) units based on age-appropriate Finnish norms (Ylikoski, 2000; Sotaniemi et al., 2012). Ten twin pairs had differing delayed verbal EM test performance. Following the Jak/Bondi actuarial neuropsychological criteria, aMCI was defined by −1 SD or poorer performance in both two tests (Jak et al., 2009). Based on this approach, we identified eight twin pairs who fulfilled a more specific and stringent criteria of discordance for EM. In addition, the mean of the two SDs constituted a continuous EM score [e.g. (−1.2 + −0.9)/2 = −1.05], which was used in the analysis including all 11 twin pairs and using continuous variables. In addition, other measures of memory performance were used to examine the association between continuous memory performance and [11C]PBR28 binding in all twin pairs. The mean of SDs of the delayed visual reproductions test from the Wechsler Memory Scale-Revised and the delayed constructional praxis savings from the CERAD-NB was used to measure delayed visual EM performance, an independent delayed free recall measure that was not used in the determination of EM discordance. The immediate verbal EM performance was assessed with the mean of SDs of the immediate word list recall from the CERAD-NB and Logical Memory immediate recall from the Wechsler Memory Scale-Revised. The free recall score of the Boston Naming test (memo-BNT) was used to measure incidental memory. In addition, we examined the association between global cognitive performance measured with the CERAD total score (Chandler et al., 2005) and [11C]PBR28 binding.
Zygosity was confirmed by genotyping multiple polymorphic markers. The study was approved by the Ethics Committee of the Hospital District of Southwest Finland in accordance with the Declaration of Helsinki, and the written informed consent was obtained from the participants.
Statistical analysis
In the eight twin pairs with the same TSPO genotype (five CC; three CT), paired t-test was used to compare the differences in [11C]PBR28 GM SUV between pairs discordant for EM or aMCI status (similar results with Wilcoxon signed-rank tests not shown). When including additional two twin pairs with differing TSPO genotype, we used linear conditional fixed effects regression with TSPO genotype as a covariate (Stata command ‘xtreg’ with the ‘fe’ option). The associations of continuous memory scores and CERAD total score with the [11C]PBR28 GM SUV were also tested using linear conditional fixed effects regression with TSPO genotype as a covariate. Two-tailed P-values <0.05 indicated statistical significance. The analyses were conducted using Stata 14.2 (Stata Corp., College Station, TX, USA). Voxel-wise comparisons in 8-mm FWHM-smoothed [11C]PBR28 SUV images between EM discordant twins with the same TSPO genotype were done using Statistical Non-Parametric Mapping (version 13) with a paired t-test design and a cluster defining threshold of P < 0.01 (corrected for family-wise error at significance level P < 0.05).
Data availability
Due to the consent given by study participants and the high degree of identifiability, data cannot be made publicly available. These data may be shared with authorized researchers, upon researcher’s request, who have IRB/ethics approval and an institutionally approved study plan.
Results
Descriptive statistics
Table 1 shows the characteristics of 11 twin pairs. Participants’ ages ranged from 72 to 77 years (median = 74), and the years of education ranged from 6 to 13 years (median = 7), reflecting the low level of formal education in the post-World War II years in Finland. In eight pairs, the twins had the same duration of education. Ten twin pairs were discordant for the primary measure of EM performance. In these pairs, nine twins with poorer delayed verbal EM performance had also poorer global cognitive performance, incidental memory performance and delayed visual EM performance as compared with their co-twins. Eight twins with poorer delayed verbal EM performance had also poorer immediate verbal EM performance as compared with their co-twins.
Table 1.
