Abstract
The prevalence of mild traumatic brain injury is highest amongst the adolescent population and can lead to complications including neuroinflammation and excitotoxicity. Also pervasive in adolescents is recreational cannabis use. Δ9-Tetrahydrocannabinol, the main psychoactive component of cannabis, is known to have anti-inflammatory properties and serves as a neuroprotective agent against excitotoxicity. Thus, we investigated the effects of Δ9-tetrahydrocannabinol on recovery when administered either prior to or following repeated mild brain injuries. Male and female Sprague-Dawley rats were randomly assigned to receive Δ9-tetrahydrocannabinol or vehicle either prior to or following the repeated injuries. Rats were then tested on a behavioural test battery designed to measure post-concussive symptomology. The hippocampus, nucleus accumbens and prefrontal cortex were extracted from all animals to examine mRNA expression changes (Bdnf, Cnr1, Comt, GR, Iba-1 and Vegf-2R). We hypothesized that, in both experiments, Δ9-tetrahydrocannabinol administration would provide neuroprotection against mild injury outcomes and confer therapeutic benefit. Δ9-Tetrahydrocannabinol administration following repeated mild traumatic brain injury was beneficial to three of the six behavioural outcomes affected by injury (reducing anxiety and depressive-like behaviours while also mitigating injury-induced deficits in short-term working memory). Δ9-Tetrahydrocannabinol administration following injury also showed beneficial effects on the expression of Cnr1, Comt and Vegf-2R in the hippocampus, nucleus accumbens and prefrontal cortex. There were no notable benefits of Δ9-tetrahydrocannabinol when administered prior to injury, suggesting that Δ9-tetrahydrocannabinol may have potential therapeutic benefit on post-concussive symptomology when administered post-injury, but not pre-injury.
Keywords: cannabis, concussion, therapeutic, development
As there is significant overlap in risk for Δ9-tetrahydrocannabinol use and mild traumatic brain injury in adolescents, and there are commonalities between injury pathology and the neuroprotective potential of Δ9-tetrahydrocannabinol, this study investigated the effects of Δ9-tetrahydrocannabinol on recovery when administered either prior to or following repeated injuries.
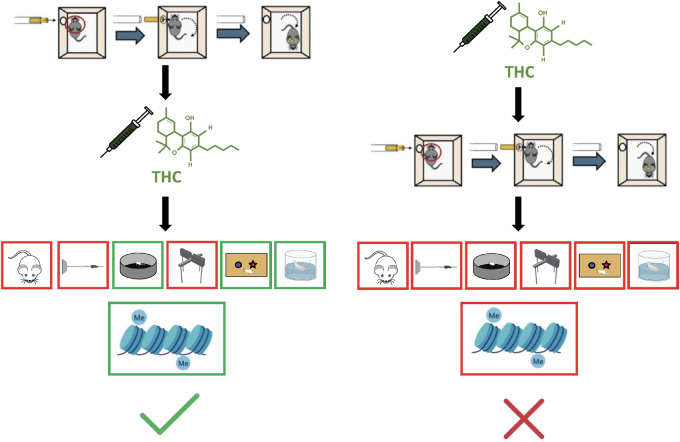
Graphical Abstract
Graphical Abstract.
Introduction
Concussions, clinically referred to as mild traumatic brain injuries (mTBIs), are the most common type of traumatic brain injury (TBI), accounting for 80% of all TBI cases (Clark and Guskiewicz, 2016). Children and youth in particular, have the highest rates of mTBI, with more than 23% of hospital-reported mTBIs being diagnosed in adolescents (McKinlay et al., 2008; Barlow et al., 2010; Halstead and Walter, 2010). mTBIs are caused by inertial forces acting on the brain at the moment of impact; these forces result in both linear and rotational acceleration of the brain that lead to brain movement within, and against the skull (Meaney and Smith, 2011). This collision results in compression, straining, and shearing of brain tissue and such brain damage can cause neuronal degeneration and many observable deficits (Meaney and Smith, 2011). The numerous long-term neurophysiological effects associated with mTBI are grouped into what is known as post-concussive symptomology (PCS) (Barlow et al., 2010). Between 10% and 50% of individuals diagnosed with concussion exhibit the persistent symptoms of PCS, which include physical, behavioural, emotional and cognitive symptoms, such as amnesia, insomnia, increased aggression, erratic behaviour and cognitive deficits (Barlow et al., 2010; Solomon et al., 2011). Furthermore, there is growing concern regarding recent evidence demonstrating that repeated mTBIs (RmTBI) can result in cumulative and lasting effects in the adolescent brain (Zhang et al., 2016; Wright et al., 2017; Yamakawa et al., 2017).
Adolescents not only have high rates of mTBIs but also of cannabis use (Barlow et al., 2010; Rotermann and MacDonald, 2018). In Canada, for example, by 18 years of age, more than 31% of youth have reported using cannabis three times or more, making it the most widely used illicit drug by the adolescent population (Arseneault et al., 2002). Δ9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, acts on cannabinoid receptors (CB1) in the central nervous system and (CB2) in the immune system (Bridgeman and Abazia, 2017). Since 1994, increasing rates of recreational THC and cannabis use has been linked to athletes at the international and collegiate levels, as well as in various amateur and leisure sports (Campos et al., 2003). In addition, the high-risk, high-intensity environment generated in sport is associated with a 10–20% increased risk for head collisions and mTBIs (Kelly et al., 2012; Gardner and Yaffe, 2015).
The rotational and linear biomechanical forces that produce mTBI typically result in diffuse damage to white matter throughout the brain as axons are particularly sensitive to stretching induced injury (Bigler, 2001; Buki and Povlishock, 2006; Fidan et al., 2018). A secondary consequence of mTBI in the initiation of neurometabolic cascades whereby cytokines are upregulated, there are changes in the subunits of N-methyl-D-aspartate receptors, broad and non-specific glutamate release, efflux of potassium ions and influxes of calcium and sodium, which causes the over-activation of ATP-dependent membrane pumps to restore ionic concentrations (Giza and Hovda, 2014; Sun et al., 2019). Although endocannabinoids and cannabinoid drugs have been thoroughly investigated in the context of mTBI [for review see Mechoulam and Shohami (2007)], there has been a void in the literature regarding THC and TBI. This is surprising given that THC, reduces excess glutamate release, offers protection against neuroinflammation and has potent antioxidative properties (Hampson et al., 1998; Hayakawa et al., 2007; Fishbein-Kaminietsky et al., 2014).
The importance of studying THC exposure in the context of mTBI is therefore apparent as there is significant overlap in risk for THC use and mTBI in adolescent populations, and there are notable commonalities between mTBI pathology and the neuroprotective potential of THC. The first study examined the therapeutic potential of THC on RmTBI, providing rats with daily injections of THC while examining PCS. The second study examined the effects of intermittent THC exposure before RmTBI, as this was ethologically relevant and could provide insight into the neuroprotective potential of THC. Using a clinically relevant model of concussion, the lateral impact device (Mychasiuk et al., 2016a, b), rats were administered RmTBIs or sham injuries, and were then subsequently tested on a behavioural test battery designed to measure post-concussion symptomatology. The hippocampus (HPC), nucleus accumbens (NAc) and prefrontal cortex (PFC) of the rats were also extracted to examine mRNA changes in the brain associated with mTBI and THC exposure. The genes Bdnf, Cnr1, Comt, GR, Iba-1 and Vegf-2R were examined to quantify the differential effects of treatment on recovery from mTBI. We hypothesized that THC would offer therapeutic benefits, reducing the severity of PCS in both groups of rats that received THC either before, or following the RmTBIs.
Materials and methods
Animals
Male and female Sprague-Dawley rats were obtained from Charles River Laboratories (QC, Canada) and arrived on postnatal day (P) 21. Rats were housed in groups of four in a temperature- and humidity-controlled husbandry room at 22–24°C with controlled humidity at ∼30%. The rats were maintained on a 12:12 light-dark cycle (lights on at 0700). Rats had ad libitum access to water and standard rat chow. All experiments were carried out in accordance with the Canadian Council of Animal Care and were approved by the University of Calgary Conjoint Faculties Research Ethics Board.
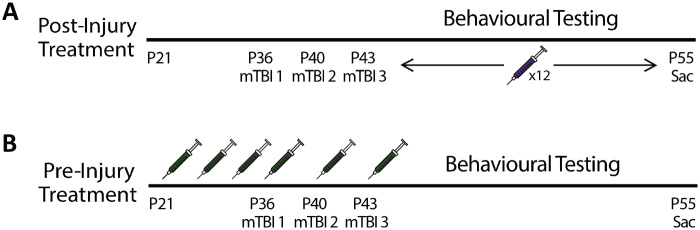
For Study 1, rats were randomly assigned to a vehicle control group (n = 12) or a post-injury treatment group (n = 20). Rats were further divided into either an RmTBI group or a sham injury group generating groups of, (i) post-THC + RmTBI (n = 5M and 5F), (ii) post-THC + sham (n = 5M and 5F), (iii) placebo + RmTBI (n = 3M and 3 F) and (iv) placebo + sham (n = 3M and 3F). The daily treatment of THC was designed to model a therapeutic paradigm whereby an individual may have been prescribed THC following RmTBI. Twelve daily intraperitoneal (I.P.) injections were administered between P44 and P54, with the first injection occurring the day after the last injury, at a dose of 1.25 mg/kg. All injections were given at least 30 min after the behavioural testing procedure for that particular day was completed. See Fig. 1A for experimental timeline.
Figure 1.
