Abstract
Early studies on long-term functional recovery after motor and premotor lesions showed better outcomes in younger monkeys than in older monkeys. This finding led to the widespread belief that brain injuries cause less impairment in children than adults. However, this view has limitations and a large body of evidence now indicates that cerebral damages can be more harmful when inflicted at young age, during critical periods of neural development. To date, this issue has been mainly investigated in the context of focal and diffuse cortical lesions. Much less is known about the potential influence of early cerebellar damages. Several studies exist in survivor of posterior fossa tumours. However, in these studies, critical confounders were not always considered and contradictory conclusions were provided. We studied the impact or early cerebellar damage on long-term functional recovery in three groups of 15 posterior fossa survivors, comparable with respect to their tumour characteristics (type, size and location) but operated at different ages: young (≤7 years), middle (>7 and ≤13 years) and older (>13 years). Daily (health-related quality of life scale, performance status scale), motor (International Cooperative Ataxia Rating Scale, Pegboard Purdue Test) and cognitive (full-scale intelligence quotient) functioning were assessed. A general linear model controlling for age at surgery, radiotherapy, preservation of deep cerebellar nuclei, tumour volume and delay between surgery and assessment was used to investigate significant variations in outcome measures. Early age at surgery, lesion of deep cerebellar nuclei and postoperative radiotherapy had a significant, independent negative influence on long-term recovery. Tumour volume and delay between surgery and assessment had no statistically detectable impact. The negative influence of early age at surgery was significant in all domains: daily functioning (health-related quality of life scale, performance status scale), motor functioning (International Cooperative Ataxia Rating Scale, Pegboard Purdue Test) and cognitive functioning (full-scale intelligence quotient). These results support the existence of an early critical period of development during which the cerebellar ‘learning machine’ is of critical importance. Although the extent to which the early deficits here observed can be reversed needs now to be established, our data plead for the implementation of prompt and intense rehabilitation interventions in children operated before 7 years of age.
Keywords: Kennard principle, posterior fossa tumour, age at surgery, recovery, quality of life
In patients with cerebellar surgical lesions, a controversy exists whether early damages to the cerebellar ‘learning machine’ are more prejudicial than late damages. Here, we show that when potential confounders are controlled for, early age at surgery (≤7 years) is an independent risk factor for poor long-term functional recovery.
Graphical Abstract
Graphical Abstract.
Introduction
Eighty years ago, Margaret Kennard showed that long-term functional recovery was better in younger monkeys than in older monkeys following experimental lesions of the motor and premotor cortices (Kennard, 1942). This finding, later coined the ‘Kennard principle’, led to the widespread belief that brain injuries cause less impairment in children than in adults (Webb et al., 1996; Johnson et al., 2003). However, as first emphasized by Kennard herself (Dennis, 2010), this view has limitations. Indeed, a large body of evidence now indicates that cerebral damages can actually be more harmful when inflicted at a young age, during critical periods of neural development (Taylor and Alden, 1997; Forsyth, 2010; Anderson et al., 2011; Krageloh-Mann et al., 2017).
To date, the impact of age on functional recovery has been mainly investigated in the context of focal and diffuse cortical injuries (Taylor and Alden, 1997; Forsyth, 2010; Anderson et al., 2011; Krageloh-Mann et al., 2017). Much less attention has been given to the issue whether the ‘Kennard principle’ holds for cerebellar lesions. This is regrettable for at least two reasons. First, the cerebellum is an essential motor and cognitive structure of the nervous system (Horne and Butler, 1995; Ito, 2006; Strick et al., 2009; Timmann et al., 2010; Stoodley, 2012; Koziol et al., 2014). Second, and perhaps more importantly, this structure is the most frequent surgical target for tumour removal in children (Kaye and Laws, 2001; McKean-Cowdin et al., 2013).
Previous studies have reported that cerebellar surgeries performed at a young age tend to produce more severe, long-lasting deficits in both the motor and cognitive domains (Dennis et al., 1996; von Hoff et al., 2008; Robinson et al., 2013; Hanzlik et al., 2015). However, the origin of this observation remains debated. It is often claimed to reflect the more deleterious impact of postoperative radiation therapies in young children (Packer et al., 1989; Hoppe-Hirsch et al., 1990; Copeland et al., 1999; Conklin et al., 2008; Moxon-Emre et al., 2014). Consistent with this explanation, several studies have identified either no effect (Steinlin et al., 2003; Konczak et al., 2005; Ronning et al., 2005) or positive effects (Levisohn et al., 2000) of age at surgery on long-term sensorimotor and cognitive functions, in non-irradiated patients. However, other studies have reported contradictory findings (Aarsen et al., 2006; Aarsen et al., 2009). In agreement with these studies, pioneering observations, in animals, have shown that early hemicerebellectomies predict higher levels of functional deficits than late hemicerebellectomies (Smith et al., 1974; Gramsbergen, 1982); although contradictory conclusions have been published (Molinari et al., 1990).
