Abstract
Familial amyloid polyneuropathy with the substitution of methionine for valine at position 30 in the TTR gene is the most common type of hereditary transthyretin amyloidosis. Although several authors have previously reported a size-dependent fibre loss, predominantly involving unmyelinated and small-diameter myelinated fibres, the mechanisms of nerve fibre loss have not been fully understood. In this study, we establish the morphometric pattern of peripheral neuropathy in patients with familial amyloid polyneuropathy and asymptomatic mutation carriers in the biopsies from our archive and correlated the pathological findings with clinical features. A total of 98 patients with familial amyloid polyneuropathy and 37 asymptomatic mutation carriers (TTR Val30Met mutation), aged between 17 and 84 years, who underwent sural nerve biopsy between 1981 and 2017 at Centro Hospitalar Universitário do Porto were studied. Thirty-one controls were included for comparison. The median age at nerve biopsy was 26.0 [interquartile range = 23.5–39.5] years for asymptomatic mutation carriers, 45.0 [35.0–60.0] years for patients with familial amyloid polyneuropathy and 44.0 [30.0–63.0] years for controls. The median duration between nerve biopsy and symptoms’ onset was 7.0 [3.3–11.8] years (range: 1–27 years) in the asymptomatic carriers. Most patients were in an earlier disease stage (93% with a polyneuropathy disability scale ≤2). Patients had loss of small and myelinated fibres compared with both asymptomatic carriers and controls (P < 0.001), whereas asymptomatic carriers showed loss of small myelinated fibres when compared with controls (P < 0.05). The loss of myelinated fibres increased with disease progression (P < 0.001), and patients in more advanced clinical stage showed more frequent amyloid deposition in the nerve (P = 0.001). There was a positive correlation between large myelinated fibre density and time to symptoms’ onset in the asymptomatic carriers that developed early-onset form of the disease (r = 0.52, P < 0.01). In addition, asymptomatic carriers with amyloid deposition already present in sural nerve biopsies developed symptoms earlier than those with no amyloid (P < 0.01). In conclusion, this study confirms that the loss of small fibre size is an initial event in familial amyloid polyneuropathy, already present in asymptomatic gene carriers, starting several years before the onset of symptoms. We show for the first time that large myelinated fibres’ loss and amyloid deposition are pathological features that correlate independently with short period to the onset of symptoms for asymptomatic carriers that developed early-onset form of the disease. These findings are therapeutically relevant, as it would allow for a better interpretation of the role of disease-modifying agents in transthyretin familial amyloid polyneuropathy.
Keywords: familial amyloid neuropathy, pathology, sural serve, transthyretin
The loss of small myelinated fibres starts several years before the onset of symptoms in familial amyloid polyneuropathy. Furthermore, we show for the first time that large myelinated fibres’ loss and amyloid deposition are pathological features that correlate independently to a shorter period to the onset of symptoms in the early-onset form of the disease.
Graphical Abstract
Graphical Abstract.
Introduction
Familial amyloid polyneuropathy is a hereditary multisystemic disease, with autosomal dominant transmission, characterized by a progressive length-dependent axonal, sensorimotor and autonomic polyneuropathy, first described by Andrade (1952). The substitution of methionine for valine at position 30 (Val30Met) on the transthyretin (TTR) gene was later identified as the cause of the original description of the disease (Saraiva et al., 1984). Currently, >150 mutations of the TTR gene are known; most of them are amyloidogenic, producing a broad range of phenotypes associated with hereditary TTR amyloidosis (ATTRv) (Planté-Bordeneuve, 2017). The original mutation described Val30Met (alternatively named pVal50Met) is the most common worldwide and is primarily associated with neuropathy (Ando et al., 2013). Disease onset can be described as bimodal, with one peak in the third to fourth decades of life (early-onset patients: <50 years old) and another peak in the sixth decade of life (late-onset patients with familial amyloid polyneuropathy; ≥50 years old) (Sequeiros et al., 1987; Ribeiro and Coutinho, 1988; Koike et al., 2009; Planté-Bordeneuve and Said, 2011). The prognosis is poor, leading to patients’ death within an average period ranging from 7 to 10 years after diagnosis, in late- and early-onset cases (Saraiva et al., 1984; Koike et al., 2012; Ando et al., 2013; Coelho et al., 2018). Pathologically is characterized by the extracellular diffuse deposition of insoluble fibril aggregates, affecting invariably and severely the peripheral nerves. Despite extensive research, the exact mechanisms for tissue damage and phenotype variability have not been fully understood (Sequeiros et al., 1987; Ribeiro and Coutinho, 1988; Coelho et al., 1994; Koike et al., 2004, 2009). Liver transplantation was introduced in 1990s (Ikeda et al., 1997; Holmgren et al., 2008) and became the first disease-modifying therapy and a standard of care for early-onset patients with predominant neuropathy phenotype; subsequently, a TTR kinetic stabilizer—tafamidis—and, more recently, two gene silencing drugs—patisiran and inotersen—were approved as pharmacological alternatives to liver transplantation (Coelho et al., 2012; Adams et al., 2018; Benson et al., 2018; Maurer et al., 2018). All treatments halt peripheral nervous system further degeneration, particularly when introduced early in disease course, and life expectancy has dramatically increased in these patients (Coelho et al., 2018). Morphometry of sural nerve biopsies is an important diagnostic tool of peripheral neuropathies as it provides quantitative and objective details and allows evaluation of the progression of disease and the treatment response (Chentanez et al., 2006; Bilego Neto et al., 2013). However, this is an invasive procedure, nowadays reserved for selected cases only. In this study, we describe the morphometric characteristics of a large ATTRv group of patients and asymptomatic gene carriers that underwent nerve biopsy in the pre-gene testing period. The establishment of the morphometric pattern (in presymptomatic subjects and at different clinical stages of the disease), as well as the identification of clinical correlations, is therapeutically relevant, as it allows a better interpretation of the role of disease-modifying agents in ATTRv with polyneuropathy.
Materials and methods
Patients
We analysed sural nerve biopsies from 98 patients with Val30Met ATTRv and 37 ATTRv asymptomatic mutation carriers, aged between 17 and 84 years, and performed between 1981 and 2017 at Centro Hospitalar Universitário do Porto—Hospital Santo António. Patients with an onset of symptoms before the age of 50 years were classified as early-onset cases, and those with an onset age of ≥50 years were classified as late-onset cases. Sural nerve biopsies from asymptomatic mutation carriers were obtained essentially in the context of reproductive counselling prior to genetic test became available in 1985 (Saraiva et al., 1984). Some cases that had doubtful and non-specific symptoms underwent sural nerve biopsy after this period. Clinical records were reviewed to confirm that no clinical or paraclinical (cardiac and EMG studies) evidence of the disease was present at the time of biopsy. To define symptom onset, patients had to develop consistent and progressive signs and/or symptoms related to the disease (Conceição et al., 2019). In all cases, including the patients who underwent sural biopsy prior to genetic testing became available, Val30Met mutation was confirmed.
Thirty-one sural nerve biopsies from subjects, matched for age and sex, who were classified as normal by experienced neuropathologists, have been selected from the Neuromuscular Database of the Neuropathology Unit of Centro Hospitalar Universitário do Porto—Hospital Santo António and used as controls. In addition, clinical records were reviewed to exclude the patients with the history of any neurological disorder that could affect the peripheral nervous system.
All aspects associated with the current study were approved by the Ethics Committee of Centro Hospitalar Universitário do Porto—Hospital Santo António.