Experimental timelines for (A) Study 1 and (B) Study 2.
For Study 2, rats were randomly assigned to a vehicle control group (n = 12) or an intermittent pre-injury treatment group (n = 20). Rats were further divided into either an RmTBI group or a sham injury group generating groups of (i) pre-THC + RmTBI (n = 5M and 5F), (ii) pre-THC + sham (n = 5M and 5F), (iii) placebo + RmTBI (n = 3M and 3F) and (iv) placebo + sham (n = 3M and 3F). Six I.P. injections of drug or vehicle were given intermittently between P29 and P43, at a dose of 1.25 mg/kg. The intermittent dosage of THC was designed to provide a realistic model of individuals that are casual consumers of THC before RmTBI and then quit when they experience concussion-related symptoms. See Fig. 1B for experimental timeline.
Experimental procedures
Drugs
THC (Sigma-Aldrich, Oakville, ON, Canada) dissolved in dimethyl sulfoxide (DMSO) (10 mg/ml) was mixed to make a 1:1:18, THC/DMSO: Tween80: Saline injection stock for a final 5% drug concentration. The vehicle was prepared similarly for a 1:1:18, DMSO: Tween80: Saline stock solution. Fresh stock was made daily. Rats were I.P. injected with a low-moderate dose of THC (1.25 mg/kg) or an equivalent volume of vehicle stock.
Lateral impact device
This clinically relevant model of concussion emulates the acceleration/deceleration forces associated with sports-related injuries using controlled speeds to deliver precise injuries (Viano et al., 2009). RmTBIs were administered 3 days apart (P36, P40 and P43), at approximately 82 Gs, which is well above the sub-concussion threshold that has been previously defined as ∼35Gs (Guskiewicz et al., 2007; Guskiewicz and Mihalik, 2011). The 3-day inter-injury interval was used as we have previously demonstrated this produces greater impairment than a single injury and is more representative of the symptomologies associated with adolescent RmTBI (Wright et al., 2017). The force exerted by the lateral impact propels the rat into a 180-degree rotation, mimicking the lateral acceleration and rotational forces experienced in sports-related injury. Before the administration of the mTBI, rats were anaesthetized with isoflurane gas until the plantar reflex was no longer present. Rats were subsequently placed in a prone position onto a Teflon board, adjacent to the lateral impact device, such that the left side of the head was facing the impactor device. A 50 g cylindrical weight was propelled at an aluminium plate placed against the rat’s head at 8.96 ± 0.12 m/s. Xylocaine was applied to the site of impact and rats were allowed to recover in a warm, clean cage before being placed back into their home cage. The time-to-right was also recorded for each rat and was measured from the time of impact to the time the rat was able to right itself to prone position from being laid on its back. The sham animals underwent the same procedure but were removed from the lateral impact device without insult.
Behavioural testing
Following RmTBI, animals were subjected to a battery of behavioural tests designed and temporally sequenced to examine the clinical symptoms associated with post-concussive syndrome (Mychasiuk et al., 2014; Mychasiuk et al., 2015).
Beam walk (P44)
Twenty-four hours following the third injury (post-injury Day 1—PID1), the beam walk task was administered. This task is designed to measure balance and motor coordination (Schallert et al., 2002). One-hundred and sixty-five centimetres in length, the beam was tapered such that it narrows towards the home cage (which was used as a reinforcing cue). The beam was raised 1 m above the ground and was equipped with safety ledges, 2 cm in length on either side of the beam to catch the rat’s leg if it slipped. Each rat performed five trials, with 1 min between each trial to reinforce the goal of the task. The final four trials were recorded and scored to determine the time to cross the beam, for each trial.
Elevated plus maze (EPM) (P46)
To measure anxiety-like behaviour, the EPM task was administered (Whishaw and Kolb, 2005). The elevated plus maze (EPM) arena consisted of two open arms and two closed arms in the form of a plus, 55 cm above the ground. At the beginning of the trial, rats were placed in the centre of the maze facing a closed arm. Each trial was recorded for 5 min and scored by a blinded observer to measure time spent in the open arms and the closed arms. More time spent in the open arms indicates decreased anxiety-like behaviour.
Novel context mismatch (P47–P50)
The novel context mismatch protocol adapted from Spanswick and Sutherland (2010) was used as a measure of short-term working memory. During the training phase (P47–P49) rats were subjected to 5 min in two discrete contexts each containing a pair of unique objects. Context A was a clear rectangular arena containing a pair of novel objects, and Context B was an opaque, blue rectangular arena containing two different novel objects. On the final day (P50), rats were first placed in Context A for 5 min, then Context B for 5 min, and an additional recorded trial in which the rats were placed in the arena from Context B, with an object from the original Context B (familiar) and an object from the original context A (novel) for 5 min after having been given time to rest in their home cage for 5 min. In the probe trial, more time spent exploring the novel object as opposed to the familiar object indicates increased memory recall. The trials were scored to measure time spent interacting with each object by a condition blind researcher.
Open field (P51)
Rats were allowed to explore a circular arena spanning 135 cm in diameter for 10 min. The task was used as a measure of locomotor activity and exploratory behaviour (Whishaw and Kolb, 2005). An overhead camera tracked movement and locomotor activity (distance travelled and velocity). Time spent in the centre of the arena was also used as an additional measure of anxiety-like behaviour.
Forced swim (P55)
The forced swim task was administered on PID12 and was used as a measure of depressive-like behaviour (Yadid et al., 2001). Rats were placed in a 30-cm diameter by 60-cm high cylindrical container filled with warm water (∼25°C) and were recorded for 7 min. The trials were scored to determine time spent immobile as a measure of depressive-like behaviour.
Tissue collection
Animals were placed under light anaesthesia using isoflurane before being euthanized via guillotine decapitation. The HPC, NAc and PFC were extracted from both hemispheres of each rat brain and quickly flash frozen on dry ice and then stored at −80°. An ear notch was also collected from each rat in order to run telomere length analysis. Blood from the trunk was collected for serum enzyme-linked immunosorbent assay (ELISA) analysis. Using serum separator tubes (BD, Franklin Labs, NJ, USA) samples were left to clot for 30 minutes at room temperature, followed by a 15-minute centrifuge at 100 g and 4 degrees. Serum was then stored at -80 degrees.
DNA and RNA extraction for real-time quantitative polymerase chain reactions
RNA was extracted from the brain regions using the Allprep RNA/DNA Mini Kit (Qiagen) according to manufacturer’s protocol. Genomic DNA was extracted from ear notch samples using the DNeasy Blood and Tissue kit (Qiagen, Germany). Concentration and purity were determined using the Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Two micrograms of RNA were reverse transcribed to make cDNA using oligo(dt)20 of the Superscript III First Strand Synthesis Supermix kit (Invitrogen, Carlsbad, CA). cDNA was diluted to 10 ng/μl and used to perform real-time quantitative polymerase chain reactions (RT-qPCR) using forward and reverse primers designed and purchased from Integrated DNA Technologies (Coalville, IA). Telomere length analysis was conducted using the protocol described elsewhere (Cawthorn, 2002; Hehar and Mychasiuk, 2016).
Brain regions and gene selection
The HPC was chosen for the role it plays in learning, memory, and emotional regulation. The response of the HPC to THC has been the subject of controversy despite this region being particularly vulnerable to stimuli. Some studies show decreased hippocampal volume following THC use, while others claim there is no effect (Yücel et al., 2016; Koenders et al., 2017). The NAc is the brain’s reward system and processes motivation, pleasure, and reinforcement, thus playing an important role in addiction and exposure to THC (Kolb and Whishaw, 2008). The PFC is one of the most vulnerable brain regions to experience-dependent changes during maturation and is important for executive functioning (Mychasiuk and Metz, 2016). Long-term consequences of mTBI experienced during adolescence are associated with deficits of PFC function and include persistent impulsiveness, and subtle deficits in psychomotor speed and visual-spatial skills (Toledo et al., 2012).
This study included six genes of interest. Brain derived neurotrophic factor (Bdnf) is a significant regulator of synaptic plasticity, is involved in neurogenesis and cellular differentiation and has shown to be modulated in response to injury (Horch and Katz, 2002; Sagarkar et al., 2017; Ferreira et al., 2018). The cannabinoid receptor 1 gene (Cnr1) codes for the CB1 receptor, and is the primary receptor for THC binding (Comings et al., 1997). Catechol-O-methyltransferase (Comt) is responsible for the degradation of catecholamine-based neurotransmitters such as dopamine, epinephrine, and norepinephrine. Comt is known to modulate the acute effects of THC on neuronal function (Bossong et al., 2019). The glucocorticoid receptor (GR) is the primary binding site for corticosterone, which is released in response to stress, and can therefore be used as an indirect marker for functioning of the stress response and the hypothalamus-pituitary-adrenal (HPA) axis (Harris et al., 2013). The ionized calcium-binding adaptor molecule 1 (Iba1) is a pro-inflammatory marker, associated with microglial activation, that has been shown to be involved in the neuroinflammatory processes that follow TBI (Sundman et al., 2015). Finally, vascular endothelial growth factor receptor 2 (Vegf-2R) mediates the effects of VEGF, promoting angiogenesis and preventing apoptosis (Blázquez et al., 2004), and changes in Vegf-2R expression have been associated with TBI outcomes (Xiong et al., 2011).