Most probably, these inconsistencies are related to experimental factors. Indeed, despite their major contribution to the field, most studies that have investigated the effect of age at surgery on functional recovery have either based their conclusions on very small samples of young patients (Konczak et al., 2005) or failed to control for important potential confounders, including tumour size, delay between surgery and assessment time and/or damages to the deep cerebellar nuclei (Steinlin et al., 2003; Ronning et al., 2005; Aarsen et al., 2006; Aarsen et al., 2009). It is the goal of the present study to address these lacunae. In general terms, this work aims to determine whether Kennard principle holds for patients with cerebellar injuries, once major potential confounders are controlled for. In keeping with previous studies, we set the cut-off for ‘young age’ at 7 years (Chin and Maruyama, 1984; Ellenberg et al., 1987; Packer et al., 1989; Chapman et al., 1995; Riva et al., 2002; Mitby et al., 2003; Conklin et al., 2008; Robinson et al., 2013). Also, we used standard tests and scales to evaluate the motor, cognitive and daily functioning of the patients. For all these domains, our results contradict Kennard principle by showing that early cerebellar damages (≤7 years old) are an independent factor for poor long-term functional recovery.
Materials and methods
Patients
Over an 18-month period, 45 patients (24 females) were recruited (see below for details). They were all operated under general anaesthesia between 2001 and 2016 by the same neurosurgeons (C.M., A.S.). Within 2 weeks of their routine follow-up appointment (annual or bi-annual), these patients were contacted (or their legal guardians for minors) and invited to participate in a long-term follow-up study, in addition to their standard clinical evaluation. They were told that this additional evaluation would last ∼2 h and require the fulfilment of a series of non-invasive cognitive and motor tests (see below). If the patients (or their legal guardians for minors) agreed, testing was scheduled on the day of their appointment and, if not possible, within the following month. For all patients, formal consent was obtained (from the patients themselves or their legal guardians for minors) according to a protocol approved by our local institutional ethical committee and in agreement with the precepts of the Declaration of Helsinki .
Inclusion criteria included: (i) having been subjected to a surgical procedure for the removal of a cerebellar tumour, at least 1 year before evaluation; (ii) exhibiting a total tumour removal with no subsequent recurrence; (iii) being free of chronic or ongoing medical treatments; (iv) suffering no transient postoperative complications capable of interfering with recovery, including mutism (this criterion was primarily established to avoid increasing clinical disparities between age group; see Supplementary Materials for details); (v) being older than 6 years at the time of evaluation (this criterion was primarily established to allow for the application of various scales used to assess clinical and cognitive status; see Supplementary Materials for details); and (vi) speaking French as mother tongue.
During the 18-month study period, 160 patients previously operated from posterior fossa tumours were scheduled for an outpatient follow-up examination. Our study sample was extracted from this population in two steps. First, we identified all patients who (i) fulfilled the inclusion criteria (see above) and (ii) were younger than 7 years at the time of surgery. We found 15 eligible children. All of them (and their legal guardians) accepted participation in the study. Second, the remaining patients’ population (n = 145) was searched to pair each young patient with a middle (>7 and ≤13 years) and older age (>13 years) peer according to tumour characteristics (type, location and volume) and radiation therapy. For these middle and older age groups, the first patient identified as compatible in the appointment list was selected. Three of the selected patients declined to participate in the study (one in the middle age group and two in the older age group). They were replaced by the next compatible individual in the list. The main demographic and clinical characteristics of the patients are summarized in Supplementary Table 1. This table also reports statistical analyses showing the comparability of the three age groups for these characteristics.
This selection procedure allowed us to reach a dual goal. First, ensure clinical comparability of the three age groups. Second, make it possible to identify potential interactions between age and the two main clinical factors known to affect recovery, namely postoperative radiation therapy and anatomical damages to the deep cerebellar nuclei. This was an important issue to address. Indeed, as observed above, it is possible that the deleterious influence of undergoing surgery at an early age is indirect and related to the fact that postoperative radiations and/or lesions of the deep cerebellar nuclei are more damaging in younger children than in older children (if this is the case, statistical analyses should reveal a significant interaction between these factors, see below).
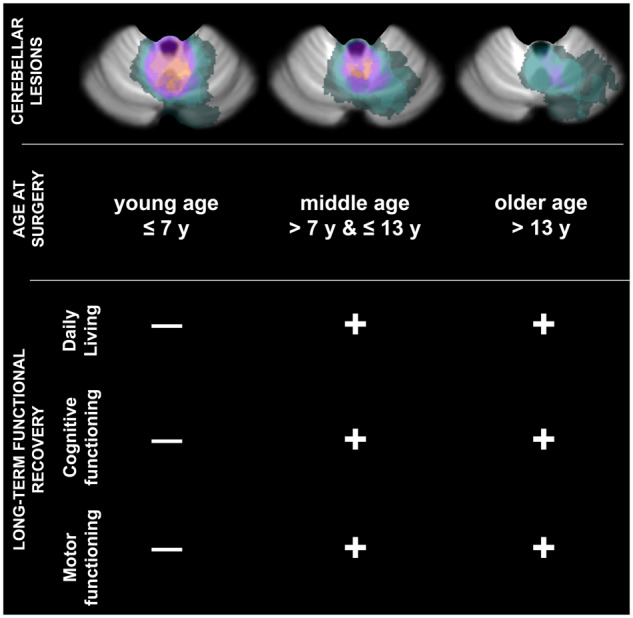
To avoid ambiguity, it must be mentioned that the vast majority of cerebellar tumours, in young children, are located within the midline (vermal) area, with or without hemispheric extension (Poretti et al., 2012; Koob and Girard, 2014). Our clinical sample reflects this epidemiological ‘bias’: all the patients of the young age group exhibited lesions encompassing the vermis with or without hemispheric extension. Due to the pairing procedure used for ensuring clinical comparability across age groups (see above), the same spatial distribution was observed in the older patients (middle, old). Supplementary Fig. 1 displays a lesion overlap map for the three age groups.
