Abstract
Objective
The understanding of systemic lupus erythematosus (SLE) and lupus nephritis (LN) pathogenesis remains incomplete. This review assessed LN development in SLE, within-LN progression and progression to end-stage renal disease (ESRD).
Methods
A keyword-based literature search was conducted, and 26 publications were included.
Results
Overall, 7–31% of patients had LN at SLE diagnosis; 31–48% developed LN after SLE diagnosis, most within 5 years. Class IV was the most commonly found LN class and had the worst prognosis. Histological transformation occurred in 40–76% of patients, more frequently from non-proliferative rather than proliferative lesions. Cumulative 5- and 10-year ESRD incidences in patients with SLE were 3% and 4%, respectively, and 3–11% and 6–19%, respectively, in patients with SLE and LN.
Conclusions
Elevated serum creatinine was identified as a predictor of worsening disease state, and progression within LN classes and from SLE/LN to ESRD. This review highlights the substantial risk for developing LN and progressing to ESRD amongst patients with SLE.
Keywords: Nephritis, renal lupus, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by autoantibody production and deposition of immune complexes with complement activation, resulting in inflammation and damage within the affected tissue.1 SLE can be diagnosed based on a multitude of clinical characteristics, including disease manifestations in the skin, joints, kidneys and central nervous system, as well as serological findings, such as ANA.2 Organ system involvement and clinical and serological presentations are highly variable, both between patients and within the same patient over time, making SLE an unpredictable and complex disease to manage.2
Patients with SLE are classified using the American College of Rheumatology (ACR) criteria where ≥4 out of 11 clinical and/or serological criteria need to be met. Given the limited sensitivity and specificity of these criteria, the Systemic Lupus International Collaborating Clinics (SLICC) research group introduced a new set of classification criteria where ≥4 of 17 criteria need to be met.3 An observational study demonstrated superiority of the SLICC criteria over ACR criteria in terms of criteria sensitivity. Furthermore, the SLICC criteria allow patients to be categorized as having SLE earlier in the disease course which may offer better management of the disease.3
Mucocutaneous involvement, musculoskeletal involvement and renal organ involvement are common in SLE.4 Almost all patients have some degree of renal involvement during the disease course, and between 40% and 70% of patients develop clinically diagnosed renal involvement, termed lupus nephritis (LN).4,5 LN is characterized by glomerular deposition of immune complexes and subsequent renal inflammation,6 and is classified by renal biopsy, the gold standard for diagnosis.7 Patients with LN present with autoantibodies to complement C1q (anti-C1q) and C3b (anti-C3b IgG); levels increase over time resulting in renal flares.8 A patient can be classified into any of six histological categories established by the World Health Organization (WHO) and later updated by the International Society of Nephrology/Renal Pathology Society (ISN/RPS), of which classes III–VI are associated with the greatest risk of long-term damage.6,9–11 Class VI is the most advanced LN stage, where patients often require kidney dialysis or transplantation.10,11
Using biomarkers to identify patient subsets allows monitoring of treatment response and detection of disease activity. Proteinuria, urine protein-to-creatinine ratio, creatinine clearance, anti-dsDNA and complement levels are current LN laboratory markers. However, they lack specificity and sensitivity for identifying renal activity and damage.12 Many novel biomarkers are under investigation, including additional autoantibodies and urinary proteomics, which might help detect renal flares earlier and assess disease progression.12 A 46-year follow-up cohort study showed a reduction in the severity of LN clinical presentation LN over time and improved long-term renal survival, potentially due to prompt diagnosis of renal involvement and treatment decisions based on renal biopsy.13
Treatment for SLE involves non-steroidal anti-inflammatory drugs (NSAIDs), antimalarials, corticosteroids, immunosuppressive agents and biological agents.14,15 However, NSAIDs are not recommended for patients with LN,14 and higher doses of corticosteroids are generally used in combination with more aggressive immunosuppression with cyclophosphamide or mycophenolate mofetil (MMF) during the induction phase, followed by maintenance treatment with MMF or azathioprine.15,16
B-cell-targeted biological therapies can control the autoimmune response associated with SLE and LN while avoiding corticosteroid use. Such therapies include belimumab, the human immunoglobulin 1λ monoclonal antibody that targets the soluble form of B lymphocyte stimulator, also known as B-cell-activating factor, and anti-CD20 monoclonal antibody rituximab, the latter of which is prescribed off-label by some physicians.17 Belimumab has been shown to improve the SLE Responder Index, compared with placebo, after 52 weeks of treatment in patients with SLE.18 Notably, despite patients with severe active LN (≥6 g/24 hours of proteinuria or serum creatinine ≥2.5 mg/dL) being excluded from Phase III trials of belimumab, as were those with other indications for intravenous cyclophosphamide treatment within six months of enrolment, improvements in renal function were seen. A systematic review of patients with SLE who had already developed LN found that 55.1% (129/234) of patients receiving belimumab exhibited renal improvement following treatment.19 Despite these therapeutic options, treatment of SLE and LN remains challenging, and without effective control of renal disease activity, patients may progress to end-stage renal disease (ESRD), which can be fatal unless dialysis or a kidney transplant is received.5,20,21
Literature review
This pragmatic review (GlaxoSmithKline study LS3178) focused on three fundamental areas, each of which comprised key research questions. First, the development of LN among patients with SLE was investigated. Questions within this category included ‘What proportion of patients with SLE have LN at initial diagnosis?’, ‘What proportion of patients with SLE go on to develop LN?’ and ‘What are the key predictors of LN development in patients with SLE?’. The second area investigated what happens within LN progression, with questions including ‘What is the proportion of patients with different LN classifications at initial biopsy?’, ‘What do we know about early renal signs and symptoms in SLE?’, ‘How does LN transition between severity stages?’ and ‘What is the time to progression within LN?’. Finally, the progression to ESRD was explored. Associated questions included ‘What proportion of patients with SLE, and patients with LN, go on to develop ESRD?’, ‘What is the time to progression to ESRD from SLE and LN?’ and ‘What are the key predictors of progression to ESRD in patients with SLE and LN?’. Twenty six articles were identified through a keyword-based literature search. The search strategy and data-extraction process used are described in Supplemental Appendix 1.
