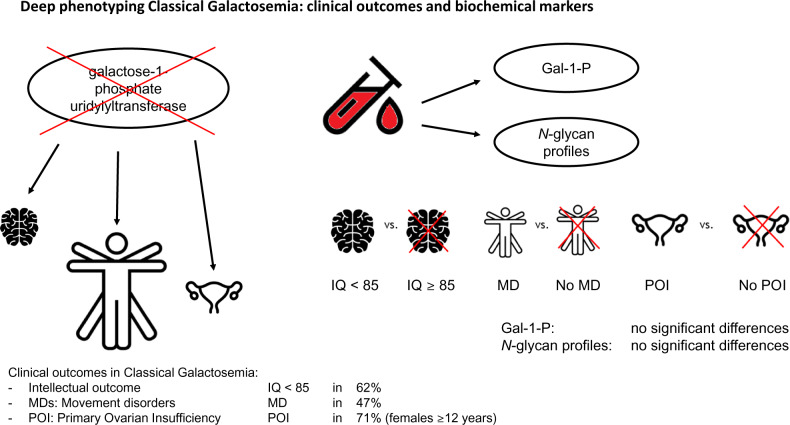
Abstract
Early diagnosis and dietary treatment do not prevent long-term complications, which mostly affect the central nervous system in classical galactosemia patients. The clinical outcome of patients is highly variable, and there is an urgent need for prognostic biomarkers. The aim of this study was first to increase knowledge on the natural history of classical galactosemia by studying a cohort of patients with varying geno- and phenotypes and second to study the association between clinical outcomes and two possible prognostic biomarkers. In addition, the association between abnormalities on brain MRI and clinical outcomes was investigated. Classical galactosemia patients visiting the galactosemia expertise outpatient clinic of the Amsterdam University Medical Centre were evaluated according to the International Classical Galactosemia guideline with the addition of an examination by a neurologist, serum immunoglobulin G N-glycan profiling and a brain MRI. The biomarkers of interest were galactose-1-phosphate levels and N-glycan profiles, and the clinical outcomes studied were intellectual outcome and the presence or absence of movement disorders and/or primary ovarian insufficiency. Data of 56 classical galactosemia patients are reported. The intellectual outcome ranged from 45 to 103 (mean 77 ± 14) and was <85 in 62%. Movement disorders were found in 17 (47%) of the 36 tested patients. In females aged 12 years and older, primary ovarian insufficiency was diagnosed in 12 (71%) of the 17 patients. Significant differences in N-glycan peaks were found between controls and patients. However, no significant differences in either N-glycans or galactose-1-phosphate levels were found between patients with a poor (intellectual outcome < 85) and normal intellectual outcome (intellectual outcome ≥ 85), and with or without movement disorders or primary ovarian insufficiency. The variant patients detected by newborn screening, with previously unknown geno- and phenotypes and currently no long-term complications, demonstrated significantly lower galactose-1-phospate levels than classical patients (P < 0.0005). Qualitative analysis of the MRI’s demonstrated brain abnormalities in 18 of the 21 patients, more severely in patients with a lower intellectual outcome and/or with movement disorders. This study demonstrates a large variability in clinical outcome, which varies from a below average intelligence, movement disorders and in females primary ovarian insufficiency to a normal clinical outcome. In our cohort of classical galactosemia patients, galactose-1-phosphate levels and N-glycan variations were not associated with clinical outcomes, but galactose-1-phosphate levels did differentiate between classical and variant patients detected by newborn screening. The correlation between brain abnormalities and clinical outcome should be further investigated by quantitative analysis of the MR images. The variability in clinical outcome necessitates individual and standardized evaluation of all classical galactosemia patients.
Keywords: classical galactosemia, GALT deficiency, clinical outcome, MRI, prognostic biomarkers
Patients with classical galactosemia frequently suffer from complications affecting the brain for which prognostic factors are lacking. In our cohort of classical galactosemia patients, we demonstrated a highly variable outcome, distinguished a subgroup of milder ‘variant patients’ and found an association between MRI abnormalities and lower intellectual outcomes and movement disorders.
Graphical Abstract
Graphical Abstract.
Introduction
Classical galactosemia (CG, OMIM 230400) is one of the more frequent inborn errors of metabolism caused by a severe deficiency of the enzyme galactose-1-phosphate uridylyltransferase (GALT, EC 2.7.7.12). In newborns with CG, the ingestion of galactose causes life-threatening illness. In the Netherlands, CG was implemented in the newborn screening (NBS) programme in 2007. Hereafter, 31 patients have been identified: 25 patients with a classical phenotype and 6 patients with previously unreported genotypes and phenotypes. Based on these findings, the incidence of CG is estimated to be 1:52 800 in the Netherlands (Welling et al., 2017b). Despite an early diagnosis by NBS and treatment with a galactose-restricted diet, patients are at risk to develop long-term complications of the central nervous system, such as abnormalities in motor and speech development, cognitive impairment and movement disorders (MDs) and ovarian insufficiency in females (Bosch, 2006; Hughes et al., 2009; Waisbren et al., 2012; Coss et al., 2013; Rubio-Gozalbo et al., 2019). There is a broad spectrum of clinical manifestations ranging from fully normal to severely impaired, even within families with identical mutations (Fridovich-Keil and Walter, 2008; Hughes et al., 2009). Unfortunately, at this time, the clinical outcome of individual patients cannot be predicted because prognostic biomarkers are lacking. This is a severe burden on parents and patients and hampers the development of new therapeutic options.
The pathophysiology of long-term complications is poorly understood. The endogenous production of significant amounts of galactose causes a persistent elevation of harmful metabolites, such as galactose-1-phosphate (Gal-1-P), which is considered a major factor (Berry et al., 1995; Holton, 1995; Fridovich-Keil and Walter, 2008). Gal-1-P has also been demonstrated to affect galactosylation (Lai et al., 2003), and N-glycan abnormalities are reported in CG patients (Fridovich-Keil and Walter, 2008; Coman et al., 2010; Coss et al., 2012). Remarkably, galactosylation abnormalities were seen both after galactose intoxication and galactose over restriction and improvement in galactosylation patterns after moderate dietary galactose relaxation could indicate that dysregulated glycosylation pathways are modifiable (Coman et al., 2010; Coss et al., 2012; Knerr et al., 2015). We hypothesize that individual differences in the extent of galactose intoxication and galactosylation abnormalities may contribute to the variability of the clinical outcome spectrum observed in CG patients. Previous studies suggested two possible predictors of clinical outcome. First, an association between Gal-1-P levels and long-term outcome in general and verbal dyspraxia in particular has been found (Robertson et al., 2000; Webb et al., 2003; Yuzyuk et al., 2018). Second, differences in glycosylation patterns of immunoglobulin G (IgG) N-glycans were found between patients and controls (Coman et al., 2010; Coss et al., 2014; Maratha et al., 2016; Stockmann et al., 2016) linked to specific glycan synthesis gene abnormalities with a proposed correlation with intellectual outcome (IQ) (Maratha et al., 2016). Also, abnormal IgG N-glycosylation, as well as both inflammatory and glycosylation gene expression in CG patients, has been correlated with fertility endocrine markers in female CG patients with ovarian insufficiency (Colhoun et al., 2018a).
The aim of this study was first to gain knowledge on the natural history of CG by performing deep phenotyping in a relatively large cohort of patients, who are assessed according to the international guideline (Welling et al., 2017a) and second to study the association between two possible predictors of clinical outcome, Gal-1-P levels and IgG N-glycan abnormalities, and long-term complications. The effect of genotype and an early initiation of treatment were also investigated, as well as the association between abnormalities on MRI and clinical outcome.
Materials and methods
Study design and recruitment
In this cohort study, clinical data were retrieved from the medical records of CG patients who visited the multidisciplinary galactosemia outpatient clinic in the Amsterdam University Medical Centre (Amsterdam UMC) or who are treated in other metabolic centres and participated in research in the Amsterdam UMC. All included patients consented to the use of their data for research purposes. Data on intellectual and neurological functioning were collected prospectively based on a predetermined standardized assessment. Serum samples, stored in the Amsterdam UMC Galactosemia Biobank after informed consent of patients and/or parents, were used to measure IgG N-glycans. The MRI was performed for research purposes, and informed consent was obtained from all included patients. The use of the serum samples and the MRI study was approved by the local institutional review board, and a waiver was given for the data collection.
Data storage
All data were stored in an electronic clinical report form in Castor EDC, a good clinical practice compliant data management system (Castor Electronic Data Capture, The Netherlands, 2018).
Inclusion and exclusion criteria
Patients with an erythrocyte GALT activity <15% of the reference mean (Shin-Bühring et al., 1976) and/or two known pathogenic variations in the GALT gene were eligible for participation in this study (Welling et al., 2017a). Patients with a second genetic diagnosis influencing clinical outcome were excluded.