Characteristics and [11C]PBR28 SUVs and VTs
| Discordant for EM performance | Discordant for at least aMCI | Zyg | TSPO | Verbal DR | Visual DR | Verbal IR | Incidental memory | CERAD total | PBR28 GM SUV | PBR28 cerebellar SUV | PBR28 GM VT | PBR28 cerebellar VT | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pair 1 | Twin 1 | Control | No | MZ | HAB | 1.2 | 1.1 | 0.9 | 13 | 87 | 1.01 | 0.81 | 2.9 | 2.3 |
| Twin 2 | Case | MZ | HAB | −0.1 | 1.0 | −0.5 | 8 | 74 | 1.06 | 1.00 | 4.3 | 3.9 | ||
| Pair 2 | Twin 1 | Control | Control | MZ | MAB | −0.7 | 0.7 | −0.5 | 4 | 72 | 0.78 | 0.54 | ||
| Twin 2 | Case | Case | MZ | MAB | −1.2 | −0.3 | −1.1 | 1 | 69 | 0.94 | 0.75 | 3.8 | 3.0 | |
| Pair 3 | Twin 1 | Control | Control | MZ | HAB | −1.0 | −0.6 | −1.1 | 4 | 67 | 1.37 | 1.21 | 4.6 | 4.0 |
| Twin 2 | Case | Case | MZ | HAB | −1.2 | 0.3 | −0.5 | 4 | 68 | 1.62 | 1.41 | 6.3 | 5.4 | |
| Pair 4 | Twin 1 | Control | No | MZ | MAB | 1.2 | 0.2 | 1.9 | 5 | 75 | 1.07 | 0.96 | 3.2 | 2.9 |
| Twin 2 | Case | MZ | MAB | −0.4 | −0.4 | −0.2 | 3 | 61 | 1.11 | 0.97 | 3.2 | 2.8 | ||
| Pair 5 | Twin 1 | Control | Control | DZ | HAB | 0.3 | 0.9 | −0.5 | 8 | 85 | 1.34 | 1.22 | 5.4 | 4.8 |
| Twin 2 | Case | Case | DZ | MAB | −3.1 | −1.5 | −4.1 | 2 | 34 | 0.71 | 0.58 | 2.8 | 2.2 | |
| Pair 6 | Twin 1 | Not discordant | DZ | HAB | −0.5 | 0.0 | −0.8 | 2 | 67 | 1.08 | 1.11 | 4.7 | 4.7 | |
| Twin 2 | DZ | HAB | −0.5 | −0.6 | 0.1 | 3 | 77 | 1.21 | 1.23 | |||||
| Pair 7 | Twin 1 | Control | Control | DZ | HAB | 2.4 | 1.3 | 2.5 | 8 | 93 | 1.14 | 0.97 | 5.2 | 4.3 |
| Twin 2 | Case | Case | DZ | HAB | −1.6 | 0.8 | −1.5 | 6 | 65 | 1.34 | 1.31 | 5.3 | 5.1 | |
| Pair 8 | Twin 1 | Control | Control | DZ | HAB | 0.6 | 0.7 | 0.8 | 11 | 80 | 0.94 | 0.94 | 2.9 | 2.9 |
| Twin 2 | Case | Case | DZ | MAB | −1.2 | −0.6 | 0.6 | 8 | 77 | 1.02 | 0.93 | |||
| Pair 9 | Twin 1 | Control | Control | DZ | MAB | 0.6 | 0.4 | 0.2 | 3 | 80 | 0.84 | 0.87 | ||
| Twin 2 | Case | Case | DZ | MAB | −2.8 | −1.3 | −2.8 | 1 | 49 | 1.22 | 1.15 | 2.7 | 2.5 | |
| Pair 10 | Twin 1 | Control | Control | DZ | HAB | 1.1 | 1.5 | 0.8 | 9 | 91 | 0.85 | 0.84 | 4.0 | 3.9 |
| Twin 2 | Case | Case | DZ | HAB | −2.1 | −1.4 | −0.2 | 1 | 68 | 1.41 | 1.24 | 5.7 | 4.8 | |
| Pair 11 | Twin 1 | Control | Control | DZ | HAB | −0.9 | 0.3 | −1.6 | 1 | 69 | 1.24 | 1.24 | 5.7 | 5.7 |
| Twin 2 | Case | Case | DZ | HAB | −1.3 | −0.5 | −1.6 | 0 | 54 | 1.26 | 1.34 | 4.3 | 4.5 | |
Case refers to the twin with poorer EM performance as compared with the control co-twin. Verbal DR is the average of SD units from the delayed word list recall from the CERAD-NB and the delayed Logical Memory recall from the WMS-R. aMCI is defined by −1 SD or poorer performance in both verbal DR tests. The visual DR is the average of SD units from the delayed visual reproductions test from WMS-R and the delayed constructional praxis savings from the CERAD-NB. The verbal IR is the average of SD units from the immediate word list recall from the CERAD and the immediate Logical Memory recall from the WMS-R. The incidental memory is the free recall score of MEMO-BNT test. PBR28 GM SUV/VT is the volume-weighted average of prefrontal cortex, parietal cortex, lateral temporal cortex, precuneus, posterior cingulate and mesial temporal cortex SUVs or VTs .