Enzyme-linked immunosorbent assays
A sandwich ELISA was run in order to measure the stress marker corticosterone and the cytokine IL-1B. The kits (AB108821 Corticosterone ELISA Kit Abcam, Cambridge, USA; BMS630/BMS630TEN- Rat IL-1B, Invitrogen-Affymetrix, Carlsbad, USA) were used according to manufacturer’s protocols. Samples were run in duplicate on a 96-well plate and were measured with the BioTek Synergy H.T. plate reader and Gen5 2.00.18 software using a path length correction algorithm. All measured samples were within the standard curve.
Statistical analyses
All statistical analysis was carried out on SPSS 25.0 for Mac. For Study #1, three-way ANOVAs were run with Sex (male; female), Injury (sham; RmTBI) and THC (vehicle; post-THC) as factors. For Study #2, three-way ANOVAs were run with Sex (male; female), Injury (sham; RmTBI) and THC (vehicle; pre-THC) as factors. Post hoc pairwise comparisons (LSD) were performed where applicable for interaction effects. Statistical significance was considered P < 0.05 and means ± standard errors are displayed for all figures.
Data availability
All data are available upon request from the corresponding author.
Results study 1
Behavioural outcomes
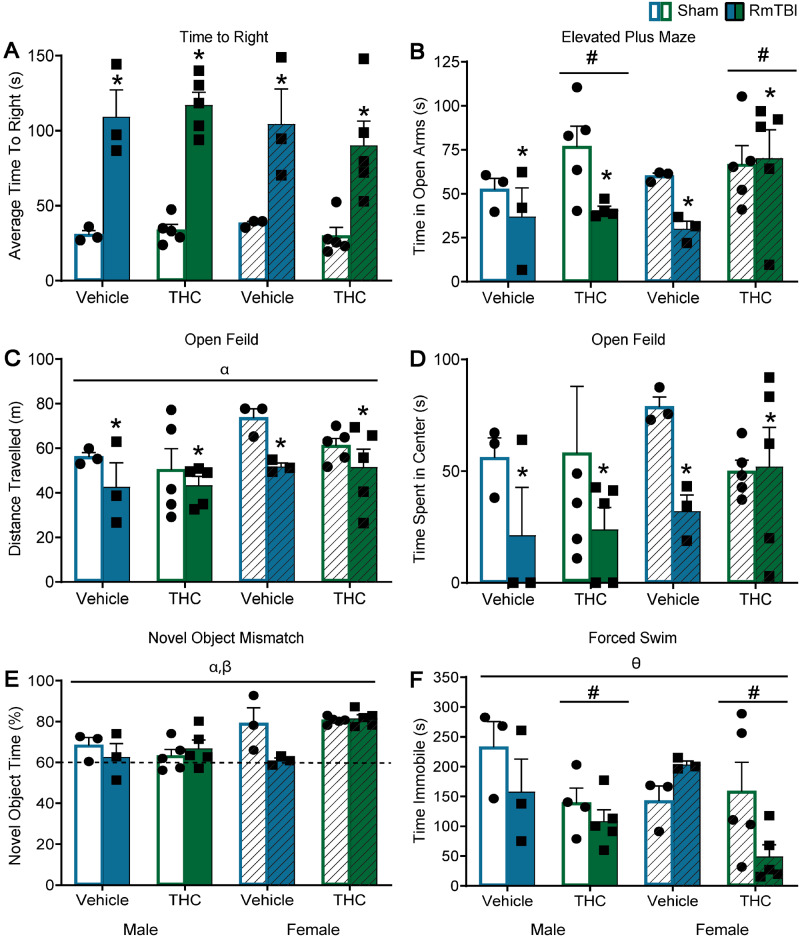
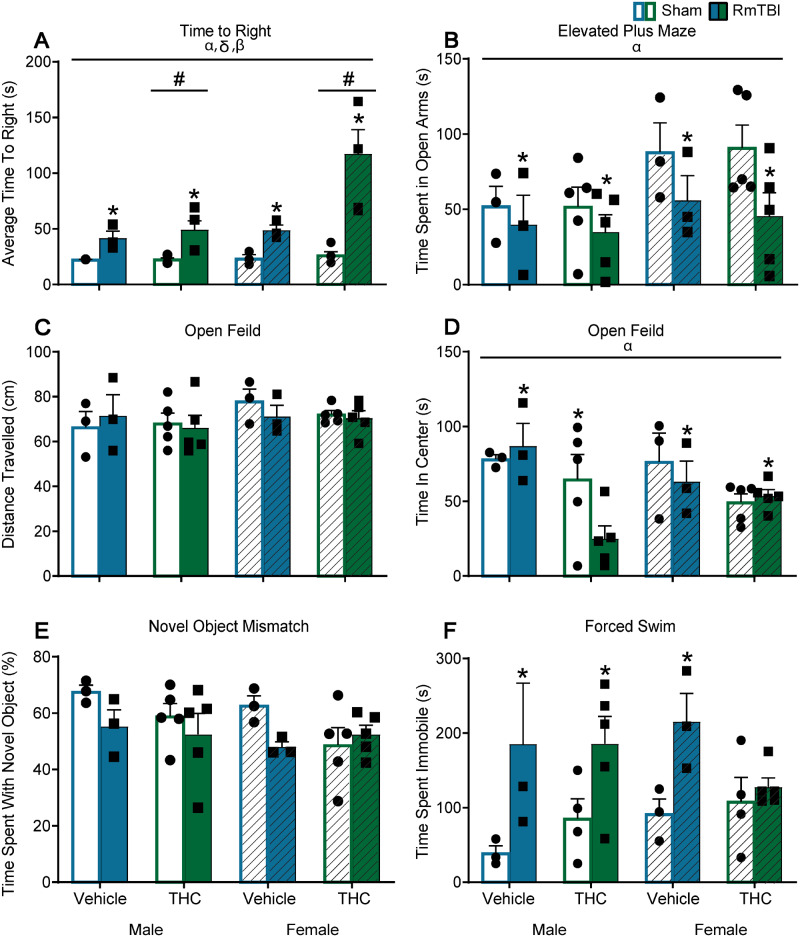
Administration of THC following RmTBI altered behaviour on 3/6 measures (EPM, novel context mismatch, and forced swim) for both sham and mTBI animals, while RmTBI produced dysfunction in 4/6 tests [time to right, EPM, open field (distance travelled and time in centre) see Fig 2B–D]. A significant TBI × THC interaction was observed in the novel context mismatch task, that was driven by the TBI group, whereby THC exposure improved performance when compared to vehicle treatment, P < 0.01, see Fig. 2E. In addition, a significant three-way interaction was identified in the forced swim task that was partially driven by female-THC animals whereby RmTBI animals spent less time immobile than sham animals, P = 0.01, and in the female-TBI group where THC animals spent less time immobile that vehicle animals P = 0.01, see Fig. 2F. See Table 1 for statistical results and Fig. 2 for graphical representation of the findings.
Figure 2.
Graphical depictions of the behavioural testing results displayed as mean ± SEM for the post-injury THC administration groups. White and white hashed bars display sham groups (males and females, respectively), while blue and green bars display RmTBI groups. Statistical significance symbols are displayed above the bars (α) indicates a main effect for sex, (*) indicates a main effect for RmTBI, (#) indicates a main effect for THC, (β) indicates a RmTBI by THC interaction and (θ) indicates a sex by RmTBI by THC three-way interaction. (A) The mean time to right following injury or sham procedures. (B) The mean time spent in the open arm of the EPM. (C and D) The mean distance travelled and time spent in the centre of the open field respectively. (E) The percentage of time spent investigating the novel object in the novel context mismatch with the dotted line representing the minimum expected level of recognition (60%). (F) The mean time spent immobile during the forced swim test.
Table 1.
Statistical results for the three-way ANOVAs for the behaviour tasks in Study #1
| Behavioural task | Effect of sex F (P) | Effect of TBI F (P) | Effect of THC F (P) | Significant interactions F (P) |
|---|---|---|---|---|
| Time to right | 0.42 (0.53) | 68.32 (0.00) | 0.23 (0.64) | N/A |
| Beamwalk | 1.12 (0.30) | 2.13 (0.16) | 0.59 (0.45) | N/A |
| EPM | 0.29 (0.59) | 4.64 (0.04) | 4.28 (0.05) | N/A |
| Novel context mismatch | 12.77 (0.00) | 2.67 (0.11) | 3.42 (0.07) | TBI × THC—6.08 (0.02) |
| Open field (distance travelled) | 4.72 (0.04) | 5.84 (0.02) | 0.73 (0.40) | N/A |
| Open field (time in centre) | 0.91 (0.35) | 4.32 (0.05) | 0.01 (0.96) | N/A |
| Forced swim | 0.64 (0.43) | 2.41 (0.16) | 7.78 (0.01) | Sex × TBI × THC—4.53 (0.04) |
Serum and telomere results
See Fig. 3 for graphical representation of these findings. The results from the three-way ANOVA for serum corticosterone demonstrated a main effect of sex, F(1,28) = 4.81, P = 0.04, but not of injury, F(1,28) = 1.79, P = 0.19, or of THC, F(1,28) = 0.73, P = 0.41, see Fig. 3A. None of the interactions were significant. The results from the three-way ANOVA for serum Il-1B failed to identify a main effect of sex, injury or THC, Ps > 0.05, but did demonstrate a significant TBI × THC interaction, F(1, 24) = 4.78, P = 0.04, see Fig. 3B. In addition, a significant TBI × THC interaction was observed in the telomere length analysis, F(1,30) = 4.33, P = 0.05, whereby TBI reduced telomere length in the vehicle group, but not in the THC group, see Fig. 3C.
Figure 3.