Clinical assessments
Patients were first submitted to a series of clinical questionnaires to measure their overall quality of life, their ability to live an independent life and the intensity of their ataxic symptoms. These evaluations were carried out by a trained physician (P.-A.B.) who was blind to patients’ clinical history and imaging results.
Health-related quality of life scale
This scale measures the self-perceived well-being of the patients. In patients older than 16 years, this dimension was assessed with a standardized questionnaire: the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (EORTC QLQ-C30) (Aaronson et al., 1993). In children younger than 16 years, health-related quality of life scale (hrQoL) was assessed with the corresponding paediatric questionnaire: the Pediatric Quality of Life Inventory (PedQol 4.0) (Varni et al., 2001). In this case, self-reports were privileged, but parent proxy report was sometimes used when the children were too young or too cognitively impaired to complete the questionnaire (Varni et al., 2007). For both scales, final mean scores between 0 and 100 were obtained after linear normalization of raw scores. Based on the previous studies in healthy adults and children populations, we used a conservative mean score of 60, as the threshold value for defining a ‘good quality of life’ (Dancey et al., 1997; van de Poll-Franse et al., 2011).
Performance status scale
Performance status (PS) scale is a standardized scale designed to evaluate patients’ ability to carry out (ordinary) daily activities. Depending on patient age, we used the Karnofsy Performance Status (≥16 years) (Sachsenheimer et al., 1992) or Lansky Scale (<16 years) (Lansky et al., 1987). The final score ranges from 0 to 100. A higher score reflects a better ability to live an independent life. The score of 70 is considered the threshold above which the patient can care for himself and is able to carry out normal daily activity independently.
International Cooperative Ataxia Rating Scale
International Cooperative Ataxia Rating Scale (ICARS) is a standardized scale designed to measure the severity of cerebellar ataxia in patients (Trouillas et al., 1997). The final score grows with disease severity from 0 to 100. For healthy subjects, ICARS score is typically below 7 (Storey et al., 2004).
Cognitive and sensorimotor assessments
These evaluations were carried out to measure fine sensorimotor functions and general intelligence of the patients. They were completed under the supervision of a licenced neuropsychologist (M.D.) who was blind to the patients’ clinical history and imaging results.
Pegboard Purdue Bimanual Assembly Task
The Pegboard Purdue Test (PegBoard) assesses upper-limb movements with a special emphasis on manual dexterity (Tiffin and Asher, 1948). The material consists of a wood board of two parallel rows of 25 holes each. The bimanual assembly task captures sensorimotor deficits irrespective of hand dominance and tumour laterality. The subject is instructed to use both hands alternatively to build as many object assemblies over a 60 s time period: put a pin in the hole with one hand, then a washer on the pin with the other hand, then a collar with the first hand, and then another a washer with the other hand. The final score is obtained through averaging of three consecutive repetitions. For each participant, this score is then evaluated as a deviation (in percentage) with respect to the mean expected performance (adjusted for age and sex).
Intelligence quotient
The full-scale intelligence quotient (FSIQ) of the Wechsler Intelligence Scale was administered as a gold standard measure of cognitive functions. The Wechsler Adult Intelligence Scale fourth edition (WAIS-IV, French version) (Wechsler, 2011) was used for patients older than 17 years. The Wechsler Intelligence Scale for Children fourth edition (WISC-IV, French version) (Wechsler, 2005) was used for patients younger than 17 years.
Tumour volume and preservation of cerebellar nuclei
For each patient, anatomical normalization of the cerebellum was performed on preoperative high resolution MRI with the SUIT toolbox of the SPM12b software (http://www.diedrichsenlab.org/imaging/suit.htm, 23 March 2020, date last accessed). Tumours were manually drawn on individual MRI and excluded from the normalization procedure. Damages to the deep cerebellar nuclei were automatically determined from normalized images by identifying areas of overlap between the tumours mesh and deep nuclei. Outcomes of this automatic procedure were visually reviewed on individual MRI by the neurosurgeons of the team (C.M., A.S., F.D.R. and P.-A.B.). To minimize the risk of false attribution, the post-surgical injury was also directly assessed on postoperative MRI (T1-weighted or T2-weighted sequences). Based on this two-step approach, a consensus was reached by the neurosurgeons for all patients.