DR, delayed recall; DZ, dizygotic; HAB, high-affinity binder (CC genotype); IR, immediate recall; MAB, mixed-affinity binder (CT genotype); MEMO-BNT; Boston Naming Test; MZ, monozygotic; [11C]PBR28 GM SUV, [11C]PBR28 gray matter standardized uptake value; VT, distribution volume (calculated with two-tissue-compartment model and metabolite-corrected arterial input function); WMS-R, Wechsler Memory Scale-Revised; Zyg, zygosity.
Pairs discordant for episodic memory
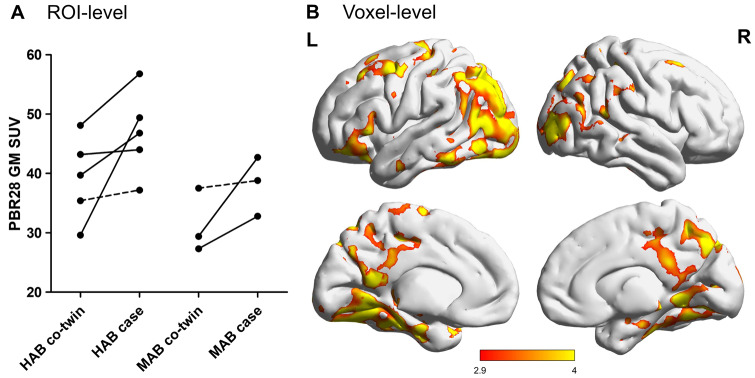
Ten pairs had discordant EM test performance, and eight pairs were identical for TSPO genotype. Twins with poorer EM as compared with their co-twins had on average 20% higher [11C]PBR28 GM binding [intra-pair difference 0.21 SUV, 95% confidence interval (CI) 0.05–0.37, P = 0.017]. The region of interest and voxel-level results are displayed in Fig. 1. When including 10 pairs and controlling for TSPO genotype, co-twins with poorer EM performance had higher [11C]PBR28 binding in the GM compared with their better-performing co-twins (intra-pair difference 0.21 SUV units, 95% CI 0.05–0.37, P = 0.016).
Figure 1.
[11C]PBR28 GM uptake in eight twin pairs discordant for EM performance and concordant for TSPO genotype. (A) [11C]PBR28 GM SUVs of twins with poorer EM performance (referred as cases) compared with their co-twins stratified into HABs and MABs. EM discordant twin pairs where case has at least aMCI based on two delayed free recall measures are shown with full lines. The GM composite SUV is the volume-weighted average of six ROIs including the prefrontal, parietal, lateral temporal, precuneus, posterior cingulate and mesial temporal cortex. (B) Statistically significant pairwise differences of [11C]PBR28 SUVs in eight discordant twin pairs at the voxel level (the 0.05 family-wise error-corrected widespread bilateral critical cluster size was 119 790). The colour bar represents T-values, in which brighter colour indicates greater T-values (T = 2.998 corresponds to cluster defining threshold of P < 0.01). HABs, high-affinity binders; MABs, mixed-affinity binders; ROIs, region of interests.