Clinical and demographic assessments
Clinical and demographic data were obtained from the database of the Corino de Andrade Unit and patients’ clinical records. The following data were collected: gender, age at the onset of symptoms, age at the time of sural nerve biopsy, the presence of family history of ATTR amyloidosis and the presence of comorbidities. Age at the onset of symptoms was established by a small group of neurologists experienced in the assistance of these patients whenever patients presented a constant and progressive set of symptoms and abnormalities in the neurological and laboratorial examinations. Isolated and intermittent non-specific symptoms were not considered as the evidence of onset. The presence of amyloid in tissues, such as nerve, skin or salivary glands, is a requested criterion for diagnosis. In the rare cases of recurrent biopsies without evidence of amyloid deposition, the diagnosis was accepted only if objective and unequivocal signs of neuropathy were present and other potential causes of neuropathy had been excluded. The family history of ATTR amyloidosis was considered to be positive in cases with one first- or second-degree relative with the diagnosis of ATTRv.
The polyneuropathy disability (PND) score, an useful disease staging tool in the evaluation of peripheral sensory and motor disturbances in patients with familial amyloid polyneuropathy (Ando et al., 2013), was calculated by reviewing patients’ medical records, to establish the stage of disease at the time of nerve biopsy. Patients with ATTRv were stratified by the following PND stages: Stage I—sensory disturbances with preserved walking capability; Stage II—sensory and motor deficits but ability to walk without any support; Stage IIIA—walk only with the help of one stick or crutch; Stage IIIB—walk with the help of two sticks or crutches; and Stage IV—confined to a wheelchair or bedridden.
Sural nerve specimens
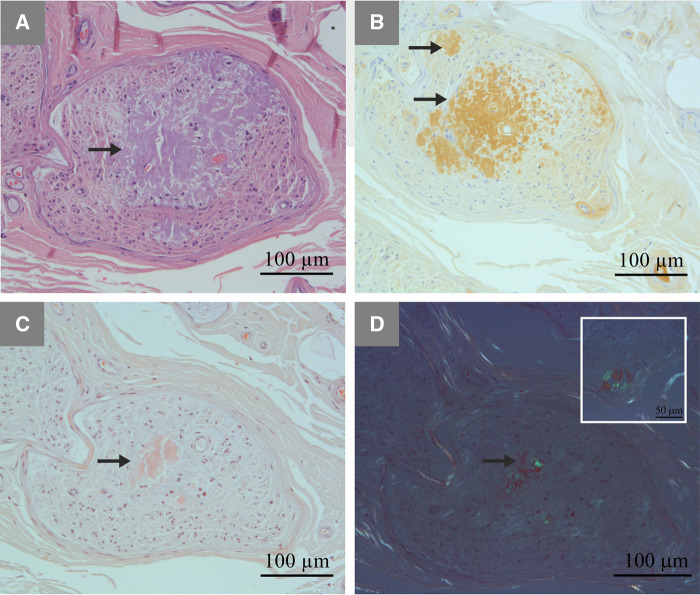
The sural nerve was exposed at the level of lateral malleolus, under local anaesthesia, and a nerve biopsy was performed (Swallow, 1968; Bevilacqua et al., 2007). The sural nerve specimens were divided into two portions. The first one was fixed in 2.5% glutaraldehyde on phosphate-buffered saline solution at pH 7.4, dehydrated with numerous alcohol passages and embedded in epoxy resin. Semithin transverse sections (1-μm thick) were cut and stained with 1% toluidine blue solution for morphometric study (Hanyu et al., 1989; Koike et al., 2004). The second portion of the specimen was fixed in 10% formalin solution and embedded in paraffin. Sections were cut by routine methods and stained with haematoxylin and eosin and Congo red. The presence of amyloid deposits was detected by Congo red staining, exhibiting an apple-green birefringence under polarized light (Fig. 1).
Figure 1.
Representative characteristics of amyloid deposition in sural nerve from a patient with familial amyloid polyneuropathy with transthyretin mutation. Amyloid deposits (arrows) seen in A haematoxylin and eosin staining, (B) immunostaining with anti-human TTR antibody (Ref A0002, 1:450; Dako) and (C and D) Congo red staining. In D, Congo red staining seen under polarized light (inset: amyloid deposition in the vessel wall).
Morphometric analysis
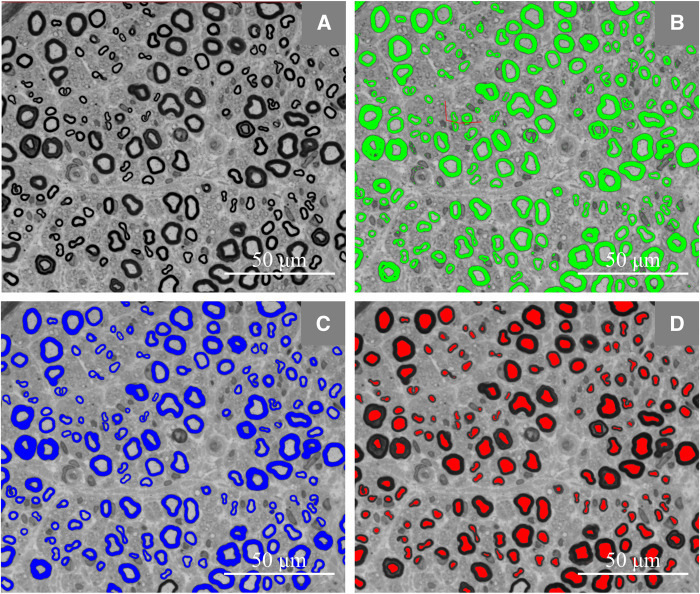
A semi-automated analysis of the density of myelinated fibres (MF) was carried out using the Leica Application Suite V4.5 and Fiber software. For each nerve, three to four digital images were captured by a digital camera (Leica MC170 HD) incorporated on a light microscope (Leica DM 4000 B), under a 63× objective, to cover the maximum nerve area (Fig. 2) and the total, the large (>7 μm), and the small (≤7 μm) MF densities (Behse, 1990; Herrmann et al., 1999) were calculated. For the calculation of the mean fibre diameter, the mean g-ratio and the ratio of small-to-large MF densities, patients with a total MF density <100/mm2 were excluded. The density of unmyelinated fibres was not assessed.
Figure 2.
Representative photograph of the semi-automated morphometry. (A–D) Representative semithin cross-section of a sural nerve’s endoneurial area from a patient with familial amyloid polyneuropathy with Val30Met transthyretin mutation. (B) Manual correction to remove the wrongly delineated areas. (C and D) Myelin sheaths in blue and axons in red.
Statistical analysis
Subjects’ data are reported as numbers and percentages for categorical variables and as median with interquartile ranges for continuous variables.
Due to asymmetrical distribution and non-homogeneity of variances of the majority of the parameters, statistical analyses were performed using non-parametric tests: Pearson’s χ2 test, Mann–Whitney test and Kruskal–Wallis test. Multiple regression was used to study the relation between time to the onset of symptoms and nerve biopsy pathological features in asymptomatic mutation gene carriers’ group. All analyses were performed using IBM SPSS® Statistics24 software. The P values of <0.05 were considered statistically significant.
Data availability
Data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Pathological findings of sural nerve biopsies
Demographic and morphometric data are summarized in Table 1. The median age at nerve biopsy was 45.0 [35.0–60.0] years for patients with ATTRv and 26.0 [23.5–39.5] years for asymptomatic mutation carriers. In patients with ATTRv, the median time from the onset of neuropathy to nerve biopsy was 2.0 [1.0–5.0] years and the median time from the onset of symptoms to death was 10.0 [6.8–13.3] years. In the asymptomatic carriers, the median duration between nerve biopsy and symptom onset was 7.0 [3.3–11.8] years, ranging from 1 to 27, and the median time from symptom onset to patients’ death was 12.0 [11.0–14.0] years. In this group, we lost the follow-up of three cases (age at biopsy: 17, 24 and 33 years) and two patients died free of symptoms at 72 and 80 years (age at biopsy: 69 years in both; cause of death: gastric carcinoma and stroke, respectively). There were no statistically differences regarding sex distribution between the groups.