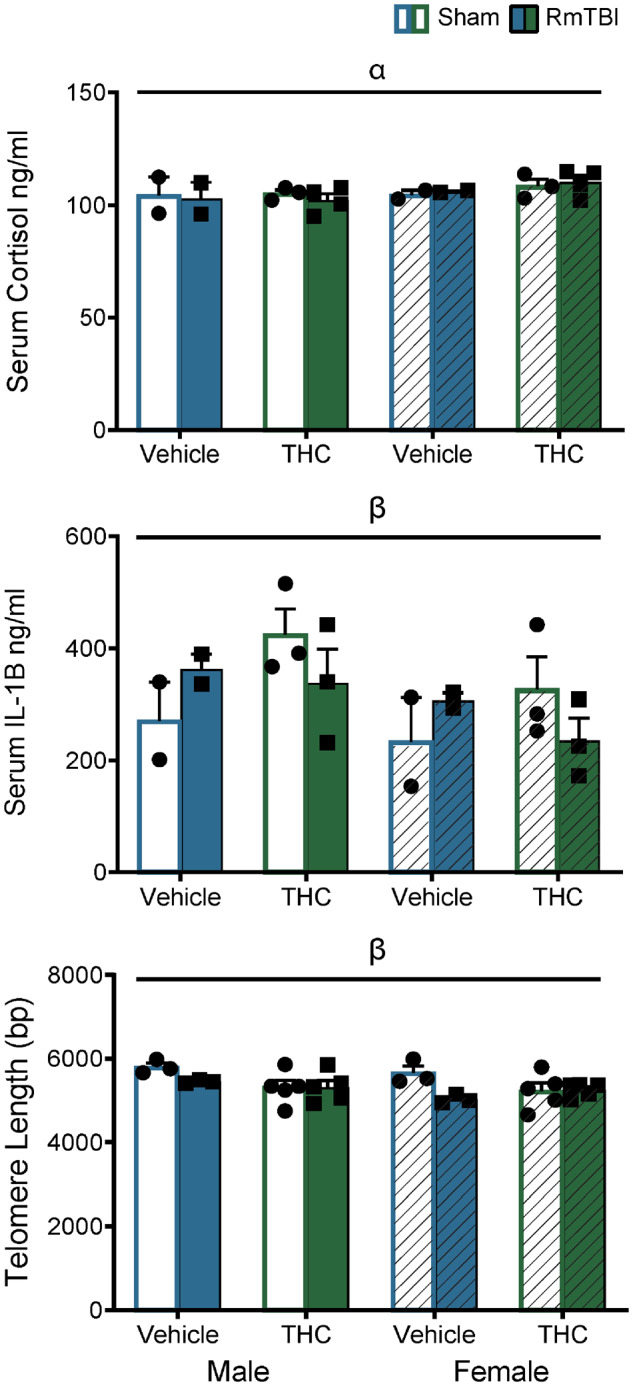
Graphical depictions of the ELISA serum cortisol and IL1B in addition to the telomere lengths displayed as means ± SEM for the post-injury THC administration groups. White and white hashed bars display sham groups (males and females, respectively), while blue and green bars display RmTBI groups. Statistical significance symbols are displayed above the bars (α) indicates a main effect for sex, (β) indicates a RmTBI by THC interaction.
Changes in mRNA expression
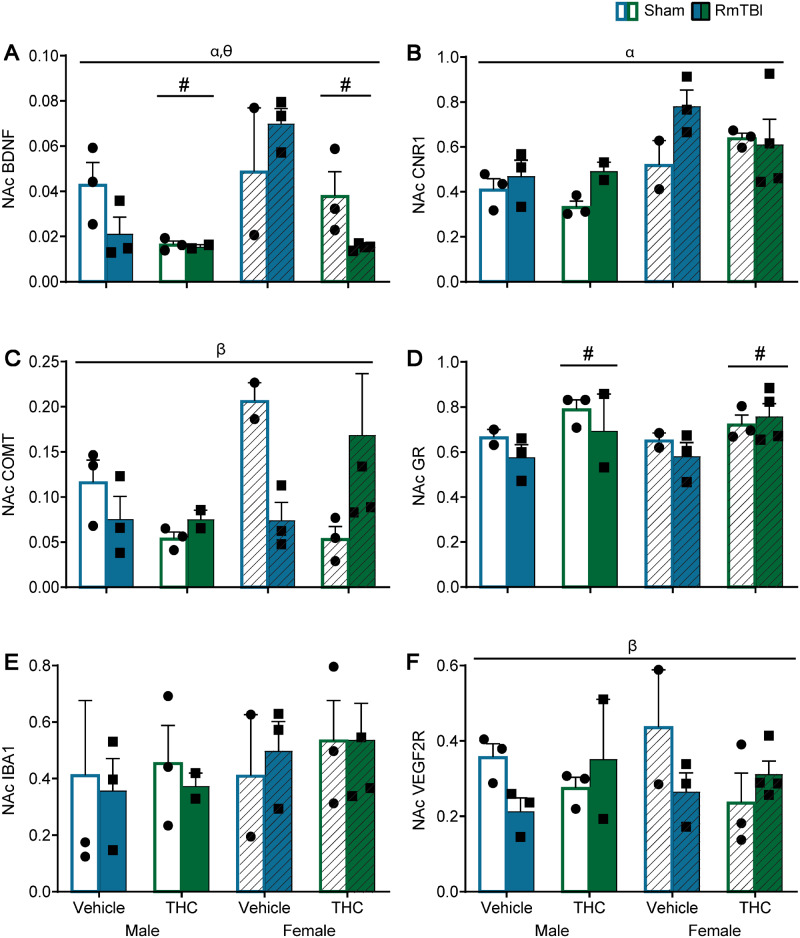
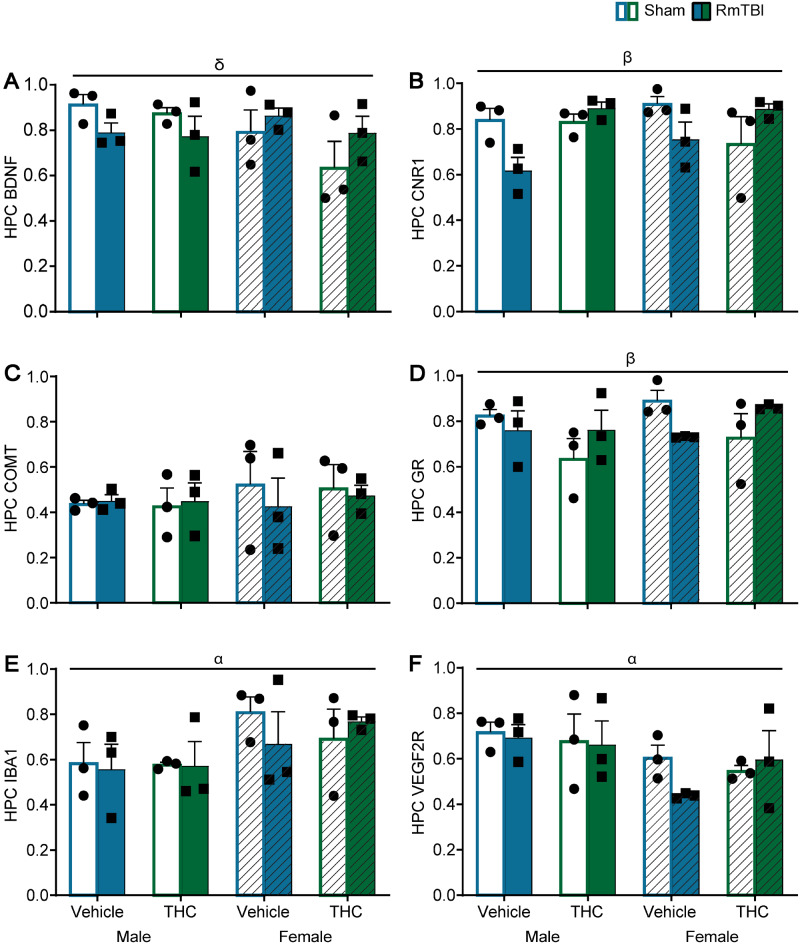
All statistical results for the mRNA three-way ANOVA results can be found in Table 2 and graphical representation found in Figs 4–6. Although there was not an abundance of main effects for TBI or THC, there were numerous TBI × THC interactions.
Table 2.