Statistical analysis
A general linear model was used to investigate the origin of significant variations in the outcome measures described above. The following predictors were entered in the model: age at surgery, three levels [younger (≤7 years), middle (>7 and ≤13 years) and older (>13 years)]; radiation therapy, two levels (no and yes); and deep cerebellar nuclei, two levels [preserved (all nuclei intact) and lesioned (nuclei lesioned)]. ‘Tumour volume’ and ‘delay between surgery and assessment’, which could not be unambiguously categorized, were entered as continuous factors in the model. All analyses were performed with the statistical software Statistica (version 8.0; StatSoft®). Duncan significant difference test was used for post hoc comparisons (Winer, 1971). Threshold for statistical significance was set at P = 0.05.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request. They are not publicly available because of ethical restrictions.
Results
General clinical outcomes
From a clinical point of view, the patients of our sample fared quite well. In majority, they reported a good quality of life (hrQoL >60; 80%) and were able to carry out daily activities independently (PS >70; 93%). At the time of the evaluation, all children below 18 years (n = 29) were enrolled in regular school programmes (seven showed academic backwardness). Among adults (n = 16), 11 owned a driving licence (in France, only adults older than 18 years can have a driving licence), 5 were working full time, 7 were enrolled in post-graduate programmes (academic or vocational), and 4 were unemployed. Severe cognitive impairments were relatively rare. Intellectual disabilities, characterized by a FSIQ score of <70, were only observed in 9% of the patients (3.9 times the proportion expected from the theoretical intelligence quotient distribution, 2.3%). Mild deficits, characterized by a FSIQ score between 70 and 85, were found in 20% of the patients (1.5 times the proportion expected from the theoretical intelligence quotient distribution, 13.6%). Still, more than half of the patients displayed detectable signs of cerebellar ataxia (ICARS >7; 51%). More than a third had difficulties performing fine distal motor tasks (PegBoard < mean −2 × SD of the norm; 36%).
This general pattern
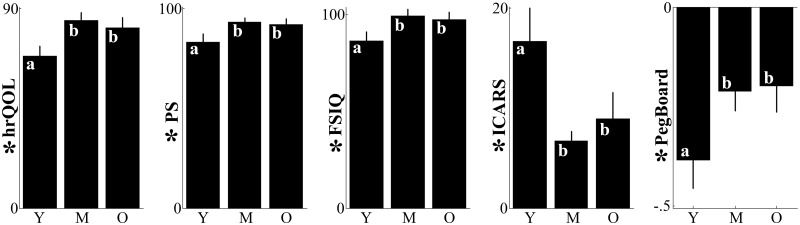
This general pattern of favourable outcomes was accompanied by a substantial level of inter-individual variability. Several factors accounted for this observation including age at surgery (see Supplementary Table 2 for statistical details). When surgery was performed before 7 years of age, all markers of functional recovery were significantly deteriorated (Fig. 1). As shown by post hoc analyses, the performance average of the young age group was systematically degraded with respect to the performance of the middle and older age groups (all Ps < 0.045). These latter two groups were not different from each other (all Ps > 0.450). Overall, young patients experienced a lower quality of life (hrQoL; F(2,31) = 4.50, P = 0.019) and a degraded ability to carry out common daily activities (PS; F(2,31) = 3.68, P = 0.037). Also, these patients exhibited stronger ataxic symptoms (ICARS; F(2,31) = 4.62, P = 0.018), a greater inability to perform fine manual movements (PegBoard; F(2,31) = 3.34, P = 0.048) and a lower intelligence quotient (FSIQ; F(2,31) = 4.39, P = 0.021). The effect of age was statistically independent of the influence of the other factors included in the model (interactions, all Ps > 0.130).
Figure 1.
Effect of age at surgery on recovery. One dependent outcome measure per column. Vertical lines display the standard errors of the means. For each panel, the symbol * before the vertical labels indicates that the effect of age is statistically significant; letters on the bars identify significant (different letters) or non-significant (same letters) post hoc difference between means. M = middle age group; O = old age group; Y = young age group.
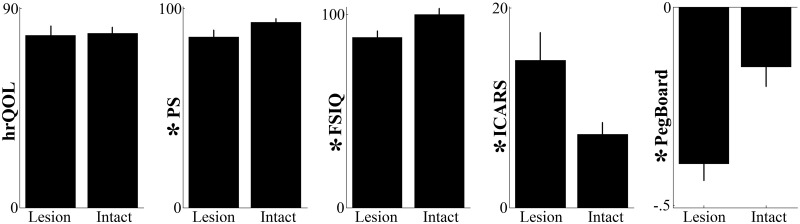
Beyond age at surgery, lesion of the deep cerebellar nuclei was also an important negative, independent (no interactions; all Ps > 0.240) predictor of functional recovery (Fig. 2). When these nuclei were injured, patients’ ability to perform regular daily activities was lessened (PS; F(1,31) = 5.60, P = 0.024), ataxic symptoms were stronger (ICARS; F(1,31) = 7.97, P = 0.008), manual dexterity was degraded (PegBoard; F(1,31) = 16.00, P = 0.0004) and intelligence quotient was diminished (FSIQ; F(1,31) = 16.66, P = 0.0003). Differences observed for the quality of life scale failed to reach statistical significance (hrQoL; F(1,31) = 1.46, P = 0.236).
Figure 2.
Effect of anatomical damages to the deep cerebellar nuclei on recovery. One dependent outcome measure per column. Vertical lines display the standard errors of the means. For each panel, the symbol * before the vertical labels indicates that the effect of lesioning the deep cerebellar nuclei is statistically significant. Intact = nuclei intact; Lesioned = nuclei lesioned.