Development of LN among patients with SLE
Proportion of patients with SLE who had LN at initial diagnosis
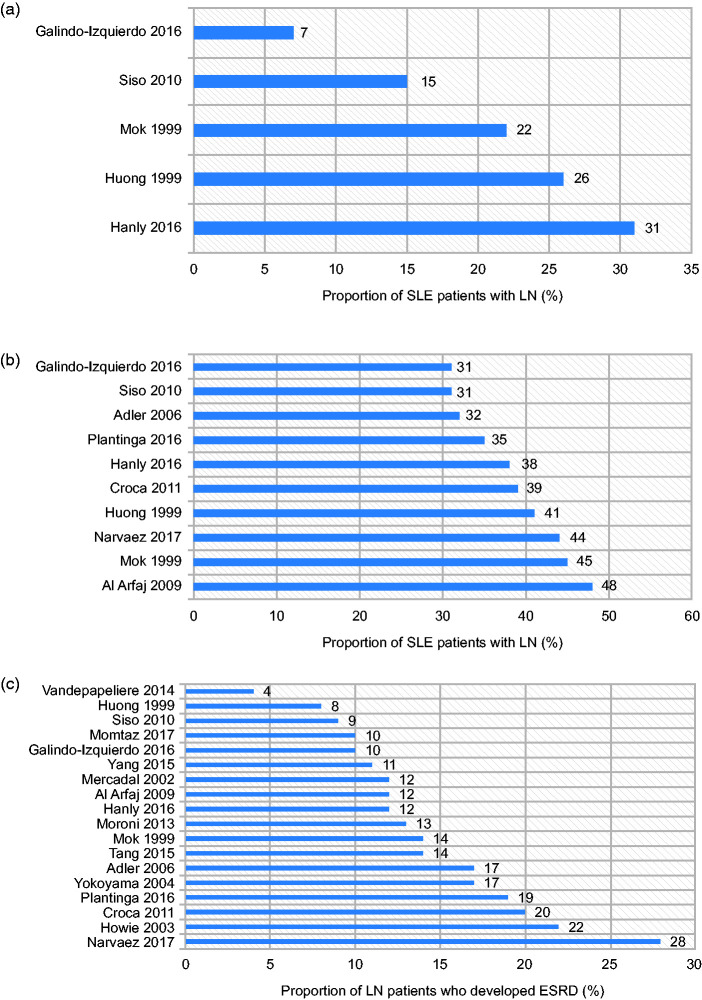
The proportion of patients with SLE who had LN at the time of their SLE diagnosis ranged from 7% to 31%22–26 (Figure 1 (a)). Patient characteristics alone did not explain the wide variation in LN rates across studies. Importantly, the lower range (7–22%) was derived from studies that relied on biopsy confirmation alone for LN,22,25,26 whereas the higher range (26–31%) was reported in studies that permitted other renal parameters in addition to biopsy confirmation.23,24
Figure 1.
Proportion of patients (a) with SLE who were diagnosed with LN at SLE diagnosis, (b) with SLE who developed LN at any time during follow-up and (c) with LN who developed ESRD.
ESRD: end-stage renal disease; LN: lupus nephritis; SLE: systemic lupus erythematosus.
Proportion of patients with SLE who ever develop LN
Two studies reported that 31–38% of patients with SLE developed LN during the course of their disease22,23 (Figure 1(b)). Hanly et al. used the SLICC international cohort, which included 1827 patients with SLE recruited from 1999 to 2012. Within this cohort, LN was diagnosed in 700 (38%) patients, 81% of whom were diagnosed at study enrolment, and a further 19% developed LN during the follow-up period (mean = 4.6 years (standard deviation (SD) = 3.4 years)).23 Galindo-Izquierdo et al. reported that of 1092 patients who developed histologically confirmed LN, 50% and 74% developed LN within 12 months and 5 years of SLE diagnosis, respectively,22 highlighting that the risk of LN is highest in the initial years following SLE diagnosis.