Statistical results for the three-way ANOVAs for mRNA expression from Study #1
| Brain region | Gene | Effect of sex, F (P) | Effect of TBI, F (P) | Effect of THC, F (P) | Significant interactions, F (P) |
|---|---|---|---|---|---|
| PFC | Bdnf | 0.01 (0.96) | 0.37 (0.55) | 2.69 (0.12) | Sex × THC—5.95 (0.03) Sex × TBI × THC—6.96 (0.02) |
| Cnr1 | 0.01 (0.91) | 0.29 (0.60) | 0.18 (0.68) | TBI × THC—7.50 (0.02) Sex × TBI × THC—5.88 (0.03) | |
| Comt | 3.65 (0.07) | 4.46 (0.05) | 11.06 (<0.01) | N/A | |
| GR | 0.64 (0.44) | 0.11 (0.75) | 2.46 (0.14) | Sex × TBI × THC—7.17 (0.01) | |
| Iba-1 | 8.25 (0.01) | 0.01 (0.94) | 2.64 (0.12) | Sex × TBI × THC—10.26 (<0.01) | |
| Vegf-2R | 0.13 (0.72) | 2.60 (0.13) | 16.31 (<0.01) | TBI × THC—5.77 (0.03) | |
| HPC | Bdnf | 1.79 (0.20) | 0.00 (0.99) | 2.05 (0.17) | Sex × TBI—4.95 (0.04) |
| Cnr1 | 0.39 (0.54) | 0.92 (0.35) | 1.72 (0.21) | TBI × THC—12.05 (<0.01) | |
| Comt | 0.44 (0.52) | 0.13 (0.72) | 0.01 (0.94) | N/A | |
| GR | 1.42 (0.25) | 0.04 (0.85) | 1.35 (0.26) | TBI × THC—6.48 (0.02) | |
| Iba-1 | 5.72 (0.02) | 0.13 (0.73) | 0.00 (0.98) | N/A | |
| Vegf-2R | 6.39 (0.02) | 0.49 (0.49) | 0.02 (0.89) | N/A | |
| NAc | Bdnf | 8.57 (0.01) | 0.87 (0.37) | 14.14 (<0.01) | Sex × TBI × THC—6.22 (0.02) |
| Cnr1 | 14.32 (<0.01) | 4.12 (0.06) | 0.23 (0.64) | N/A | |
| Comt | 2.51 (0.13) | 0.10 (0.75) | 1.12 (0.31) | TBI × THC—7.31 (0.01) | |
| GR | 0.01 (0.95) | 1.38 (0.26) | 6.93 (0.02) | N/A | |
| Iba-1 | 0.69 (0.42) | 0.01 (0.92) | 0.24 (0.63) | N/A | |
| Vegf-2R | 0.08 (0.78) | 0.75 (0.40) | 0.27 (0.61) | TBI × THC—6.15 (0.03) |
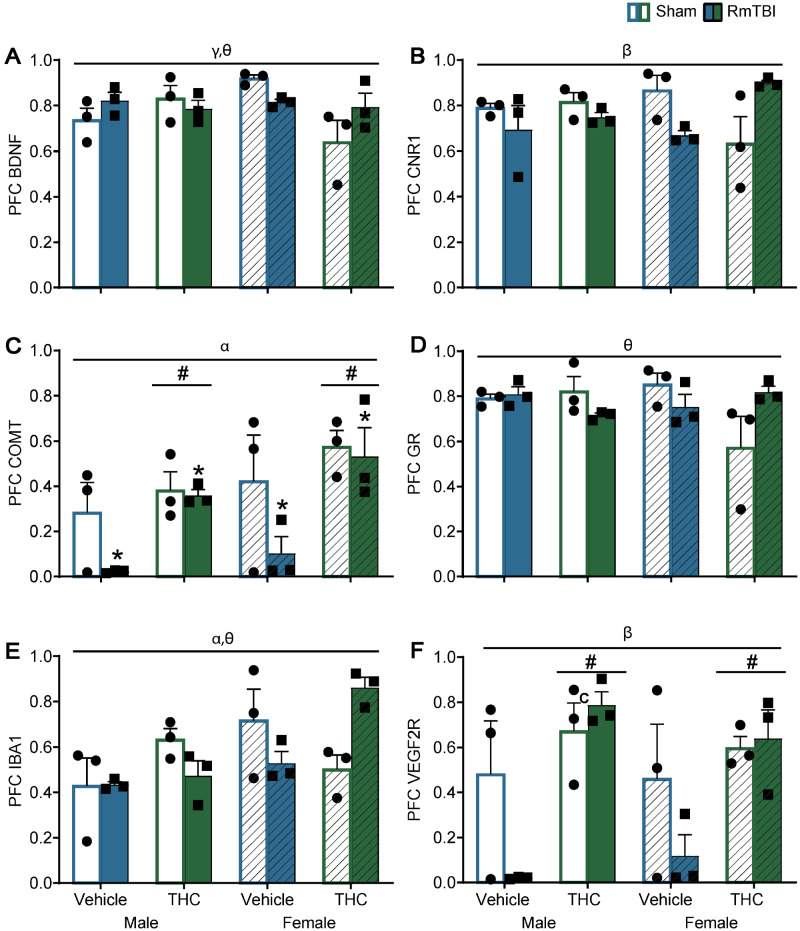
Figure 4.
Graphical depictions of the RT-qPCR results in the PFC as mean ± SEM for the post-injury THC administration groups. White and white hashed bars display sham groups (males and females, respectively), while blue and green bars display RmTBI groups. Statistical significance symbols are displayed above the bars (α) indicates a main effect for sex, (*) indicates a main effect for RmTBI, (#) indicates a main effect for THC, (β) indicates a RmTBI by THC interaction, (γ) indicates a sex by THC interaction and (θ) indicates a sex by RmTBI by THC three-way interaction.
Figure 6.
Graphical depictions of the RT-qPCR results in the nucleus accumbens displayed as mean ± SEM for the post-injury THC administration groups. White and white hashed bars display sham groups (males and females, respectively), while blue and green bars display RmTBI groups. Statistical significance symbols are displayed above the bars (α) indicates a main effect for sex, (#) indicates a main effect for THC, (β) indicates a RmTBI by THC interaction and (θ) indicates a sex by RmTBI by THC three-way interaction.
In the PFC, the sex by THC interaction for Bdnf was driven by females whereby animals in the THC group exhibited lower levels of Bdnf than vehicle rats P < 0.01. The sex by TBI by THC interaction for Bdnf was driven by the sham rats whereby males differed from females in the vehicle and in the THC groups, P = 0.02, see Fig. 4A. For Cnr1 the TBI by THC interaction was moderated by the vehicle group where RmTBI animals exhibited significant reductions in Cnr1 compared to sham animals, P = 0.03. The sex by TBI by THC interaction for Cnr1 was driven by females whereby vehicle sham animals displayed higher levels of Cnr1 expression compared to THC sham animal P = 0.04, and the THC group where females with RmTBI also exhibited higher levels of Cnr1 than THC sham animals P = 0.01, see Fig. 4B. For the GR, the three-way interaction was driven largely by females, where sham-vehicle animals had higher levels of GR than sham THC rats P < 0.01, and in the female-THC group where RmTBI animals exhibited higher levels of GR than sham animals, P = 0.01. With respect to Iba-1, the three-way interaction was driven by the injury groups whereby male and female rats in the sham-vehicle group differed significantly P = 0.02, and also in the TBI–THC groups, P < 0.01, see Fig. 4D. In addition, the sham and RmTBI animals in the female-THC group exhibited significantly different levels of Iba-1 expression, P < 0.01, see Fig. 4E. Finally, for Vegf-2R the TBI by THC interaction was driven by animals in the vehicle group, where RmTBI rats exhibited significant reductions in Vegf-2R compared to sham animals P < 0.01, see Fig. 4F.
In the HPC, the significant sex by TBI interaction for Bdnf was driven by the sham animals where the males displayed higher levels of Bdnf than the females, P = 0.02, see Fig. 5A. For Cnr1, the TBI by THC interaction was identified in the vehicle groups where RmTBI animals have a reduction in Cnr1 compared to sham animals, P < 0.01, and in the TBI animals whereby THC increased Crn1 compared to vehicle, P < 0.01, see Fig. 5B. Finally, for GR, the TBI by THC interaction was moderated by the sham group, where animals exposed to THC had reductions in GR expression compared to animals in the vehicle group, P = 0.01, see Fig. 5D.
Figure 5.
Graphical depictions of the RT-qPCR results in the hippocampus displayed as mean ± SEM for the post-injury THC administration groups. White and white hashed bars display sham groups (males and females, respectively), while blue and green bars display RmTBI groups. Statistical significance symbols are displayed above the bars (α) indicates a main effect for sex, (β) indicates a RmTBI by THC interaction and (δ) indicates a sex by RmTBI interaction.
With respect to the NAc, the three-way interaction for Bdnf was largely driven by the RmTBI-vehicle animals where females exhibited significant elevations in Bdnf compared to males, P < 0.01, and in the female-RmTBI animals where THC significantly reduced Bdnf, P < 0.01, see Fig. 5A. The TBI by THC interaction for Comt was driven by the sham animals, whereby rats in the vehicle group exhibited significant increases in Comt levels compared to animals that received THC, P = 0.01, see Fig. 5C. Lastly, the TBI by THC interaction for Vegf-2R was driven by the sham animals whereby THC significantly decreased Vegf-2R levels as compared to vehicle treated animals, P = 0.03, see Fig. 5F.
Results study 2
Behavioural outcomes
See Table 3 for statistical results and Fig. 7 for graphical representation of the findings. Intermittent administration of THC prior to RmTBI did not alter behaviour with the exception of time to right, while mTBI produced dysfunction in 4/6 tests (time to right, beamwalk, EPM, forced swim, Fig. 7A, B and F, respectively) and there was a trend towards an effect in the novel context mismatch task (Fig. 7E). There was also a significant Sex × THC interaction in the time spent in the centre of the open field whereby females exposed to THC spent more time in the centre of the open field than males in the THC groups, P < 0.01, and where males treated with vehicle spent more time in the centre than males treated with THC, P = 0.01, see Fig. 7D.
Table 3.