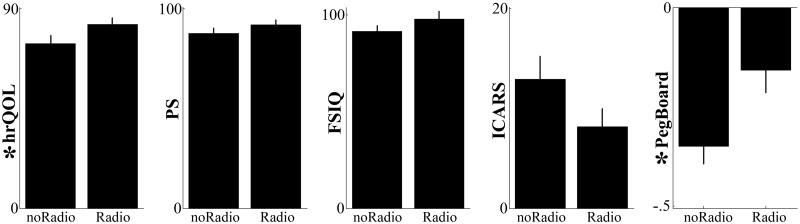
Finally, a significant, independent (no interactions; all Ps > 0.130) negative influence of postoperative radiotherapy was observed for fine motor abilities (PegBoard; F(1,31) = 4.84, P = 0.035) and quality of life (hrQoL; F(1,31) = 4.34, P = 0.0.45). Negative trends were also identified for cognitive outcomes (FSIQ), ataxic symptoms (ICARS) and regular daily activities (PS) (Fig. 3). However, none reached statistical significance (all Fs(1,31) < 2.43, Ps > 0.130).
Figure 3.
Effect of postoperative radiotherapy on recovery. One dependent outcome measure per column. Vertical lines display the standard errors of the means. For each panel, the symbol * before the vertical labels indicates that the effect of postoperative radiotherapy is statistically significant. NoRadio = no radiotherapy; Radio = radiotherapy.
Tumour volume and the time elapsed from surgery to assessment had no statistically detectable impact on the measured variables (all Fs(1,31) < 3.61, Ps > 0.065).
Discussion
To sum up, the main aim of the present study was to clarify the debated issue whether young age at surgery is, in itself, an independent predictive factor of poor functional recovery in survivors of posterior fossa tumours. Our results provide a positive answer to this question. Indeed, we observe that patients operated before 7 years of age show degraded outcomes with respect to patients operated after this age. This negative influence is observed across all the dependent measures considered in this study: daily functioning (hrQoL, PS), motor functioning (ICARS, PegBoard), and cognitive functioning (FSIQ).
Two complementary lines of evidence may account for the identified relationship between early cerebellar lesions and impaired recovery: (i) the cerebellum plays a central role in motor and cognitive learning (Ito, 2006; Koziol et al., 2014; Sokolov et al., 2017) and (ii) during early childhood, sensitive periods cascade to lay the foundations for future acquisitions (Anderson et al., 2011). Within this framework, it may be speculated that early lesions of the cerebellum, a structure seen as a ‘broad learning machine’ (Ito, 2006), damage the development of cardinal functional skills (motor and cognitive) upon which later acquisitions are built.
Although the extent to which this negative impact of early cerebellar lesions can be reversed needs now to be established, our results clearly plead for the implementation of heavy rehabilitation programmes in children operated before 7 years of age. Evidence exist that these programmes are efficient at remediating existing deficits and preventing gradual deterioration (Castellino et al., 2014; Olson and Sands, 2016). With regard to this latter point, it is worth noting that we found no sign of functional worsening over time. A similar observation was reported in previous studies (Copeland et al., 1999; Konczak et al., 2005; von Hoff et al., 2008). Others, however, have described a progressive worsening (Hoppe-Hirsch et al., 1990; Dennis et al., 1996; Aarsen et al., 2006; Conklin et al., 2008). We cannot rule out the possibility that our follow-up duration (5 years on average) was too short to allow for such a gradual deterioration to reach a detectable threshold. Alternatively, it is also possible that our study was not sensitive enough for identifying longitudinal changes, due to the high inter-subject variability inherent to cross-sectional designs. As a consequence, the absence of progressive functional worsening here observed should be considered cautiously.
Another interesting finding of the present study concerns the potential impact of tumour volume on functional recovery. The existing literature provides conflicting observations on this issue. Some studies have reported a significant relation (Catsman-Berrevoets et al., 1999; Law et al., 2012), while others, like the present one, found no association (Steinlin et al., 2003; Konczak et al., 2005; Kuper et al., 2013). It is tempting to speculate that this divergence reflects the failure of positive studies to control for the anatomical status of the deep cerebellar nuclei. Indeed, the risk for these nuclei to be damaged increases with tumour volume. Ours and previous studies that have taken this factor into account found no effect of tumour volume on recovery (Konczak et al., 2005; Kuper et al., 2013). This finding is not unexpected in light of ablation experiments showing, in monkeys, that large lesions involving different lobes of the cerebellar cortex produce very mild, often undetectable, deficits as long as the deep nuclei are spared (Dow and Moruzzi, 1958). From a surgical point of view, this observation supports aggressive gross-total resections as long as the cerebellar nuclei can be preserved. When this is not the case, the possibility of near total resections protecting these nuclei should be considered (e.g. medulloblastomas; Thompson et al., 2016). When complete resections are advised (e.g. ependymomas; Guyotat et al., 2009), a special emphasis should be put on post-surgical rehabilitation procedures.