In seven single-centre studies, 31–48% of patients with SLE developed LN (Figure 1(b)). In a study where 180/436 (41%) patients with SLE had renal involvement, 36% presented with renal involvement after SLE was diagnosed, just 31% of whom developed renal abnormalities more than 5 years after their SLE diagnosis.24 The longest time elapsing between SLE diagnosis and renal involvement onset was 19 years.24 However, occurrence of renal involvement so long after SLE diagnosis was generally uncommon. Mok et al. studied 406 patients with SLE from 1976 to 1997. Of these, 183 patients developed biopsy-proven LN.25 The majority of patients (78%) did not have LN at the time of their SLE diagnosis. However, the mean time between the diagnosis of SLE and the development of renal disease was just 52.6 months ((standard error (SE)) = 5.0 months).25 Siso et al. reported that of the 190 patients with biopsy-proven LN, 87 (46%) patients developed LN after SLE diagnosis, but only 25 (13%) of these patients were diagnosed with LN more than 5 years after SLE diagnosis.26
Key predictors of LN development in patients with SLE
Among patients with SLE who developed LN, there were proportionally more males and younger-aged patients compared with those who did not develop LN. This was demonstrated in a study in which 9% of patients with SLE but not LN at diagnosis were males compared with 16% in the group of patients who also had LN at the time of their diagnosis (p < 0.001).23 Further, the mean ages of the LN patients compared with non-LN patients in this study were 31.3 years (SD = 11.9 years) and 36.9 years (SD = 13.6 years), respectively (p < 0.001).23 Similarly, a retrospective cohort analysis conducted by Galindo-Izquierdo et al. identified a significantly higher risk of developing LN among men than women (odds ratio (OR) = 2.57, 95% confidence interval (CI) 2.02–3.29, p < 0.001).22 Compared to patients ≥50 years of age, the ORs for developing LN in patients aged <16 years of age and 16–50 years of age were 6.06 (95% CI 4.29–8.56, p < 0.001) and 2.52 (95% CI 1.91–3.32, p < 0.001), respectively.22
A number of studies also identified patients of African or Asian race23,27 or Hispanic ethnicity22,23 having an increased risk of developing LN compared with Caucasian patients. One study showed that Hispanic ethnicity was independently associated with a higher risk of renal disease than Caucasian ethnicity (OR = 1.85, 95% CI 1.37–2.51, p < 0.001), after adjusting for sex and age.22 Another study demonstrated a significant difference between the proportion of black patients who developed LN compared with white patients (44% (33/74) vs. 22% (58/258), respectively).27
LN
Early renal involvement in SLE
Data on signs of renal involvement prior to LN diagnosis were limited. In general, worsening renal parameters were indicated in most of the studies as the primary reason for biopsy. These included unexplained haematuria (particularly dysmorphic erythrocytes), cellular casts and/or proteinuria >0.5 ± 1.0 g/day.26,28 However, the evolution of renal function in the period prior to biopsy has not been extensively studied.
Proportion of patients with different LN classifications at initial biopsy
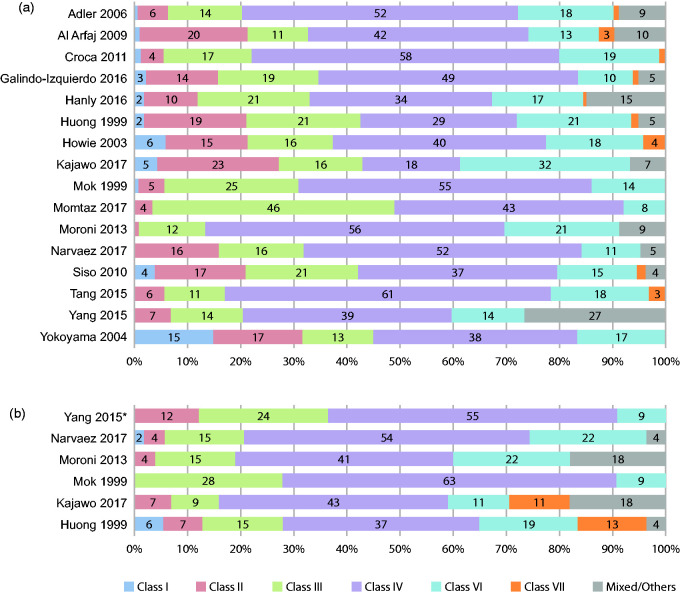
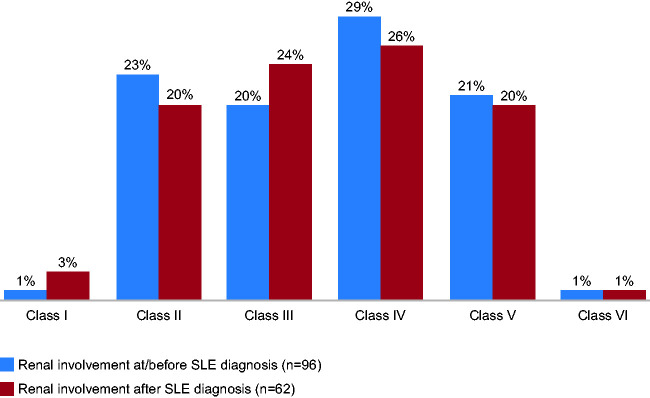
Class IV nephritis (diffuse proliferative glomerulonephritis) was the most frequently reported LN classification at initial biopsy22–36 (Figure 2 (a)) and at repeat biopsy24,25,28,32,34,35 (Figure 2(b)). The classes reported least frequently at initial biopsy were class I and class VI (Figure 2(a)). In two studies, class IV LN was further divided into class IV-S (segmental: where >50% of the involved glomeruli have segmental lesions) and class IV-G (global (diffuse): where >50% of the involved glomeruli have global (diffuse) lesions), and both studies reported a higher proportion of patients with class IV-G compared with class IV-S (20% vs. 13% and 28% vs. 10%, Hanly and Yokoyama, respectively).23,36 Histological distribution at initial biopsy was similar whether renal involvement occurred before or after SLE diagnosis24,26 (Figure 3 ). At first biopsy, the most commonly reported mixed classifications were class IV/V, with prevalence ranging from 1% to 15%,22,23,30,32,34,35 and class III/V, with prevalence ranging from 1% to 11%.22,23,30,34,35
Figure 2.