Statistical results for the three-way ANOVAs for the behaviour tasks in Study #2
| Behavioural task | Effect of sex, F (P) | Effect of TBI, F (P) | Effect of THC, F (P) | Significant interactions, F (P) |
|---|---|---|---|---|
| Time to right | 6.33 (0.02) | 25.99 (0.00) | 6.18 (0.02) | Sex × TBI—4.92 (0.04) TBI × THC—5.29 (0.03) |
| Beamwalk | 8.71 (0.01) | 5.01 (0.03) | 0.03 (0.86) | N/A |
| EPM | 5.18 (0.03) | 5.65 (0.02) | 0.08 (0.77) | N/A |
| Novel context mismatch | 2.01 (0.17) | 3.52 (0.07) | 1.77 (0.19) | N/A |
| Open Field (distance travelled) | 2.54 (0.12) | 0.23 (0.68) | 0.09 (0.77) | N/A |
| Open field (time in center) | 4.43 (0.04) | 1.67 (0.21) | 2.05 (0.17) | Sex × THC—5.50 (0.03) |
| Forced swim | 0.22 (0.65) | 14.62 (<0.01) | 0.06 (0.82) | N/A |
Figure 7.
Graphical depictions of the behavioural testing results displayed as mean ± SEM for the pre-injury THC administration groups. White and white hashed bars display sham groups (males and females, respectively), while blue and green bars display RmTBI groups. Statistical significance symbols are displayed above the bars (α) indicates a main effect for sex, (*) indicates a main effect for RmTBI, (#) indicates a main effect for THC, (β) indicates a RmTBI by THC interaction and (δ) indicates a sex by RmTBI interaction. (A) The mean time to right following injury or sham procedures. (B) The mean time spent in the open arm of the EPM. (C and D) The mean distance travelled and time spent in the centre of the open field, respectively. (E) The percentage of time spent investigating the novel object in the novel context mismatch. (F) The mean time spent immobile during the forced swim test.
Serum and telomere results
The results from the three-way ANOVA for serum corticosterone failed to demonstrate any main effects, but did identify a significant TBI × THC interaction, F(1,23) = 5.38, P = 0.04. The results from the three-way ANOVA for serum Il-1B failed to identify any main effects or significant interactions, Ps > 0.05. The three-way ANVOA for telomere length did, however, identify a main effect of sex, F(1,31) = 5.38, P = 0.03, and a main effect of Injury, F(1,31) = 12.71, P < 0.01, but not of THC, F(1,31) = 0.17, P = 0.69; none of the interactions were significant. Telomere length was significantly reduced in response to RmTBI and in males compared to females.
Given that there were no significant effects of intermittent pre-injury THC exposure on behavioural outcomes, nor any significant TBI × THC interactions, despite significant main effects of RmTBI, we did not examine changes in mRNA expression for Study #2.
Discussion
The objective of this study was to observe the effects of THC on the adolescent brain in the context of RmTBI. Specifically, the study aimed to examine the efficacy of THC as a therapeutic for remediation of PCS, and also investigate whether or not intermittent THC exposure prior to RmTBI would affect recovery. We hypothesized that THC would have both therapeutic and neuroprotective potency against the negative consequences of mTBI. The results of this study suggest that THC exposure post-injury has some beneficial potential and warrants further investigation.
Study 1—therapeutic potential of THC for RmTBI
Although the risks associated with THC use in adolescence have been a topic of significant debate, substantial evidence has suggested that THC is a potent antioxidant with neuroprotective properties (Hampson et al., 1998, 2000). In this study, RmTBI affected 4/6 of the behaviours examined, with therapeutic administration of THC following RmTBI producing beneficial outcomes in three of these measures. In vehicle treated animals, RmTBI increased loss of consciousness, increased anxiety-like behaviour in both the EPM and the open field, reduced exploratory behaviour and produced sex-dependent effects on depressive-like behaviours (reducing depression in males and increasing depression in females). Interestingly, daily administration of THC reduced anxiety in the EPM and reduced depressive-like behaviour in the forced swim task. These findings are in line with previous literature demonstrating that THC (at lower doses) reduces anxiety and emotional responses to psychosocial stress (Crippa et al., 2009; Childs et al., 2017), and suggests that THC may offer therapeutic potential for the treatment of RmTBI-induced anxiety. In addition, daily administration of THC mitigated RmTBI-induced impairments in short-term working memory. This is consistent with prior studies demonstrating that THC administration improves cognitive performance (Suliman et al., 2018). In summary, results from the behavioural test battery suggest that daily administration of THC following RmTBI has potential therapeutic efficacy for the treatment of PCS.
Telomeres are evolutionarily conserved sequences of repetitive DNA at the ends of chromosomes that maintain the integrity of the genome and protect the DNA from oxidative stress (Zhu et al., 2011). While telomere shortening has been recognized as a marker of biological aging and neurodegeneration (Klapper et al., 2001), research in our laboratory has demonstrated that telomere shortening is a characteristic of mTBI and can be used as a prognostic tool in rodent models (Hehar and Mychasiuk, 2016; Wright et al., 2018). Consistent with our previous studies, RmTBI reduced telomere length but interestingly, there was no RmTBI-induced reduction in telomere length for animals who were administered THC providing further support for the therapeutic benefits of THC.
The analyses of mRNA expression changes across the brain regions revealed numerous important neurological alterations associated with THC and RmTBI at the molecular level. First, we did not see any THC-induced changes in hippocampal Bdnf expression. This is important as evidence suggests that exogenous THC can affect long-term synaptic plasticity and remodel neurocircuitry (Gerdeman and Lovinger, 2003), and Bdnf has recently been implicated as a critical moderator of cannabinoid-mediated neurogenesis (Ferreira et al., 2018). We did, however, see downregulation of Bdnf in the PFC and NAc of THC treated rats. This is contradictory to previous studies that have found an upregulation of Bdnf in the NAc and ventral tegmental area of adult rats who were chronically administered THC (Butovsky et al., 2005). As studies have found that serum Bdnf levels are associated with whether or not individuals have a history of cannabis use (D’Souza et al., 2009), the timing of sample collection, age of the rats and history of exposure likely contribute to the discrepant changes in Bdnf expression identified in this study.
The numerous interactions identified in mRNA expression changes of Cnr1 are of particular relevance given that chronic THC exposure is known to alter the expression of Cnr1 (Zhuang et al., 1998) and cannabinoid receptor activation is critical for attenuation of brain damage following injury (Mechoulam and Shohami, 2007; Fishbein-Kaminietsky et al., 2014). Exactly how the endocannabinoid system assists in neuroprotection and acute neuronal injury is not fully understood. However, the activation of CB1 receptors by THC causes an alteration in GABA/glutamergic neurotransmission, inhibition of acetylcholine release and modulates immune responses and the release of inflammatory mediators (Pacher et al., 2006; Boggs et al., 2018). Moreover, CB1 receptors located on post-synaptic neurons control N-methyl-D-aspartate ion channel activity and therefore, provide protection against acute excitotoxicity (Paloczi et al., 2018). Given these functions of CB1 receptors, THC appeared to provide therapeutic benefit with respect to expression of Cnr1 in the HPC of both males and females; while Cnr1 mRNA levels were reduced in RmTBI animals treated with vehicle, they were increased in RmTBI animals treated with THC. This therapeutic benefit was also identified in the PFC, but only for female rats treated with THC.
TBI is believed to causes disruption to the catecholaminergic system, specifically because these neurons in the PFC are vulnerable to axonal injury, metabolic stress and mechanical injury, which often results in cognitive impairment (Jenkins et al., 2016). In addition, and of importance for this study, Comt modulates the acute neuronal effects of THC and is involved in the release of striatal dopamine (Bossong et al., 2019). The changes in Comt expression in the PFC were consistent with both of these concepts; RmTBI reduced Comt expression in vehicle animals but not THC animals, suggesting that THC administration provided therapeutic restoration of TBI-induced reductions in Comt expression. THC also altered expression of Comt in the NAc, corroborating evidence that Comt modulates THC-induced dopamine release in the striatum.
We examined GR expression because it can be used as a measurable indicator of the stress response. The endocannabinoid system is intricately involved in regulating the stress response and numerous studies have demonstrated that THC can directly modulate the HPA axis and glucocorticoid release (Manzanares et al., 1999). While current literature indicates that endocannabinoid signalling suppresses HPA activity (Hill and Tasker, 2012), stressful stimuli such as a traumatic brain injuries do the opposite and activate this system (Grundy et al., 2001). In this study, we found that while sham animals exposed to THC had reductions in GR mRNA expression in the HPC, RmTBI animals exhibited GR expression levels equivalent to vehicle-sham animals, suggesting that THC may have provided neuroprotection. These alterations in GR expression may have also contributed to the reduced anxiety-like behaviour on the EPM and open field tasks. Interestingly, GR expression was upregulated in the NAc in response to THC in both sham and RmTBI. Given the NAc and HPC work synergistically in feedback loops involved in drug administration and stress (Piazza and Le Moal, 1998), the reductions of GR in the HPC may be associated with the increased expression in NAc. To our knowledge, this is the first study to examine changes in GR expression in the NAc following THC exposure and this finding warrants further investigation.
In addition, neuroinflammation, such as microglial activation, is hypothesized to play a significant role in the pathophysiology of mTBI (Patterson and Holahan, 2012). Microglial activation has been found to persist in areas remote from the area of focal damage after TBI and has been related to various cognitive and behavioural impairments (Ramlackhansingh et al., 2011). We examined Iba1 mRNA expression as an indicator of neuroinflammation and microglial activation and found that THC exposure actually increased Iba-1 mRNA expression in the PFC and had no effect in the HPC or NAc. While the expression of Iba-1 is typically upregulated in microglia following TBI (Ito et al., 1998), in this study, THC administration did not confer therapeutic benefit.