Our results also confirm the well-established impact of post-surgical radiotherapy on functional recovery (see ‘Introduction’ section). However, this effect was only significant for fine motor abilities (PegBoard) and the self-perceived well-being of the patients (hrQoL). Only a trend was found for ICARS, FSIQ and PS. Radiotherapy protocols might explain these results. Indeed, past studies involving standard craniospinal irradiation have almost unanimously reported long-term intellectual deficits after radiotherapy (Packer et al., 1989; Hoppe-Hirsch et al., 1990; Palmer et al., 2003). However, more recent researches have described more favourable outcomes in the context of less aggressive protocols involving lower dose radiations (e.g. 25 Gy rather than 36 Gy) (Grill et al., 1999; Moxon-Emre et al., 2014), hyperfractionated radiations (Gupta et al., 2012; Kennedy et al., 2014) or radiations circumscribed to the posterior fossa (Fouladi et al., 2005; von Hoff et al., 2008). In our cohort, 88% of the 25 patients exposed to radiotherapy were submitted to these less aggressive protocols.
In summary, our results support the conclusion that cerebellar lesion has a stronger negative impact on long-term functional recovery when inflicted at a young age. This finding pleads for the implementation of prompt and intense rehabilitation interventions in children with posterior fossa tumours, operated before 7 years of age. Further studies are now required to confirm this pioneering observation and extend it to more diverse clinical populations including, in particular, patients with hemispheric lesions. Indeed, all our patients had tumours centred on the midline (vermal) region, either purely or with some degree of hemispheric extension. This bias reflects the over-representation of midline tumours in young children (Poretti et al., 2012; Koob and Girard, 2014) and, in this sense, ensues directly from the goal of this study. Whether our findings can be generalized to subpopulations of patients with more lateral lesion needs to be investigated. In the same way, it will be important to determine whether our findings can be extended to children and adolescent populations with cerebellar mutism. Also, finer assessments in larger population samples will need to be undertaken to isolate more precisely the critical period during which cerebellar lesions are especially deleterious.
Funding
This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Agence Nationale de la Recherche (ANR-12-BSV4-0018-01; ANR-16-CE37-0017-01) and the programme ‘Laboratoire d’Excellence’ of the University of Lyon (Labex Cortex; ANR-11-LABX-0042) within the national programme ‘Investissements d’Avenir’ of the Agence Nationale de la Recherche (ANR-11-IDEX-0007).
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- FSIQ =
full-scale intelligence quotient
- hrQoL =
health-related quality of life scale
- ICARS =
International Cooperative Ataxia Rating Scale
- PegBoard =
Pegboard Purdue Test
- PS =
performance status
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76. [DOI] [PubMed] [Google Scholar]
- Aarsen FK, Paquier PF, Arts WF, Van Veelen ML, Michiels E, Lequin M, et al. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol 2009; 27: 3526–32. [DOI] [PubMed] [Google Scholar]
- Aarsen FK, Paquier PF, Reddingius RE, Streng IC, Arts WF, Evera-Preesman M, et al. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer 2006; 106: 396–402. [DOI] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, Wood A.. Do children really recover better? Neurobehavioural plasticity after early brain insult. [Review. Brain 2011; 134: 2197–221. [DOI] [PubMed] [Google Scholar]
- Castellino SM, Ullrich NJ, Whelen MJ, Lange BJ.. Developing interventions for cancer-related cognitive dysfunction in childhood cancer survivors. J Natl Cancer Inst 2014; 106: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsman-Berrevoets CE, Van Dongen HR, Mulder PGH, y Geuze DP, Paquier PF, Lequin MH.. Tumour type and size are high risk factors for the syndrome of “cerebellar” mutism and subsequent dysarthria. J Neurol Neurosurg Psychiatry 1999; 67: 755–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA, Waber DP, Bernstein JH, Pomeroy SL, LaVally B, Sallan SE, et al. Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: role of age and perioperative factors. J Child Neurol 1995; 10: 209–12. [DOI] [PubMed] [Google Scholar]
- Chin HW, Maruyama Y.. Age at treatment and long-term performance results in medulloblastoma. Cancer 1984; 53: 1952–8. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Li C, Xiong X, Ogg RJ, Merchant TE.. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol 2008; 26: 3965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland DR, deMoor C, Moore BD 3rd, Ater JL.. Neurocognitive development of children after a cerebellar tumor in infancy: a longitudinal study. J Clin Oncol 1999; 17: 3476–86. [DOI] [PubMed] [Google Scholar]
- Dancey J, Zee B, Osoba D, Whitehead M, Lu F, Kaizer L, et al. Quality of life scores: an independent prognostic variable in a general population of cancer patients receiving chemotherapy. The National Cancer Institute of Canada Clinical Trials Group. Qual Life Res 1997; 6: 151–8. [DOI] [PubMed] [Google Scholar]