Patient groups
For analyses, patients were divided into three groups:
Classical phenotypes: patients with two pathogenic GALT gene mutations with an erythrocyte GALT enzyme activity below the limit of quantitation of the enzyme assay (<3.3%; <1.1 μmol/h.g Hb). Patients with a classical phenotype, hereafter classical patients, can be divided into screened patients [detected after NBS or family screening (FS) because of an older sibling with CG with dietary treatment started immediately after birth] and non-screened patients (diagnosed before the implementation of NBS, after a clinical presentation with CG-related symptoms).
Variant patients: patients detected since the introduction of NBS in 2007 (Welling et al., 2017b). These patients with previously unknown genotypes and phenotypes were asymptomatic at the time of diagnosis and have residual GALT enzyme activities up to 10% in erythrocytes and erythrocyte Gal-1-P levels below the detection limit (<0.05 μmol/g Hb) under treatment.
Homozygous p. Ser135Leu patients: patients with GALT deficiency in erythrocytes but residual GALT enzyme activity in other tissues that may improve clinical outcome (Lai et al., 1996).
Clinical outcome measures
Patients were assessed according to the International CG guideline (Welling et al., 2017a). In patients who received a standardized age-specific intelligence test, the IQ was used as derivative of intelligence (Welsink-Karssies et al., 2019). The used intelligence tests were the Bayley Scales of Infant and Toddler Development, the Wechsler Preschool and Primary Scale of Intelligence, the Wechsler Intelligence Scale for Children and the Wechsler Adult Intelligence Scale. A poor IQ was defined as an IQ of <85, and a normal IQ was defined as an IQ of ≥85. Adult patients of whom no IQ was available and who were unable to live independently due to cognitive impairment were considered to have a poor IQ.
The neurological examination to assess the presence or absence of MDs and standardized screening for tremors (Fahn–Tolosa–Marin Clinical Rating Scale for Tremor, scores ranging from 0 to 84) (Fahn et al., 1993) and dystonia (Fahn–Marsden Rating Scale, scores ranging from 0 to 120) (Burke et al., 1985) were performed by one (paediatric) neurologist (M.E.) and documented for all patients. Since the rating scales are not validated in children, scores of paediatric patients <12 years were not reported in this article.
Data on speech development and the development of gross and fine motor skills reported by the treating physician were retrieved from the medical records. Information on the presence or absence of primary ovarian insufficiency (POI) was retrieved from the medical records of female patients.
Gal-1-P measurement in erythrocytes
Before studying the association between Gal-1-P and clinical outcomes, we evaluated the stability of erythrocyte Gal-1-P (hereafter Gal-1-P) in our cohort of classical patients. We found relatively stable Gal-1-P levels from the age of 12 months (Supplementary Fig. 1), and therefore, measurements performed before the age of 12 months were excluded. The most recent Gal-1-P levels reported in this article were measured by gas chromatography–mass spectrometry and were <0.82 μmol/g Hb in diet adherent patients. To evaluate Gal-1-P as a prognostic biomarker, only dietary adherent patients were included. Considering that the most recent Gal-1-P levels of the classical patients were within a narrow range, lifetime Gal-1-P was also evaluated in this subgroup.
The method to measure Gal-1-P changed from spectrophotometry (Gitzelmann, 1969) to gas chromatography–mass spectrometry in September 2016; therefore, lifetime Gal-1-P was defined as the mean of all Gal-1-P levels measured between 12 months of age until September 2016. Patients with a minimum of five measurements were included in the analysis. The Gal-1-P levels measured by spectrophotometry were <0.58 μmol/g Hb in diet adherent patients.
IgG N-glycan measurement
Analysis of serum IgG N-glycans was performed by the National Institute for Bioprocessing Research and Training (Dublin, Ireland) using automated glycan preparation method linked to ultra-performance liquid chromatography as previously described (Colhoun et al., 2018b). The percentage areas of 28 high-resolution IgG N-glycan peaks (GPs) were quantified in patients and healthy controls (Stockmann et al., 2016). Based on previous research, main glycans were assigned and N-glycan features were calculated including agalactosylated (G0), monogalactosylated (G1) and digalactosylated (G2) structures, resulting G ratios G0/G1, G0/G2 as well as core fucosylated bisected neutral glycans, afucosylated bisected neutral glycans and total bisected glycans (Pucic et al., 2011; Stockmann et al., 2013; Maratha et al., 2016). The results of the adult CG patients were compared with the 79 adult control samples, previously reported by Maratha et al. (2016), and the results of the paediatric CG patients in our cohort were compared with nine paediatric control samples, previously reported by Coss et al. (2014).
MRI protocol
An open-bore 3.0-T MRI scanner (Tesla Philips Ingenia scanner) with a 32-channel head coil was used to perform MRI of the brain. The MRI protocol included three-dimensional T1-weighted and fluid-attenuated inversion recovery sequences with isotropic voxels.
Image analysis
All brain MR images were scored by one neuro-radiologist (S.D.R.) blinded for the clinical outcome of patients. The MR images were evaluated for focal white matter abnormalities and abnormalities of the basal ganglia, thalamus and cortex. The Fazekas scale was used to quantify white matter abnormalities. Cerebellar atrophy was assessed in the cerebellum and vermis. Cerebral atrophy was scored with the use of the global cortical atrophy (GCA) scale. White matter abnormalities and atrophy were scored if the MR images deviated from the existing age-based standards.
Statistical analysis
SPSS version 25 (SPSS Inc. Chicago, IL, USA) was used to perform all statistical analyses. Median and ranges were presented since data followed a non-normal distribution. Descriptive statistics of patients and MR images were reported. To determine if there were statistically significant differences in continuous variables and proportions between two groups, the Mann–Whitney U-test and chi-square tests (or Fisher’s exact test) were used, respectively. Considering the differences between the N-glycans profiles of children and adults (Pucic et al., 2012), both groups were analysed separately. The Spearman’s rank coefficient test was used to test for associations, and in case of a significant association, linear regression or logistic regression was used where appropriate to test for correlations. P-values <0.05 were considered statistically significant. If multiple tests were carried out regarding a single hypothesis, the results were corrected using the Bonferroni–Holm method.
Data availability
The data that support the findings of this study are available upon reasonable request from the corresponding author.
Results
In total, 70 out of 77 CG patients visiting the Amsterdam UMC consented to the use of their clinical data. Four patients were excluded because of a second diagnosis influencing clinical outcome, and 10 patients were excluded because of missing data. Demographics and clinical outcome data of 56 patients are presented in Table 1 on an individual level and in Table 2 on a group level.
Table 1.