Table 1.
Demographic, clinical and morphometric features of patients with ATTR-FAP, asymptomatic mutation carriers and control subjects
| Features | Controls (n = 31) | Asymptomatic mutation carriers (n = 37) | Patients with ATTR-FAP |
||
|---|---|---|---|---|---|
| All patients (n = 98) | Early-onset cases (n = 60) | Late-onset cases (n = 38) | |||
| Male sex, % (n) | 58.1 (18) | 48.6 (18) | 55.1 (54) | 51.7 (31) | 60.5 (23) |
| Age at onseta (year) | NA | 34.5 [28.5–48.8]c | 41.0 [31.0–55.5] | 34.0 [29.0–41.0] | 58.0 [53.0–63.0] |
| Age at nerve biopsya (year) | 44.0 [30.0–63.0] | 26.0 [23.5–39.5] | 45.0 [35.0–60.0] | 37.0 [30.3–43.8] | 63.0 [58.0–68.0] |
| Pathology of the sural nervea | |||||
| Total myelinated fibre density (n/mm2) | 9104 [7982–9967] | 7507 [6945–8322] | 917 [367–2179] | 1257 [478–2740] | 669 [235–1416] |
| Large myelinated fibre density (n/mm2) | 3760 [3348–4682] | 4346 [3716–4978] | 592 [156–1761] | 793 [277–1895] | 319 [112–965] |
| Small myelinated fibre density (n/mm2) | 4777 [3937–5993] | 3219 [2854–3938] | 260 [128–722] | 313 [165–838] | 221 [77–511] |
| Ratio small/largeb | 1.2 [1.0–1.6] | 0.8 [0.6–0.9] | 0.6 [0.3–1.1]d | 0.5 [0.3–0.8]e | 0.8 [0.2–1.6]f |
| Fibre diameterb (μm) | 7.4 [7.0–7.8] | 8.6 [8.1–9.0] | 8.5 [7.6–9.8]d | 8.9 [8.0–10.1]e | 7.9 [7.1–9.7]f |
| g-Ratiob | 0.54 [0.49–0.55] | 0.50 [0.46–0.54] | 0.50 [0.46–0.53]d | 0.50 [0.46–0.52]e | 0.50 [0.46–0.52]f |
ATTR-FAP = familial amyloid polyneuropathy with ATTR mutation; TTR = transthyretin[AuthorQuery id="AQ16" rid="16"]?>; NA = not applicable.
Values are expressed as median [interquartile range].
Results are presented for patients with total myelinated fibre densities ≥100/mm2.
n = 32 (asymptomatic ATTR mutation carriers who developed symptoms during 27 years of clinical follow-up).
n = 87 (excluded cases with a total myelinated fibre density <100/mm2).
n = 54 (excluded cases with a total myelinated fibre density <100/mm2).
n = 33 (excluded cases with a total myelinated fibre density <100/mm2).
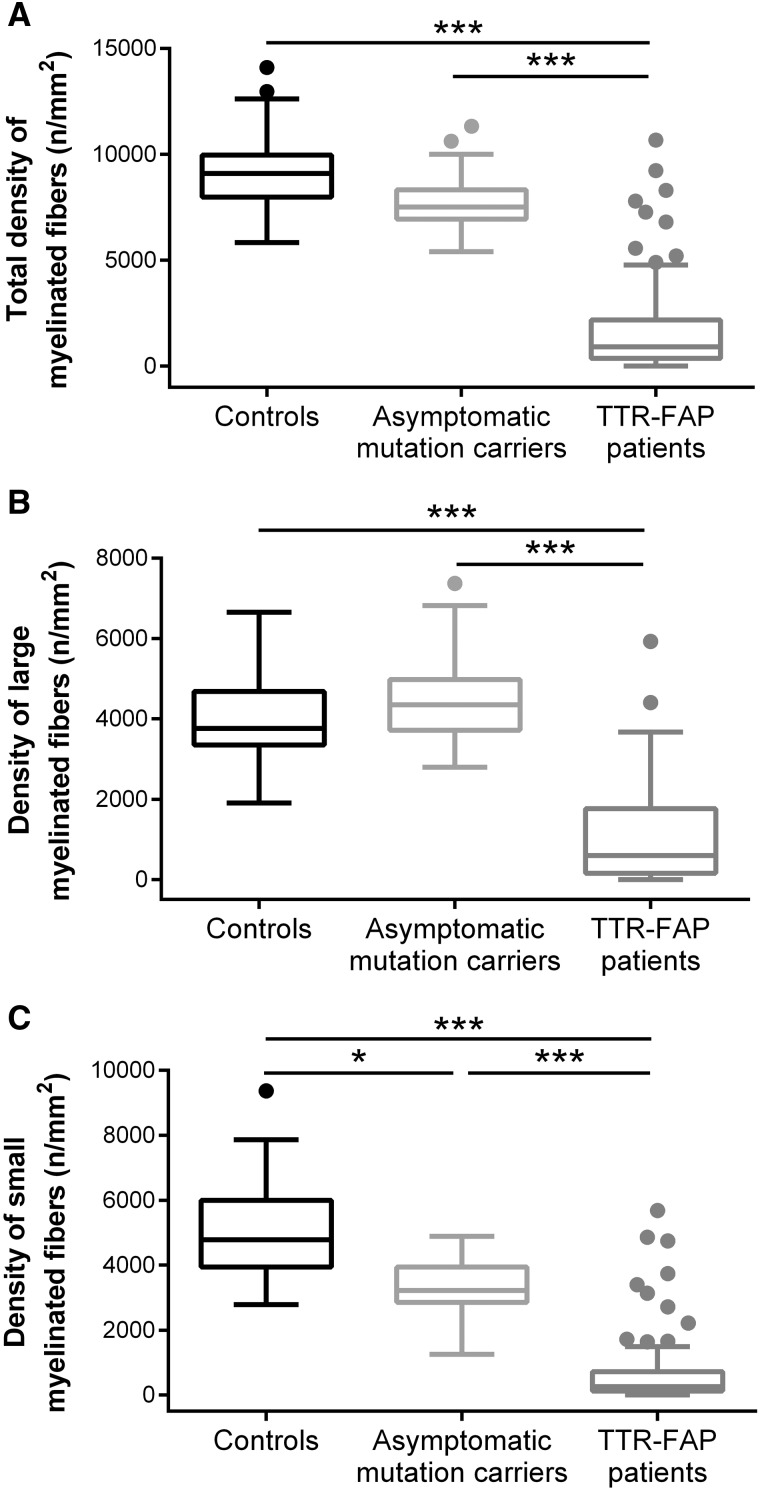
Patients with ATTRv had a total MF density of 917 [367–2179] fibres/mm2, exhibiting the loss of large (P < 0.001) and small (P < 0.001) MF when compared with controls and asymptomatic mutation carriers (Fig. 3). These patients also presented greater MF diameters (P < 0.001) and lower median of g-ratio (P = 0.01) compared with controls.
Figure 3.
Density of myelinated fibres in controls, asymptomatic mutation carriers and patients with ATTR-FAP. Patients with ATTR-FAP had reduced (A) total myelinated fibre density, corresponding to loss of both (B) large and (C) small myelinated fibres when compared with controls and asymptomatic mutation carriers. (C) The asymptomatic mutation carriers presented the depletion of small myelinated fibres compared with controls. Controls, n = 31; asymptomatic mutation carriers, n = 37; patients with ATTR-FAP, n = 98. *P ≤ 0.05, ***P ≤ 0.001 (Kruskal–Wallis test). ATTR-FAP = familial amyloid polyneuropathy with transthyretin mutation.