WHO/ISN/RPS histological class distribution (%*) across studies at (a) initial biopsy and (b) repeat biopsy. WHO classification (class I = normal kidney; class II = mesangial glomerulonephritis; class III = focal segmental proliferative glomerulonephritis; class IV = diffuse proliferative glomerulonephritis; class V = membranous glomerulonephritis; class VI = chronic sclerosing glomerulonephritis); ISN/RPS classification (class I = minimal mesangial LN; class II = mesangial proliferative LN; class III = focal LN; class IV = diffuse LN; class V = membranous LN; class VI = advanced sclerosis LN; ‘other’ ISN/RPS categories include: class III (A) = active lesions: focal proliferative LN; class III (A/C) = active and chronic lesions: focal proliferative and sclerosing LN; class III (C) = chronic inactive lesions with glomerular scars: focal sclerosing LN; class IV-S (A) = active lesions: diffuse segmental proliferative LN; class IV-G (A) = active lesions: diffuse global proliferative LN; class IV-S (A/C) = active and chronic lesions: diffuse segmental proliferative and sclerosing LN; class IV-G (A/C) = active and chronic lesions: diffuse global proliferative and sclerosing LN; class IV-S (C) = chronic inactive lesions with scars: diffuse segmental sclerosing LN; class IV-G (C) = chronic inactive lesions with scars: diffuse global sclerosing LN).37
*Percentages calculated based on the number of patients with available data.
ISN/RPS: International Society of Nephrology/Renal Pathology Society; LN: lupus nephritis; WHO: World Health Organization.
Figure 3.
Onset of renal involvement within each WHO classification (%).
WHO: World Health Organization.
Transition between LN histological classes
A SLR conducted by Narvaez et al. identified 10 studies analysing 686 patients with LN who had undergone a repeat biopsy because of a LN flare.28 Histological transformation from one LN class to another occurred in 40–76% of cases, and the time interval between first and second biopsies varied from 6 months to 4.2 years.28 Similar results have been reported elsewhere. Histological transformation was observed in approximately 51–56% of patients who underwent a second biopsy.24,26,34 These biopsies were conducted due to immunological features suggestive of high disease activity or renal relapse or to assess the outcome of induction therapy. A retrospective study found histological transformation at repeat biopsy in as many as 94% of patients. However, this was a small study in which only 18 patients had a second biopsy.38 Histological transformations were more common from non-proliferative lesions (class II and class V) compared with proliferative lesions (class III and class IV).28,32,38–40
Time to progression within LN
Data on time to progression within LN were scarce, most likely because transformation between LN classes is not sequential. Limited evidence from a retrospective multi-centre study suggested that the median time to histological transformation with LN is 32 months (range 11–305 months).38
Progression to ESRD
Proportion of patients with SLE who develop ESRD
Overall, the proportion of patients with SLE who develop ESRD increases as time from diagnosis increases. An analysis of the Georgia Lupus Registry by Plantinga et al., which included 344 newly diagnosed patients with SLE, found that incidence rates/1000 patient years for ESRD were 13.8 in black patients and 3.3 in white patients.41 The corresponding 5-year cumulative incidences were 6.4% and 2.5% among black and white patients, respectively.41 Similar results were observed in an international cohort of patients with SLE, whereby cumulative incidences of ESRD at 5 and 10 years were 3.3% and 4.3%, respectively, amongst all included patients.23
Proportion of patients with LN who develop ESRD
Within the primary studies analysed, 4–28% of patients with LN developed ESRD22–31,33–36,39–41 (Figure 1(c)). The cumulative incidence of developing ESRD at 5, 10 and 15 years ranged from 3% to 11%, 6% to 19% and 19% to 25%, respectively.21 Incidences reported within SLRs were at the higher end of the ranges seen within the primary studies, with 11–12%, 17–18% and 22–26% reported for 5, 10 and 15 years, respectively.21
Time to progression from SLE/LN to ESRD
Limited data are available relating to the time to progression from SLE to ESRD. In one study, the median time to ESRD from SLE diagnosis was approximately 4.1 years among patients who progressed during the course of the study. In this case, >75% of the population were black. These patients were an estimated four times more likely than white patients to progress to ESRD after adjusting for age.41 The high proportion of black patients in this study may reflect the higher incidence of SLE in these individuals compared with white patients.42 One study reported data regarding time to progression from LN to ESRD. A cohort of 154 patients with LN was assessed in which 20% of the patients went on to develop ESRD, with an average time from onset of renal disease to ESRD of 7.5 years.31 In this study, there were no differences in the ethnic distribution, with 36.7% of patients of Afro-Caribbean origin, 26.7% Caucasian and 23.3% Asian developing ESRD. A lower percentage of black patients compared with the study by Plantinga may have resulted in longer time to ESRD. This is further supported by the finding that patients of Afro-Caribbean origin had significantly higher ESRD rate within 5 years of LN diagnosis (p = 0.001).31 Overall, the race and/or ethnicity of the study population should be considered when interpreting and comparing time to ESRD between studies.