Finally, RmTBI is believed to pose a threat to Vegf-2R expression, angiogenesis and tissue repair, with inhibition of Vegf-2R being associated with worse TBI outcomes, including greater lesion size and decreased neurogenesis (Xiong et al., 2011). Given that the cerebral vasculature is crucial for the delivery of oxygen and other nutrients to the brain, functional impairment and dysregulation of this system following TBI may inhibit recovery (Wendel et al., 2019). In both the PFC and NAc, RmTBI significantly reduced Vegf-2R mRNA expression levels in vehicle-treated animals, but this RmTBI-induced reduction was absent in THC-treated animals. Similar to the results identified for Comt and Crn1, post-injury THC administration appears to have provided some therapeutic benefit.
Neuroprotective potential of THC prior to RmTBI
Given that cannabis use is gaining popularity in adolescents, and is now more common than cigarette use (Chadwick et al., 2013), we sought to examine the effects of intermittent THC exposure prior to RmTBI on PCS symptom resolution. This intermittent dosage of THC was designed to provide a realistic model of individuals that are casual consumers of THC before RmTBI and then quit when they experience concussion-related symptoms. Despite numerous injury related impairments (loss of consciousness, beamwalk, increased anxiety- and depressive-like behaviour), intermittent THC use only affected loss of consciousness and time spent in the centre of the open field for female rats, failing to influence any of the other behavioural measures examined. While there is currently conflicting literature regarding early life THC exposure and neurodevelopmental outcomes [for review see Jacobus and Tapert (2014); Lorenzetti et al. (2016)], our study found very little evidence for behavioural impairments in adolescence associated with intermittent THC use at this developmental time period. It is possible that the six injections over 20 days was not frequent enough, or at a high enough dosage, to produce lasting changes, as many of the human studies do report greater behavioural and neurological impairment in heavy cannabis users (Lorenzetti et al., 2016; Koenders et al., 2017). The sex-dependent THC effects in time to right and open field, do however suggest that females may be more sensitive to the effects of THC at this time period. This finding is consistent with accumulating evidence demonstrating that sex is an important modulator of cannabinoid sensitivity resulting in a sexually dimorphic endocannabinoid system (Struik et al., 2018). Overall however, we failed to support our hypothesis that THC prior to RmTBI conferred neuroprotection. Moreover, as this regimen of THC exposure failed to produce significant changes in behaviour or produce significant interactions with our RmTBI model, we did not investigate changes in mRNA expression in this cohort.
Conclusions and limitations
These studies demonstrated that THC before and after RmTBI differentially affected outcomes. While the results indicate that there are some therapeutic benefits of THC when administered after RmTBI, further investigation regarding this association is warranted. To gain a better understanding of the identified effects, it would be important to allow these adolescent rats to mature and then assess various cognitive and emotional measures in adulthood to obtain a comprehensive picture of the long-term effects of adolescent THC exposure. Future studies would also benefit from testing THC at multiple doses and via different administration routes, such as vaporized cannabis exposure, in order to replicate human experiences in an ecologically valid manner (McLaughlin, 2018). In summary, we did not find evidence to support our hypothesis that THC prior to RmTBI provided neuroprotection. However, we did find that therapeutic administration of THC following RmTBI was beneficial for short-term working memory, and anxiety- and depressive-like behaviours. In addition, chronic administration of THC following the injuries appeared to have beneficial effects on many of the genes examined in the PFC, HPC and NAc. Overall, this study suggests that THC has potential therapeutic efficacy for the treatment of RmTBI-induced symptomology but requires additional examination.
Acknowledgements
The authors would like to thank Melinda Wang and Haley Vecchiarelli for their expertise and help with experimental design.
Funding
The authors would like to thank the Alberta Children’s Hospital Research Institute, Canadian Institute of Health Research (Grant # PJT-153051), and National Scientific and Engineering Research Council (Grant # 1304881) for their financial contributions.
Competing Interests
The authors report no competing interests.
Glossary
- Bdnf =
brain derived neurotrophic factor
- CB =
cannabinoid receptors
- Cnr1 =
cannabinoid receptor 1
- Comt =
catechol-O-methyltransferase
- EPM =
elevated plus maze
- ELISA =
enzyme-linked immunosorbent assay
- GR =
glucocorticoid receptor
- HPC =
hippocampus
- Iba-1 =
ionized calcium-binding adaptor molecule 1
- NAc =
nucleus accumbens
- mTBI =
mild traumatic brain injury
- PCS =
post-concussive symptomology
- PFC =
prefrontal cortex
- P =
postnatal day
- PID =
post-injury day
- RT-qPCR =
real-time quantitative polymerase chain reactions
- RmTBI =
repeated mild traumatic brain injury
- TBI =
traumatic brain injury
- THC =
Δ9-tetrahydrocannabinol
- Vegf-2R =
vascular endothelial growth factor receptor 2
References
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE.. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. Br Med J 2002; 325: 1212–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow K, Crawford S, Stevenson A, Sandhu S, Belanger F, Dewey D.. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics 2010; 126: e374–81. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Quantitative magnetic resonance imaging in traumatic brain injury. J Head Trauma Rehabil 2001; 16: 117–34. [DOI] [PubMed] [Google Scholar]
- Blázquez C, González-Feria L, Álvarez L, Haro A, Casanova ML, Guzmán M.. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res 2004; 64: 5617–23. [DOI] [PubMed] [Google Scholar]
- Boggs D, Nguyen J, Morgenson D, Taffe M, Ranganathan M.. Clinical and preclinical evidence for functional interactions of cannabidiol and △9-tetrahydrocannabinol. Neuropsychopharmacology 2018; 43: 142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, van Hell HH, Schubart CD, van Saane W, Iseger TA, Jager G, et al. Acute effects of △9-tetrahydocannabinol (THC) on resting state brain function and their modulation by COMT genotype. Eur Neuropsychopharmacol 2019; 29: 766–76. [DOI] [PubMed] [Google Scholar]
- Bridgeman M, Abazia D.. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T 2017; 42: 180–8. [PMC free article] [PubMed] [Google Scholar]
- Buki A, Povlishock JT.. All roads lead to disconnection?—traumatic axonal injury revisited. Acta Neurochir (Wien) 2006; 148: 181–93; discussion 193–4. [DOI] [PubMed] [Google Scholar]
- Butovsky E, Juknat A, Goncharov I, Elbaz J, Eilam R, Zangen A, et al. In vivo up-regulation of brain derived neurotrophic factor in specific brain areas by chronic exposure to delta-tetrahydrocannabinol. J Neurochem 2005; 93: 802–11. [DOI] [PubMed] [Google Scholar]
- Campos D, Yonamine M, de Moraes Moreau R.. Marijuana as doping in sports. Sports Med 2003; 33: 395–9. [DOI] [PubMed] [Google Scholar]
- Cawthorn R. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick B, Miller M, Hurd Y.. Cannabis use during adolescent development susceptibility to psychiatric illness. Front Psychiatry 2013; 4: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Lutz J, de Wit H.. Dose-related effects of delta-9-THC on emotional responses to acute psychosocial stress. Drug Alcohol Depend 2017; 177: 136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M, Guskiewicz K, Sports-related traumatic brain injury In: Laskowitz D, Grant G, editors. Translational research in traumatic brain injury. Boca Raton, FL: CRC Press/Taylor and Francis Group; 2016. [PubMed] [Google Scholar]
- Comings D, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, et al. Cannabinoid receptor gene (CNR1): association with i.v. drug use. Mol Psychiatry 1997; 2: 161–8. [DOI] [PubMed] [Google Scholar]
- Crippa J, Zuardi A, Martin-Santos R, Bhattacharyya A, Atakan Z, McGuire P, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol Clin Exp 2009; 24: 515–23. [DOI] [PubMed] [Google Scholar]
- D’Souza D, Pittman B, Perry E, Simen A.. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology 2009; 202: 569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F, Ribeiro F, Rodrigues R, Sebastiao A, Xapelli S.. Brain-derived neurotrophic factor (BDNF) role in cannabinoid-mediated neurogenesis. Front Cell Neurosci 2018; 12: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidan E, Foley LM, New LA, Alexander H, Kochanek PM, Hitchens TK, et al. Metabolic and structural imaging at 7 Tesla after repetitive mild traumatic brain injury in immature rats. ASN Neuro 2018; 10: 175909141877054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein-Kaminietsky M, Gafni M, Sarne Y.. Ultralow doses of cannabinoid drugs protect the mouse brain from inflammation‐induced cognitive damage. J Neurosci Res 2014; 92: 1669–77. [DOI] [PubMed] [Google Scholar]
- Gardner R, Yaffe K.. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 2015; 66: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger D.. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol 2003; 140: 781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Hovda DA.. The new neurometabolic cascade of concussion. Neurosurgery 2014; 75: S24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy P, Harbuz M, Jessop D, Lightman S, Sharples P.. The hypothalamo-pituitary-adrenal axis response to experimetnal traumatic brain injury. J Neurotrau 2001; 18: 1373–81. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K, Mihalik J.. Biomechanics of sports concussion: quest for the elusive injury threshold. Exerc Sport Sci Rev 2011; 39: 4–11. [DOI] [PubMed] [Google Scholar]
- Guskiewicz K, Mihalik J, Shankar V, Marshall S, Crowell D, Oliaro S, et al. Measurement of head impacts in collegiate football players: relationship between head impact biomechanics and acute clinical outcome after concussion. Neurosurg 2007; 61: 1244–53. [DOI] [PubMed] [Google Scholar]