The asymptomatic mutation carriers presented the depletion of small MF (P = 0.041) and greater mean MF diameter (P < 0.001) compared with controls. The g-ratio did not differ from the control group.
The medians of the ratios of small-to-large MF densities of both patients with ATTRv and asymptomatic carriers were lower than that of controls (in both cases P < 0.001), meaning that most of these subjects had a higher proportion of large MF than small MF in relation to controls. In Fig. 4 representative examples of the morphometric analysis of the different groups are detailed.
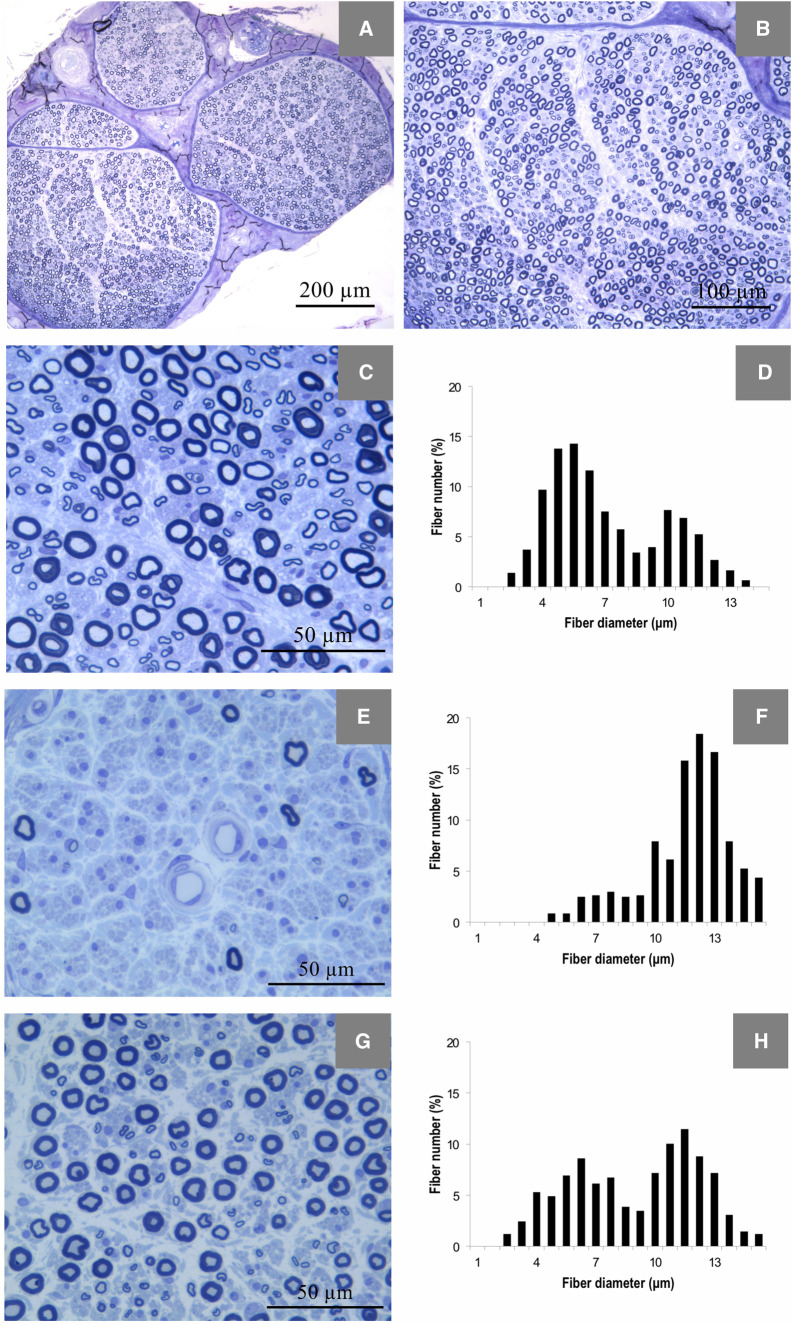
Figure 4.
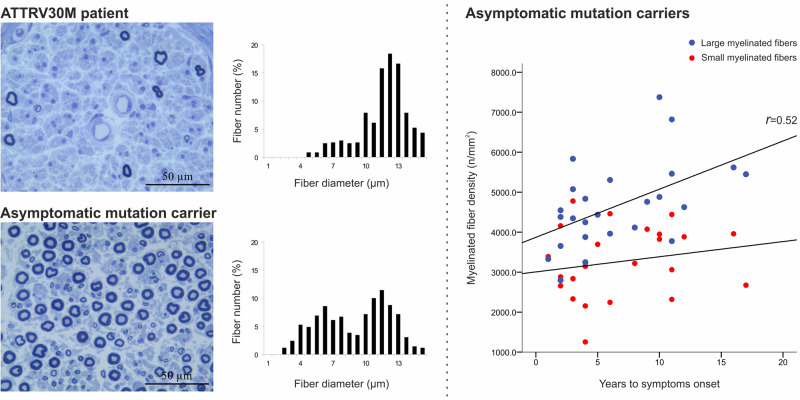
Morphometric findings in sural nerve specimens. Representative example photomicrographs of 1 μm transversal sections of sural nerve (stained with toluidine blue) from a control (A–C), a patient with familial amyloid polyneuropathy with transthyretin mutation (E) and an asymptomatic mutation carrier (G). In E, there is a severe depletion of both small and large myelinated fibres, and in G, there is a moderate loss of small myelinated fibres. Graphics show representative examples of diameter frequency histograms of myelinated fibres in sural nerve from a control (D), exhibiting a typical bimodal distribution of fibre diameters, with higher proportion of small myelinated fibre than of large myelinated fibres, a patient with familial amyloid polyneuropathy with transthyretin mutation (F), exhibiting loss of the bimodal distribution of fibre diameters, and an asymptomatic mutation carrier (H), presenting a higher peak of large than of small myelinated fibres, in contrast to the fibre size distribution in controls.
Amyloid deposition was found in a significantly lower proportion of asymptomatic carriers than that of ATTRv (32 versus 86%, respectively; P < 0.001).
Clinicopathological correlations
Patients with familial amyloid polyneuropathy with transthyretin mutation
Correlation between myelinated fibres density, amyloid deposition and clinical stages
The distribution of patients with ATTRv by clinical stages is summarized in Table 2.
Table 2.
Clinical stages of patients with ATTR-FAP according to PND score
| All patients (n = 92) | Early-onset cases (n = 55) | Late-onset cases (n = 37) | |
|---|---|---|---|
| PND score, % (n) | |||
| Stage I | 48.9 (45) | 58.2 (32) | 35.1 (13) |
| Stage II | 44.6 (41) | 36.4 (20) | 58.8 (21) |
| Stage IIIa | 3.3 (3) | 1.8 (1) | 5.4 (2) |
| Stage IIIb | 2.2 (2) | 1.8 (1) | 2.7 (1) |
| Stage IV | 1.1 (1) | 1.8 (1) | 0 |
ATTR-FAP = familial amyloid polyneuropathy with transthyretin mutation.
The PND score was assessed in 92 patients: 48.9% had only sensory disturbances (Stage I), 44.6% exhibited impaired walking capability but without requiring any support (Stage II), 5.5% were able to walk only with help (Stage III) and one patient (1%) was confined to a wheelchair at the time of nerve biopsy (Stage IV). To establish a correlation between the total MF density and the clinical stage, we considered only patients with ATTRv at Stages I and II of PND, due to the low number of patients in other clinical stages.
Patients in clinical Stage I of PND score presented a median density of 2168 [1057–4266] MF/mm2, which revealed to be higher than that of the patients in clinical Stage II of PND score (443 [173–696] MF/mm2; P < 0.001). The same difference between the two clinical stages was also observed for the large (P < 0.001) and small MF densities (P < 0.001). The median time between the onset of symptoms and nerve biopsy was higher in patients with Stage II than in patients with Stage I (4.0 [2.0–6.5] versus 1.0 [1.0–2.5] years, respectively; P < 0.001).