Key predictors of ESRD in patients with SLE/LN
The most commonly reported independent clinical laboratory predictor for ESRD in patients with SLE alone or LN was high serum creatinine (>1.5 mg/dL) at disease onset (from sources reporting both LN at presentation or at biopsy). Other factors indicating an increased risk for ESRD included: hypocomplementaemia; class III, IV and VI LN; higher chronicity index; high systolic blood pressure; older age; male sex; and black race. 22,26,30,34,35
Summary of disease progression, transformation and severity
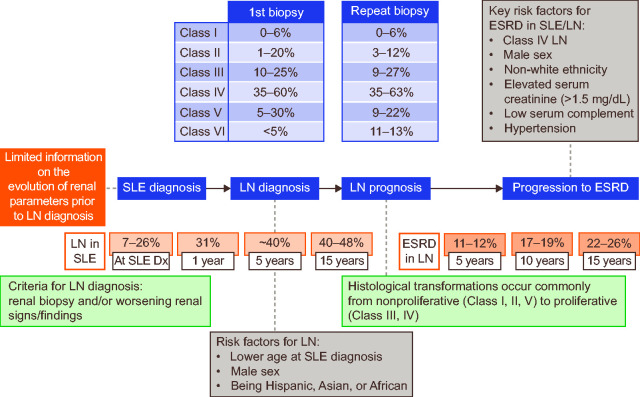
Figure 4 provides an overview of how the data identified as part of this pragmatic review can be placed within an overall map of disease progression, transformation and severity within SLE, LN and ESRD.
Figure 4.
Mapping of disease progression from SLE to LN and ESRD.
Dx: diagnosis; ESRD: end-stage renal disease; LN: lupus nephritis; SLE: systemic lupus erythematosus.
Figure reproduced under the terms of the Creative Commons CC-BY-NC licence from Justyna Amelio, Kerry Gairy, Anadi Mahajan, Gavneet Kaur, Damon Bass, Roger Abramino Levy, David Roth. Mapping disease severity and progression of renal involvement in patients with systemic lupus erythematosus (Abstract 87). Lupus Sci Med 2019; 6: A63.43
Discussion
This work mapped the disease pathway, including topics relevant to targeting disease management: renal involvement and renal progression in patients with SLE, different stages of renal involvement and the proportion of patients who progress between classes, as well as timing of progression and risk factors associated with progression.
Across the publications included, the proportion of patients with SLE who had LN at the time of their SLE diagnosis varied from 7% to 31%, and 31–48% of patients with SLE developed LN at some point in their disease course.22–28,30,31,41 It was noted that there was a higher risk of worse outcomes during the earlier stages of SLE, with most patients who went on to develop LN doing so within the first 5 years following their SLE diagnosis.22–26 Data on the predictors of LN development among patients with SLE were limited. However, it was suggested that among patients who did develop LN, there was a higher proportion of male and younger-aged patients than in those who did not develop LN. Additionally, an increased risk of LN development was shown in patients of African or Asian race, or Hispanic ethnicity, compared with Caucasian patients.
Next, within-LN progression was explored. This topic was selected based on the diverse clinical manifestations and histological patterns of renal injury associated with LN, warranting further clarification of the disease pathway. The majority of studies suggested that patients with class IV had the worst prognosis, and class IV was consistently the most frequent histopathological class of LN, at both first and repeat biopsies.22–36 However, it should be noted that there is the chance of selection bias in these studies due to the type of patients who undergo biopsy. A renal biopsy is recommended for diagnosis of LN. However, a second biopsy is still considered controversial due to its invasive nature, in spite of studies highlighting the benefits of a second biopsy in better understanding a patient’s response to treatment.44,45 The second biopsy, when performed, is almost invariably due to lack of treatment response. Hence, patients who underwent biopsies were more likely to have a worse clinical picture and/or be more refractory to treatment than those who were not biopsied. Indeed, biopsies were not always conducted in all patients with LN, and repeat biopsies were uncommon. In patients who did undergo repeat biopsies, histopathological transformation between LN classes was common (40–76% of patients with LN), but this varied according to the class of LN and was more common from non-proliferative than from proliferative classes.24,26,28,34 This study found that data on time to LN transformation were scarce, possibly because the transition between LN stages is not sequential.