- Halstead M, Walter K; The Council on Sports Medicine and Fitness. Sport-related concussion in children and adolescents. Pediatrics 2010; 126: 597–615. [DOI] [PubMed] [Google Scholar]
- Hampson A, Grimaldi M, Axelrod J, Wink D.. Cannabidiol and (-) delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci USA 1998; 95: 8268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson A, Grimaldi M, Lolic M, Wink D, Rosenthal R, Axelrod J.. Neuroprotective anitoxidants from marijuana. Ann N Y Acad Sci 2000; 899: 274–82. [PubMed] [Google Scholar]
- Harris A, Holmes M, Kloet E, Chapman K, Seckl J.. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behavior. Psychoneuroendocrinology 2013; 38: 648–58. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Irie K, Fujioka M, et al. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor‐independent myeloperoxidase‐inhibiting mechanism. J Neurochem 2007; 102: 1488–96. [DOI] [PubMed] [Google Scholar]
- Hehar H, Mychasiuk R.. The use of telomere length as a predictive biomarker for injury prognosis in juvenile rats following a concussion/mild traumatic brain injury. Neurobiol Dis 2016; 87: 11–8. [DOI] [PubMed] [Google Scholar]
- Hill M, Tasker J.. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience 2012; 204: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch H, Katz L.. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci 2002; 5: 1177–84. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S.. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol Brain Res 1998; 57: 1–9. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert S.. Effects of cannabis on the adolescent brain. Curr Pharm Des 2014; 20: 2186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins P, Mehta M, Sharp D.. Catecholamines and cognition after trauamtic brain injury. Brain 2016; 139: 2345–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Amerson E, Barth J.. Mild traumatic brain injury: lessons learned from clinical, sports, and combat concussions. Rehabil Res Pract 2012; 2012: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper W, Parwaresch R, Krupp G.. Telomere biology in human aging and aging syndromes. Mech Ageing Dev 2001; 122: 695–712. [DOI] [PubMed] [Google Scholar]
- Koenders L, Lorenzetti V, de Haan L, Suo C, Vingerhoets WAM, van den Brink W, et al. Longitudinal study of hippocampal volumes in heavy cannabis users. J Psychopharmacol 2017; 31: 1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Whishaw I.. Fundamentals of human neuropsychology. 6th edn New York, NY: Worth Publishers; 2008. [Google Scholar]
- Lorenzetti V, Alonso-Lana S, Youssef G, Verdejo-Garcia A, Suo C, Cousijn J, et al. Adolescent cannabis use: what is the evidence for functional brain alteration? Curr Pharm Des 2016; 22: 6353–65. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Fuentes JA.. Opiod and cannabinoid receptor-mediated regulation of the increase in adrenocorticotropin hormone and corticosterone plasma concentrations induced by central administration of delta(9)-tetrahydrocannabinol in rats. Brain Res 1999; 839: 173–9. [DOI] [PubMed] [Google Scholar]
- McKinlay A, Grace R, Horwood L, Fergusson D, Ridder E, MacFarlane M.. Prevalence of traumatic brain injury among children, adolescents, and young adults: prospective evidence from a birth cohort. Brain Injury 2008; 22: 175–81. [DOI] [PubMed] [Google Scholar]
- McLaughlin R. Toward a translationally relevant preclinical model of cannabis use. Neuropsychopharmacology 2018; 43: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney DF, Smith DH.. Biomechanics of concussion. Clin Sports Med 2011; 30: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Shohami E.. Endocannabinoids and traumatic brain injury. Mol Neurobiol 2007; 36: 68–74. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Farran A, Esser MJ.. Assessment of an experimental rodent model of pediatric mild traumatic brain injury. J Neurotrau 2014; 31: 1–9. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Ma I, Candy S, Esser MJ.. The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J Neurosci Methods 2016. a; 257: 168–78. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Ma I, Candy S, Esser MJ.. Reducing the time interval between concussion and voluntary exercise restores motor impairment, short-term memory, and alterations to gene expression. Eur J Neurosci 2016. b; 44: 2407–17. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Ma I, Esser MJ.. Dietary intake alters behavioural recovery and gene expression profiles in the brain of juvenile rats that have experienced a concussion. Front Behav Neurosci 2015; 9: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Metz GA.. Epigenetic and gene expression changes in the adolescent brain: what have we learned from animal models? Neurosci Behav Rev 2016; 70: 189–97. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G.. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 2006; 58: 389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloczi J, Varga Z, Hasko G, Pacher P.. Neuroprotection in oxidative stress-related neurodegenerative diseases: role of endocannabinoid system modulation. Antioxid Redox Signal 2018; 29: 75–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson Z, Holahan M.. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci 2012; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza P, Le Moal M.. The role of stress in drug self-administration. Trends Pharmacol Sci 1998; 19: 67–74. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol 2011; 70: 374–83. [DOI] [PubMed] [Google Scholar]
- Rotermann M, MacDonald R.. Analysis of trends in the prevalence of cannabis use in Canada, 1985 to 2015. Health Rep 2018; 29: 10–20. [PubMed] [Google Scholar]
- Sagarkar S, Bhamburkar T, Shelkar G, Choudhary A, Kokare D, Sakharkar A.. Minimal traumatic brain injury causes persistent changes in DNA methylation at BDNF promoters in rat amygdala: a possible role in anxiety-like behaviors. Neurobiol Dis 2017; 106: 101–9. [DOI] [PubMed] [Google Scholar]
- Schallert T, Woodlee M, Fleming S, Disentangling multiple types of recovery from brain injury In: Krieglstein J, Klumpp S, editors. Pharmacology of cerebral ischemia. Stuttgart: Medpharm Scientific Publishers; 2002. p. 201–16. [Google Scholar]
- Solomon G, Ott S, Lovell M.. Long-term neurocognitive dysfunction in sports: what is the evidence? Clin Sports Med 2011; 30: 165–77. [DOI] [PubMed] [Google Scholar]
- Spanswick S, Sutherland R.. Object/context-specific memory deficits associated with loss of hippocampal granule cells after adrenalectomy in rats. Learn Mem 2010; 17: 241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struik D, Sanna F, Fattore L.. The modulating role of sex and anabolic-androgenic steroid hormones in cannabinoid sensitivity. Front Behav Neurosci 2018; 12: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliman N, Taib C, Moklas M, Basir R.. Delta-9-tetrahydrocannabinol (△9-THC) induce neurogenesis and improve cognitive performances of male Sprague Dawley rats. Neurotox Res 2018; 33: 402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Bai L, Niu X, Wang Z, Yin B, Bai G, et al. Elevated serum levels of inflammation-related cytokines in mild traumatic brain injury are associated with cognitive performance. Front Neurol 2019; 10: 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundman M, Doraiswamy P, Morey R.. Neuroimaging assessment of early and late neurobiological sequelae of traumatic brain injury: implications for CTE. Front Neurosci 2015; 9: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo E, Lebel A, Becerra L, Minster A, Linnman C, Maleki N, et al. The young brain and concussion: imaging as a biomarker for diagnosis and prognosis. Neurosci Behav Rev 2012; 36: 1510–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viano D, Hamberger A, Bolouri A, Saljo A.. Concussion in professional football: Animal model of brain injury—part 15. Neurosurgery 2009; 64: 1162–73. [DOI] [PubMed] [Google Scholar]
- Wendel K, Lee J, Affeldt B, Hamer M, Harahap-Carillo I, Pardo C, et al. Corpus callosum vasculature predicts white matter microsctructure abnormalities after pediatric mild traumatic brain injury. J Neurotrau 2019; 36: 152–64. doi: 10.1089/neu.2018.5670. [DOI] [PubMed] [Google Scholar]
- Whishaw I, Kolb B.. The behavior of the laboratory rat: a handbook with tests. New York, NY: Oxford University Press; 2005. [Google Scholar]
- Wright DK, O'Brien TJ, Mychasiuk R, Shultz SR.. Telomere length and diffusion MRI as biomarkers for experimental mild traumatic brain injury. Neuroimage Clin 2018; 18: 315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DK, O'Brien TJ, Shultz SR, Mychasiuk R.. Sex matters: repetitive mild traumatic brain injury in adolescent rats. Ann Clin Transl Neurol 2017; 4: 640–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhang Y, Mahmood A, Meng Y, Qu C, CHopp M.. Erythropoietin mediates neurobehavioral recovery and neurovascular remodeling following traumatic brain injury in rats by increasing expression of vascular endothelial growth factor. Transl Stroke Res 2011; 2: 619–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid G, Overstreet D, Zangen A.. Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res 2001; 896: 43–7. [DOI] [PubMed] [Google Scholar]
- Yamakawa G, Lengkeek C, Salberg S, Spanswick S, Mychasiuk R.. Behavioural and pathophysiological outcomes associated with caffeine consumption and repetitive mild traumatic brain injury (RmTBI) in adolescent rats. PLoS One 2017; 12: e0187218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Lorenzetti V, Suo C, Zalesky A, Fornito A, Takagi MJ, et al. Hippocampal harms, protection, and recovery following regular cannabis use. Transl Psychiatry 2016; 6: e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Sing D, Rugg C, Feeley B, Senter C.. The rise of concussions in the adolescent population. Orthop J Sports Med 2016; 4: 2325967116662458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Belcher M, van der Harst P.. Healthy aging and disease: role for telomere biology? Clin Sci 2011; 120: 427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S-y, Kittler J, Grigorenko EV, Kirby MT, Sim LJ, Hampson RE, et al. Effects of long-term exposure to delta9-THC on expression of cannabinoid receptor (CB1) mRNA in different brain regions. Mol Brain Res 1998; 62: 141–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request from the corresponding author.