Overview of included patients, demographics and clinical outcome
| Pt ID | Gender | Group | GALT_1/GALT_2 | GALT activity, % | IQ | Tremor/ TRS | Dystonia/ DRS | POI | BMD Z-score ≤ −2 SD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Non-screened | p.Gln188Arg/400delT | <3.3 | – | – | – | NA | No |
| 2 | F | Non-screened |
p.Gln188Arg/p.Lys285Asn p.Lus285Asn |
<3.3 | 77 | – | – | No | – |
| 3 | M | Non-screened | p.Gln188Arg/p.Ser135Trp | <3.3 | >85 | – | – | NA | – |
| 4 | M | Non-screened | −/− | <3.3 | – | – | – | NA | – |
| 5 | F | Non-screened | p.Ser135Trp/p.Arg51Gln | <3.3 | 78 | – | – | Yes | Yes |
| 6 | M | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | – | Yes/7 | No/− | NA | – |
| 7 | F | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | 78 | – | – | Yes | No |
| 8 | F | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | – | Yes/− | No/− | Yes | No |
| 9 | M | Non-screened | p.Gln188Arg/p.Lys127Glu | <3.3 | 70 | – | – | NA | – |
| 10 | M | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | 74 | – | – | NA | No |
| 11 | M | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | 45 | Yes/38 | Yes/6 | NA | No |
| 12d | F | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | 81 | Yes/4 | No/0 | Yes | No |
| 13 | M | Non-screened | c.329-2A>C/c.329-2A>C | <3.3 | – | Yes/− | No/− | NA | Yes |
| 14 | F | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | 83 | – | – | Yes | No |
| 15 | F | Non-screened | p.Gln188Arg/p.Ser135Trp | <3.3 | 57 | – | – | Yes | No |
| 16 | F | Non-screened | p.Gln188Arg/p.Gln188Arg | – | 53 | No/4 | No/0 | Yes | No |
| 17 | F | Non-screened | c.329-2A>C/c.329-2A>C | <3.3 | – | No/− | No/− | Yes | No |
| 18 | F | Non-screened | p.Ser135Leu/p.Ser135Leu | <3.3 | 71 | No/1 | No/0 | No | – |
| 19 | F | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | 82 | No/0 | No/0 | ? | No |
| 20c | F | Non-screened | p.Gln188Arg/p.Leu195Pro | <3.3 | 88 | Yes/5 | No/0 | Yes | No |
| 21 | F | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | 71 | – | – | ? | No |
| 22 | M | Non-screened | p.Gln188Arg/p.Gln188Arg | – | 91 | – | – | NA | No |
| 23 | M | Non-screened | p.Gln188Arg/p.Lys195Pro | <3.3 | 93 | No/0 | No/0 | NA | No |
| 24 | M | Non-screened | p.Gln188Arg/p.Ser135Trp | <3.3 | 98 | No/0 | No/0 | NA | No |
| 25 | F | Non-screened | p.Arg205*/p.Trp316* | <3.3 | 97 | – | – | No | Yes |
| 26a | M | Non-screened | p.Gln188Arg/p.Lys285Asn | <3.3 | 76 | Yes/7 | No/0 | NA | No |
| 27a | M | Non-screened | p.Gln188Arg/p.Lys285Asn | <3.3 | 86 | Yes/6 | No/0 | NA | No |
| 28 | F | Non-screened | p.Ser135Leu/p.Ser135Leu | 3.9 | 61 | No/0 | No/0 | No | No |
| 29 | M | Non-screened | p.Gln188Arg/p.Ser135Trp | 3.9 | 65 | Yes/6 | No/0 | NA | No |
| 30e | M | Non-screened | p.Gln188Arg/c.377 + 7A>C | <3.3 | 87 | No/3 | No/0 | NA | No |
| 31 | M | Non-screened | p.Gln188Arg/p.Lys285Asn | <3.3 | 49 | Yes/29 | Yes/4 | NA | No |
| 32 | M | Non-screened | p.Gln188Arg/p.Leu195Pro | <3.3 | 103 | Yes/8 | No/0 | NA | No |
| 33b | F | Non-screened | p.Arg148Gln/p.Trp316* | <3.3 | 88 | Yes/13 | No/0 | ? | No |
| 34 | F | Non-screened | p.Gln188Arg/p.Gln188Arg | <3.3 | 46 | Yes/− | No/− | – | No |
| 35f | F | Screened, FS | p.Gln188Arg/p.Gln188Arg | <3.3 | 77 | Yes/4 | No/0 | Yes | No |
| 36 | M | Screened, FS | p.Gln188Arg/p.Lys127Glu | <3.3 | 61 | – | – | NA | No |
| 37 | F | Screened, FS | p.Gln188Arg/p.Arg148Gln | <3.3 | – | – | – | No | – |
| 38f | F | Screened, FS | p.Gln188Arg/p.Gln188Arg | <3.3 | 71 | – | – | Yes | No |
| 39d | F | Screened, FS | p.Gln188Arg/p.Gln188Arg | <3.3 | 61 | Yes/34 | Yes/1 | Yes | Yes |
| 40c | F | Screened, FS | p.Gln188Arg/p.Leu195Pro | <3.3 | 52 | Yes/10 | Yes/2 | ? | – |
| 41e* | M | Screened, FS | p.Gln188Arg/c.377 + 7A>C | <3.3 | 95 | – | – | NA | Yes |
| 42b* | M | Screened, FS | p.Arg148Gln/p.Trp316* | <3.3 | 68 | Yes/− | No/− | NA | No |
| 43* | F | Screened, NBS | p.Gln188Arg/p.Leu195Pro | <3.3 | 82 | No/− | No/− | – | – |
| 44* | F | Screened, NBS | p.Gln188Arg/p.Gln188Arg | <3.3 | 64 | No/− | No/− | – | – |
| 45* | M | Screened, NBS | p.Gln188Arg/p.Gln188Arg | <3.3 | 89 | No/− | No/− | NA | – |
| 46* | M | Screened, NBS | p.Gln188Arg/p.Gln188Arg | <3.3 | 64 | No/− | No/− | NA | – |
| 47* | F | Screened, NBS | p.Gln188Arg/p.Gln188Arg | <3.3 | 70 | No/− | No/− | – | – |
| 48* | M | Screened, NBS | p.Ser135Leu/p.*380Argext*50 | <3.3 | 95 | No/− | No/− | NA | – |
| 49* | F | Screened, NBS | p.Gln188Arg/p.Gln188Arg | <3.3 | 83 | – | – | – | – |
| 50g* | F | Variant | p.Val128lle/p.Val128lle | 8.7 | 86 | No/− | No/− | – | – |
| 51g* | F | Variant | p.Val128lle/p.Val128lle | 8.7 | 89 | No/− | No/− | – | – |
| 52* | M | Variant | p.Gln188Arg/p.Met219Lys | 7.2 | 96 | No/− | No/− | NA | – |
| 53* | F | Variant | p.Gln188Arg/c.1-96T>G | 3.6 | 86 | No/− | No/− | – | – |
| 54g* | M | Variant | p.Val128lle/p.Val128lle | 9.3 | 91 | No/− | No/− | NA | – |
| 55* | M | Variant | p.Arg201His/p.Arg201His | 8.9 | – | – | – | NA | – |
| 56g* | F | Variant | p.Val128lle/p.Val128lle | 6.3 | – | – | – | – | – |
*Age <12 years.
Pt ID: patient ID; F: female; M: male; FS: family screening; IQ: intelligence quotient; TRS: tremor rating scale; DRS: dystonia rating scale; BMD: bone mineral density; NA: not applicable; -: data not available; ?: data unclear; a, b, c, d, e, f: sibs, patients are ordered by group and listed by decreasing age; grey shades: age <12 years; bold: patients diagnosed late.
Table 2.
Patient groups and clinical outcomes
| n | All patients (n = 56) | n | Classical patients (n = 47) | n | Variant patients (n = 7) | n | Homozygous p.Ser135Leu (n = 2) | |
|---|---|---|---|---|---|---|---|---|
| Gender | 56 | 47 | ||||||
| Male | 26 | 23 | 3 | |||||
| Female | 30 | 24 | 4 | 2 | ||||
| Age (years) | 56 | 18.0 (0–48) | 47 | 21.0 (4–48) | 7 | 5.0 (0–7) | 2 | 16/23 |
| Paediatric patients (<18 years) | 27 | 19 | 7 | 1 | ||||
| Adult patients (≥18 years) | 29 | 28 | 1 | |||||
| GALT activity (%) | 54 | 45 | 7 | 2 | ||||
| <3.3 | 45 | 44 | 1 | |||||
| ≥3.3 | 9 | 1 | 7 | 1 | ||||
| IQ | 47 | 78 (45–103) | 40 | 77 (45–103) | 5 | 89 (86–96) | 2 | 61/71 |
| IQ < 85 | 29 | 27 | 2 | |||||
| IQ ≥ 85 | 18 | 13 | 5 | |||||
| Neurology | 36 | 29 | 5 | 2 | ||||
| MDs, no | 19 | 12 | 5 | 2 | ||||
| MDs, yes | 17 | 17 | ||||||
| TRS | 20 | 5.5 (0–38) | 18 | 6.0 (0–38) | 2 | 0/1 | ||
| DRS | 20 | 0.0 (0–6) | 18 | 0.0 (0–6) | 2 | 0/0 | ||
| Endocrinology (females ≥12 years) | 21 | 19 | 2 | |||||
| Puberty induced, no | 11 | 9 | 2 | |||||
| Puberty induced, yes | 8 | 8 | ||||||
| POI, no | 5 | 3 | 2 | |||||
| POI, yes | 12 | 12 | ||||||
| Gal-1-P (μmol/g Hb) in erythrocytesa | 50 | 0.35 (0.0–0.70) | 43 | 0.37 (0.16–0.70) | 7 | 0.0 (0–0.13) | 2 | 0.14/0.13 |
| Galactitol (mmol/mol creatinine) in urinea | 34 | 120 (0–311) | 25 | 140 (109–311) | 7 | 10 (0.0–97) | 2 | 69/58 |
Data reported in median and ranges.
Most recent value is presented.
IQ: intelligence quotient; TRS: tremor rating scale; DRS: dystonia rating scale.
Previously unreported mutations detected since the implementation of NBS (Welling et al., 2017b) were found in 7 of the 55 patients, and the homozygous p. Ser135Leu mutation was found in 2 of the 55 patients (Table 1). In 9 of the 54 patients, erythrocyte GALT enzyme activity was above the limit of quantitation of the enzyme assay (>3.3%; >1.1 μmol/h.g Hb) and ranged from 3.6% to 9.3%.
Diagnosis and dietary treatment
Data on diagnosis were available in 50 patients. Twenty-five patients were diagnosed in the first six weeks of life because of CG-related symptoms, 12 patients were diagnosed after NBS and 10 patients were diagnosed after family screening. In 9 of the 10 family screening diagnosed patients, no CG-related symptoms were reported in the newborn period and data were missing in one patient. Three patients were diagnosed late (Table 1): two homozygous p. Ser135Leu patients were diagnosed at age 7 months and 10 years because of feeding difficulties and cataract and because of visual impairment due to cataract, respectively. The third patient was diagnosed at age 9 months after a diagnostic delay, and information regarding CG-related symptoms is missing.