Finally, data on progression from SLE or LN to ESRD were analysed. The cumulative incidence of ESRD in patients with SLE at 5 and 10 years was low (3% and 4%, respectively). Obviously, this was higher in patients with LN, ranging from 3% to 11% and 6% to 19%, respectively, increasing further to 19–25% at 15 years.21 In comparison, <6% of patients with type 1 diabetes and nephropathy are reported to develop ESRD.46 Importantly, the risk of developing ESRD in patients with SLE after 10 and 15 years decreased notably between 1970 and 2010 in developed countries, potentially due to increased use of angiotensin-converting enzyme inhibitors, immunosuppressive therapy use, better serological monitoring,25 improved treatment access, earlier diagnosis and improved treatment management.21,25 Again, data on time to progression from SLE/LN to ESRD were sparse. However, the study did find several important risk factors for progression from SLE/LN to ESRD, including male sex and non-Caucasian race/ethnicity. Elevated baseline serum creatinine was an important predictor of worsening progression, in terms of both transition between LN classes (based on data for performing repeat biopsies) and ESRD.22,26,30,34,35 The results pertaining to higher risk of disease progression from SLE to LN and from SLE/LN to ESRD within patients of black and Asian race and Hispanic ethnicity are similar to reports in the wider literature,47,48 including studies that consider the socio-economic factors involved in the increased risk of disease progression.48 Furthermore, it has been suggested that independent scoring of fibrinoid necrosis, fibrous crescents and interstitial fibrosis/tubular atrophy should also be included when assessing risk of progressive renal dysfunction in patients with LN.50
There are a number of design limitations that should be considered when interpreting the findings from this review. First, some relevant publications may have been missed, as the search strategy involved keyword-based and non-systematic bibliographic searches. Second, although predefined research questions and selection criteria were used to select 26 publications for inclusion, ultimately the selection was subjective and open to bias. Third, the data presented do not reflect the patient perspective. As renal symptoms can be silent but have severe long-term consequences, the patient experience will be an important consideration in any future research in this area. Finally, most studies included in this review were conducted in specialist referral centres, and therefore reported rates of nephritis may be higher than in the community. It is also important to note that out of the 26 studies, only 10 had 100% of patients with LN-proven by biopsy (11 studies if this criterion is extended to those in which ≥80% of patients had LN-proven biopsy). The remaining 16 studies either reported varying lower proportions of patients with biopsy-confirmed LN or it was unclear whether biopsy was used to confirm the LN diagnosis.
Despite these limitations, this review provides valuable information regarding disease progression in SLE, which may be useful when considering prognosis and relevant treatment options. The study also identified a number of gaps within the literature, including a lack of information pertaining to early signs of nephritis and robust data on class transformations (as repeat biopsies are not commonly conducted in earlier stages). Moreover, characterization of time to progression within LN stages was difficult, as most patients with LN had class IV LN at diagnosis. Data on the time to progression from SLE and LN to ESRD were also lacking. These clinically relevant data gaps should be taken into consideration when planning future epidemiological research including patients with SLE, and will be valuable when posing specific questions to be addressed in more directed systematic literature reviews.
Conclusions
This pragmatic review highlighted that although 30–60% of patients with SLE do not develop LN, of those who do, up to 90% do so within 5 years of SLE diagnosis, indicating that the risk of LN is higher in the initial years following SLE diagnosis. Risk factors for developing LN include lower age and SLE diagnosis, male sex and being of Hispanic, Asian or black race. Furthermore, histological transformation within LN is common. However, further studies are required to understand the time to and risk factors for this better. Up to 1/20 patients with SLE and up to one quarter of patients with LN go on to develop ESRD, highlighting the substantial risk for disease progression and worsening within a short time frame in SLE.
Supplemental Material
Supplemental material, sj-pdf-1-lup-10.1177_0961203320932219 for Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: a pragmatic review mapping disease severity and progression by Anadi Mahajan, Justyna Amelio, Kerry Gairy, Gavneet Kaur, Roger A Levy, David Roth and Damon Bass in Lupus
Acknowledgements
Medical writing support was provided by Emma Hargreaves, MA, and Jennie McLean, PhD, of Fishawack Indicia Ltd, UK, and was funded by GlaxoSmithKline.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: J.A., D.B., K.G., R.A.L. and D.R. are employees of GlaxoSmithKline and hold shares in the company. A.M. and G.K. are employees of Bridge Medical Pvt. Ltd.
Funding
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study (LS3178) was funded by GlaxoSmithKline, and GlaxoSmithKline commissioned Bridge Medical to conduct the pragmatic literature review.
Data availability
All data relevant to the study are included in the manuscript or uploaded as supplementary information.
ORCID iD
Justyna Amelio https://orcid.org/0000-0002-3130-5101
Supplemental material
Supplemental material for this article is available online.