Six twin pairs identical for TSPO genotype had available VT results. Twins with poorer EM had on average 16% higher [11C]PBR28 GM VT compared with their better-performing co-twins, but the result did not reach statistical significance (intra-pair difference 0.58 ml/cm3, 95% CI −0.74 to 1.90, P = 0.31). The delivery rate constant (K1) of cortical GM was similar between twins with poorer EM compared with their better-performing twins with mean K1 values 0.194 (SD 0.063) and 0.199 (SD 0.037) ml/min/ml, respectively.
Pairs discordant for at least amnestic mild cognitive impairment-level impairment
Eight twin pairs were discordant for at least aMCI. In the six pairs with the same genotype, twins with aMCI had on average 25% higher [11C]PBR28 GM binding compared with their healthy siblings (intra-pair difference 0.26 SUV, 95% CI 0.06–0.46, P = 0.019). When TSPO genotype in eight pairs was controlled, twins with aMCI had higher [11C]PBR28 binding compared with their co-twins (intra-pair difference 0.26 SUV units, 95% CI 0.07–0.45, P = 0.014).
In the four pairs with identical TSPO genotype and available VT values, twins with aMCI had on average 11% higher [11C]PBR28 GM VT compared with their better-performing co-twins, but the result did not reach statistical significance (intra-pair difference 0.54 ml/cm3, 95% CI −1.86 to 2.94, P = 0.53).
Analyses with continuous episodic memory scores
The association between continuous EM score (delayed verbal recall) and [11C]PBR28 GM binding was examined within all 11 twin pairs: one SD lower performance was associated with 0.08 SUV units (95% CI −0.15 to −0.01, P = 0.027) higher [11C]PBR28 binding. Next, we analysed if the within-twin pair differences in other EM and global cognition measures were related to within-twin pair differences in [11C]PBR28 binding. There was a negative visual EM-[11C]PBR28 GM binding association: one SD lower performance was associated with 0.13 SUV units (95% CI −0.26 to 0.00, P = 0.044) higher [11C]PBR28 GM binding. There was also a negative incidental memory-[11C]PBR28 GM binding association: one point lower free recall score from the memo-BNT was associated with 0.05 SUV units (95% CI −0.09 to 0.00, P = 0.048) higher [11C]PBR28 binding. In contrast, the associations of immediate verbal EM score (B = −0.03, 95% CI −0.12 to 0.06, P = 0.44) and total CERAD score (B = 0.00, 95% CI −0.02 to 0.01, P = 0.56) with [11C]PBR28 GM binding were not found to be statistically significant.
Discussion
We found that twins with poorer EM had higher [11C]PBR28 uptake in cortical GM regions compared with their better-performing co-twins. The increase in TSPO binding was seen especially in the posterior cingulate/precuneus, parietal cortex, temporal cortex and anterior frontal lobe (Fig. 1), i.e. in many areas typically showing early accumulation of beta-amyloid in Alzheimer’s disease. Furthermore, twins with at least an aMCI-level impairment had higher [11C]PBR28 uptake compared with their intact co-twins. Despite the small sample size, the differences in [11C]PBR28 SUV values were statistically significant and showed consistency in region of interest and voxel-level analyses. [11C]PBR28 VT values were not available for all twin pairs. Twins with poorer EM had higher [11C]PBR28 VT, but the difference was not statistically significant probably due to the limited sample size. We also detected a statistically significant associations between the continuous measures of delayed verbal EM, delayed visual EM and incidental memory performance and the [11C]PBR28 SUV. On the contrary, there were no statistically significant associations between the immediate verbal EM or global cognitive performance with the [11C]PBR28 SUV.