Considering the amyloid deposition, all 41 patients in clinical Stage II of PND score exhibited amyloid in their nerve biopsy and 35 of the 45 patients in clinical Stage I of PND score exhibited amyloid in their nerve biopsy (P = 0.001). Comparing the MF density of patients with ATTRv according to the presence or absence of amyloid deposition (10 without amyloid versus 76 with amyloid), the group with no amyloid showed higher total (P = 0.001), large (P = 0.001) and small (P = 0.001) MF densities than those with positive amyloid (Table 3). Disease duration and age at biopsy were similar in both groups. There was a tendency for the age at disease onset to be lower in the group with amyloid (P = 0.051).
Table 3.
Myelinated fibre densities of patients with ATTR-FAP in Stage ≤II of PND according to the presence or absence of amyloid deposits in sural nerve biopsy
| Patients with ATTR-FAP without amyloid deposits (n = 10) | Patients with ATTR-FAP with amyloid deposits (n = 76) | P-value | |
|---|---|---|---|
| Pathology of the sural nervea | |||
| Total myelinated fibre density (n/mm2) | 6008 [1591–8157] | 755 [321–2087] | 0.001 |
| Large myelinated fibre density (n/mm2) | 3015 [935–3853] | 469 [119–1608] | 0.001 |
| Small myelinated fibre density (n/mm2) | 2926 [345–3988] | 242 [111–592] | 0.001 |
Statistical analyses were performed using the Mann–Whitney U test. ATTR-FAP = familial amyloid polyneuropathy with transthyretin mutation.
Values are expressed as median [interquartile range].
Early- versus late-onset cases (myelinated fibres density and clinical stages)
In the early-onset cases, the median density of total MF was 1257 [478–2740] fibres/mm2 (21% of age-matched control density; P < 0.001). Their median density of large MF was 793 [277–1895] fibres/mm2 (29% of age-matched control density; P < 0.001), and that of small MF was 313 [164–838] fibres/mm2 (14% of age-matched control density; P < 0.001). In late-onset cases, the median density of total MF was 669 [235–1416] fibres/mm2 (17% of age-matched control density; P < 0.001), the median density of large MF was 319 [112–965] fibres/mm2 (22% of age-matched control density; P < 0.001) and that of small MF was 221 [77–511] fibres/mm2 (14% of age-matched control density; P < 0.001). Considering the age-associated fibre loss, early- and late-onset cases did not differ regarding fibre densities (Kruskal–Wallis test with the groups: early onset, late onset and age-matched controls for early-onset cases and age-matched controls for late-onset cases).
When considering patients at Stages ≤III of PND, the distribution of PND stages differed between these two groups: 61.5% of the early-onset patients were at Stage I at the time of nerve biopsy and 38.5% of the late-onset cases were at Stage I at the time of nerve biopsy (P = 0.034), who were predominantly in advanced clinical stages. The late-onset cases presented a higher median time gap from symptoms to nerve biopsy than did early-onset patients (3.0 [1.8–6.0] versus 2.0 [1.0–4.0], respectively); however this difference did not reach statistical significance. In early-onset cases, a weak negative correlation was found between the time from the onset of symptoms to nerve biopsy and the total and large MF densities (r = −0.361, P = 0.005 and r = −0.439, P = 0.001; respectively). This correlation was not found in the late-onset group.
No statistically significant differences were observed regarding the presence of amyloid deposition between early- and late-onset cases (92 versus 82%, respectively).
A positive family history was found in a higher proportion of early-onset cases than in late-onset cases (82 versus 44%, respectively; P < 0.001).
Asymptomatic mutation carriers
Correlation between myelinated fibre density, amyloid deposition and time to disease onset
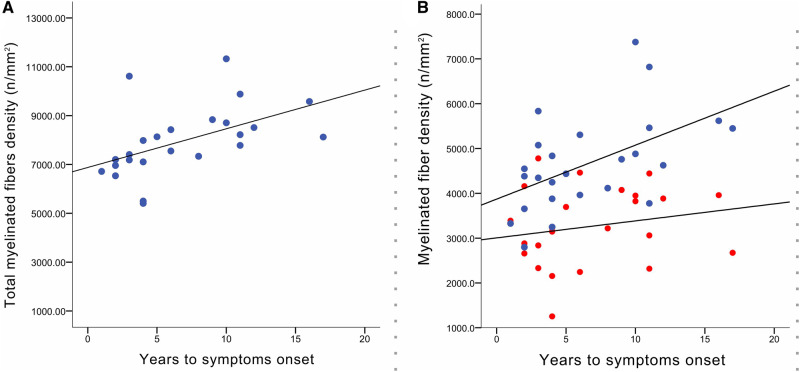
No correlation was found between any of the morphometric variables analysed and the time between nerve biopsy and disease onset. Considering the subgroup who developed early-onset form of the disease, there was a positive correlation between total MF density and time to disease onset (r = 0.52; P < 0.01). As shown in Fig. 5A, a higher density of MF at the time of nerve biopsy was associated with a longer period free-of-disease. Interestingly, when analysing this correlation according to the MF size, this correlation was only significant for large MF density (Fig. 5B).
Figure 5.
Correlation between myelinated fibre density and time to the onset of symptoms. (A) Correlation between the total myelinated fibre density and the time to the onset of symptoms in ATTR-FAP who developed early form of disease (r = 0.52; P = 0.008). (B) Correlation between the large (blue) and small (red) myelinated fibres densities, and the time to the onset of symptoms in ATTR-FAP who developed early form of disease. A positive correlation was observed only between the large myelinated fibres density and the time to disease onset (r = 0.52; P = 0.008). ATTR-FAP = familial amyloid polyneuropathy with transthyretin mutation.
Twelve subjects presented amyloid in their sural nerve biopsy (32%). These cases developed symptoms earlier (the median time to disease onset 3.5 [2.0–5.5] years) compared with the asymptomatic subjects without amyloid deposition (10.5 [5.0–17.0] years; P = 0.003). This group also showed younger age at symptom onset (32.5 [27.8–34.3] years in the amyloid group than in the non-amyloid group (38.0 [30.8–57.3] years; P = 0.039). Interestingly, when considering only the cases who developed the early-onset form of the disease, the time to disease onset remained shorter for the subjects with amyloid deposition (3.5 [2.0–5.0] versus 8.0 [4.0–11.0] years; P = 0.041), despite the similar age of onset between the groups (32.5 [27.8–34.3] versus 33 [28.0–39.0] years). Nevertheless, two patients with amyloid deposits in nerve biopsy started developing symptoms >10 years later. The two patients who died free of symptoms did not have amyloid in the sural biopsy. The median of the ratios of small-to-large MF densities was lower in the amyloid-positive group than in the amyloid-negative counterpart (P < 0.05). No differences were found in the other parameters.
A multiple regression model, with the time gap from nerve biopsy to disease onset as the dependent variable and MF density, amyloid deposition and sex as predictors, showed that all these variables had a statistically significant contribution, with an explanatory power of R2 = 56% of the observed variance. Moreover, amyloid deposition and male sex had negative coefficients, meaning that the time gap between nerve biopsy and disease onset decreased with the increasing of the values of amyloid deposition and was smaller for the male sex. On the other hand, a higher MF density implied an increase in the dependent variable.