References
- 1.Teruel M, Sawalha AH. Epigenetic variability in systemic lupus erythematosus: what we learned from genome-wide DNA methylation studies. Curr Rheumatol Rep 2017; 19: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhn A, Bonsmann G, Anders H-J, Herzer P, Tenbrock K, Schneider M. The diagnosis and treatment of systemic lupus erythematosus. Dtsch Arztebl Int 2015; 112: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ines L, Silva C, Galindo M, et al. Classification of systemic lupus erythematosus: Systemic Lupus International Collaborating Clinics versus American College of Rheumatology Criteria. A comparative study of 2,055 patients from a real-life, international systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken) 2015; 67: 1180–1185. [DOI] [PubMed] [Google Scholar]
- 4.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Manifestations of systemic lupus erythematosus. Maedica (Buchar) 2011; 6: 330–336. [PMC free article] [PubMed] [Google Scholar]
- 5.Beckwith H, Lightstone L. Rituximab in systemic lupus erythematosus and lupus nephritis. Nephron Clin Pract 2014; 128: 250–254. [DOI] [PubMed] [Google Scholar]
- 6.Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol 2016; 12: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moroni G, Quaglini S, Radice A, et al. The value of a panel of autoantibodies for predicting the activity of lupus nephritis at time of renal biopsy. J Immunol Res 2015; 2015: 106904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol 2017; 12: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiremitci S, Ensari A. Classifying lupus nephritis: an ongoing story. ScientificWorldJournal 2014: 580620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inderjeeth CA, Habib P, Sharma C, Nossent J. Systemic lupus erythematosus: reducing life-threatening progression. Med Today 2018; 19: 31–41. [Google Scholar]
- 11.Lau WL, Appel GB. Lupus nephritis in 2017: an update. ASN Kidney News 2017; 9: 17–18. [Google Scholar]
- 12.Mok CC. Biomarkers for lupus nephritis: a critical appraisal. J Biomed Biotechnol 2010; 2010: 638413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moroni G, Vercelloni PG, Quaglini S, et al. Changing patterns in clinical-histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann Rheum Dis 2018; 77: 1318–1325. [DOI] [PubMed] [Google Scholar]
- 14.Amissah-Arthur MB, Gordon C. Contemporary treatment of systemic lupus erythematosus: an update for clinicians. Ther Adv Chronic Dis 2010; 1: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tunnicliffe DJ, Singh‐Grewal D, Kim S, Craig JC, Tong A. Diagnosis, monitoring, and treatment of systemic lupus erythematosus: a systematic review of clinical practice guidelines. Arthritis Care Res 2015; 67: 1440–1452. [DOI] [PubMed] [Google Scholar]
- 16.Bertsias G, Ioannidis J, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis 2008; 67: 195–205. [DOI] [PubMed] [Google Scholar]
- 17.Rydén-Aulin M, Boumpas D, Bultink I, et al. Off-label use of rituximab for systemic lupus erythematosus in Europe. Lupus Sci Med 2016; 3: e000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, Phase 3 trial. Lancet 2011; 377: 721–731. [DOI] [PubMed] [Google Scholar]
- 19.Sciascia S, Radin M, Yazdany J, et al. Efficacy of belimumab on renal outcomes in patients with systemic lupus erythematosus: a systematic review. Autoimmun Rev 2017; 16: 287–293. [DOI] [PubMed] [Google Scholar]
- 20.Inda‐Filho A, Neugarten J, Putterman C, Broder A. Improving outcomes in patients with lupus and end stage renal disease. Sem Dial 2013; 26: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tektonidou MG, Dasgupta A, Ward MM. Risk of end‐stage renal disease in patients with lupus nephritis, 1971–2015: a systematic review and Bayesian meta-analysis. Arthritis Rheum 2016; 68: 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galindo-Izquierdo M, Rodriguez-Almaraz E, Pego-Reigosa JM, et al. Characterization of patients with lupus nephritis included in a large cohort from the Spanish Society of Rheumatology registry of patients with systemic lupus erythematosus (RELESSER). Medicine 2016; 95: e2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanly JG, O’Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatol 2016; 55: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huong D, Papo T, Beaufils H, et al. Renal involvement in systemic lupus erythematosus. A study of 180 patients from a single center. Medicine 1999; 78: 148–166. [DOI] [PubMed] [Google Scholar]
- 25.Mok CC, Wong RW-S, Lau CS. Lupus nephritis in Southern Chinese patients: clinicopathologic findings and long-term outcome. Am J Kidney Dis 1999; 34: 315–323. [DOI] [PubMed] [Google Scholar]
- 26.Sisó A, Ramos-Casals M, Bové A, et al. Outcomes in biopsy-proven lupus nephritis: evaluation of 190 white patients from a single center. Medicine 2010; 89: 300–307. [DOI] [PubMed] [Google Scholar]
- 27.Adler M, Chambers S, Edwards C, Neild G, Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatol 2006; 45: 1144–1147. [DOI] [PubMed] [Google Scholar]