In previous studies using [11C]PBR28, higher binding has been detected in Alzheimer’s disease dementia and MCI (Lyoo et al., 2015; Kreisl et al., 2017; Fan et al., 2018; Schain et al., 2018), while others have not found statistically significant group differences compared with healthy controls (Dani et al., 2018; Femminella et al., 2019). The negative findings are not explained by smaller sample sizes. When using absolute quantification, higher binding was detected only if [11C]PBR28 VT values were corrected for the free fraction of radioligand in plasma (Lyoo et al., 2015; Kreisl et al., 2017), whereas in some cases, there was no statistically significant group difference in [11C]PBR28 VT between Alzheimer’s disease and healthy individuals (Dani et al., 2018; Schain et al., 2018; Femminella et al., 2019). Some studies have detected higher [11C]PBR28 binding in Alzheimer’s disease using the SUV ratio method with cerebellar GM as a pseudo-reference region (Lyoo et al., 2015; Kreisl et al., 2017). Studies using other second-generation TSPO ligands than [11C]PBR28 show similar results: most studies have found higher cortical binding in Alzheimer’s disease dementia but results on the group differences between individuals with MCI and healthy controls are more conflicting (see Supplementary Table 1 for compilation of previous TSPO PET studies).
We used SUV as the primary outcome measure because arterial input function was not available for all twins. [11C]PBR28 SUV is moderately associated with VT and has high test–retest reliability (Nair et al., 2016; Matheson et al., 2017). SUV has been used to analyse [11C]PBR28 data, for example in a study that identified a significant association between the Alzheimer’s disease-linked PARP1 gene and [11C]PBR28 PET (Kim et al., 2013). However, the pairwise SUV differences could be biased by differential peripheral TSPO expression, tracer metabolism or tracer delivery to tissue. At least intra-pair differences in cerebral perfusion did not account for those in [11C]PBR28 SUV because there was no difference in the available K1 values. In addition, intra-pair differences in VT, which is independent of perfusion, seemed to support the SUV results. The quantification of specific binding of TSPO tracers is challenging as there is no brain region devoid of specific TSPO binding sites. Cerebellum has been used as a pseudo-reference region in individuals with Alzheimer’s disease and in healthy individuals to calculate the SUV ratio of [11C]PBR28 uptake (Lyoo et al., 2015). We did not apply SUV ratio because twins with poorer EM performance had higher [11C]PBR28 cerebellar SUVs compared with their co-twins (intra-pair difference 0.21 SUV, 95% CI 0.11–0.32, P = 0.002, n = 8 pairs; 0.60 ml/cm3, 95% CI −0.51 to 1.71, P = 0.22, n = 6 pairs; Table 1). Some previous studies have also found higher TSPO PET signal in the cerebellum in Alzheimer’s disease as compared with healthy controls (Fan et al., 2018; Bradburn et al., 2019), while others have not (Lyoo et al., 2015; Schain et al., 2018). Non-invasive quantification of [11C]-R-PK11195 is achieved with the supervised clustering algorithm, which identifies a cluster of reference voxels that have kinetic behaviour resembling that of normal GM. However, it is debatable whether this procedure is suited for the high-affinity tracer [11C]PBR28 (Rizzo et al., 2019; Zanotti-Fregonara et al., 2019).
Another challenge related to TSPO PET imaging is the unclear cellular nature of TSPO PET signal. Human post-mortem brain studies indicate that upregulated TSPO response originates mainly from microglia in Alzheimer’s disease, but astrocytes may also contribute (Venneti et al., 2009). However, TSPO binding sites are also present particularly in vascular cells in the CNS (Tomasi et al., 2008). Upregulated TSPO response may reflect either or both protective and detrimental neuroinflammatory processes.
Most previous cross-sectional studies have found that higher TSPO binding is associated with worse cognitive performance, which has typically been measured with Mini-Mental State Examination, while almost as often studies have not detected a correlation between TSPO binding and cognitive performance (Supplementary Table 1). On the contrary, the largest TSPO PET studies of Alzheimer’s disease individuals detected that higher cortical TSPO binding was cross-sectionally associated with better Mini-Mental State Examination score (Hamelin et al., 2016, 2018). The first longitudinal results suggest that increase in cortical TSPO PET signal is associated with decline in global cognition, function and GM volume in Alzheimer’s disease (Kreisl et al., 2016; Hamelin et al., 2018). Fewer studies have examined the association of TSPO binding particularly with EM performance. One study found that worse delayed word list recall score was associated with higher TSPO binding in the precuneus in Alzheimer’s disease (Passamonti et al., 2018), while two studies did not find a correlation with delayed verbal EM test scores (Kreisl et al., 2017; Knezevic et al., 2018).