In 32 of the 50 patients, of whom 7 patients were diagnosed by NBS, CG-related symptoms were reported at diagnosis. The most frequently reported symptoms were jaundice (81%), elevated liver enzymes (58%), clinical suspicion of sepsis (52%, positive blood culture 35%), feeding difficulties (45%), vomiting (33%) and coagulopathy (30%). In the non-screened patients diagnosed because of critical illness (with the exclusion of the three late diagnosed patients), the lactose free, galactose-restricted diet was started at the median age of 10 days (4–39) with the confirmation of diagnosis at a median age of 11 days (4–46). In patients detected by NBS, the diet was started at a median age of 7 days (5–8) with the confirmation of diagnosis at a median age of 8 days (6–11).
In 52 of the 56 patients, strict dietary adherence was reported, while 4 of the 56 patients did not adhere to the diet in the past or at the most recent visit.
Deep phenotyping: the clinical outcome spectrum
Motor and speech development
The development of gross and fine motor skills was abnormal in 9 (21%) of the 43 and 5 (12%) of the 41 patients with available data, respectively. Speech and language development was abnormal in 25 (58%) of the 43 patients, with a language delay in 11 (44%) of the 25 patients, a speech defect in 4 (16%) of the 25 patients and a combination of both in 7 (28%) of the 25 patients. In three (12%) patients, the speech and language development problems were not further specified. The frequency of abnormal motor, speech and language development did not differ between classical non-screened and classical screened patients.
Ophthalmology
In 12 of the 49 patients, cataract was present at the most recent ophthalmic evaluation, 11 of the 49 had cataract in the past, which had resolved, and 26 of the 49 patients have never been diagnosed with cataract. The most frequently reported description was stable, nuclear cataract without visual impairment.
General intelligence
In 47 patients, IQ testing was performed. As a group, the included patients demonstrated an overall below average but highly variable intelligence with the IQ ranging between 45 and 103 (mean 78, 14 SD). Three adult patients were unable to live independently due to cognitive impairment. A total of 31 patients had a poor IQ, and 19 patients had a normal IQ. There was no significant difference in IQ between classical non-screened and classical screened patients (Table 3).
Table 3.
Clinical outcomes of non-screened and screened classical patients
| N | Classical non-screened patients (n = 32) | n | Classical screened patients (n = 15) | P-value | |
|---|---|---|---|---|---|
| Gender | 32 | 15 | |||
| Male | 17 | 6 | 0.534 | ||
| Female | 15 | 9 | |||
| Age (years) | 32 | 24.5 (11–48) | 15 | 10.0 (4–32) | 0.002 |
| Paediatric patients (<18 years) | 9 | 10 | 0.024 | ||
| Adult patients (≥18 years) | 23 | 5 | |||
| GALT activity (%) | 30 | 15 | |||
| <3.3 | 29 | 15 | 1.000 | ||
| ≥3.3 | 1 | ||||
| IQ | 26 | 78.0 (45–103) | 14 | 71.0 (52–95) | 0.421 |
| IQ < 85 | 16 | 10 | 1.000 | ||
| Q ≥ 85 | 10 | 4 | |||
| Neurology | 19 | 10 | |||
| MDs, no | 6 | 6 | 0.236 | ||
| Ds, yes | 13 | 4 | |||
| TRS | 15 | 6.0 (0–38) | 3 | 10.0 (4–34) | 0.311 |
| DRS | 15 | 0.0 (0–6) | 3 | 1.0 (0–2) | 0.104 |
| Endocrinology (females ≥ 12 years) | 14 | 5 | |||
| Puberty induced, no | 7 | 2 | 0.620 | ||
| Puberty induced, yes | 5 | 3 | |||
| POI, no | 2 | 1 | 1.000 | ||
| POI, yes | 9 | 3 | |||
| Gal-1-P (μmol/g Hb) in erythrocytesa | 28 | 0.36 (0.24–0.62) | 15 | 0.40 (0.16–0.70) | 0.221 |
| Galactitol (mmol/mol creatinine) in urinea | 14 | 128 (109–168) | 11 | 175 (113–311) | 0.204 |
Data reported in median and ranges.
Most recent value is presented.
IQ: intelligence quotient; TRS: tremor rating scale; DRS: dystonia rating scale.
Bold numbers defining as statistically significant.
Neurology
In 36 of the 56 patients, an examination by a (paediatric) neurologist including a standardized screening for tremors and dystonia was performed at the outpatient clinic visit. Neurologic complaints were self-reported by 10 of the 36 patients (four adults) or their parents (six children) and ranged from a poor handwriting to symptoms interfering with activities in daily life. In 17 of the 36 (9/22 children, 8/14 adults), the neurologic examination was abnormal of whom 12 patients had a subtle tremor, 1 patient had an evident tremor and 4 patients demonstrated both an evident tremor and dystonia.
Of the 12 patients with a subtle tremor, 9 had an action tremor, 2 had a postural and action tremor and 1 patient had a postural and intention tremor. Of the five patients with an evident tremor, two patients had a combined action and postural tremor and two patients had an action tremor only and in one patient the nature of the tremor was not specified.
Twenty patients aged 12 years and older underwent standardized screening for tremors and dystonia by the previously described rating scales (Table 1).
Neurologic comorbidity was reported in patients with and without MDs. The most severely affected patient had a complex MD with a dystonic tremor and spastic paraparesis. This patient had a meningitis in the neonatal period and suffers from epileptic seizures (tonic–clonic and focal with impaired consciousness) treated with multiple anti-epileptic drugs. Neurological comorbidity was reported in two other patients with an MD [epilepsy (n = 1) and neonatal meningitis (n = 1)] and in two patients without an MD [traumatic brain injury leading to a skull fracture with increased intracerebral pressure (n = 1) and neonatal meningitis (n = 1)].
In patients with an MD, the motor development was abnormal in 42% and the speech development was abnormal in 75%, which is in contrast to patients without an MD in whom motor and speech development were abnormal in 16% and 38%, respectively.
The frequency of MDs did not differ between classical non-screened and classical screened patients (Table 3).
Magnetic Resonance Imaging
A total of 21 CG patients (8–47 years, 9 males and 12 females) underwent MRI of the brain, and in 18 of the 21 patients, brain abnormalities were found (Table 4).
Table 4.
Brain MRI abnormalities in patients
| Pt ID | Fazekas | Cerebellum | Vermis | Cerebrala | Frontala | Parietala | Temporala | Occipitala | IQ | MD |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2 | + | + | 1–2 | 2 | 2 | 1 | 1 | 77 | – |
| 5 | 1 | + | + | 1 | 1 | 2 | 0 | 1 | 78 | – |
| 7 | 1 | ± | ± | 1 | 1 | 1 | 1 | 0 | 78 | – |
| 35 | 1 | − | − | 0 | 0 | 1 | 0 | 0 | 77 | Yes |
| 36 | 0 | − | + | 1 | 1 | 1 | 1 | 1 | 61 | – |
| 12 | 0 | + | + | 0–1 | 1 | 1 | 0 | 0 | 81 | Yes |
| 14 | 1 | − | + | 0 | 0 | 0 | 0 | 0 | 83 | – |
| 38 | 1 | − | ± | 0–1 | 1 | 1 | 0 | 0 | 71 | – |
| 39 | 1 | + | + | 1 | 1 | 1 | 1 | 1 | 61 | Yes |
| 19 | 0 | − | + | 0 | 0 | 1 | 0 | 0 | 82 | No |
| 20 | 0 | − | + | 0 | 0 | 1 | 0 | 0 | 88 | Yes |
| 24 | 0 | − | − | 0 | 0 | 0–1 | 0 | 0 | 98 | No |
| 22 | 1 | − | − | 0 | 0 | 1 | 0 | 0 | 91 | – |
| 23 | 1 | − | − | 0 | 0 | 0 | 0 | 0 | 93 | No |
| 40 | 0 | + | + | 0 | 0 | 1 | 0 | 0 | 52 | Yes |
| 28 | 0 | − | + | 0 | 0 | 1 | 0 | 0 | 61 | No |
| 31 | 0 | ± | + | 0–1 | 1 | 1 | 0 | 0 | <50 | Yes |
| 42 | 0 | − | − | 0–1 | 1 | 1 | 0 | 0 | 68 | Yes |
GCA scale (scores ranging from 0 to 3 per region).
+: atrophy is present; −: atrophy is absent; ±: minor atrophy; IQ: intelligence quotient; -: data not available; Pt ID: patient ID corresponds with Table 1. Patients are listed by decreasing age.
The supratentorial white matter appeared normal in 12 patients (median age 17.0 years, 8–31). In 9 of the 21 patients (median age 26.0 years, 18–47), focal deep white matter abnormalities were found, most frequently in the frontoparietal region, with confluent white matter abnormalities in 1 patient. In none of the patients, focal abnormalities were found in the infratentorial white matter, basal ganglia, thalamus and cortex.