- 28.Narváez J, Ricse M, Gomà M, et al. The value of repeat biopsy in lupus nephritis flares. Medicine 2017; 96: e7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howie A, Turhan N, Adu D. Powerful morphometric indicator of prognosis in lupus nephritis. QJM 2003; 96: 411–420. [DOI] [PubMed] [Google Scholar]
- 30.Al Arfaj AS, Khalil N, Al Saleh S. Lupus nephritis among 624 cases of systemic lupus erythematosus in Riyadh, Saudi Arabia. Rheumatol Int 2009; 29: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 31.Croca SC, Rodrigues T, Isenberg DA. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatol 2011; 50: 1424–1430. [DOI] [PubMed] [Google Scholar]
- 32.Kajawo S, Botha F, Okpechi I. Clinico-pathological features of repeat renal biopsies in patients with lupus nephritis at Groote Schuur Hospital, Cape Town. Lupus 2017; 26: 1339–1346. [DOI] [PubMed] [Google Scholar]
- 33.Momtaz M, Fayed A, Wadie M, et al. Retrospective analysis of nephritis response and renal outcome in a cohort of 928 Egyptian lupus nephritis patients: a university hospital experience. Lupus 2017; 26: 1564–1570. [DOI] [PubMed] [Google Scholar]
- 34.Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C. Progressive improvement of patient and renal survival and reduction of morbidity over time in patients with lupus nephritis (LN) followed for 20 years. Lupus 2013; 22: 810–818. [DOI] [PubMed] [Google Scholar]
- 35.Tang Y, Zhang X, Ji L, et al. Clinicopathological and outcome analysis of adult lupus nephritis patients in China. Int Urol Nephrol 2015; 47: 513–520. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama H, Wada T, Hara A, et al. The outcome and a new ISN/RPS 2003 classification of lupus nephritis in Japanese. Kidney Int 2004; 66: 2382–2388. [DOI] [PubMed] [Google Scholar]
- 37.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004; 15: 241–250. [DOI] [PubMed] [Google Scholar]
- 38.Collado MV, Dorado E, Rausch S, et al. Long-term outcome of lupus nephritis class II in Argentine patients: an open retrospective analysis. J Clin Rheumatol 2016; 22: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercadal L, Du Montcel ST, Nochy D, et al. Factors affecting outcome and prognosis in membranous lupus nephropathy. Nephrol Dial Transplant 2002; 17: 1771–1778. [DOI] [PubMed] [Google Scholar]
- 40.Vandepapelière J, Aydin S, Cosyns J-P, Depresseux G, Jadoul M, Houssiau F. Prognosis of proliferative lupus nephritis subsets in the Louvain Lupus Nephritis inception Cohort. Lupus 2014; 23: 159–165. [DOI] [PubMed] [Google Scholar]
- 41.Plantinga L, Lim SS, Patzer R, et al. Incidence of end‐stage renal disease among newly diagnosed systemic lupus erythematosus patients: the Georgia Lupus Registry. Arthritis Care Res 2016; 68: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petri M. Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2002; 16: 847–858. [DOI] [PubMed] [Google Scholar]
- 43.Kaur G, Bass D, Abramino Levy R and Roth D. Mapping disease severity and progression of renal involvement in patients with systemic lupus erythematosus (Abstract 87). Lupus Sci Med 2019; 6: A63. [Google Scholar]
- 44.Pakozdi A, Pyne D, Sheaff M, Rajakariar R. Utility of a repeat renal biopsy in lupus nephritis: a single centre experience. Nephrol Dial Transplant 2018; 33: 507–513. [DOI] [PubMed] [Google Scholar]
- 45.Zickert A, Sundelin B, Svenungsson E, Gunnarsson I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med 2014; 1: e000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mollsten A, Svensson M, Waernbaum I, et al. Cumulative risk, age at onset, and sex-specific differences for developing end-stage renal disease in young patients with type 1 diabetes: a nationwide population-based cohort study. Diabetes 2010; 59: 1803–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sánchez E, Comeau ME, Freedman BI, et al. Identification of novel genetic susceptibility loci in African American lupus patients in a candidate gene association study. Arthritis Rheum 2011; 63: 3493–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan KE, Wooten C, Schmeckpeper BJ, Goldman D, Petri MA. A promoter polymorphism of tumor necrosis factor α associated with systemic lupus erythematosus in African-Americans. Arthritis Rheum 1997; 40: 2207–2211. [DOI] [PubMed] [Google Scholar]
- 49.Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 2003; 18: 2039–2046. [DOI] [PubMed] [Google Scholar]
- 50.Rijnink EC, Teng YO, Wilhelmus S, et al. Clinical and histopathologic characteristics associated with renal outcomes in lupus nephritis. Clin J Am Soc Nephrol 2017; 12: 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-lup-10.1177_0961203320932219 for Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: a pragmatic review mapping disease severity and progression by Anadi Mahajan, Justyna Amelio, Kerry Gairy, Gavneet Kaur, Roger A Levy, David Roth and Damon Bass in Lupus
Data Availability Statement
All data relevant to the study are included in the manuscript or uploaded as supplementary information.