Our results supported specifically the presence of a negative relationship between cortical TSPO binding and the delayed recall measures of EM performance. First, twin discordance and within-twin pair differences in the verbal delayed free recall performance were associated with TSPO binding. Second, there was also a negative association between the delayed visual recall, measure that was not used for the classification of discordance and TSPO binding. Third, there was no significant association between the immediate verbal recall or global cognitive performance and TSPO binding. Delayed recall measures have been found as more sensitive in the early Alzheimer’s disease stages in comparison to immediate recall measures (Chen et al., 2000; Elias et al., 2000; Saxton et al., 2004), although not always (Bilgel et al., 2014). Immediate and delayed recall measures have at least partly different neural correlates and genetic influences (Wolk and Dickerson, 2011; Kremen et al., 2014). EM measures are more sensitive in the early stages of Alzheimer’s disease than global cognitive screening measures that typically detect impairment later during the Alzheimer’s disease continuum (Chen et al., 2000; Bilgel et al., 2014). Our different results with the immediate and delayed recall measures of EM and finding no association between global cognition and TSPO binding may support increased neuroinflammation in the context of early Alzheimer’s disease. However, the small sample size restricts us from drawing strong conclusions. In addition, we found a negative association between incidental memory performance and TSPO binding. Other studies have indicated that poorer incidental memory differentiates those with mild Alzheimer’s disease from cognitively normal individuals (Karrasch et al. 2010; Kontaxopoulou et al. 2018).
We detected negative cognition–neuroinflammation associations based on multiple memory tests. However, our study was cross-sectional and, therefore, it is not possible to conclude whether neuroinflammation is a primary pathology that leads to poorer memory performance or a secondary response to other pathologies. Finally, the small number of twin pairs did not allow us to analyse the result separately in monozygotic and dizygotic twin pairs.
In conclusion, we found in a matched case–control twin study that increased cortical neuroinflammation measured with [11C]PBR28 PET was associated with poorer delayed EM performance.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We are grateful for the study participants for their cooperation. We thank the personnel of Turku PET Centre and the Neurology Research group. We thank Markus Matilainen, PhD, from the Turku PET Centre for statistical consultation, Kristiina Saanakorpi and Ulla Kulmala-Gråhn for conducting telephone interviews and Alexandra Elsing, Mikko Kolehmainen, Pasi Roslund, Carina Saarela and Sirkku Turunen for conducting neuropsychological testing.
Funding
This work was supported by the Sigrid Juselius Foundation (to J.O.R.), Finnish State Research Funding (to J.O.R., N.L. and J.T.) and Academy of Finland (133193 and 310962 to J.O.R.; 265240, 308248 and 312073 to J.K.; and 314639 and 320109 to E.V.). N.L. was supported by the Finnish Cultural Foundation, Päivikki and Sakari Sohlberg Foundation, Yrjö Jahnsson Foundation, Turku University Foundation and Finnish Brain Foundation. J.T. was supported by the Alfred Kordelin Foundation, Instrumentarium Science Foundation, Orion Research Foundation, Paulo Foundation and Päivikki and Sakari Sohlberg Foundation.
Competing interests
J.O.R. serves as a neurology consultant for Clinical Research Services Turku (CRST Oy). All other authors report no competing interests.