In 4 patients, no atrophy was detected, 12 patients demonstrated both cerebral and cerebellar atrophy, 4 patients demonstrated cerebral atrophy only and 1 patient demonstrated cerebellar atrophy only. Cerebral atrophy varied from minimal atrophy in one region of the brain to atrophy in multiple regions, up to a GCA score of 2 per region. Parietal (16/17) and frontal (9/17) were the most affected cerebral regions, and the vermis was the most affected cerebellar region (13/17).
All patients who underwent an MRI received IQ testing. The median IQ was 78 (49–98). Six of the 21 patients had a normal IQ (IQ ≥ 85). Thirteen out of 21 patients were examined by a neurologist, who diagnosed an MD in eight patients.
The presence of white matter abnormalities was not associated with IQ nor MDs.
Patients with a poor IQ (IQ < 85) had significantly more cerebral atrophy (higher GCA scores) than patients with a normal IQ (P = 0.011). The IQ (as continuous measure) was associated with cerebral atrophy (the GCA score) (P = 0.014). The presence of cerebellar atrophy was significantly higher in patients with a poor IQ (P = 0.014), and the IQ (as a continuous measure) was associated with cerebellar atrophy (P = 0.028).
MDs were associated with GCA scores (P = 0.041), and patients with MDs demonstrated significantly higher GCA scores than patients without MDs (P = 0.048). Patients with MDs did not demonstrate a higher frequency of cerebellar atrophy.
The frequency of MRI abnormalities (white matter abnormalities, cerebral and cerebellar atrophy) did not differ between classical non-screened and classical screened patients.
Endocrinology
Of 21 out of 30 females aged 12 years and older at the time of data collection, data on the endocrinological outcome were available. During the most recent visit, the menarche had occurred in all patients. The median age at puberty induction (n = 8) was 12 years (11–15 years) with the menarche at a median age of 15 years (12–17 years), while a spontaneous menarche (n = 11) occurred at a median age of 13.5 years (12–16 years). The diagnosis POI was reported in 12 of the 17 females and is uncertain in four. All females with POI received hormone replacement therapy at the most recent visit. Both p. Ser135Leu homozygous patients had a normal puberty development and have no POI. The frequency of POI did not differ between classical non-screened and classical screened patients (Table 3).
Bone health
In 36 of the 56 patients, the results of a dual-energy X-ray absorptiometry scan were available. In total, 3 of the 17 children (18%) and 2 of the 19 adults (11%) had a bone mineral density ≤−2 SD at the most recent dual-energy X-ray absorptiometry scan. The Z-scores of the femoral neck and lumbar spine did not significantly differ between males and females nor between children and adults.
A history of fractures was reported in 7 of the 30 patients. In these patients, the bone mineral density was ≤−2 SD in two patients and normal in three patients and two patients were too young to undergo a dual-energy X-ray absorptiometry scan. All reported fractures were preceded by a trauma.
NBS-detected variant patients
A recently identified group of patients present in this cohort are the seven NBS-detected variant patients (Tables 1 and 2) of whom four are siblings. Six variant patients have erythrocyte Gal-1-P levels below the detection limit (<0.05 μmol/g Hb) and normal urine galactitol levels on a galactose-restricted diet, while one patient with a residual erythrocyte GALT activity of 3.6% (just above the limit of quantitation) demonstrated variable Gal-1-P levels ranging from below the detection limit to 0.13 μmol/g Hb as the highest measured level and galactitol levels above the normal range. The most recent Gal-1-P and galactitol levels were significantly lower in the variant patients when compared with the classical patients (P < 0.0005).
In the four patients with the highest GALT enzyme activities (8.7–9.3%) and normal metabolites on a galactose-restricted diet, the diet was relaxed to a maximum daily allowance of 1200 mg galactose (50 ml dairy product) in three patients and 2400 mg galactose (100 ml dairy product) in one patient. The dietary relaxation did not increase the Gal-1-P or galactitol levels in any of these patients.
The assessment of two variant patients was limited due to their young age (4 and 23 months). In the assessed patients, the development of gross and fine motor skills was normal. The speech development was normal in all but one patient, who suffered from persistent adenoiditis for whom two adenoidectomies were required in the first 2 years of life. Currently, none of the variant patients demonstrates long-term complications.
Homozygous p. Ser135Leu patients
In spite of a late diagnosis and late onset of a galactose-restricted diet, both patients do not demonstrate POI or MDs. However, both patients demonstrated a poor IQ with an IQ well below 85. Both patients have lower Gal-1-P and galactitol levels than classical patients (Table 1) and at times Gal-1-P levels even below the detection limit despite dietary incompliance in one patient.
Possible predictors of clinical outcome
The most recent Gal-1-P level
The most recent Gal-1-P level measured by gas chromatography–mass spectrometry was available in 50 patients. There were no significant differences in Gal-1-P levels between children and adults nor between males and females. The Gal-1-P levels between patients with a poor and normal IQ and with and without MDs and POI were not significantly different. Linear regression analysis indicated that Gal-1-P was not a significant predictor for IQ (P = 0.90). Logistic regression to evaluate if Gal-1-P was able to predict POI and MDs was considered unreliable due to broad confidence intervals.
In the screened patient group, patients with a normal IQ (n = 9) had significantly lower Gal-1-P levels than patients with a poor IQ (n = 10) (P = 0.017). This group includes the variant patients with higher GALT activities in erythrocytes and significantly lower Gal-1-P levels than the classical patients (P < 0.0005). There was a significant negative correlation between the Gal-1-P levels and erythrocyte GALT activity (P < 0.0005). In the screened classical patients, there was no significant difference in Gal-1-P levels between patients with a poor and normal IQ.
In the screened patient group, the Gal-1-P levels between patients with and without MDs and POI demonstrated no significant differences.
Lifetime Gal-1-P
The lifetime Gal-1-P level measured by spectrophotometry was available in 29 patients. The average number of Gal-1-P measurements per patient was 14 (5–59). The lifetime Gal-1-P levels between classical patients with a poor and normal IQ and with and without MDs and POI were not significantly different.
To investigate whether lifetime Gal-1-P was stable from 12 months, the association with age was tested, demonstrating a significant negative correlation between age and lifetime Gal-1-P (P = 0.043). The inclusion of patients with <5 Gal-1-P measurements did not change this finding.
N-glycan profiles
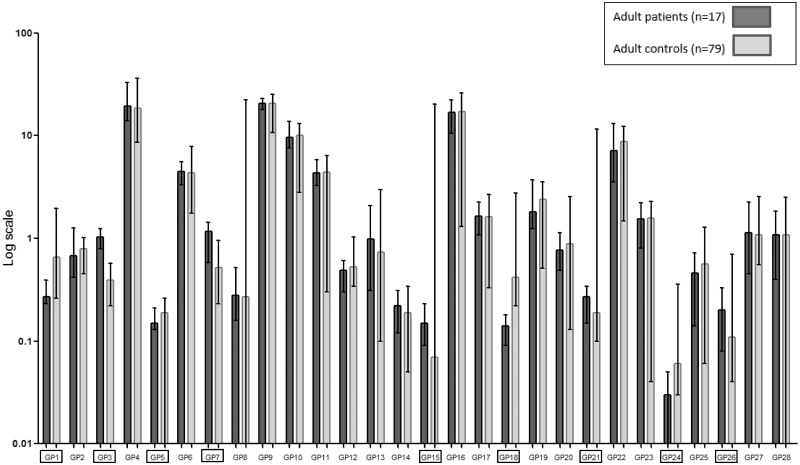
In total, 9 of the 28 GPs were significantly different when the samples of adult CG patients were compared with the control samples (P ≤ 0.001). From these nine GPs, a major trend emerges that the bisecting glycans increase in CG patients (GP3, GP21 and GP26, Fig. 1). Also, an increase in afucosylated bisected glycans was observed when compared with controls (Table 5 and Supplementary Fig. 2A). There were no significant differences in GPs, G ratios or IgG N-glycan features (core fucosylation and bisected GlcNAcylation) between adult patients with a poor and normal IQ and patients with or without MDs or POI (data not shown).
Figure 1.
IgG N-GPs adult patients and controls. The percentage areas of 28 IgG N-GPs, quantified in adult galactosemia patients and controls as described in Stockmann et al. (2016). Data reported in median (ranges) with error bars. ▭ Significantly different GPs (P≤0.001), as shown in Table 5.
Table 5.