Discussion
In this study, we established the morphometric pattern of peripheral neuropathy in ATTRV30M amyloidosis. The earliest lesions found in the peripheral nerve in ATTRv are the degeneration of unmyelinated and small MF, simultaneously with, although in a somewhat erratic manner, amyloid deposition (Melo-Pires et al., 2014). Several authors have previously reported a size-dependent fibre loss in ATTRv (Dyck and Lambert, 1969; Thomas and King, 1974; Guimaraes et al., 1988; Koike et al., 2004), but the precise mechanism of nerve fibre depletion has not yet been fully clarified. Ischaemia from the obliteration or dysfunction of small vessels (Hanyu et al., 1989), mechanical compression (Dyck and Lambert, 1969) and toxic effects (Reilly and Staunton, 1996) are some of the adduced mechanisms, whereby endoneurial amyloid deposits cause neuropathy. As expected, our results revealed that patients with ATTRv have the loss of small and large MF compared with controls and asymptomatic mutation carriers (Dyck and Lambert, 1969; Thomas and King, 1974; Guimaraes et al., 1988; Sobue et al., 1990), in agreement with the clinical manifestation of sensorimotor polyneuropathy in patients with ATTRv. Furthermore, using a larger sample, we showed that the loss of MF increases with disease progression in the earlier stages of the disease (PND I versus PND II) and that patients in more advanced clinical stage showed more frequently amyloid deposition in the nerve (Hanyu et al., 1989; Said and Planté-Bordeneuve, 2009). In addition, and similar to a previous description by (Koike et al. (2008), we found that the density of large MF negatively correlated with the duration of neuropathic symptoms only in early-onset patients. A g-ratio value between 0.5 and 0.7 reflects a theoretical optimal value for the conduction velocity of nerve impulses (Thomas, 1984). Values <0.5 may indicate the presence of degenerated nerve fibres, whereas values >0.7 denote the presence of demyelinated nerve fibres or regenerated fibres with thinner myelin sheath (Thomas, 1984). We found that patients with ATTRv had lower mean g-ratio than controls, supporting axonal degenerative changes in MF (Coimbra and Andrade, 1971; Hanyu et al., 1989; Ando et al., 2005). Despite lower numbers of patients with ATTRv with no amyloid in the sural biopsy, there was a significant higher density of MF in this group when compared with the amyloid-positive group. In a previous study, no clear relationship between the amount of endoneurial amyloid deposition and the degree of fibre loss was found (Guimaraes et al., 1988). In the present study, only presence or absence of amyloid in the sural nerve biopsy was registered and only earlier clinical stages were considered for the analysis. Our findings suggest a possible direct role of amyloid deposition in disease pathogenesis, at least at this disease stage. Interestingly, it has been recently proposed that a direct insult of Schwann cells by amyloid fibrils plays an important role in small-diameter nerve fibre loss in early-onset cases (Koike et al., 2016). However, it cannot be excluded that amyloid deposition can represent a marker of disease progression, appearing because of the amyloidogenic mechanism and not being the causative insult. Several studies have reinforced the idea that the differences in clinical features between patients with early- and late-onset ATTRv correlate well with their pathological differences (Koike et al., 2004, 2009; Ando et al., 2005). Previous studies reported severe fibre loss in late-onset patients, particularly large MF, when compared with early-onset patients (Koike et al., 2004). In this larger sample, if we consider age-associated fibre loss, no differences between the two groups were found. Contrary to what is described in Japanese patients, in which late-onset cases revealed early impairment of all sensory modalities (Ikeda et al., 1987; Koike et al., 2002), in Portuguese patients, such clinical differences are not that pronounced and a high proportion of the late-onset cases present with neuropathic features of sensory dissociation and autonomic dysfunction. Furthermore, and unlike in Japan, it is not possible to establish a clear division of Val30Met patients from endemic foci and non-endemic areas. This is in agreement with a previous Japanese work reporting that late-onset cases in endemic foci have clinical features similar to early-onset cases (Koike et al., 2002). The absence of major clinical differences between early- and late-onset cases together with the neuropathological findings suggests that the differences in fibre density between early- and late-onset patients seen in Portuguese patients with ATTRv may only reflect the baseline starting point when ATTR amyloidosis pathological insult (oligomeric or fibrillar) emerges. Family history was positive in a lower proportion of the late-onset patients, a fact that may lead to a delay in diagnosis and poorer patient outcomes at diagnosis and the time of nerve biopsy (Planté-Bordeneuve et al., 2007; Suhr et al., 2009; Adams et al., 2012; Koike et al., 2012). In our sample, despite similar time gap between disease onset and nerve biopsy, the late-onset group was associated with more severe polyneuropathy disease score at nerve biopsy. Different and additional mechanisms must be present to explain this clinical difference.
An earlier work has already disclosed abnormal pathological findings in >50% of asymptomatic carriers (Leite et al., 1988). In the present study, we extended the number of asymptomatic mutation carriers studied and clearly demonstrated in a longer follow-up that the loss of small MF is already present several years before the onset of symptoms. The lower ratio of small-to-large MF in both symptomatic and asymptomatic ATTRv subjects, compared with controls, reinforces that small MF are early and preferentially involved in this neuropathy. Our findings support the recent reported changes in imaging studies with magnetic resonance neurography and peripheral nerve cross-sectional areas assessed through ultrasonography in asymptomatic ATTRv subjects, confirming that the pathological mechanisms start in a pre-symptomatic stage (Kollmer et al., 2017; Podnar et al., 2017). Approximately one-third of the asymptomatic mutation carriers showed amyloid deposits in their sural nerve biopsies. Contrary to the symptomatic ATTRv group, we did not find differences in MF densities between the subjects with and without amyloid deposits in the sural nerve. Nevertheless, the ratio of small-to-large MF densities of amyloid-positive group was lower. The precise molecular mechanism underlying TTR fibrillogenesis is not fully understood. The current hypothesis is that point mutations cause TTR tetramer instability allowing its dissociation to non-native monomers, which can self-associate (Quintas et al., 2001). Soluble monomers aggregate into insoluble multimeric forms leading to amyloid fibrils with the characteristic β-pleated sheet cross structure (Quintas et al., 2001). The pathological findings in the asymptomatic gene carriers without visible amyloid deposits support the importance of other factors in addition to mature fibrils in this disorder. The toxicity of non-fibrillar ATTR aggregates has been shown by several studies (Sousa et al., 2001; Andersson et al., 2002; Reixach et al., 2004). Considering a biological continuum, our data suggest that fibrillar amyloid seems to add a pathological insult in the second phase of the disease.
Despite extensive knowledge of epidemiological and genetic factors, it remains impossible to predict the age at the onset of clinical symptoms in asymptomatic mutation gene carriers (Ando et al., 2013). Furthermore, in this population, true disease onset is difficult to differentiate from some subjective complaints related to anxiety due to the knowledge of developing such devastating disease at unknown time in the future. For the first time, we described a longitudinal follow-up study showing a positive correlation between the MF density and the time to disease onset in the asymptomatic mutated gene carriers that developed early-onset form of ATTRv amyloidosis. Surprisingly, considering that small MF loss is initially affected in the disease process, our results suggest that they occur in an independent way of the time for disease progression and the turning point to become symptomatic seems to be the loss of large MF. Moreover, it strengthens the fact that late-onset cases are more heterogeneous in terms of clinical presentation and pathological features (Koike et al., 2004) and also in terms of prediction for disease onset regarding nerve fibre loss. In early-onset cases, the negative correlation of both density of large MF (present study and Koike et al., 2008) and electrophysiological indices (Koike et al., 2008), with the duration of neuropathic symptoms, suggests a biological continuum between a pre-symptomatic stage and earlier clinical stages. The presence of amyloid in the biopsy was also associated with a short period to disease onset. However, it should be noted that two asymptomatic gene carriers with amyloid in the biopsy develop symptoms >10 years later. These patients are two sisters from a larger sibship with five affected subjects with ages at onset between 30 and 48 years. The first symptoms appeared at the age of 33 years (biopsy with 22 years old) and 35 years (biopsy with 25 years old). Both patients had simultaneous complaints of neuralgic pain and loss of temperature and pain sensation in the feet and legs, urinary retention with urinary tract infections and digestive problems, such as early satiety, recurrent nausea and vomiting, occasional diarrhoea and unintentional loss of weight. The family is from a region with a high prevalence of the disease and more than four generations were observed at our centre. The mean age of onset of all 35 patients from this family, observed and registered a tour centre, is 37 years. The association of male sex to a shorter time to disease onset in asymptomatic mutation carriers is in line to the known differences in the average age of onset of the disease seen in Portugal, where women were found to have a significantly later onset than men (Sousa et al., 1995). Our study has some limitations, namely, their retrospective nature and the absence of the morphometric analysis of the unmyelinated fibres. In addition, the control group was not composed from cases completely free from neurological symptoms (although mostly unspecific) and genetic testing for Val30Met mutation was not performed. However, these cases were selected and blinded through the neuropathological report archive database and, subsequently, the clinical data were revised. The clinical setting related to the biopsy together with the follow-up makes the possibility of a neurological condition affecting the peripheral nerve very unlikely.