Glossary
- aMCI =
amnestic mild cognitive impairment
- CERAD-NB =
Consortium to Establish a Registry for Alzheimer’s disease neuropsychological battery
- CI =
confidence interval
- EM =
episodic memory
- GM =
grey matter
- SUV =
standardized uptake value
- TSPO =
translocator protein
- VT =
distribution volume
- [11C]PBR28 =
[11C]N-acetyl-N-(2-methoxybenzyl)-2-phenoxy-5-pyridinamine
References
- Bilgel M, An Y, Lang A, Prince J, Ferrucci L, Jedynak B, et al. Trajectories of Alzheimer disease-related cognitive measures in a longitudinal sample. Alzheimers Dement 2014; 10: 735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburn S, Murgatroyd C, Ray N.. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Ageing Res Rev 2019; 50: 1–8. [DOI] [PubMed] [Google Scholar]
- Chandler MJ, Lacritz LH, Hynan LS, Barnard HD, Allen G, Deschner M, et al. A total score for the CERAD neuropsychological battery. Neurology 2005; 65: 102–6. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M.. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology 2000; 55: 1847–53. [DOI] [PubMed] [Google Scholar]
- Collste K, Forsberg A, Varrone A, Amini N, Aeinehband S, Yakushev I, et al. Test–retest reproducibility of [11C]PBR28 binding to TSPO in healthy control subjects. Eur J Nucl Med Mol Imaging 2016; 43: 173–83. [DOI] [PubMed] [Google Scholar]
- Dani M, Wood M, Mizoguchi R, Fan Z, Walker Z, Morgan R, et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 2018; 141: 2740–54. [DOI] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB.. The preclinical phase of Alzheimer disease. Arch Neurol 2000; 57: 808–13. [DOI] [PubMed] [Google Scholar]
- Fan Z, Dani M, Femminella GD, Wood M, Calsolaro V, Veronese M, et al. Parametric mapping using spectral analysis for 11C-PBR28 PET reveals neuroinflammation in mild cognitive impairment subjects. Eur J Nucl Med Mol Imaging 2018; 45: 1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femminella GD, Dani M, Wood M, Fan Z, Calsolaro V, Atkinson R, et al. Microglial activation in early Alzheimer trajectory is associated with higher gray matter volume. Neurology 2019; 92: e1331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin L, Lagarde J, Dorothée G, Leroy C, Labit M, Comley RA, et al. ; The Clinical IMABio3 Team. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain 2016; 139: 1252–64. [DOI] [PubMed] [Google Scholar]
- Hamelin L, Lagarde J, Dorothée G, Potier MC, Corlier F, Kuhnast B, et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain 2018; 141: 1855–70. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B.. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 2015; 16: 358–72. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009; 17: 368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrasch M, Myllyniemi A, Latvasalo L, Söderholm C, Ellfolk U, Laine M.. The diagnostic accuracy of an incidental memory modification of the Boston Naming Test (memo-BNT) in differentiating between normal aging and mild Alzheimer’s disease. Clin Neuropsychol 2010; 24: 1355–64. [DOI] [PubMed] [Google Scholar]
- Kim S, Nho K, Risacher SL, Inlow M, Swaminathan S, Yoder KK, et al. PARP1 gene variation and microglial activity on [11C]PBR28 PET in older adults at risk for Alzheimer’s disease. Multimodal Brain Image Anal 2013; 8159: 150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic D, Verhoeff NPL, Hafizi S, Strafella AP, Graff-Guerrero A, Rajji T, et al. Imaging microglial activation and amyloid burden in amnestic mild cognitive impairment. J Cereb Blood Flow Metab 2018; 38: 1885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontaxopoulou D, Beratis IN, Fragkiadaki S, Pavlou D, Andronas N, Yannis G, et al. Exploring the profile of incidental memory in patients with amnestic mild cognitive impairment and mild Alzheimer’s disease. J Alzheimers Dis 2018; 65: 617–27. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, Liow JS, Snow J, Page E, Jenko KJ, et al. Distinct patterns of increased translocator protein in posterior cortical atrophy and amnestic Alzheimer’s disease. Neurobiol Aging 2017; 51: 132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, Liow JS, Wei M, Snow J, Page E, et al. 11C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol Aging 2016; 44: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 2013; 136: 2228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Panizzon MS, Franz CE, Spoon KM, Vuoksimaa E, Jacobson KC, et al. Genetic complexity of episodic memory: a twin approach to studies of aging. Psychol Aging 2014; 29: 404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren N, Kaprio J, Rinne JO, Vuoksimaa E.. Immediate verbal recall and familial dementia risk: population-based study of over 4000 twins. J Neurol Neurosurg Psychiatry 2019; 90: 90–7. [DOI] [PubMed] [Google Scholar]
- Lyoo CH, Ikawa M, Liow J-S, Zoghbi SS, Morse CL, Pike VW, et al. Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J Nucl Med 2015; 56: 701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson GJ, Plavén-Sigray P, Forsberg A, Varrone A, Farde L, Cervenka S.. Assessment of simplified ratio-based approaches for quantification of PET [11C]PBR28 data. EJNMMI Res 2017; 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Veronese M, Xu X, Curtis C, Turkheimer F, Howard R, et al. Test-retest analysis of a non-invasive method of quantifying [11C]-PBR28 binding in Alzheimer’s disease. EJNMMI Res 2016; 6: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Rodríguez PV, Hong YT, Allinson KSJ, Bevan-Jones WR, Williamson D, et al. PK11195 binding in Alzheimer disease and progressive supranuclear palsy. Neurology 2018; 90: e1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo G, Veronese M, Tonietto M, Bodini B, Stankoff B, Wimberley C, et al. Generalization of endothelial modelling of TSPO PET imaging: considerations on tracer affinities. J Cereb Blood Flow Metab 2019; 39: 874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology 2004; 63: 2341–7. [DOI] [PubMed] [Google Scholar]
- Schain M, Zanderigo F, Ogden RT, Kreisl WC.. Non-invasive estimation of [11C]PBR28 binding potential. Neuroimage 2018; 169: 278–85. [DOI] [PubMed] [Google Scholar]
- Sotaniemi M, Pulliainen V, Hokkanen L, Pirttilä T, Hallikainen I, Soininen H, et al. CERAD-neuropsychological battery in screening mild Alzheimer’s disease. Acta Neurol Scand 2012; 125: 16–23. [DOI] [PubMed] [Google Scholar]
- Tomasi G, Edison P, Bertoldo A, Roncaroli F, Singh P, Gerhard A, et al. Novel reference region model reveals increased microglial and reduced vascular binding of 11C-(R)-PK11195 in patients with Alzheimer’s disease. J Nucl Med 2008; 49: 1249–56. [DOI] [PubMed] [Google Scholar]
- Tuisku J, Plavén-Sigray P, Gaiser EC, Airas L, Al-Abdulrasul H, Brück A, et al. ; HRRT [11C ]PBR28 Study Group. Effects of age, BMI and sex on the glial cell marker TSPO—a multicentre [11C]PBR28 HRRT PET study. Eur J Nucl Med Mol Imaging 2019; 46: 2329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wang G, Hamilton RL, Mathis CA, Klunk WE, et al. PK11195 labels activated microglia in Alzheimer’s disease and in vivo in a mouse model using PET. Neurobiol Aging 2009; 30: 1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC.. Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage 2011; 54: 1530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski R. The Relationship of Neuropsychological Functioning with Demographic Characteristics, Brain Imaging Findings, and Health in Elderly Individuals [Internet]. 2000. http://urn.fi/urn:ISBN:952-91-2144-X (12 March 2020, date last accessed).
- Zanotti-Fregonara P, Kreisl WC, Innis RB, Lyoo CH.. Automatic extraction of a reference region for the noninvasive quantification of translocator protein in brain using 11C-PBR28. J Nucl Med 2019; 60: 978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the consent given by study participants and the high degree of identifiability, data cannot be made publicly available. These data may be shared with authorized researchers, upon researcher’s request, who have IRB/ethics approval and an institutionally approved study plan.