N-glycans of adult patients
| CG patients (n = 17) | Controls (n = 79) | P-value | Main glycansa | |
|---|---|---|---|---|
| GPsb | ||||
| GP1c | 0.27 (0.23–0.39) | 0.66 (0.26–1.95) | <0.0005 | FA1 |
| GP3d | 1.04 (0.79–1.25) | 0.39 (0.22–0.57) | <0.0005 | A2B |
| GP5c | 0.15 (0.13–0.21) | 0.19 (0.01–0.26) | 0.001 | M5 |
| GP7d | 1.17 (0.58–1.44) | 0.52 (0.23–0.95) | <0.0005 | A2[3]G1 |
| GP15d | 0.15 (0.09–0.23) | 0.07 (0.00–20.25) | <0.0005 | FA2G2 |
| GP18c | 0.14 (0.09–0.18) | 0.42 (0.22–2.75) | <0.0005 | FA2G1S1 |
| GP21d | 0.27 (0.15–0.34) | 0.19 (0.10–11.50) | <0.0005 | A2BG2S1 |
| GP24c | 0.03 (0.01–0.05) | 0.06 (0.03–0.36) | <0.0005 | ? |
| GP26d | 0.20 (0.08–0.33) | 0.11 (0.04–0.70) | <0.0005 | A2BG2S2 |
| Glycan featuresa | ||||
| Bn | 1.57 (1.19–1.69) | 0.85 (0.44–22.50) | <0.0005 | Afucosylated neutral glycans |
Data reported in median and ranges. Only significant differences are shown.
Main glycans were assigned and N-glycan features calculated as described in Pucic et al. (2011).
The percentage areas of 28 IgG N-GPs, quantified as described in Stockmann et al. (2016).
cDecreased in CG patients (when compared with controls).
dIncreased in CG patients (when compared with controls).
Bn: afucosylated bisected neutral glycans; ?: unknown.
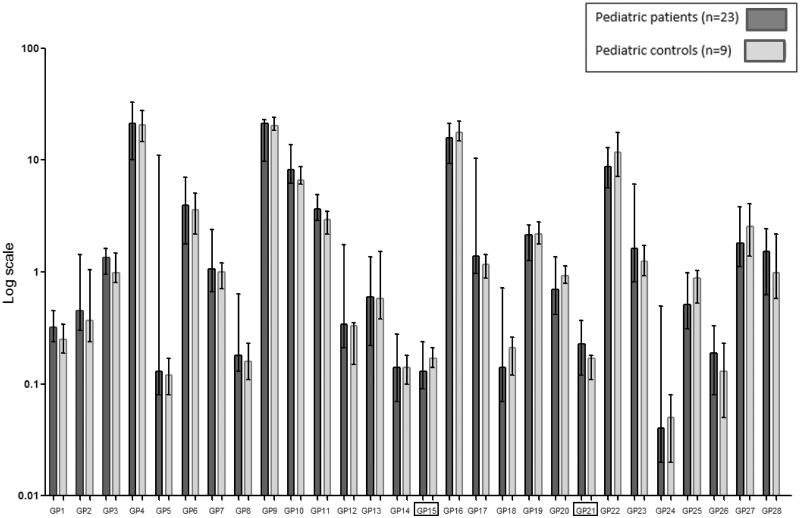
In the paediatric patients, GP15 (containing core fucosylated biantennary digalactosylated glycan FA2G2 as the major glycan) was significantly decreased and GP21 (containing bisected digalactosylated monosialylated glycan A2BG2S1 as the major glycan) was significantly increased when compared with the nine control samples (P ≤ 0.001, Fig. 2, individual data shown in Supplementary Fig. 3A and B). The core fucosylated bisected neutral glycans, afucosylated bisected neutral glycans and total bisected glycans were significantly higher in paediatric patients when compared with controls (Table 6 and Supplementary Fig. 2B). There were no significant differences in GPs or G ratios between paediatric patients with a poor and normal IQ and with and without MDs. Both core fucosylated bisected neutral glycans and total bisected glycans were significantly higher in paediatric patients with MDs (Supplementary Table 1). The exclusion of the variant patients and homozygous p. Ser135Leu patients did not change any of these results.
Figure 2.
IgG N-GPs paediatric patients and controls. The percentage areas of 28 IgG N-GPs, quantified in paediatric galactosemia patients and controls as described in Stockmann et al. (2016). Data reported in median (ranges) with error bars. ▭ Significantly different GPs (P≤0.001), as shown in Table 6. Individual data shown in Supplementary Fig. 3A and B.
Table 6.
N-glycans of paediatric patients
| CG patients (n = 23) | Controls (n = 9) | P-value | Main glycansa | |
|---|---|---|---|---|
| GPsb | ||||
| GP15c | 0.13 (0.09–0.24) | 0.17 (0.14–0.21) | 0.001 | FA2G2 |
| GP21c | 0.23 (0.12–0.37) | 0.17 (0.11–0.18) | <0.0005 | A2BG2S1 |
| Glycan featuresa | ||||
| FBnc | 9.55 (7.40–18.80) | 8.31 (5.72–10.25) | 0.022 | Core fucosylated bisected glycans |
| Bnc | 1.66 (1.33–2.38) | 1.25 (1.09–1.73) | 0.002 | Afucosylated neutral glycans |
| Bc | 14.78 (11.35–29.49) | 12.32 (9.06–13.93) | 0.001 | Total bisected glycans |
Data reported in median and ranges. Only significant differences are shown.
Main glycans were assigned and N-glycan features calculated as described in Pucic et al. (2011).
The percentage areas of 28 IgG N-GPs, quantified as described in Stockmann et al. (2016), Coss et al. (2016).
cDecreased in CG patients (when compared with controls).
dIncreased in CG patients (when compared with controls).
FBn: core fucosylated bisected neutral glycans; FBn: GP6+11+12+17; Bn: afucosylated bisected neutral glycans; Bn: GP3+8+14; B: total bisected glycans; B: GP3+6+8+11+12+14+17+21+23+26+28.
Discussion
The aim of this cohort study, which includes >30% of all Dutch CG patients, was to perform deep phenotyping and to investigate the association between long-term complications and possible predictors of clinical outcome. The results of our study reflect the broad clinical outcome spectrum of CG with an overall below average intelligence and the frequent occurrence of MDs, brain abnormalities on MRI and POI in females.
The neurological examination revealed MDs in 47% of patients, which is comparable to the results of Kuiper et al. (2019) but less frequent than reported by Rubio-Agusti et al. (2013). This finding might be explained by the fact that the latter reported on adults only, and it confirms the lower frequency of MDs in children, which has also been reported previously (Kuiper et al., 2019). Interestingly, tremors were more frequently and dystonia less frequently observed in our cohort when compared with previous studies (Rubio-Agusti et al., 2013; Kuiper et al., 2019). Unlike other cohorts, ataxia was not found in our patients.
Patients with a poor IQ and/or MDs demonstrated more brain abnormalities on MRI than patients without MDs and a higher IQ. The finding that the presence of cerebellar atrophy is associated with IQ is in accordance with previous research that suggested that the cerebellum might be involved in cognition (Rapoport et al., 2000; Timmann et al., 2010).
Puberty was induced in 42% of the females aged 12 years and older, and POI was diagnosed in 71%. This frequency is lower when compared with other studies (Waisbren et al., 2012; Coss et al., 2013), possibly because our cohort also includes two homozygous p. Ser135Leu patients without POI.
A low bone mineral density (Z-score ≤ −2 SD) was reported in 14% of the patients, compared with 2.3% in the general population. This finding is in line with previous estimations (van Erven et al., 2017). We found a higher frequency of low bone mineral density in children than in adults, suggesting later maturation as previously reported in CG (Waggoner et al., 1990) and improvement with age. Considering that no fragility fractures were reported in our cohort, the clinical relevance of the higher incidence of low bone mineral density in CG patients remains uncertain.
Our cohort consists of classical, homozygous p. Ser135Leu and NBS-detected variant patients. In the largest group, the classical patients, the patients with identical genotypes and biochemical outcomes demonstrated highly variable clinical outcomes. The frequency of long-term complications did not differ between screened and non-screened classical patients. Thus, early diagnosis and initiation of treatment does not explain the differences in clinical outcome, which is in line with previous research (Fridovich-Keil and Walter, 2008; Hughes et al., 2009; Kuiper et al., 2019). The highly variable clinical outcome spectrum of CG patients and the diversity in genotypes, phenotypes and biochemistry underline the need for predictors of clinical outcome.
In our cohort, Gal-1-P and N-glycans as possible predictors of clinical outcome were investigated. Both lifetime and the most recent Gal-1-P levels of patients with and without long-term complications were not significantly different. This seems to be in contrast to the findings of Yuzyuk et al. (2018) who evaluated lifetime Gal-1-P. In the screened patients in our cohort, we did find significantly lower Gal-1-P levels in patients with a normal IQ. However, this resulted directly from the inclusion of the NBS-detected variant patients with a different biochemical profile. In the cohort reported by Yuzyuk et al., the lifetime Gal-1-P levels of patients with a normal long-term outcome were indeed lower. However, some of these patients are comparable to the variant patients in our cohort with higher residual GALT activities and lower Gal-1-P levels. In our cohort, the most recent Gal-1-P levels were able to discriminate between classical and variant patients, but both the lifetime and the most recent Gal-1-P levels were not able to discriminate between classical patients with a poor and normal clinical outcome.