- Dennis M. Margaret Kennard (1899-1975): not a ‘principle’ of brain plasticity but a founding mother of developmental neuropsychology. Cortex 2010; 46: 1043–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, Hetherington CR, Greenberg ML.. Neuropsychological sequelae of the treatment of children with medulloblastoma. J Neurooncol 1996; 29: 91–101. [DOI] [PubMed] [Google Scholar]
- Dow RS, Moruzzi G.. The physiology and pathology of the cerebellum. Minneapolis: Minnesota University Press; 1958. [Google Scholar]
- Ellenberg L, McComb JG, Siegel SE, Stowe S.. Factors affecting intellectual outcome in pediatric brain tumor patients. Neurosurgery 1987; 21: 638–44. [DOI] [PubMed] [Google Scholar]
- Forsyth RJ. Back to the future: rehabilitation of children after brain injury. Arch Dis Child 2010; 95: 554–9. [DOI] [PubMed] [Google Scholar]
- Fouladi M, Gilger E, Kocak M, Wallace D, Buchanan G, Reeves C, et al. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J Clin Oncol 2005; 23: 7152–60. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A. The effects of cerebellar hemispherectomy in the young rat. I. Behavioral sequelae. Behav Brain Res 1982; 6: 85–92. [DOI] [PubMed] [Google Scholar]
- Grill J, Renaux VK, Bulteau C, Viguier D, Levy-Piebois C, Sainte-Rose C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys 1999; 45: 137–45. [DOI] [PubMed] [Google Scholar]
- Gupta T, Jalali R, Goswami S, Nair V, Moiyadi A, Epari S, et al. Early clinical outcomes demonstrate preserved cognitive function in children with average-risk medulloblastoma when treated with hyperfractionated radiation therapy. Int J Radiat Oncol Biol Phys 2012; 83: 1534–40. [DOI] [PubMed] [Google Scholar]
- Guyotat J, Metellus P, Giorgi R, Barrie M, Jouvet A, Fevre-Montange M, et al. Infratentorial ependymomas: prognostic factors and outcome analysis in a multi-center retrospective series of 106 adult patients. Acta Neurochir 2009; 151: 947–60. [DOI] [PubMed] [Google Scholar]
- Hanzlik E, Woodrome SE, Abdel-Baki M, Geller TJ, Elbabaa SK.. A systematic review of neuropsychological outcomes following posterior fossa tumor surgery in children. Childs Nerv Syst 2015; 31: 1869–75. [DOI] [PubMed] [Google Scholar]
- Hoppe-Hirsch E, Renier D, Lellouch-Tubiana A, Sainte-Rose C, Pierre-Kahn A, Hirsch JF.. Medulloblastoma in childhood: progressive intellectual deterioration. Child’s Nerv Syst 1990; 6: 60–5. [DOI] [PubMed] [Google Scholar]
- Horne MK, Butler EG.. The role of the cerebello-thalamo-cortical pathway in skilled movement. Prog Neurobiol 1995; 46: 199–213. [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol 2006; 78: 272–303. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Rose FD, Brooks BM, Eyers S.. Age and recovery from brain injury: legal opinions, clinical beliefs and experimental evidence. Pediatr Rehabil 2003; 6: 103–9. [DOI] [PubMed] [Google Scholar]
- Kaye AH, Laws ER.. Brain tumors. London: England Harcourt; 2001. [Google Scholar]
- Kennard MA. Cortical reorganization of motor function: studies on series of monkeys of various ages from infancy to maturity. Arch NeurPsych 1942; 48: 227–40. [Google Scholar]
- Kennedy C, Bull K, Chevignard M, Culliford D, Dorr HG, Doz F, et al. Quality of survival and growth in children and young adults in the PNET4 European controlled trial of hyperfractionated versus conventional radiation therapy for standard-risk medulloblastoma. Int J Radiat Oncol Biol Phys 2014; 88: 292–300. [DOI] [PubMed] [Google Scholar]
- Konczak J, Schoch B, Dimitrova A, Gizewski E, Timmann D.. Functional recovery of children and adolescents after cerebellar tumour resection. Brain 2005; 128: 1428–41. [DOI] [PubMed] [Google Scholar]
- Koob M, Girard N.. Cerebral tumors: specific features in children. Diagn Interv Imaging 2014; 95: 965–83. [DOI] [PubMed] [Google Scholar]
- Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum 2014; 13: 151–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krageloh-Mann I, Lidzba K, Pavlova MA, Wilke M, Staudt M.. Plasticity during early brain development is determined by ontogenetic potential. Neuropediatrics 2017; 48: 66–71. [DOI] [PubMed] [Google Scholar]
- Kuper M, Doring K, Spangenberg C, Konczak J, Gizewski ER, Schoch B, et al. Location and restoration of function after cerebellar tumor removal-a longitudinal study of children and adolescents. Cerebellum 2013; 12: 48–58. [DOI] [PubMed] [Google Scholar]
- Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR.. The measurement of performance in childhood cancer patients. Cancer 1987; 60: 1651–6. [DOI] [PubMed] [Google Scholar]
- Law N, Greenberg M, Bouffet E, Taylor MD, Laughlin S, Strother D, et al. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro Oncol 2012; 14: 1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD.. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain 2000; 123: 1041–50. [DOI] [PubMed] [Google Scholar]
- McKean-Cowdin R, Razavi P, Barrington-Trimis J, Baldwin RT, Asgharzadeh S, Cockburn M, et al. Trends in childhood brain tumor incidence, 1973-2009. J Neurooncol 2013; 115: 153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitby PA, Robison LL, Whitton JA, Zevon MA, Gibbs IC, Tersak JM, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer 2003; 97: 1115–26. [DOI] [PubMed] [Google Scholar]