In summary, we were able to demonstrate the initial involvement of small MF in ATTRV30M, starting in pre-symptomatic stage, the axonal nature of the neuropathy and the correlation of the fibre loss with the disease progression in the earlier clinical stages. We found that large MF loss and amyloid deposition are pathological features that correlated independently to a shorter period to the onset of symptoms in the early-onset form of the disease. We hypothesized that amyloid deposition can have a direct role in disease mechanism in the earlier symptomatic stages. Unmyelinated and small MF loss starts independently of fibrillar amyloid deposition, with the latter probably adding direct insult to an ongoing process, accelerating disease onset and progression. At this stage, large MF start becoming involved.
We believe that this study will be therapeutically relevant, as it will allow a better interpretation of the role of disease-modifying agents in ATTR amyloidosis. It reinforces the need of starting treatment early in ATTRv amyloidosis and, consequently, the development of disease biomarkers for a peri-symptomatic earlier diagnosis. Supported by these data, it will also enable to get further insight from study treatment effects looking to the subgroup of patients. Late-onset forms may respond differently to treatment (different underlying mechanisms can be responsible for disease progression—no relation of large MF density with disease duration in patients with ATTRv or time to disease onset in ATTRv asymptomatic mutation carriers). In the early-onset form of the disease, the evidence of affection of large MF at the beginning, for instance supported by nerve conduction studies (Sousa et al., 2019), could reflect a different disease stage and underlying pathophysiology already in course. This study also highlights that the identification of amyloid via biopsy is still important information for the interpretation of the pathophysiology of the disease, as it was associated to severe MF loss in patients with ATTRv and associated to an earlier disease onset in ATTRv asymptomatic gene carriers. A tissue biopsy may be obtained from non-specific sites (e.g. salivary gland and abdominal fat pad), without significant morbidity, potentially adding valuable information for treatment effect interpretation of different classes of drugs.
Competing interests
M.M.-P. has received support from Pfizer to attend scientific meetings and has received honoraria to provide scientific lectures. R.T. has received support from Pfizer to attend scientific meetings and has received honoraria to provide scientific lectures. T.C. received financial support from Alnylam, Ionis and Pfizer to attend scientific meetings and personal fees from Alnylam and Pfizer to provide scientific lectures.
Glossary
- ATTRv
hereditary transthyretin amyloidosis
- MF
myelinated fires
- PND
polyneuropathy disability
- TTR
transthyretin
- Val30Met
substitution of methionine for valine at position 30
References
- Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang C-C, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018; 379: 11–21. [DOI] [PubMed] [Google Scholar]
- Adams D, Lozeron P, Theaudin M, Mincheva Z, Cauquil C, Adam C, et al. Regional difference and similarity of familial amyloidosis with polyneuropathy in France. Amyloid 2012; 19: 61–4. [DOI] [PubMed] [Google Scholar]
- Andersson K, Olofsson A, Nielsen EH, Svehag SE, Lundgren E.. Only amyloidogenic intermediates of transthyretin induce apoptosis. Biochem Biophys Res Commun 2002; 294: 309–14. [DOI] [PubMed] [Google Scholar]
- Ando Y, Coelho T, Berk JL, Cruz MW, Ericzon BG, Ikeda S, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis 2013; 8: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando Y, Nakamura M, Araki S.. Transthyretin-related familial amyloidotic polyneuropathy. Arch Neurol 2005; 62: 1057–62. [DOI] [PubMed] [Google Scholar]
- Andrade C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain 1952; 75: 408–27. [DOI] [PubMed] [Google Scholar]
- Behse F. Morphometric studies on the human sural nerve. Acta Neurol Scand Suppl 1990; 132: 1–38. [PubMed] [Google Scholar]
- Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 2018; 379: 22–31. [DOI] [PubMed] [Google Scholar]
- Bevilacqua NJ, Rogers LC, Malik RA, Armstrong DG.. Technique of the sural nerve biopsy. J Foot Ankle Surg 2007; 46: 139–42. [DOI] [PubMed] [Google Scholar]
- Bilego Neto AP, Silveira FB, Rodrigues da Silva GA, Sanada LS, Fazan VP.. Reproducibility in nerve morphometry: comparison between methods and among observers. Biomed Res Int 2013; 2013: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chentanez V, Cha-Oumphol P, Kaewsema A, Agthong S, Huanmanop T.. Morphometric data of normal sural nerve in Thai adults. J Med Assoc Thai 2006; 89: 670–4. [PubMed] [Google Scholar]
- Coelho T, Inês M, Conceição I, Soares M, de Carvalho M, Costa J.. Natural history and survival in stage 1 Val30Met transthyretin familial amyloid polyneuropathy. Neurology 2018; 91: e1999–2009. [DOI] [PubMed] [Google Scholar]
- Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Planté-Bordeneuve V, Lozeron P, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 2012; 79: 785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho T, Sousa A, Lourenco E, Ramalheira J.. A study of 159 Portuguese patients with familial amyloidotic polyneuropathy (FAP) whose parents were both unaffected. J Med Genet 1994; 31: 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra A, Andrade C.. Familial amyloid polyneuropathy: an electron microscope study of the peripheral nerve in five cases. II. Nerve fibre changes. Brain 1971; 94: 207–12. [DOI] [PubMed] [Google Scholar]
- Conceição I, Damy T, Romero M, Galán L, Attarian S, Luigetti M, et al. Early diagnosis of ATTR amyloidosis through targeted follow-up of identified carriers of TTR gene mutations. Amyloid 2019; 26: 3–9. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Lambert EH.. Dissociated sensation in amylidosis. Compound action potential, quantitative histologic and teased-fiber, and electron microscopic studies of sural nerve biopsies. Arch Neurol 1969; 20: 490–507. [DOI] [PubMed] [Google Scholar]
- Guimaraes A, Pinheiro AV, Leite I.. Sural nerve biopsy in familial amyloidotic polyneuropathy: a morphological and morphometric polyneuropathy In: Isobe T, editor. Amyloid and amyloidosis. Boston, MA: Springer; 1988. p. 493–8. [Google Scholar]
- Hanyu N, Ikeda S, Nakadai A, Yanagisawa N, Powell HC.. Peripheral nerve pathological findings in familial amyloid polyneuropathy: a correlative study of proximal sciatic nerve and sural nerve lesions. Ann Neurol 1989; 25: 340–50. [DOI] [PubMed] [Google Scholar]
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC.. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999; 53: 1634–40. [DOI] [PubMed] [Google Scholar]