Previous research reported a stabilization in Gal-1-P within a year (Walter et al., 1999; Yuzyuk et al., 2018). However, the evaluation of lifetime Gal-1-P in our cohort of classical patients demonstrated a significant negative correlation between lifetime Gal-1-P and age. This indicates a more gradual but further decline in Gal-1-P after the age of 12 months. As it is currently unclear if and when Gal-1-P stabilizes, caution is required for its use as prognostic biomarker.
The evaluation of N-glycan profiles demonstrated N-glycan variations in CG patients with significant differences between patients and controls for various N-GPs. In our cohort, adult patients showed more differentiation between N-GPs when compared with controls than paediatric patients. N-GP GP21, containing bisecting glycans, as well as afucosylated bisected neutral glycans, was found to be significantly higher in CG patients when compared with controls in both paediatric and adult patients. This observation conflicts with previous findings (Maratha et al., 2016), where this glycan feature was found to be decreased but does correlate with the more recently published study (Colhoun et al., 2018a) where MGAT3 gene expression is significantly upregulated in CG patients. MGAT3 gene encodes β-1,4-mannosyl-glycoprotein 4-β-N-acetylglucosaminyltransferase, which adds bisecting GlcNAcs to N-glycans. Possible limitations in all studies are the small sample sizes, and the patients from these studies may have different phenotypes including ethnicity and GALT variants. In contrast to previous research, G ratios were not informative in our cohort of patients (Coss et al., 2012; Coss et al., 2014).
In our cohort, both core fucosylated bisected neutral glycans and total bisected glycans were significantly higher in paediatric patients with MDs and there may be link to glycan dysregulation in the central nervous system. No other significant differences in N-glycans between patients with and without long-term complications were found in our cohort, and it remains unclear if (ongoing) galactosylation abnormalities may be able to predict clinical outcome in CG (Coss et al., 2014; Maratha et al., 2016; Stockmann et al., 2016). At this moment, the clinical relevance of the (main) glycan abnormalities found in CG patients is unclear and future research is needed to investigate this further.
Limitations
Even though 56 patients is one of the larger reported CG cohorts, patient numbers are small due to the rarity of the disorder. Furthermore, missing data posed a challenge in the statistical analyses. The use of tremor and dystonia rating scales is hampered because of the lack of control data and age-dependent scales, particularly in the paediatric population where higher scores might represent immature movements due to incomplete brain maturation instead of pathology.
In this study, the fluid-attenuated inversion recovery sequence was used to assess focal white matter abnormalities on MRI. In certain areas of the brain, most notably deep grey matter and infratentorial structures, T2-weighted images are known to have a higher sensitivity. Unfortunately, this sequence was not available in our MRI protocol.
Even though plasma glycans in individuals have been shown to be stable, a high level of variability has been observed within populations and age-, gender-, environmental- and physiological influences have been reported (Gornik et al., 2009; Knezevic et al., 2009). Moreover, the N-glycan profiles consist of multiple variables, which require correction for multiple testing.
Moreover, in this study, both erythrocyte Gal-1-P and serum IgG N-glycans were investigated as these are easy to collect and relatively non-invasive when compared with other tissues. The question remains whether Gal-1-P and N-glycans in the affected tissues such as brain and ovaries would provide different results. As Gal-1-P is trapped into the cells due to its charged nature, the Gal-1-P levels measured in erythrocytes may not represent the Gal-1-P levels in the affected tissues. Also, serum IgG N-glycans may not represent glycosylation patterns in the affected tissues.
The elimination of possible confounders such as age and genetic heterogeneity in the N-glycan analyses and genetic heterogeneity in the Gal-1-P analysis reduced the sample size even further, and therefore, definitive conclusions on the prognostic value of Gal-1-P and N-glycans cannot be drawn.
Strengths
In this study, we included >30% of the total Dutch CG population. The clinical assessment based on the CG guideline enabled a standardized evaluation, and patients with a second (genetic) diagnosis influencing clinical outcome were excluded. A prospective study design was used for the intelligence testing and neurological examination. Moreover, the neurological examination was performed by one (paediatric) neurologist and complemented with tremor and dystonia rating scales.
Since variant patients and p. Ser135Leu homozygous patients differ from classical CG patients in genotypes, biochemistry and clinical outcomes, which may influence the results, analyses, were repeated after the exclusion of the homozygous p. Ser135Leu and variant patients.
Future perspectives
In our cohort, we demonstrated a highly variable clinical outcome with a frequency of long-term complications comparable to previous studies. A long-term complication that has yet received limited attention in the literature is MDs. A standardized neurological evaluation is warranted in all patients, but especially in children with a delay in motor and speech development. Importantly, the impact of the MDs on daily functioning and the possible progressiveness of MDs with age should be investigated.
We hypothesized that the variability in clinical outcome in CG patients is caused by differences in the extent of galactose intoxication and galactosylation abnormalities. However, the results indicate that Gal-1-P and N-glycans were not able to predict clinical outcome in our cohort. This may well be the result of limited power and underlines the need for international collaboration to increase patient numbers in studies evaluating predictors of clinical outcome. Importantly, age variability and stability of Gal-1-P should be further investigated.
A remarkable group in our cohort are the variant patients detected since the introduction of NBS with previously unknown genotypes and different clinical and biochemical phenotypes. Currently, all patients with enzyme activities <15% are treated (Welling et al., 2017a); however the question remains if the variant patients with higher erythrocyte GALT activity (up to 10%) are indeed patients in need of strict dietary treatment, especially since galactose over restriction might be harmful (Knerr et al., 2015). After the first months of life, the variant patients demonstrated mostly normal Gal-1-P levels even after galactose allowance in some. To determine the optimal treatment of this group, which constitutes 14% of patients detected by NBS in the Netherlands (Welling et al., 2017b), further studies of galactose tolerance are warranted.
To develop prognostic biomarkers, improving our understanding of galactose metabolism and the underlying mechanism of long-term complications is crucial. Future studies focusing on galactose metabolism at cell level preferably in the affected tissues and whole body metabolism could provide more insight. In addition, more research into the brain abnormalities on MRI and the association with clinical outcome may be of value. Visible lesions on MRI are relatively scarce in CG patients and may not satisfyingly explain clinical outcome. The use of diffusion-weighted imaging in CG patients has revealed white matter pathology that correlated with (cognitive) outcome (Timmers et al., 2015). The use of quantitative MR techniques may contribute to the investigation of pathology in the normal appearing white matter and grey matter, and therefore, quantitative analysis of the MR images in this study will be performed.
Conclusion
In this study, the deep- phenotyping of a representative cohort of CG patients demonstrated a large variability in clinical outcome. In our cohort, Gal-1-P levels did discriminate between classical and NBS-detected variant patients. However, both Gal-1-P and IgG N-glycans were not associated with long-term complications. The variability in clinical outcome necessitates individual and standardized evaluation of all CG patients. Future studies to increase knowledge and understanding of the pathophysiology of CG and its long-term complications are needed to determine the cause of the broad clinical outcome spectrum in CG.
Funding
This study was supported by grants of Stichting Steun Emma, Stofwisselkracht and The Galactosemia Foundation. R.S. acknowledges funding from the Science Foundation Ireland Starting Investigator Research grant (13/SIRG/2164). The source of funding had no involvement in the study design, data collection, analysis and interpretation, reporting of the results and in the decision to submit the paper for publication.
Competing interests
M.E. received unrestricted research grants from Minoryx, Vertex and SwanBio. C.E.M.H. is involved in premarketing studies with Sanofi, Protalix and Idorsia in the field of lysosomal storage disorders. She reports no conflicts of interest in relation to the current study. A.M.B. received a speakers fee from Nutricia and was a member of an advisory board of Biomarin. All other authors declared no conflict of interest.
Supplementary Material
Glossary
- CG
classical galactosemia
- GALT
galactose-1-phosphate uridylyltransferase
- Gal-1-P
galactose-1-phosphate
- GCA
global cortical atrophy
- GPs
glycan peaks
- IgG
immunoglobulin G
- IQ
intellectual quotient
- NBS
newborn screening
- MD
movement disorder
- POI
primary ovarian insufficiency
References
- Berry GT, Nissim I, Lin Z, Mazur AT, Gibson JB, Segal S.. Endogenous synthesis of galactose in normal men and patients with hereditary galactosaemia. Lancet 1995; 346: 1073–4. [DOI] [PubMed] [Google Scholar]
- Bosch AM. Classical galactosaemia revisited. J Inherit Metab Dis 2006; 29: 516–25. [DOI] [PubMed] [Google Scholar]
- Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J.. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985; 35: 73–7. [DOI] [PubMed] [Google Scholar]
- Castor Electronic Data Capture. Amsterdam, The Netherlands: Ciwit BV; 2018.