- Molinari M, Petrosini L, Gremoli T.. Hemicerebellectomy and motor behaviour in rats. II. Effects of cerebellar lesion performed at different developmental stages. Exp Brain Res 1990; 82: 483–92. [DOI] [PubMed] [Google Scholar]
- Moxon-Emre I, Bouffet E, Taylor MD, Laperriere N, Scantlebury N, Law N, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol 2014; 32: 1760–8. [DOI] [PubMed] [Google Scholar]
- Olson K, Sands SA.. Cognitive training programs for childhood cancer patients and survivors: a critical review and future directions. Child Neuropsychol 2016; 22: 509–36. [DOI] [PubMed] [Google Scholar]
- Packer RJ, Sutton LN, Atkins TE, Radcliffe J, Bunin GR, D'Angio G, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg Pediatr 1989; 70: 707–13. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Gajjar A, Reddick WE, Glass JO, Kun LE, Wu S, et al. Predicting intellectual outcome among children treated with 35-40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology 2003; 17: 548–55. [DOI] [PubMed] [Google Scholar]
- Poretti A, Meoded A, Huisman TA.. Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging 2012; 35: 32–47. [DOI] [PubMed] [Google Scholar]
- Riva D, Giorgi C, Nichelli F, Bulgheroni S, Massimino M, Cefalo G, et al. Intrathecal methotrexate affects cognitive function in children with medulloblastoma. Neurology 2002; 59: 48–53. [DOI] [PubMed] [Google Scholar]
- Robinson KE, Fraley CE, Pearson MM, Kuttesch JF Jr, Compas BE.. Neurocognitive late effects of pediatric brain tumors of the posterior fossa: a quantitative review. J Int Neuropsychol Soc 2013; 19: 44–53. [DOI] [PubMed] [Google Scholar]
- Ronning C, Sundet K, Due-Tonnessen B, Lundar T, Helseth E.. Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg 2005; 41: 15–21. [DOI] [PubMed] [Google Scholar]
- Sachsenheimer W, Piotrowski W, Bimmler T.. Quality of life in patients with intracranial tumors on the basis of Karnofsky’s performance status. J Neurooncol 1992; 13: 177–81. [DOI] [PubMed] [Google Scholar]
- Smith RL, Parks T, Lynch G.. A comparison of the role of the motor cortex in recovery from cerebellar damage in young and adult rats. Behav Biol 1974; 12: 177–98. [DOI] [PubMed] [Google Scholar]
- Sokolov AA, Miall RC, Ivry RB.. The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci 2017; 21: 313–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlin M, Imfeld S, Zulauf P, Boltshauser E, Lovblad KO, Ridolfi Luthy A, et al. Neuropsychological long-term sequelae after posterior fossa tumour resection during childhood. Brain 2003; 126: 1998–2008. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 2012; 11: 352–65. [DOI] [PubMed] [Google Scholar]
- Storey E, Tuck K, Hester R, Hughes A, Churchyard A.. Inter-rater reliability of the International Cooperative Ataxia Rating Scale (ICARS). Mov Disord 2004; 19: 190–2. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA.. Cerebellum and nonmotor function. Annu Rev Neurosci 2009; 32: 413–34. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Alden J.. Age-related differences in outcomes following childhood brain insults: an introduction and overview. J Int Neuropsychol Soc 1997; 3: 555–67. [PubMed] [Google Scholar]
- Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B, Gururangan S, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol 2016; 17: 484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin J, Asher EI.. The Purdue Pegboard: norms and studies of reliability and validity. J Appl Psychol 1948; 32: 234–47. [DOI] [PubMed] [Google Scholar]
- Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, et al. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex 2010; 46: 845–57. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 1997; 145: 205–11. [DOI] [PubMed] [Google Scholar]
- van de Poll-Franse LV, Mols F, Gundy CM, Creutzberg CL, Nout RA, Verdonck-de Leeuw IM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer 2011; 47: 667–75. [DOI] [PubMed] [Google Scholar]
- Varni JW, Limbers CA, Burwinkle TM.. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL (TM) 4.0 Generic Core Scales. Health Qual Life Outcomes 2007; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW, Seid M, Kurtin PS.. PedsQL™ 4.0: reliability and validity of the pediatric quality of life Inventory™ Version 4.0 generic core scales in healthy and patient populations. Med Care 2001; 39: 800–12. [DOI] [PubMed] [Google Scholar]
- von Hoff K, Kieffer V, Habrand JL, Kalifa C, Dellatolas G, Grill J.. Impairment of intellectual functions after surgery and posterior fossa irradiation in children with ependymoma is related to age and neurologic complications. BMC Cancer 2008; 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C, Rose FD, Johnson DA, Attree EA.. Age and recovery from brain injury: clinical opinions and experimental evidence. Brain Inj 1996; 10: 303–10. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children—fourth edition. French Version. Paris: ECPA/Pearson; 2005. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale—fourth edition. French Version. Paris: ECPA/Pearson; 2011. [Google Scholar]
- Winer BJ. Statistical principles in experimental design. New York: Mc Graw-Hill; 1971. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request. They are not publicly available because of ethical restrictions.