- Holmgren G, Steen L, Ekstedt J, Groth CG, Ericzon BG, Eriksson S, et al. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30). Clin Genet 2008; 40: 242–6. [DOI] [PubMed] [Google Scholar]
- Ikeda S-I, Hanyu N, Hongo M, Yoshioka J, Oguchi H, Yanagisawa N, et al. Hereditary generalized amyloidosis with polyneuropathy. Brain 1987; 110: 315–37. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Takei Y, Yanagisawa N, Matsunami H, Hashikura Y, Ikegami T, et al. Peripheral nerves regenerated in familial amyloid polyneuropathy after liver transplantation. Ann Intern Med 1997; 127: 618–20. [DOI] [PubMed] [Google Scholar]
- Koike H, Ando Y, Ueda M, Kawagashira Y, Iijima M, Fujitake J, et al. Distinct characteristics of amyloid deposits in early- and late-onset transthyretin Val30Met familial amyloid polyneuropathy. J Neurol Sci 2009; 287: 178–84. [DOI] [PubMed] [Google Scholar]
- Koike H, Ikeda S, Takahashi M, Kawagashira Y, Iijima M, Misumi Y, et al. Schwann cell and endothelial cell damage in transthyretin familial amyloid polyneuropathy. Neurology 2016; 87: 2220–9. [DOI] [PubMed] [Google Scholar]
- Koike H, Kawagashira Y, Iijima M, Yamamoto M, Hattori N, Tanaka F, et al. Electrophysiological features of late-onset transthyretin Met30 familial amyloid polyneuropathy unrelated to endemic foci. J Neurol 2008; 255: 1526–33. [DOI] [PubMed] [Google Scholar]
- Koike H, Misu K, Ikeda S, Ando Y, Nakazato M, Ando E, et al. Type I (transthyretin Met30) familial amyloid polyneuropathy in Japan: early- vs late-onset form. Arch Neurol 2002; 59: 1771–6. [DOI] [PubMed] [Google Scholar]
- Koike H, Misu K, Sugiura M, Iijima M, Mori K, Yamamoto M, et al. Pathology of early- vs late-onset TTR Met30 familial amyloid polyneuropathy. Neurology 2004; 63: 129–38. [DOI] [PubMed] [Google Scholar]
- Koike H, Tanaka F, Hashimoto R, Tomita M, Kawagashira Y, Iijima M, et al. Natural history of transthyretin Val30Met familial amyloid polyneuropathy: analysis of late-onset cases from non-endemic areas. J Neurol Neurosurg Psychiatry 2012; 83: 152–8. [DOI] [PubMed] [Google Scholar]
- Kollmer J, Sahm F, Hegenbart U, Purrucker JC, Kimmich C, Schonland SO, et al. Sural nerve injury in familial amyloid polyneuropathy: MR neurography vs clinicopathologic tools. Neurology 2017; 89: 475–84. [DOI] [PubMed] [Google Scholar]
- Leite I, Coutinho P, Pinheiro AV, Guimaraes A, Saraiva MJM, Costa PP.. Familial amyloid polyneuropathy (Portuguese type): study of asymptomatic carriers In: Isobe T, editor. Amyloid and amyloidosis. Boston, MA: Springer; 1988. p. 429–34. [Google Scholar]
- Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–16. [DOI] [PubMed] [Google Scholar]
- Melo-Pires M, Taipa R, Guimaraes A.. Familial amyloid polyneuropathy In: Vallet J-M, Weiss J, Keohane K, editors. Peripheral nerve disorders—pathology and genetics. West Sussex: Wiley-Blackwel; 2014. p. 131–7. [Google Scholar]
- Planté-Bordeneuve V. Transthyretin familial amyloid polyneuropathy: an update. J Neurol 2017; 265: 976–83. [DOI] [PubMed] [Google Scholar]
- Planté-Bordeneuve V, Ferreira A, Lalu T, Zaros C, Lacroix C, Adams D, et al. Diagnostic pitfalls in sporadic transthyretin familial amyloid polyneuropathy (TTR-FAP). Neurology 2007; 69: 693–8. [DOI] [PubMed] [Google Scholar]
- Planté-Bordeneuve V, Said G.. Familial amyloid polyneuropathy. Lancet Neurol 2011; 10: 1086–97. [DOI] [PubMed] [Google Scholar]
- Podnar S, Sarafov S, Tournev I, Omejec G, Zidar J.. Peripheral nerve ultrasonography in patients with transthyretin amyloidosis. Clin Neurophysiol 2017; 128: 505–11. [DOI] [PubMed] [Google Scholar]
- Quintas A, Vaz DC, Cardoso I, Saraiva MJ, Brito RM.. Tetramer dissociation and monomer partial unfolding precedes protofibril formation in amyloidogenic transthyretin variants. J Biol Chem 2001; 276: 27207–13. [DOI] [PubMed] [Google Scholar]
- Reilly MM, Staunton H.. Peripheral nerve amyloidosis. Brain Pathol 1996; 6: 163–77. [DOI] [PubMed] [Google Scholar]
- Reixach N, Deechongkit S, Jiang X, Kelly JW, Buxbaum JN.. Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc Natl Acad Sci U S A 2004; 101: 2817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro I, Coutinho P.. Late-onset forms of familial amyloid polyneuropathy (Portuguese type): a reappraisal In: Isobe T, editor. Amyloid and amyloidosis. Boston, MA: Springer; 1988. p. 429–34. [Google Scholar]
- Said G, Planté-Bordeneuve V.. Familial amyloid polyneuropathy: a clinico-pathologic study. J Neurol Sci 2009; 284: 149–54. [DOI] [PubMed] [Google Scholar]
- Saraiva MJ, Birken S, Costa PP, Goodman DS.. Amyloid fibril protein in familial amyloidotic polyneuropathy, Portuguese type. Definition of molecular abnormality in transthyretin (prealbumin). J Clin Invest 1984; 74: 104–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeiros J, Saraiva MJ, Opitz JM, Reynolds JF.. Onset in the seventh decade and lack of symptoms in heterozygotes for the TTRMet30 mutation in hereditary amyloid neuropathy-type I (Portuguese, Andrade). Am J Med Genet 1987; 27: 345–57. [DOI] [PubMed] [Google Scholar]
- Sobue GEN, Nakao N, Murakami KEN, Yasuda T, Sahashi KO, Mitsuma T, et al. Type I familial amyloid polyneuropathy patological study of peripheral nervous system. Brain 1990; 113: 903–19. [DOI] [PubMed] [Google Scholar]
- Sousa A, Coelho T, Barros J, Sequeiros J.. Genetic epidemiology of familial amyloidotic polyneuropathy (FAP)-type I in Póvoa do Varzim and Vila do Conde (north of Portugal). Am J Med Genet 1995; 60: 512–21. [DOI] [PubMed] [Google Scholar]
- Sousa AP, Valdrez K, Cardoso M, Anselmo J, Freitas M, Ferreira H, et al. The diagnostic utility of neurophysiologic tests for early diagnostic of transthyretin familial amyloid polyneuropathy. Amyloid 2019; 26: 70.. [DOI] [PubMed] [Google Scholar]
- Sousa MM, Cardoso I, Fernandes R, Guimaraes A, Saraiva MJ.. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am J Pathol 2001; 159: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhr OB, Andersen O, Aronsson T, Jonasson J, Kalimo H, Lundahl C, et al. Report of five rare or previously unknown amyloidogenic transthyretin mutations disclosed in Sweden. Amyloid 2009; 16: 208–14. [DOI] [PubMed] [Google Scholar]
- Swallow D. The fibre size and content of the radial and sural nerves. J Neurol Neurosurg Psychiatry 1968; 31: 464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. Microscopic anatomy of the peripheral nervous system In: Dyck P, Thomas P, editors. Peripheral neuropathy. London: Saunders; 1984. p. 39–91. [Google Scholar]
- Thomas PK, King RH.. Peripheral nerve changes in amyloid neuropathy. Brain 1974; 97: 395–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study are available from the corresponding author upon reasonable request.