- Colhoun HO, Rubio Gozalbo EM, Bosch AM, Knerr I, Dawson C, Brady J, et al. Fertility in classical galactosaemia, a study of N-glycan, hormonal and inflammatory gene interactions. Orphanet J Rare Dis 2018a; 13: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colhoun HO, Treacy EP, MacMahon M, Rudd PM, Fitzgibbon M, O'Flaherty R, et al. Validation of an automated ultraperformance liquid chromatography IgG N-glycan analytical method applicable to classical galactosaemia. Ann Clin Biochem 2018b; 55: 593–603. [DOI] [PubMed] [Google Scholar]
- Coman DJ, Murray DW, Byrne JC, Rudd PM, Bagaglia PM, Doran PD, et al. Galactosemia, a single gene disorder with epigenetic consequences. Pediatr Res 2010; 67: 286–92. [DOI] [PubMed] [Google Scholar]
- Coss KP, Byrne JC, Coman DJ, Adamczyk B, Abrahams JL, Saldova R, et al. IgG N-glycans as potential biomarkers for determining galactose tolerance in classical galactosaemia. Mol Genet Metab 2012; 105: 212–20. [DOI] [PubMed] [Google Scholar]
- Coss KP, Doran PP, Owoeye C, Codd MB, Hamid N, Mayne PD, et al. Classical Galactosaemia in Ireland: incidence, complications and outcomes of treatment. J Inherit Metab Dis 2013; 36: 21–7. [DOI] [PubMed] [Google Scholar]
- Coss KP, Hawkes CP, Adamczyk B, Stockmann H, Crushell E, Saldova R, et al. N-glycan abnormalities in children with galactosemia. J Proteome Res 2014; 13: 385–94. [DOI] [PubMed] [Google Scholar]
- Fahn S, Tolosa E, Marín C.. Clinical rating scale for tremor In Parkinson’s disease and movement disorders. Baltimore: Williams & Wilkins; 1993. [Google Scholar]
- Fridovich-Keil JL, Walter JH.. Galactosaemia chapter 72. In The online metabolic and molecular bases of inherited disease, OMMBID. New York: McGraw Hill; 2008. [Google Scholar]
- Gitzelmann R. Estimation of galactose-I-phosphate in erythrocytes: a rapid and simple enzymatic method. Clin Chim Acta 1969; 26: 313–6. [DOI] [PubMed] [Google Scholar]
- Gornik O, Wagner J, Pucic M, Knezevic A, Redzic I, Lauc G.. Stability of N-glycan profiles in human plasma. Glycobiology 2009; 19: 1547–53. [DOI] [PubMed] [Google Scholar]
- Holton JB. Effects of galactosemia in utero. Eur J Pediatr 1995; 154: S77–81. [DOI] [PubMed] [Google Scholar]
- Hughes J, Ryan S, Lambert D, Geoghegan O, Clark A, Rogers Y, et al. Outcomes of siblings with classical galactosemia. J Pediatr 2009; 154: 721–6. [DOI] [PubMed] [Google Scholar]
- Knerr I, Coss KP, Kratzsch J, Crushell E, Clark A, Doran P, et al. Effects of temporary low-dose galactose supplements in children aged 5-12 y with classical galactosemia: a pilot study. Pediatr Res 2015; 78: 272–9. [DOI] [PubMed] [Google Scholar]
- Knezevic A, Polasek O, Gornik O, Rudan I, Campbell H, Hayward C, et al. Variability, heritability and environmental determinants of human plasma N-glycome. J Proteome Res 2009; 8: 694–701. [DOI] [PubMed] [Google Scholar]
- Kuiper A, Grunewald S, Murphy E, Coenen MA, Eggink H, Zutt R, et al. Movement disorders and nonmotor neuropsychological symptoms in children and adults with classical galactosemia. J Inherit Metab Dis 2019; 42: 451–8. [DOI] [PubMed] [Google Scholar]
- Lai K, Langley SD, Khwaja FW, Schmitt EW, Elsas LJ.. GALT deficiency causes UDP-hexose deficit in human galactosemic cells. Glycobiology 2003; 13: 285–94. [DOI] [PubMed] [Google Scholar]
- Lai K, Langley SD, Singh RH, Dembure PP, Hjelm LN, Elsas LJ. 2nd,. A prevalent mutation for galactosemia among black Americans. J Pediatr 1996; 128: 89–95. [DOI] [PubMed] [Google Scholar]
- Maratha A, Stockmann H, Coss KP, Estela Rubio-Gozalbo M, Knerr I, Fitzgibbon M, et al. Classical galactosaemia: novel insights in IgG N-glycosylation and N-glycan biosynthesis. Eur J Hum Genet 2016; 24: 976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O, et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics 2011; 10: M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucic M, Muzinic A, Novokmet M, Skledar M, Pivac N, Lauc G, et al. Changes in plasma and IgG N-glycome during childhood and adolescence. Glycobiology 2012; 22: 975–82. [DOI] [PubMed] [Google Scholar]
- Rapoport M, van Reekum R, Mayberg H.. The role of the cerebellum in cognition and behavior: a selective review. J Neuropsychiatry Clin Neurosci 2000; 12: 193–8. [DOI] [PubMed] [Google Scholar]
- Robertson A, Singh RH, Guerrero NV, Hundley M, Elsas LJ.. Outcomes analysis of verbal dyspraxia in classic galactosemia. Genet Med 2000; 2: 142–8. [DOI] [PubMed] [Google Scholar]
- Rubio-Agusti I, Carecchio M, Bhatia KP, Kojovic M, Parees I, Chandrashekar HS, et al. Movement disorders in adult patients with classical galactosemia. Mov Disord 2013; 28: 804–10. [DOI] [PubMed] [Google Scholar]
- Rubio-Gozalbo ME, Haskovic M, Bosch AM, Burnyte B, Coelho AI, Cassiman D, et al. The natural history of classic galactosemia: lessons from the GalNet registry. Orphanet J Rare Dis 2019; 14: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin-Bühring Y, Osang M, Ziegler R, Schaub J.. A method for galactose-1-phosphate uridyltransferase assay and the separation of its isozymes by DEAE-cellulose column chromatography. Clin Chim Acta 1976; 70: 371–7. [DOI] [PubMed] [Google Scholar]
- Stockmann H, Adamczyk B, Hayes J, Rudd PM.. Automated, high-throughput IgG-antibody glycoprofiling platform. Anal Chem 2013; 85: 8841–9. [DOI] [PubMed] [Google Scholar]
- Stockmann H, Coss KP, Rubio-Gozalbo ME, Knerr I, Fitzgibbon M, Maratha A, et al. IgG N-glycosylation galactose incorporation ratios for the monitoring of classical galactosaemia. JIMD Rep 2016; 27: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, et al. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex 2010; 46: 845–57. [DOI] [PubMed] [Google Scholar]
- Timmers I, Zhang H, Bastiani M, Jansma BM, Roebroeck A, Rubio-Gozalbo ME.. White matter microstructure pathology in classic galactosemia revealed by neurite orientation dispersion and density imaging. J Inherit Metab Dis 2015; 38: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erven B, Welling L, van Calcar SC, Doulgeraki A, Eyskens F, Gribben J, et al. Bone health in classic galactosemia: systematic review and meta-analysis. JIMD Rep 2017; 35: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner DD, Buist NR, Donnell GN.. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis 1990; 13: 802–18. [DOI] [PubMed] [Google Scholar]
- Waisbren SE, Potter NL, Gordon CM, Green RC, Greenstein P, Gubbels CS, et al. The adult galactosemic phenotype. J Inherit Metab Dis 2012; 35: 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JH, Collins JE, Leonard JV, Chiswick M, Marcovitch H.. Recommendations for the management of galactosaemia. UK Galactosaemia Steering Group. Arch Dis Child 1999; 80: 93–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AL, Singh RH, Kennedy MJ, Elsas LJ.. Verbal dyspraxia and galactosemia. Pediatr Res 2003; 53: 396–402. [DOI] [PubMed] [Google Scholar]
- Welling L, Bernstein LE, Berry GT, Burlina AB, Eyskens F, Gautschi M, et al. ; Network Galactosemia. International clinical guideline for the management of classical galactosemia: diagnosis, treatment, and follow-up. J Inherit Metab Dis 2017a; 40: 171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling L, Boelen A, Derks TG, Schielen PC, de Vries M, Williams M, et al. Nine years of newborn screening for classical galactosemia in the Netherlands: Effectiveness of screening methods, and identification of patients with previously unreported phenotypes. Mol Genet Metab 2017b; 120: 223–8. [DOI] [PubMed] [Google Scholar]
- Welsink-Karssies MM, Oostrom KJ, Hermans ME, Hollak CEM, Janssen MCH, Langendonk JG, et al. Classical Galactosemia: neuropsychological and psychosocial functioning beyound intellectual abilities. Orphanet J Rare Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzyuk T, Viau K, Andrews A, Pasquali M, Longo N.. Biochemical changes and clinical outcomes in 34 patients with classic galactosemia. J Inherit Metab Dis 2018; 41: 197–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author.